Abstract
Background
Representative prognostic data by clinical characteristics for lung cancer is not yet available in China. This study aimed to calculate the survival of lung cancer patients with different pathological evaluations, explore their predictive effects and provide information for prognosis improvement.
Methods
In this multicenter cohort study, primary lung cancer patients diagnosed in 17 hospitals at three distinct levels in China between 2011–2013 were enrolled and followed up till 2020. Overall survival and lung cancer specific survival were calculated by Kaplan-Meier method. Cox proportional hazards model was applied to assess the effects of predictors of lung cancer survival.
Results
Of all the 7,311 patients, the 5-year overall and lung cancer specific survival rates were 37.0% and 41.6%, respectively. For lung cancer patients at stages I, II, III, and IV, the 5-year overall survival rates were 76.9%, 56.1%, 32.6%, and 21.4%, respectively; the lung cancer specific survival rates were 82.3%, 59.7%, 37.2%, and 26.4%, respectively. Differences of survival for each stage remained significant between histological classifications (P<0.01). The 5-year overall survival rates for patients with squamous cell carcinoma, adenocarcinoma (AC), and small cell carcinoma were 36.9%, 43.3% and 27.9%, respectively; the corresponding disease-specific rates were 41.5%, 48.6% and 31.0%, respectively. Such differences were non-statistically significant at advanced stages (P=0.09). After multivariate adjustments, stage and classification remained independent predictors for the survival of lung cancer.
Conclusions
The prognosis of lung cancer varied with the pathological stages and histological classifications, and had room for improvement. Stage was the strongest predictor, so efforts on early detection and treatment are needed.
Keywords: Lung neoplasms, neoplasm staging, histological classification, survival analysis, multicenter study
Introduction
Lung cancer ranks first in both incidence and mortality among all cancers in China (1), and has caused heavy disease and economic burden on the Chinese population (2,3). The prognosis of lung cancer patients was poor, with the 5-year survival rates less than 20% (4-6). By reflecting the development of tumor, pathological stage and histological classification were considered as important tools for physicians to make comprehensive decision and to predict the patients’ prognosis (7-9). The 5-year survival rate serves as a key indicator for evaluating the severity of disease, as well as the local medical level (6,10,11). Thus, monitoring the survival of patients with different clinical characteristics will be able to evaluate the prognosis of local cancer patients, provide reference for health policy makers to understand the disease structure of local lung cancer patients and assess the effectiveness of current measures on cancer prevention and control.
Up to now, there have been several reports on the survival of lung cancer patients in Chinese population (12-17) but they were mostly single-center studies with poor representativeness. A survival analysis based on 17 cancer registries in China with long-term follow-up (6) assessed the 5-year survival rates and the trends for all cancer types in recent years, but it lacked the specific survival in pathological stages and histological classifications. Hence, there is still a lack of large-scale survival analysis by pathological evaluation for Chinese lung cancer patients.
The purpose of this study was to describe the survival of lung cancer patients in China based on data from 17 hospitals, as well as to compare the 5-year survival rates among patients with different pathological stages and histological classifications, and to investigate the possible factors of the prognosis among lung cancer patients. The findings in this study may help to provide basic data for evaluating the prognosis of Chinese cancer patients and for cancer control strategies. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-240/rc) (18).
Methods
Study design and study population
This multi-center, prospective cohort study was conducted in 17 hospitals from 6 provinces of China (3 in North China: Beijing, Henan, Hebei provinces; and 3 in South China: Guangdong, Zhejiang, and Hubei provinces), covering 6 provincial hospitals, 7 municipal hospitals, and 4 county hospitals. Considering that cancer patients tended to visit specialized or large general hospitals for treatment (19), we chose the local largest specialized hospitals and general hospitals and also selected corresponding county-level hospitals to cover patients first diagnosed with lung cancer as many as possible.
All the patients met the following criteria were included: (I) diagnosed with primary lung cancer (according to the third edition of the International Classification of Diseases for Oncology topography, ICD-10: C34.0-C34.9) in the selected hospitals; (II) the date of first diagnosis was between January 1st, 2011 and December 31st, 2013; (III) local residents. The patients were excluded if meeting any of the exclusion criteria: (I) diagnosed with multiple primary cancer or metastatic cancer; (II) having received any treatment before admission to the selected hospitals; (III) incomplete requiring information for defining the survival status and survival time. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Cancer Hospital, Chinese Academy of Medical Sciences (No. NCC-006288) and informed consent was taken from all the patients.
Clinical data collection and definition
For all lung cancer patients, personal and clinical information were extracted from medical records of the involved hospitals by trained investigators, including sociodemographic information (e.g., age, sex), investigation on risk factors (smoking, alcohol assumption, related disease history, family history of lung cancer), and clinical information (pathological stage, histological classification, lesion site, the size and extent of the primary tumor, lymph node involvement, status of distant metastasis, and treatment). This process was carried out through health information system (HIS) using EpiData software (Version 3.1).
Individual who had smoked ≥100 cigarettes in lifetime but had quitted smoking was defined as a former smoker, and drinkers were individuals who had alcohol assumption ≥1 time per week. Participants were defined as having related disease history if they were diagnosed with respiratory diseases, such as tuberculosis, chronic bronchitis, emphysema, asthma, and silicosis/pneumoconiosis. Having family history refers to participants with first-degree or second-degree relatives diagnosed with any type of lung cancer. Body Mass Index (BMI) was calculated based on the height and weight values, and was categorized into 3 groups according to the World Health Organization (WHO) standard, which were underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), and overweight (≥25 kg/m2) (20). Histological classification was confirmed by pathological examination and classified according to the histological classification criteria of lung and pleural tumors issued by the WHO in 2015 (21). Cancer stages were ascertained based on the 7th American Joint Committee on Cancer (AJCC) TNM classification (22). Treatments included but were not limited to surgery, chemotherapy, radiotherapy and targeted therapy.
Follow-up of the enrolled lung cancer patients
All patients were followed up to December 31st, 2020. The vital status of each patient was confirmed annually, and detailed information on all deaths was collected, including the death date, primary cause of death and its ICD-10 coding. If patients cannot be found through the medical records, data was obtained by referring to the national cancer registry system and death cause registry system. Finally, the survival status and survival time of each patient were calculated. Survival time referred to the span between the diagnosis date and the date of last contact or deaths.
Statistical analysis
Baseline characteristics of involved patients were described by subgroups of pathological stage and histological classification. Also, associations of these pathological evaluations with above-mentioned features were examined using Chi square (or Fisher’s exact test), respectively. The 5-year cumulative survival rates for each pathological stage and histological classification were calculated by Kaplan-Meier method, and the differences between the survival curves were tested by log rank test. A multivariable Cox proportional hazards regression model was applied to explore the predictive effects of the two key variables on the prognosis of lung cancer. Before all the statistical analysis, missing data were dealt with depending on its proportion. For variables containing less than 15% of missing values, analysis was conducted after exclusion of patients with incomplete information. For variables with large but acceptable missing data, results were reported after multiple imputation (with 5 imputations) by the chained equations method (MICE). Furthermore, two sensitivity analyses were conducted, by treating missing values through excluding patients with a less than 6- and 12-month follow-up, to verify the reliability of primary findings in this article. All statistical analysis was conducted using R software (Version 4.1.0). P<0.05 (two-sided) was considered statistically significant.
Results
Characteristics of enrolled lung cancer patients
A total of 7,419 participants were diagnosed with lung cancer in the involved hospitals between 2011 and 2013. After excluding patients with incomplete follow-up information (n=86), logical error (n=1), duplicate records (n=10), or a diagnosis of stage 0 (n=11), 7,311 participants were included for the present analysis (Figure 1). The median age at diagnosis of all the patients was 60 with a range of 20 to 96. Males, smokers (including current smokers and former smokers), drinkers, and overweight participants accounted for 67.2%, 54.2%, 31.2%, and 20.6%, respectively. As for health status, there were 1,654 (22.6%) patients having lung-related diseases and 283 (3.9%) with relatives diagnosed with lung cancer.
Figure 1.
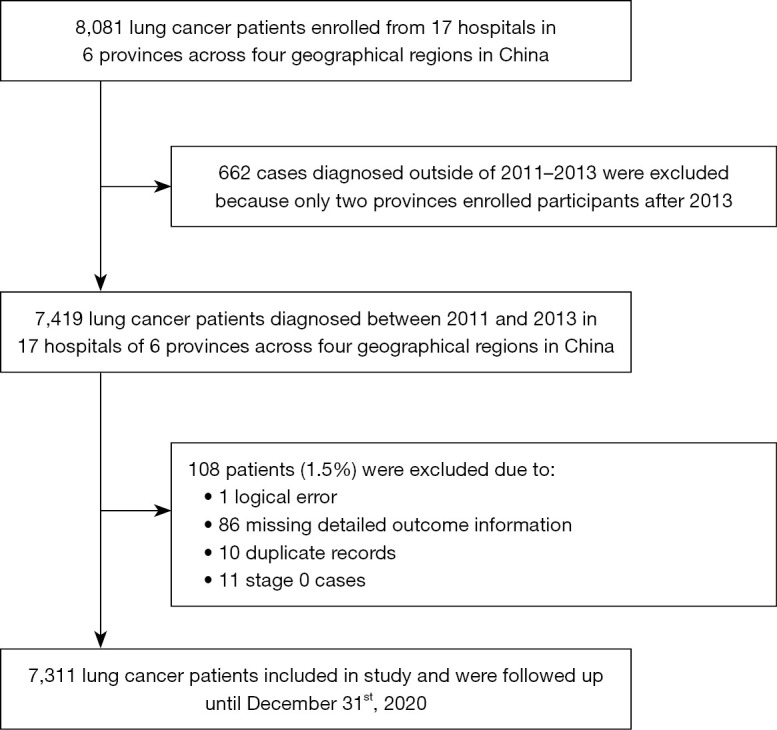
Flowchart of the study population.
Among those reporting specific pathological stage, there were 1,067 (19.2%), 374 (6.7%), 1,431 (25.8%), and 2,674 (48.2%) lung cancer patients diagnosed with stage I, stage II, stage III and stage IV, respectively. Moreover, as is shown in Table 1, in comparison with patients in the early stages (stage I and stage II), those in advanced stages (stage III and stage IV) tended to be males, current smokers, alcohol consumers, underweight, participants with a diagnosis of small cell lung carcinoma (SCLC), or not receiving treatment. Age, disease history, family history, and lesion sites also varied in different pathological stages (P<0.01).
Table 1. Baseline characteristics of 7,311 enrolled lung cancer patients by pathological stage.
| Characteristic | Total† | Pathological stage | P value§ | ||||
|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | Unknown‡ | |||
| Age | <0.01 | ||||||
| <60 years | 3,401 (46.5) | 459 (43.0) | 166 (44.4) | 680 (47.5) | 1,346 (50.3) | 750 (42.5) | |
| ≥60 years | 3,910 (53.5) | 608 (57.0) | 208 (55.6) | 751 (52.5) | 1,328 (49.7) | 1,015 (57.5) | |
| Sex | <0.01 | ||||||
| Male | 4,912 (67.2) | 582 (54.5) | 256 (68.4) | 1,052 (73.5) | 1,751 (65.5) | 1,271 (72.0) | |
| Female | 2,399 (32.8) | 485 (45.5) | 118 (31.6) | 379 (26.5) | 923 (34.5) | 494 (28.0) | |
| Smoking | <0.01 | ||||||
| Never-smoker | 3,283 (44.9) | 624 (58.5) | 150 (40.1) | 554 (38.7) | 1,249 (46.7) | 706 (40.0) | |
| Current-smoker | 3,148 (43.1) | 330 (30.9) | 166 (44.4) | 696 (48.6) | 1,118 (41.8) | 838 (47.5) | |
| Former-smoker | 812 (11.1) | 108 (10.1) | 54 (14.4) | 175 (12.2) | 275 (10.3) | 200 (11.3) | |
| Unknown‡ | 68 (0.9) | 5 (0.5) | 4 (1.1) | 6 (0.4) | 32 (1.2) | 21 (1.2) | |
| Alcohol assumption | <0.01 | ||||||
| No | 4,968 (68.0) | 797 (74.7) | 241 (64.4) | 891 (62.3) | 1,861 (69.6) | 1,178 (66.7) | |
| Yes | 2,281 (31.2) | 259 (24.3) | 127 (34.0) | 533 (37.2) | 790 (29.5) | 572 (32.4) | |
| Unknown‡ | 62 (0.8) | 11 (1.0) | 6 (1.6) | 7 (0.5) | 23 (0.9) | 15 (0.8) | |
| Disease history | <0.01 | ||||||
| No | 5,547 (75.9) | 648 (60.7) | 278 (74.3) | 1,115 (77.9) | 2,169 (81.1) | 1,337 (75.8) | |
| Yes¶ | 1,654 (22.6) | 411 (38.5) | 93 (24.9) | 301 (21.0) | 470 (17.6) | 379 (21.5) | |
| Unknown‡ | 110 (1.5) | 8 (0.7) | 3 (0.8) | 15 (1.0) | 35 (1.3) | 49 (2.8) | |
| Family history | <0.01 | ||||||
| No | 6,553 (89.6) | 916 (85.8) | 325 (86.9) | 1,222 (85.4) | 2,432 (90.9) | 1,658 (93.9) | |
| Yes†† | 283 (3.9) | 77 (7.2) | 13 (3.5) | 52 (3.6) | 87 (3.3) | 54 (3.1) | |
| Unknown‡ | 475 (6.5) | 74 (6.9) | 36 (9.6) | 157 (11.0) | 155 (5.8) | 53 (3.0) | |
| Body mass index‡‡ | <0.01 | ||||||
| Underweight | 342 (4.7) | 28 (2.6) | 15 (4.0) | 75 (5.2) | 136 (5.1) | 88 (5.0) | |
| Normal | 3,372 (46.1) | 398 (37.3) | 163 (43.6) | 602 (42.1) | 1,242 (46.4) | 967 (54.8) | |
| Overweight | 1,508 (20.6) | 280 (26.2) | 94 (25.1) | 289 (20.2) | 440 (16.5) | 405 (22.9) | |
| Unknown‡ | 2,089 (28.6) | 361 (33.8) | 102 (27.3) | 465 (32.5) | 856 (32.0) | 305 (17.3) | |
| Histological classification | <0.01 | ||||||
| Squamous cell carcinoma | 1,579 (21.6) | 201 (18.8) | 130 (34.8) | 430 (30.0) | 388 (14.5) | 430 (24.4) | |
| Adenocarcinoma | 3,335 (45.6) | 704 (66.0) | 183 (48.9) | 558 (39.0) | 1,344 (50.3) | 546 (30.9) | |
| Small cell carcinoma | 938 (12.8) | 51 (4.8) | 20 (5.3) | 212 (14.8) | 310 (11.6) | 345 (19.5) | |
| Others§§ | 518 (7.1) | 71 (6.7) | 32 (8.6) | 115 (8.0) | 178 (6.7) | 122 (6.9) | |
| Unknown‡ | 941 (12.9) | 40 (3.7) | 9 (2.4) | 116 (8.1) | 454 (17.0) | 322 (18.2) | |
| Lesion site | <0.01 | ||||||
| Right | 3,971 (54.3) | 636 (59.6) | 200 (53.5) | 800 (55.9) | 1,414 (52.9) | 921 (52.2) | |
| Left | 3,184 (43.6) | 428 (40.1) | 168 (44.9) | 625 (43.7) | 1,192 (44.6) | 771 (43.7) | |
| Both | 49 (0.7) | 1 (0.1) | 5 (1.3) | 5 (0.3) | 26 (1.0) | 12 (0.7) | |
| Unknown‡ | 107 (1.5) | 2 (0.2) | 1 (0.3) | 1 (0.1) | 42 (1.6) | 61 (3.5) | |
| Treatment | <0.01 | ||||||
| No | 954 (13.0) | 42 (3.9) | 18 (4.8) | 158 (11.0) | 365 (13.6) | 371 (21.0) | |
| Yes¶¶ | 6,307 (86.3) | 1,023 (95.9) | 356 (95.2) | 1,268 (88.6) | 2,289 (85.6) | 1,371 (77.7) | |
| Unknown‡ | 50 (0.7) | 2 (0.2) | 0 (0.0) | 5 (0.3) | 20 (0.7) | 23 (1.3) | |
| Sample size | 7,311 | 1,067 | 374 | 1,431 | 2,674 | 1,765 | |
Categorical data are shown as n (%). †, the total proportion may not be 100 due to the rounding; ‡, unknown data were not included in the statistical tests; §, P values were calculated by Chi square/Fisher exact test; ¶, referring to lung diseases, such as tuberculosis, chronic bronchitis, emphysema, asthma, and silicosis/pneumoconiosis; ††, referring to participants with first-degree or second-degree relatives diagnosed with any type of lung cancer; ‡‡, body mass index was calculated based on the height and weight values, and was categorized in to 3 groups according to the WHO standard, which were underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), and overweight (≥25 kg/m2); §§, including large cell carcinoma, carcinoid, sarcomatoid carcinoma, and adenosquamous carcinoma; ¶¶, Including surgery, chemotherapy, radiotherapy and targeted therapy.
Among the patients with definite histological classification, adenocarcinoma (AC, 52.4%) was the most common type, followed by squamous cell carcinoma (SCC, 24.8%), SCLC (14.7%) and other types of lung cancer (8.1%). Except for the family history (P=0.55), the distributions of the above-mentioned characteristics varied in subgroups of histological classification among enrolled lung cancer patients (P<0.01; Table S1).
OS and LCSS by pathological stage and histological classification
During a median of 100.5 months of follow-up, we documented 4,842 all-cause deaths till December 31st, 2020, among which 4,185 patients died from lung cancer. There were 912 participants lost to follow-up, and the follow-up rate was 87.5%. The median survival time of all the included participants was 32.1 months [95% confidence interval (95% CI): 30.6–33.4 months]. Of the 7,311 patients, the 5-year OS rate was 37.0% (95% CI: 35.9–38.1%), and the 5-year LCSS rate was 41.6% (95% CI: 40.5–42.8%).
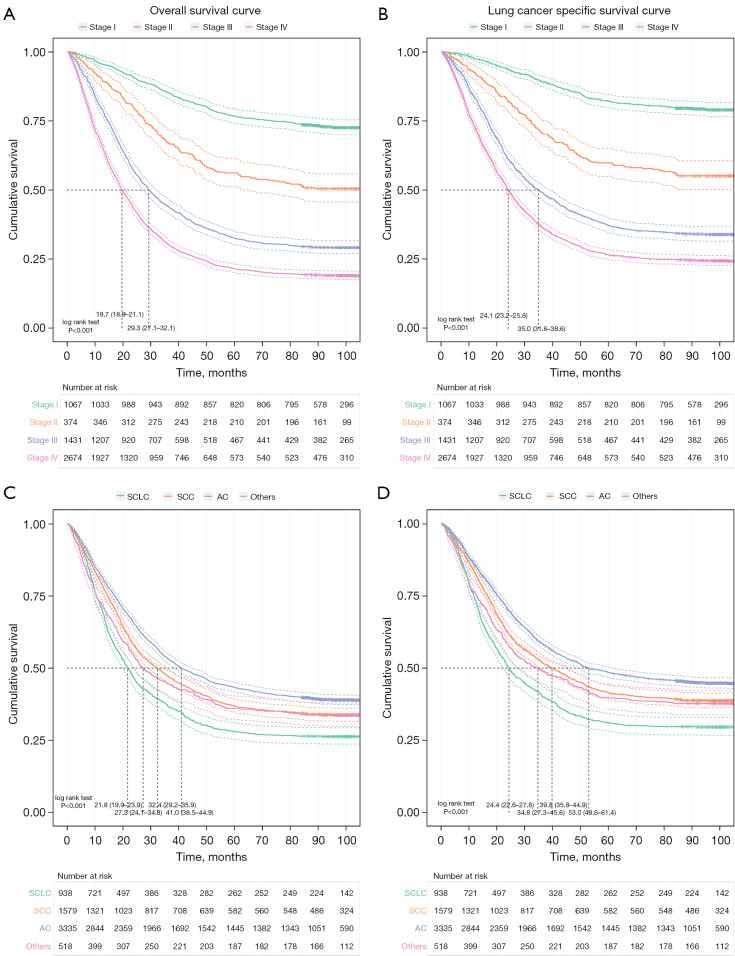
As is shown in Figure 2, the Kaplan-Meier curves among pathological stages were different significantly (P<0.01). For patients diagnosed with stage I, II, III, and IV lung cancer, the 5-year OS rates were 76.9% (95% CI: 74.4–79.5%), 56.1% (95% CI: 51.3–61.4%), 32.6% (95% CI: 30.2–35.1%), and 21.4% (95% CI: 19.9–23.0%), respectively; the 5-year LCSS rates were 82.3% (95% CI: 79.9–84.6%), 59.7% (95% CI: 54.9–65.0%), 37.2% (95% CI: 34.7–40.0%), and 26.4% (95% CI: 24.7–28.3%), respectively.
Figure 2.
Survival curves of lung cancer patients with specific diagnosis of pathological stage/histological classification. (A) Overall survival curves of patients by pathological stage. (B) Lung cancer specific survival curves of patients by pathological stage. (C) Overall survival curves of patients by histological classification. (D) Lung cancer specific survival curves of patients by histological classification. AC, adenocarcinoma; SCC, squamous cell carcinoma; SCLC, small cell lung carcinoma.
There were significant variations between the Kaplan-Meier curves of each histological classification (P<0.01). For patients diagnosed with SCC, AC, SCLC and other types of lung cancer, the 5-year OS rates were 36.9% (95% CI: 34.6–39.3%), 43.3% (95% CI: 41.7–45.0%), 27.9% (95% CI: 25.2–31.0%), and 36.1% (95% CI: 32.2–40.5%), respectively; the 5-year LCSS rates were 41.5% (95% CI: 39.0–44.1%), 48.6% (95% CI: 46.8–50.4%), 31.0% (95% CI: 28.0–34.2%), and 39.9% (95% CI: 35.7–44.5%), respectively.
Among patients with each classification of lung cancer, the prognosis at early stages was better than at advanced stages (P<0.01, Table 2). Except for the 5-year OS rates at advanced stages, the survival rates between different histological classifications varied significantly in the subgroups (P<0.05). Besides, for both the 5-year OS and LCSS rates, there were also significant differences between patients with other clinical characteristics (Detailed information was presented in Table 3).
Table 2. Subgroup survival analysis for lung cancer patients with early- and advanced stages.
| Stages | Sample sizes | OS | LCSS | |||||
|---|---|---|---|---|---|---|---|---|
| All death cases | 5-year OS rates† | P value‡ | Lung cancer death cases | 5-year LCSS rates† | P value‡ | |||
| Early Stages¶ | 1,392 | 444 | <0.01 | 348 | <0.01 | |||
| Small cell carcinoma | 71 | 39 | 47.9 (37.6–61.0) | 33 | 51.9 (41.2–65.5) | |||
| Squamous cell carcinoma | 331 | 151 | 60.4 (55.4–65.9) | 116 | 66.6 (61.5–72.0) | |||
| Adenocarcinoma | 887 | 217 | 79.0 (76.4–81.8) | 166 | 83.5 (81.1–86.0) | |||
| Others†† | 103 | 37 | 69.9 (61.6–79.3) | 33 | 73.1 (64.9–82.3) | |||
| Advanced Stages ‡‡ | 3,535 | 2725 | 0.09 | 2,306 | <0.05 | |||
| Small cell carcinoma | 522 | 404 | 24.3 (20.9–28.3) | 350 | 28.7 (24.8–33.1) | |||
| Squamous cell carcinoma | 818 | 604 | 28.0 (25.1–31.2) | 515 | 33.1 (29.8–36.6) | |||
| Adenocarcinoma | 1,902 | 1496 | 25.6 (23.7–27.6) | 1,249 | 30.7 (28.6–33.0) | |||
| Others | 293 | 221 | 25.6 (21.1–31.1) | 192 | 29.6 (24.6–35.7) | |||
| Overall | 4,927§§ | 3169 | 37.0 (35.9–38.1) | – | 2,654 | 41.6 (40.5–42.8) | – | |
†, survival rates were calculated by Kaplan-Meier method and were shown as rate (%) and its 95% CIs; ‡, P values were calculated by Log Rank test; ¶, including stage I and stage II; ††, including large cell carcinoma, carcinoid, sarcomatoid carcinoma, and adenosquamous carcinoma; ‡‡, including stage III and stage IV; §§, patient with specific information on both pathological stage and histological classification. OS, overall survival; LCSS, lung cancer specific survival; CIs, confidence intervals.
Table 3. Univariate analysis on associations between considered variables and survival status of lung cancer patients.
| Variables | Person-years | OS | LCSS | |||||
|---|---|---|---|---|---|---|---|---|
| 5-year OS rate† | All death cases | P value‡ | 5-year LCSS rate† | Lung cancer death cases | P value‡ | |||
| Age | <0.01 | <0.01 | ||||||
| <60 years | 176,909.9 | 40.2 (38.6–41.9) | 2,135 | 44.5 (42.8–46.3) | 1,865 | |||
| ≥60 years | 179,394.0 | 34.1 (32.7–35.7) | 2,707 | 39.0 (37.5–40.7) | 2,320 | |||
| Sex | <0.01 | <0.01 | ||||||
| Male | 227,239.9 | 34.2 (32.9–35.6) | 3,373 | 38.9 (37.5–40.3) | 2,912 | |||
| Female | 129,064.0 | 42.6 (40.7–44.6) | 1,469 | 47.1 (45.1–49.2) | 1,273 | |||
| Smoking | <0.01 | <0.01 | ||||||
| Never-smoker | 171,811.2 | 41.2 (39.6–43.0) | 2,043 | 46.9 (45.2–48.7) | 1,708 | |||
| Current-smoker | 146,038.2 | 34.2 (32.6–35.9) | 2,155 | 37.0 (35.3–38.7) | 1,978 | |||
| Former-smoker | 35,534.8 | 30.8 (27.8–34.1) | 595 | 39.0 (35.5–42.9) | 453 | |||
| Alcohol assumption | <0.01 | <0.01 | ||||||
| No | 246,121.2 | 38.0 (36.7–39.4) | 3,242 | 43.4 (42.0–44.8) | 2,746 | |||
| Yes | 107,441.1 | 34.9 (33–36.9) | 1,554 | 38.1 (36.1–40.2) | 1,394 | |||
| Disease history | 0.1 | 0.3 | ||||||
| No | 270207.1 | 36.1 (34.8–37.4) | 3,708 | 40.8 (39.5–42.2) | 3,189 | |||
| Yes§ | 81363.5 | 40.4 (38.1–42.8) | 1,055 | 45.1 (42.7–47.7) | 920 | |||
| Family history | <0.01 | 0.08 | ||||||
| No | 315,133.9 | 36.6 (35.5–37.8) | 4,369 | 41.7 (40.4–42.9) | 3,734 | |||
| Yes¶ | 15,075.9 | 43.5 (38.1–49.6) | 169 | 46.6 (41.0–52.9) | 154 | |||
| Body mass index†† | <0.01 | <0.01 | ||||||
| Underweight | 14,755.4 | 30.4 (25.9–35.7) | 244 | 31.3 (26.7–36.7) | 237 | |||
| Normal | 161,445.8 | 36.0 (34.4–37.7) | 2,263 | 37.4 (35.8–39.1) | 2,160 | |||
| Overweight | 79,770.3 | 42.6 (40.2–45.2) | 929 | 43.7 (41.3–46.3) | 885 | |||
| Lesion site | 0.04 | 0.03 | ||||||
| Right | 198,403.0 | 38.5 (37.0–40.1) | 2,565 | 43.2 (41.6–44.8) | 2,215 | |||
| Left | 152,796.7 | 35.8 (34.2–37.5) | 2,149 | 40.5 (38.8–42.3) | 1,855 | |||
| Both | 2,205.1 | 30.6 (20.1–46.7) | 36 | 32.5 (21.5–49.1) | 34 | |||
| Pathological stage | <0.01 | <0.01 | ||||||
| Stage I | 85,813.9 | 76.9 (74.4–79.5) | 287 | 82.3 (79.9–84.6) | 211 | |||
| Stage II | 24,711.0 | 56.1 (51.3–61.4) | 185 | 59.7 (54.9–65.0) | 161 | |||
| Stage III | 67,019.1 | 32.6 (30.2–35.1) | 1,013 | 37.2 (34.7–40.0) | 879 | |||
| Stage IV | 94,143.8 | 21.4 (19.9–23.0) | 2,166 | 26.4 (24.7–28.3) | 1,804 | |||
| Histological classification | <0.01 | <0.01 | ||||||
| Squamous cell carcinoma | 78,571.7 | 36.9 (34.6–39.3) | 1,046 | 41.5 (39.0–44.1) | 906 | |||
| Adenocarcinoma | 179,832.5 | 43.3 (41.7–45.0) | 2,029 | 48.6 (46.8–50.4) | 1,716 | |||
| Small cell carcinoma | 38,329.6 | 27.9 (25.2–31.0) | 692 | 31.0 (28.0–34.2) | 626 | |||
| Others‡‡ | 24,842.3 | 36.1 (32.2–40.5) | 344 | 39.9 (35.7–44.5) | 304 | |||
| Treatment | <0.01 | <0.01 | ||||||
| No | 37,202.7 | 26.2 (23.6–29.1) | 719 | 28.2 (25.5–31.3) | 672 | |||
| Yes§§ | 317,004.6 | 38.7 (37.5–39.9) | 4,087 | 43.8 (42.5–45.1) | 3,479 | |||
| Grade of the hospital | 0.04 | <0.01 | ||||||
| Provincial hospital | 316,787.4 | 39.4 (38.2–40.6) | 3,944 | 40.5 (39.2–41.7) | 3,792 | |||
| Municipal hospital | 34,900.3 | 27.4 (24.7–30.5) | 702 | 60.7 (56.8–64.9) | 272 | |||
| County hospital | 4,616.2 | 8.1 (5.1–12.7) | 196 | 19.1 (13.0–27.9) | 121 | |||
| Area of the hospital | 0.02 | <0.01 | ||||||
| North China | 222,431.3 | 38.3 (36.9–39.8) | 2,886 | 39.7 (38.3–41.2) | 2,747 | |||
| South China | 133,872.5 | 34.8 (33.1–36.6) | 1,956 | 45.1 (43.2–47.2) | 1,438 | |||
†, survival rates were calculated by Kaplan-Meier method and were shown as rate (%) and its 95% CIs; ‡, P values were calculated by Log Rank test; §, referring to lung diseases, such as tuberculosis, chronic bronchitis, emphysema, asthma, and silicosis/pneumoconiosis; ¶, referring to participants with first-degree or second-degree relatives diagnosed with any type of lung cancer; ††, BMI was calculated based on the height and weight values, and was categorized in to 3 groups according to the WHO standard, which were underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), and overweight (≥25 kg/m2); ‡‡, including large cell carcinoma, carcinoid, sarcomatoid carcinoma, and adenosquamous carcinoma. §§, Including Surgery, chemotherapy, radiotherapy and targeted therapy. OS, overall survival; LCSS, lung cancer specific survival; CIs, confidence interval.
Multivariate Cox regression analysis
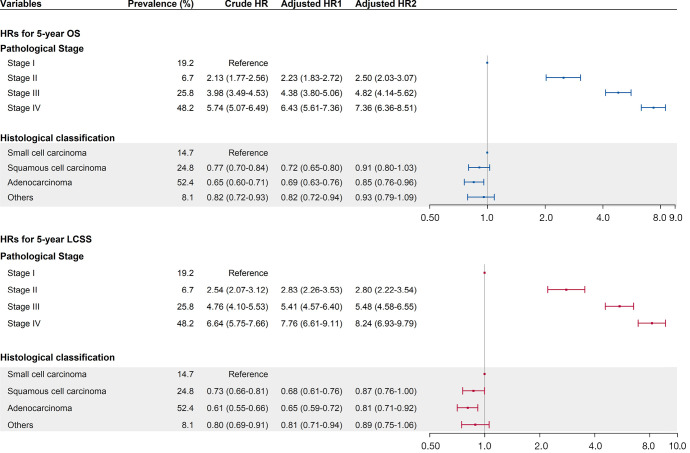
Multivariate cox regression analyses for OS and LCCS are shown in Figure 3. After adjustments by demographic characteristics, lifestyles and information on diagnosis and treatment, the pathological stage proved to be the strongest factor in predicting the risk level of mortality among all the considered variables [hazard ratios (HRs) of other variables were not shown]. Compared with the stage I patients, patients with a diagnosis of stage II, stage III and stage IV lung cancer had a 1.50-fold (HR =2.50, 95% CI: 2.03–3.07), 3.82-fold (HR =4.82, 95% CI: 4.14–5.62), and 6.36-fold (HR =7.36, 95% CI: 6.36–8.51) increased risk of all-cause death, respectively. Similar associations were also observed for LCSS. The risks of LCSS for more advanced stages were higher than stage I, with HR values of 2.80 (95% CI: 2.22–3.54), 5.48 (95% CI: 4.58–6.55), and 8.24 (95% CI: 6.93–9.79), respectively.
Figure 3.
Cox regression analysis on associations between clinical characteristics and the survival of enrolled lung cancer patients. [Notes: The forest plot refers to the HRs in the cox regression analysis adjusted by all the considered variables (adjusted HR2). Adjusted HR1: Cox proportional hazards models were adjusted by age at diagnosis, sex, smoking, alcohol assumption, history of respiratory diseases, family history of lung cancer, and BMI. Adjusted HR2: Cox proportional hazards models were further adjusted by pathological stage (for histological classification)/histological classification (for pathological stage), lesion site, treatment, grade of the hospital and location of the hospital. LCSS, lung cancer specific survival; OS, overall survival; HR, hazard ratio.
In comparison with SCLC, the risks of OS and LCSS decreased for non-SCLCs. Such association remained in different multivariable cox models. Specifically, SCC and AC faced a 0.13-fold (HR =0.87, 95% CI: 0.76–1.00) and 0.19-fold (HR =0.81, 95% CI: 0.71–0.92) declined risk of lung cancer specific death, respectively. Meanwhile, the risks of OS for AC were lower than SCLC, with an HR of 0.85 (95% CI: 0.76–0.96), while such associations were not-statistically significant for SCC. In addition, age and smoking were also identified as independent prognostic factors for OS and LCSS of lung cancer patients.
Sensitivity analysis
If the patients being followed up less than six or twelve months were excluded, for both pathological stages and histological classifications, the values of 5-year OS and LCSS rates did not vary materially from findings of the primary analysis among each group, which suggests the robustness of the reported results in this study (See details in Figure S1).
Discussion
To our knowledge, this is the first large-scale multicenter hospital-based study reporting survival of primary lung cancer among Chinese patients with different pathological evaluations. Although several published studies have reported the survival of each pathological stage (16,23-27) for Chinese patients with lung cancer, they were based on data from a single region or target on specific population. Also, studies on the prognosis of each histological classification were limited, and most were focused on one type of lung cancer (28,29). In this work, the estimated overall 5-year observed survival rates of 7,311 lung cancer patients were 37.0% and 41.6%, respectively. The prognosis of patients diagnosed with different cancer stages and classifications varied significantly, with the 5-year observed survival rates increasing with lower stages and a diagnosis with non-SCLCs. In addition, aging, smoking, advanced stages and SCLC were considered independent risk factors for all-cause or cause-specific mortality based on multivariate analysis.
This study showed higher 5-year survival rates and longer median survival time, in comparison with previously reported data on Chinese patients in the same period (13,14,17,30). There may be several reasons for such findings. First, among the enrolled patients without unclear clinical information, those who smoked without quitting, diagnosed with advanced stages, and had a pathological diagnosis of SCLC accounted for 43.5%, 74.0% and 14.7%, which were less than the corresponding values in other studies. The smaller proportion of risk factors for prognosis might have led to the increase in survival rates reported in this study. Second, a systematic survey (2) on death causes conducted in 31 provinces in mainland China demonstrated distinct regional discrepancies in cause-specific mortality across provinces. For all the covered provinces, the mortality from lung cancer was no higher than the national level, which was consistent with the better survival found in this study. It might be because Beijing, Zhejiang, and Guangdong provinces were in the high tier of economic development across China, which were equipped with the soundest medical insurance system and more well-educated local residents (31). Third, compared with data obtained from cancer registries (12,14,32-34), hospital-sourced studies tended to overestimate the overall survival rates because there was an inevitable selection bias in hospital-based study where the diagnostic and treatment rates were significantly higher than in the general population. For example, those who went to the hospital were more likely to have a stronger willingness to treatment and capacity to pay, and thus to receive better medical consultation and rehabilitation guidance, which emphasized the significance of active treatment. In addition, some studies reported higher survival in other regions (15,35,36), and the leading reason might be the decreases in the proportions of advanced lung cancer (stage III and stage IV).
According to cancer statistics from the third cycle of Global surveillance of trends in cancer survival program (CONCORD-3), 5-year age-standardized net survival among most countries was in the range of 10–19%, while it peaked in Japanese patients diagnosed with lung cancer during 2010–2014 (32.9%) (37). The corresponding rate was 28.1% for the Chinese-American, based on 18 registries of the Surveillance, Epidemiology, and End Results Program (SEER) between 2011 and 2017 (5). Nevertheless, direct comparisons of survival of lung cancer patients among countries should be treated with caution considering the differences in the applied indexes. Five-year OS rates by pathologic stage, reported by the International Association for the Study of Lung Cancer (IASLC) based on 94,708 cases from 16 countries (38), were close to the results in this study. The former rates were adjusted to simulate database from registries, indicating that there was still a gap between China and the developed regions since the proportion of receiving treatment was much higher than the general population.
Although the overall prognosis of lung cancer patients remained unsatisfactory in China, patients diagnosed with early stages had a relatively acceptable survival. Such a trend has been observed by some studies conducted in China, but most focused on the specific types of lung cancer (23), young patients (24), or single-centered studies (25), which suggests a lack of evidence based on a representative population in China. Similar results were also reported by national surveys in developed countries (4,5,39), demonstrating an over 50% improvement in the 5-year age-standardized survival from Stage IV to Stage I. Given that pathological stage was the strongest factor for the prediction of prognosis among all the considered variables, health-policy makers can alleviate the death burden caused by lung cancer through multiple efforts on raising the early detection rate. For example, given that the proportion of patients with a diagnosis of stage I lung cancer in the LDCT-based screening group could be twice times higher than in the non-screening group (40,41), as well as that lung cancer cases at stage I only accounted for 17.3% in China (19), there was still a great potential to improve the OS of lung cancer patients by early interventions through screening.
The prognosis of SCLC was worse than that of AD and SCC, mainly due to its tendency of dissemination (9), limited breakthrough agents and rapid development of resistance (7,42), which was consistent with findings in the published articles (9,28,43). In this study, the 5-year OS and LCSS rates varied significantly in each histological classification among patients at early stages, while it remained non-statistically significant for advanced stages. It was likely that efficient treatments were available for non-SCLCs at earlier stages, including surgery and stereotactic body radiation therapy, but the survival of SCLC and non-SCLCs approached at late stages caused by the restricted choices of treatment, increasing toxicity, and worse tolerance of patients (7). Hence, to achieve significant progress in improving patients’ prognosis, effective measures should be adopted to detect cancer in early stages and timely interventions should be taken to inhibit the progression of the tumor, which has become one of the consensuses in the aspect of cancer diagnosis and treatment. In addition, age was also an independent predictor, possibly due to the decline in physical function and poor tolerance in the elders, which have been proved in former studies (44-46). Continued smoking was an essential adverse predictor for prognosis by increasing risks of second primary tumors and poorer quality of life (47). Although former smokers faced a higher risk of OS, smoking cession after diagnosis would improve the survival(48) and the duration of cessation should be long enough (49), suggesting an urgency to quit smoking.
This study was based on 17 hospitals from six provinces in different classes. The management of participants enrolled from hospitals was easier because of the completeness and integrity of requiring information, contributing to a high response rate. Also, the patients were followed up by both active and passive methods to achieve an acceptable follow-up rate. Meanwhile, the data on the cancer staging was not routinely collected by cancer registries (19), so the stage information at population level was only available from multi-centered hospital-based surveys. The main findings in this work implies the vital significance to promote early detection and early treatment.
There are also some limitations in this study. First, the hospitals located in Western China were not covered, and the selected hospitals were mostly core hospitals with the aim of enrolling local lung cancer patients as many as possible. The diversity in the level of economic development, health care conditions and the proportion of patients receiving treatment might result in the survival reported being overestimated. Besides, the results of this work provided valuable information of the survival situations in Central and East China, but was not able to reflect a national-level data. Second, it was not a population-based study, so the relative survival rates have not been calculated. Considering the possible differences in the life expectancy of study population at baseline, direct comparisons on the prognosis of lung cancer patients between this work and other studies should be treated with caution. Third, missing values existed in the raw data, which might lead to bias in the HR estimations. Nevertheless, similar associations were detected in different cox regression models, which verified the predictive effects of pathological evaluations.
Conclusions
In summary, this study estimated the 5-year survival rates of the Chinese lung cancer patients by pathological characteristics. It demonstrates that both the OS and LCSS rates decreased significantly with the advance of cancer stages, and stage at diagnosis was the most influential prognostic factor for lung cancer. Histological classification was also associated with the prognosis, with the SCLC facing higher risks at early stages. These findings suggest that more attention should to be paid on the early diagnosis and reasonable treatment of lung cancer patients.
Supplementary
The article’s supplementary files as
Acknowledgments
We gratefully thank the following hospitals for their cooperation with regard to providing, sorting, and verifying the cancer data: the Fourth Hospital of Hebei Medical University, the First Hospital of Shijiazhuang, Cixian Cancer Hospital, the Beijing Cancer Hospital, Cancer Hospital of the Chinese Academy of Medical Sciences, the First People’s Hospital of Dongcheng, Henan Cancer Hospital, the People’s Hospital of Jiyuan, the Third People’s Hospital of Luoyang, Dongfang Hospital of Luoyang, Zhejiang Cancer Hospital, Haining Traditional Chinese Medicine Hospital, Zhejiang Jinhua Guangfu Hospital, Hubei Cancer Hospital, the First Hospital of Danjiangkou, the First People’s Hospital of Xiangyang, the People’s Hospital of Xiaolan, and the People's Hospital of Zhongshan.
Funding: This work was supported by Sanming Project of Medicine in Shenzhen (Grant No. SZSM201911015) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Grant No. 2019PT320027).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Cancer Hospital, Chinese Academy of Medical Sciences (No. NCC-006288) and informed consent was taken from all the patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-240/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-240/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-240/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-240/coif). The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;394:1145-58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Y, Chen W, Wang X, et al. Contemporary trends on expenditure of hospital care on total cancer and its subtypes in China during 2008-2017. Chin J Cancer Res 2021;33:627-36. 10.21147/j.issn.1000-9604.2021.05.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Office for National Statistics. Cancer survival in England: adult, stage at diagnosis and childhood - patients followed up to 2018. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancersurvivalinengland/latest, based on 12 August 2019 data submission.
- 5.Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2018, National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
- 6.Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555-67. 10.1016/S2214-109X(18)30127-X [DOI] [PubMed] [Google Scholar]
- 7.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 8.Jeong O, Jung MR, Kang JH, et al. Prognostic Performance of Preoperative Staging: Assessed by Using Multidetector Computed Tomography-Between the New Clinical Classification and the Pathological Classification in the Eighth American Joint Committee on Cancer Classification for Gastric Carcinoma. Ann Surg Oncol 2020;27:545-51. 10.1245/s10434-019-07845-3 [DOI] [PubMed] [Google Scholar]
- 9.Beadsmoore CJ, Screaton NJ. Classification, staging and prognosis of lung cancer. Eur J Radiol 2003;45:8-17. 10.1016/S0720-048X(02)00287-5 [DOI] [PubMed] [Google Scholar]
- 10.Karanikolos M, Ellis L, Coleman MP, et al. Health systems performance and cancer outcomes. J Natl Cancer Inst Monogr 2013;2013:7-12. 10.1093/jncimonographs/lgt003 [DOI] [PubMed] [Google Scholar]
- 11.McSorley ST, Watt DG, Horgan PG, et al. Postoperative Systemic Inflammatory Response, Complication Severity, and Survival Following Surgery for Colorectal Cancer. Ann Surg Oncol 2016;23:2832-40. 10.1245/s10434-016-5204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Yu L, Na J, et al. Survival of Cancer Patients in Northeast China: Analysis of Sampled Cancers from Population-Based Cancer Registries. Cancer Res Treat 2017;49:1106-13. 10.4143/crt.2016.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He M, Li B, Du J, et al. Clinical Characteristics and Survival of Lung Cancer Patients in Chongqing, 2001-2018. China Cancer 2020;29:865-70. [Google Scholar]
- 14.Wang Y, Bi Y, Wang Z, et al. Survival analysis of lung cancer patients in Shandong Province, China. Chinese Journal of Health Statistics 2018;35:111-3,6.
- 15.Li R, Zhang M, Cheng Y, et al. Using Population-Based Cancer Registration Data and Period Analysis to Accurately Assess and Predict 5-Year Relative Survival for Lung Cancer Patients in Eastern China. Front Oncol 2021;11:661012. 10.3389/fonc.2021.661012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YM, Lin KC, Tsai CM, et al. Survival status of veterans with lung cancer is poorer than that among civilians due to age and sex differences: a study of Chinese veterans in Taiwan. J Chin Med Assoc 2008;71:286-93. 10.1016/S1726-4901(08)70124-2 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Wang X, Gu X, et al. Survival analysis of lung cancer in Affiliated Tumor Hospital of Xinjiang Medical University 2010—2015. Journal of Modern Oncology 2018;26:208-11. [Google Scholar]
- 18.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng H, Ran X, An L, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health 2021;6:e877-87. 10.1016/S2468-2667(21)00157-2 [DOI] [PubMed] [Google Scholar]
- 20.Expert Consultation WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157-63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 23.Fan H, Shao ZY, Xiao YY, et al. Incidence and survival of non-small cell lung cancer in Shanghai: a population-based cohort study. BMJ Open 2015;5:e009419. 10.1136/bmjopen-2015-009419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Yang F, Li X, et al. Characteristics, survival, and risk factors of Chinese young lung cancer patients: the experience from two institutions. Oncotarget 2017;8:89236-44. 10.18632/oncotarget.19183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Zhang G, Li C, et al. Prognostic factors and survival of patients with small cell lung cancer in a northeastern Chinese population. Thorac Cancer 2013;4:143-52. 10.1111/j.1759-7714.2012.00149.x [DOI] [PubMed] [Google Scholar]
- 26.Li J, He J, Zhang Y, et al. Survival in Lung Cancer among Female Never-smokers in Rural Xuanwei and Fuyuan Counties in Eastern Yunnan Province, China. Zhongguo Fei Ai Za Zhi 2019;22:477-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang W, Shao W, Jiang G, et al. Chinese multi-institutional registry (CMIR) for resected non-small cell lung cancer: survival analysis of 5,853 cases. J Thorac Dis 2013;5:726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu LL, Li CW, Lin WK, et al. Incidence and survival analyses for occult lung cancer between 2004 and 2015: a population-based study. BMC Cancer 2021;21:1009. 10.1186/s12885-021-08741-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y, Sun Y. Development and validation of nomograms for predicting overall and cancer-specific survival in young patients with non-small cell lung cancer. J Thorac Dis 2020;12:1404-16. 10.21037/jtd.2020.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang C, Shen Y, Zhang Z, et al. Analysis of Incidence and Survival Rate of Cancer Among Residents in Haining City from 2003 to 2015. China Cancer 2018;27:267-72.29514610 [Google Scholar]
- 31.National Bureau of Statistics of China. 2021 China Statistical Yearbook. Available online: http://www.stats.gov.cn/tjsj/ndsj/2021/indexch.htm
- 32.Wei K, Liang Z, Cen H. Net Survival of Cancers in Zhongshan City, Guangdong Province, 1995-2009. China Cancer 2016;25:747-51. [Google Scholar]
- 33.Zhu J, Zhang Y, Chen Y, et al. Analysis on Lung Cancer Survival from 2001 to 2007 in Qidong, China. Chinese Journal of Lung Cancer 2011;14:23-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M, Wu C, Gong Y, et al. Survival analysis of patients with lung cancer in Shanghai. China Oncology 2017;27:326-33.
- 35.Wang Z, Zhang Y, Mo M, et al. Survival report of 7753 surgical lung cancer patients from a large single hospital-based cancer registry: results based on the 8th edition of the TNM classification of lung cancer. China Oncology 2020;30:321-7. [Google Scholar]
- 36.Zhang Z, Guo F, Cui Y, et al. The study on improvement of survival for lung cancer surgically intervened in PUMC Hospital. Chinese Journal of Lung Cancer 2005;8:124-8. [DOI] [PubMed] [Google Scholar]
- 37.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 39.Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Qian F, Teng J, et al. Community-based lung cancer screening with low-dose CT in China: Results of the baseline screening. Lung Cancer 2018;117:20-6. 10.1016/j.lungcan.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 41.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 42.Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244-55. 10.1158/1078-0432.CCR-14-2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gedvilaitė V, Danila E, Cicėnas S, et al. Lung Cancer Survival in Lithuania: Changes by Histology, Age, and Sex From 2003-2007 to 2008-2012. Cancer Control 2019;26:1073274819836085. 10.1177/1073274819836085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang YJ, Huang JY, Lin CH, et al. Survival and Treatment of Lung Cancer in Taiwan between 2010 and 2016. J Clin Med 2021;10:4675. 10.3390/jcm10204675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casal-Mouriño A, Ruano-Ravina A, Torres-Durán M, et al. Lung cancer survival in never-smokers and exposure to residential radon: Results of the LCRINS study. Cancer Lett 2020;487:21-6. 10.1016/j.canlet.2020.05.022 [DOI] [PubMed] [Google Scholar]
- 46.Huang LL, Hu XS, Wang Y, et al. Survival and pretreatment prognostic factors for extensive-stage small cell lung cancer: A comprehensive analysis of 358 patients. Thorac Cancer 2021;12:1943-51. 10.1111/1759-7714.13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jassem J. Tobacco smoking after diagnosis of cancer: clinical aspects. Transl Lung Cancer Res 2019;8:S50-8. 10.21037/tlcr.2019.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheikh M, Mukeriya A, Shangina O, et al. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study. Ann Intern Med 2021;174:1232-9. 10.7326/M21-0252 [DOI] [PubMed] [Google Scholar]
- 49.Shima T, Kinoshita T, Uematsu M, et al. How long is cessation of preoperative smoking required to improve postoperative survival of patients with pathological stage I non-small cell lung cancer? Transl Lung Cancer Res 2020;9:1924-39. 10.21037/tlcr-20-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as