Key Points
Question
What are the sociodemographic and clinical characteristics of patients with long COVID and persistent olfactory dysfunction?
Findings
In this cross-sectional study of 219 patients with long COVID and neurologic symptoms, 64% had olfactory dysfunction, with the highest prevalence among women, adults, and outpatients. Patients with olfactory dysfunction may develop severe olfactory loss (hyposmia or anosmia) that may persist for more than 1 year after the onset of symptoms.
Meaning
This study suggests that olfactory dysfunction in patients with long COVID may become permanent.
Abstract
Importance
Determining the characteristics, type, and severity of olfactory dysfunction in patients with long COVID is important for the prognosis and potential treatment of the affected population.
Objective
To describe the sociodemographic and clinical features of patients with long COVID who develop persistent olfactory dysfunction.
Design, Setting, and Participants
This cross-sectional study, conducted at a rehabilitation center at a public university in the Amazon region of Brazil between September 9, 2020, and October 20, 2021, comprised 219 patients with long COVID and self-reported neurologic symptoms. Of these 219 patients, 139 received a diagnosis of chronic olfactory dysfunction, as confirmed by the Connecticut Chemosensory Clinical Research Center (CCCRC) test.
Exposure
Clinical diagnosis of long COVID.
Main Outcomes and Measures
Electronic case report forms were prepared for the collection of sociodemographic and clinical data. Patients’ sense of smell was evaluated via a CCCRC test, and the association of olfactory dysfunction with aspects of daily life was recorded using a questionnaire.
Results
Of the 219 patients included in the study, 164 (74.9%) were women, 194 (88.6%) were between 18 and 59 years of age (mean [SD] age, 43.2 [12.9] years), 206 (94.1%) had more than 9 years of education, and 115 (52.5%) had a monthly income of up to US $192.00. In the study group, 139 patients (63.5%) had some degree of olfactory dysfunction, whereas 80 patients (36.5%) had normosmia. Patients with olfactory dysfunction had a significantly longer duration of long COVID symptoms than those in the normosmia group (mean [SD], 242.7 [101.9] vs 221.0 [97.5] days; P = .01). Among patients with anosmia, there was a significant association between olfactory dysfunction and daily activities, especially in terms of impairment in hazard detection (21 of 31 patients [67.7%]), personal hygiene (21 of 31 patients [67.7%]), and food intake (21 of 31 patients [67.7%]). Univariable logistic regression analyses found that ageusia symptoms were associated with the occurrence of olfactory dysfunction (odds ratio [OR], 11.14 [95% CI, 4.76-26.07]; P < .001), whereas headache (OR, 0.41 [95% CI, 0.22-0.76]; P < .001) and sleep disorders (OR, 0.48 [95% CI, 0.26-0.92]; P = .02) showed an inverse association with the occurrence of olfactory dysfunction.
Conclusions and Relevance
Olfactory dysfunction is one of the most important long-term neurologic symptoms of COVID-19, with the highest prevalence seen among women, adults, and outpatients. Patients with olfactory dysfunction may experience persistent severe hyposmia or anosmia more than 1 year from the onset of symptoms, suggesting the possibility of the condition becoming a permanent sequela.
This cross-sectional study describes the sociodemographic and clinical features of patients with long COVID who develop persistent olfactory dysfunction.
Introduction
Long COVID can be described as a set of symptoms, signs, or abnormal laboratory test parameters persisting for 2 weeks or more after the onset of COVID-19.1,2,3,4 In total, 55 long-term effects of COVID-19 have been identified, with fatigue, lung dysfunction, abnormal chest radiograph results, neurologic disorders, and anosmia being the most common.5 Olfactory dysfunction is among the most prevalent neurologic symptoms among patients with long COVID, and persistent anosmia has been reported in 23% of patients with acute COVID-19.5,6,7
To date, little is known regarding the long-term course of olfactory dysfunction associated with COVID-19, and the question of whether it is completely reversible remains unclear.8,9 However, chronic olfactory disorders are associated with disturbances in eating behavior, depression, and a general reduction in quality of life.10,11,12 Individuals with chronic olfactory dysfunction report difficulties with cooking, maintaining health and nutritional status, personal hygiene, and social relationships10,13 and are 3 times more likely to experience hazardous events, such as smoke, delayed detection of gas leaks, and spoiled food.14
The characteristics, type, and severity of olfactory dysfunction are important in determining prognosis and potential treatment.15,16 This study aimed to describe the sociodemographic and clinical features of patients with long COVID who developed persistent olfactory dysfunction and its features and the association of persistent olfactory dysfunction with daily life activities.
Methods
Study Population
This cross-sectional study was conducted among individuals enrolled in a follow-up program for long COVID at a public university in Belém, in the Amazon region of Brazil. The genetic heterogeneity and admixture of the Amazon population are characteristics that overlap the concepts of isolated races; therefore, race and ethnicity were not discussed in this study. Patients had long COVID and a history of typical symptoms of acute COVID-19 with positive nasal swab reverse transcription–polymerase chain reaction results and presented with long-duration symptoms.2 None of the study participants had been immunized prior to SARS-CoV-2 infection. This study was approved by the Ethics Committee on Research with Human Beings of the State University of Pará and followed the ethical principles of the Declaration of Helsinki.17 All participants provided written informed consent. This observational, cross-sectional, quantitative, descriptive, and analytical study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline18 for cross-sectional studies and the Standards of Reporting of Neurological Disorders (STROND) checklist—a guideline for reporting of incidence and prevalence in neuroepidemiology studies.19
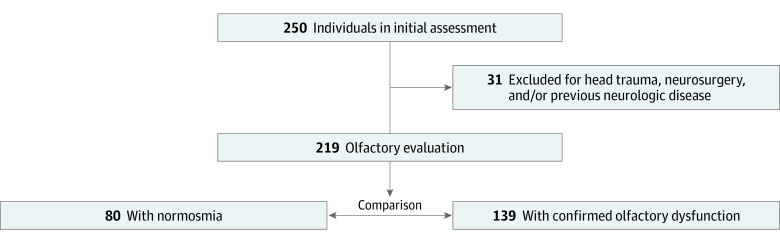
Participants were registered using an electronic form (Google Forms; Google Corp) and were later contacted via telephone for a formal neurologic consultation with a multidisciplinary team. Patients (n = 250) older than 18 years were evaluated between September 9, 2020, and October 20, 2021. Of these 250 patients, 31 were excluded because of a previous history of head trauma, neurologic disease, or neurosurgery, resulting in a sample size of 219 patients. Among the 219 patients with confirmed long-term neurologic symptoms, 139 presented with chronic olfactory dysfunction associated with SARS-CoV-2 infection (Figure).
Figure. Flowchart of the Study.
Study Design, Data Collection, and Procedures
We collected sociodemographic and clinical data, including variables such as educational level, sex, income, health history (diabetes, allergic rhinitis, and smoking), reported symptoms (headache, depression, anxiety, insomnia, tingling in extremities, mild cognitive disorder, tiredness, dyspnea, weakness, and pain), anosmia, and ageusia after COVID-19 from an electronic medical record (Microsoft Access; Microsoft Corp).
We evaluated patients’ sense of smell with the Connecticut Chemosensory Clinical Research Center (CCCRC) test. The CCCRC test solutions were developed by the pharmacology laboratory of Cosmopolita College, Belém, Brazil, following the recommendations of Cain et al20 and Fenólio et al.21 The CCCRC test is composed of 2 subtests: a threshold test and an odor identification test.
For the threshold test, a test kit comprising 8 bottles was used; 7 bottles contained different dilutions of butanol (n-butyl alcohol) in distilled water at the following butanol concentrations: 0.005%, 0.01%, 0.05%, 0.1%, 0.4%, 1%, and 4%; and 1 bottle contained only distilled water. The procedure consisted of 2 alternatives with a forced choice between the butanol concentration and distilled water. The concentration of the first solution with 4 correct identifications was considered the perceptual threshold for the identification of the smell of butanol. The odor identification test consisted of identification through inhalation of 8 common substances to be identified from a 16-item list.
The CCCRC score for each nostril is calculated by adding the score of the threshold and odor identification tests. The score can range between 0 and 7. The final score was arrived by taking the mean of the scores of both nostrils. The final score classified the participants as having anosmia (0-1.75), severe hyposmia (2-3.75), moderate hyposmia (4-4.75), mild hyposmia (5-5.75), and normosmia (6-7) (eFigure in the Supplement).
In addition, we evaluated the association of olfactory dysfunction with daily life activities using a questionnaire that listed domains in which olfactory function plays a major role according to previous studies: (1) In your opinion, did the olfactory dysfunction affect your personal hygiene? Yes or no? (personal hygiene: ability to keep the body clean and sensing of body odors); (2) In your opinion, did the olfactory dysfunction affect your food intake? Yes or no? (food intake: ability and behavior related to eating); (3) In your opinion, did the olfactory dysfunction affect your preparation of food ability? Yes or no? (preparation of food: ability and behavior related to cooking); (4) In your opinion, did the olfactory dysfunction affect your hazard detection ability? Yes or no? (hazard detection: ability to detect environmental hazards [eg, spoiled food, gas leak, fire, or smoke]); (5) In your opinion, did the olfactory dysfunction affect your work? Yes or no? (work: activities related to the development, production, delivery, or management of objects or services); and (6) In your opinion, did the olfactory dysfunction affect your social relations? Yes or no? (social relations: activities that involve social interaction with others, including family, friends, peers, and community members). The questionnaire was self-completed, and patients were instructed to mark only domains directly affected by COVID-19–associated olfactory dysfunction.
Statistical Analysis
Descriptive results are presented as mean (SD) values for continuous variables and frequencies and percentages for categorical variables. Data normality was assessed using the D’Agostino-Pearson K2 test. Parametric variables were assessed with the 2-sample t test, and nonparametric variables were assessed with the Mann-Whitney test or the χ2 test. Univariable and multivariable logistic models estimated the odds ratios (ORs) that were used to evaluate the association between olfactory dysfunction and the independent variables (female sex, age, no hospital admittance, headache, sleep disorder, depression, anxiety, ageusia, tingling of extremities, mild cognitive disorder, fatigue, and time from onset symptoms) of the sample. All P values were from 2-sided tests, adjusted for multiple comparisons, and results were deemed statistically significant at P ≤ .05. Statistical analysis was performed using GraphPad Prism, version 5.0 software (GraphPad Software).
Results
Of the 219 patients in the study, 164 (74.9%) were women, 194 (88.6%) were between 18 and 59 years of age (mean [SD] age, 43.2 [12.9] years), 206 (94.1%) had more than 9 years of education, and 115 (52.5%) had a monthly income of up to US $192 (Table 1). A total of 80 patients (36.5%) had normosmia, and 139 (63.5%) had some degree of olfactory dysfunction.
Table 1. Comparison of Sociodemographic and Clinical Features Among Study Population According to the CCCRC Classification.
| Feature | Patients, No. (%) | P valuea | ||
|---|---|---|---|---|
| General (N = 219) | Normosmia (n = 80) | Olfactory dysfunction (n = 139) | ||
| Age, y | ||||
| 18-59 | 194 (88.6) | 67 (83.8) | 127 (91.4) | .12 |
| ≥60 | 25 (11.4) | 13 (16.3) | 12 (8.6) | |
| Sex | ||||
| Female | 164 (74.9) | 62 (77.5) | 102 (73.4) | .49 |
| Male | 55 (25.1) | 18 (22.5) | 37 (26.6) | |
| Years of education | ||||
| ≤9 | 13 (5.9) | 7 (8.8) | 6 (4.3) | .23 |
| >9 | 206 (94.1) | 73 (91.3) | 133 (95.7) | |
| Monthly income, US$ | ||||
| ≤192.00 | 115 (52.5) | 39 (48.8) | 76 (54.7) | .39 |
| >192.00 | 104 (47.5) | 41 (51.3) | 63 (45.3) | |
Abbreviation: CCCRC, olfactory test of Connecticut Chemosensory Clinical Research Center.
Considering normosmia × olfactory dysfunction groups.
Table 2 provides the clinical characteristics of the participants with olfactory dysfunction grouped according to the CCCRC test score, as well as the comparison between the normosmia and olfactory dysfunction groups. There was no significant difference in the hospitalization rates between the normosmia group and the olfactory dysfunction groups (16 of 80 [20.0%] vs 19 of 139 [13.7%]; P = .21). The olfactory dysfunction group had a significantly longer duration from symptom onset than the normosmia group (mean [SD], 242.7 [101.9] vs 221.0 [97.5] days; P = .01), and the olfactory dysfunction group had a higher proportion of participants with neurologic symptoms for more than 6 months than the normosmia group (110 of 139 [79.1%] vs 51 of 80 [63.8%]; P = .01). More patients with normosmia than patients with olfactory dysfunction had headache (43 of 80 [53.8%] vs 52 of 139 [37.4%]; P = .01), sleep disorder (29 of 80 [36.3%] vs 32 of 139 [23.0%]; P = .03), and anxiety (36 of 80 [45.0%] vs 30 of 139 [21.6%]; P < .001) symptoms, whereas the olfactory dysfunction group had a higher proportion of patients with ageusia (83 of 139 [59.7%] vs 19 of 80 [23.8%]; P < .001).
Table 2. Clinical Findings Among Study Population According to CCCRC Classification.
| Characteristic | Patients, No. (%) | P value | Olfactory dysfunction, No. (%) of patients | ||||
|---|---|---|---|---|---|---|---|
| Normosmia (n = 80) | Olfactory dysfunction (n = 139) | Mild hyposmia (n = 21) | Moderate hyposmia (n = 23) | Severe hyposmia (n = 64) | Anosmia (n = 31) | ||
| Hospitalization | |||||||
| Yes | 16 (20.0) | 19 (13.7) | .21 | 5 (23.8) | 4 (17.4) | 4 (6.3) | 6 (19.4) |
| No | 64 (80.0) | 120 (86.3) | 16 (76.2) | 19 (82.6) | 60 (93.8) | 25 (80.6) | |
| Time from symptom onset, mean (SD), d | 221.0 (97.5) | 242.7 (101.9) | .01a | 264.4 (119.4) | 246.2 (105.6) | 243.9 (101.7) | 222.7 (87.2) |
| Time from symptom onset, mo | |||||||
| ≤6 | 29 (36.3) | 29 (20.9) | .01b | 3 (14.3) | 5 (21.7) | 14 (21.9) | 7 (22.6) |
| >6 | 51 (63.8) | 110 (79.1) | 18 (85.7) | 18 (78.3) | 50 (78.1) | 24 (77.4) | |
| Health history | |||||||
| Allergic rhinitis | 12 (15.0) | 33 (23.7) | .12 | 4 (19.0) | 6 (26.1) | 14 (21.9) | 9 (29.0) |
| Smoking | 3 (3.8) | 5 (3.6) | .95 | 0 | 0 | 2 (3.1) | 3 (9.7) |
| Diabetes | 8 (10.0) | 9 (6.5) | .34 | 2 (9.5) | 0 | 6 (9.4) | 1 (3.2) |
| Long COVID symptoms | |||||||
| Ageusia | 19 (23.8) | 83 (59.7) | <.001b | 12 (57.1) | 12 (52.2) | 39 (60.9) | 21 (67.7) |
| Fatigue | 31 (38.8) | 67 (48.2) | .17 | 15 (71.4) | 9 (39.1) | 33 (51.6) | 10 (32.3) |
| Cognitive disorder | 40 (50.0) | 62 (44.6) | .44 | 13 (61.9) | 9 (39.1) | 27 (42.2) | 13 (41.9) |
| Headache | 43 (53.8) | 52 (37.4) | .01b | 13 (61.9) | 11 (47.8) | 20 (31.3) | 8 (25.8) |
| Weakness | 14 (17.5) | 31 (22.3) | .39 | 10 (47.6) | 4 (17.4) | 13 (20.3) | 4 (12.9) |
| Dyspnea | 14 (17.5) | 27 (19.4) | .72 | 6 (28.6) | 4 (17.4) | 9 (14.1) | 8 (25.8) |
| Paresthesias | 16 (20.0) | 35 (25.2) | .38 | 8 (38.1) | 3 (13.0) | 18 (28.1) | 6 (19.4) |
| Sleep disorder | 29 (36.3) | 32 (23.0) | .03b | 8 (38.1) | 5 (21.7) | 14 (21.9) | 5 (16.1) |
| Anxiety | 36 (45.0) | 30 (21.6) | <.001b | 8 (38.1) | 5 (21.7) | 13 (20.3) | 4 (12.9) |
| Depression | 11 (13.8) | 17 (12.2) | .74 | 5 (23.8) | 5 (21.7) | 6 (9.4) | 1 (3.2) |
Abbreviation: CCCRC, olfactory test of Connecticut Chemosensory Clinical Research Center.
Calculated using a 2-sample t test with a threshold for statistical significance of P ≤ .05.
Calculated using a χ2 test with a threshold for statistical significance of P ≤ .05.
Hazard detection, personal hygiene, and food preparation were the domains of daily life most frequently associated with olfactory dysfunction. The anosmia group reported impairments more frequently than the other olfactory dysfunction subgroups, with significant differences in personal hygiene (anosmia, 21 of 31 [67.7%]; mild hyposmia, 7 of 21 [33.3%]; moderate hyposmia, 13 of 23 [56.5%]; severe hyposmia, 29 of 64 [45.3%]), food intake (anosmia, 21 of 31 [67.7%]; mild hyposmia, 7 of 21 [33.3%]; moderate hyposmia, 9 of 23 [39.1%]; severe hyposmia, 24 of 64 [37.5%]), and hazard detection (anosmia, 21 of 31 [67.7%]; mild hyposmia, 7 of 21 [33.3%]; moderate hyposmia, 14 of 23 [60.9%]; severe hyposmia, 31 of 64 [48.4%]) (Table 3).
Table 3. Long-term Association of Olfactory Dysfunction With Domains of Daily Life.
| Domain | Olfactory dysfunction (n = 139) | Hyposmia, No. (%) of patients | Anosmia (n = 31) | P valuea | ||
|---|---|---|---|---|---|---|
| Mild (n = 21) | Moderate (n = 23) | Severe (n = 64) | ||||
| Personal hygiene | 70 (50.4) | 7 (33.3) | 13 (56.5) | 29 (45.3) | 21 (67.7) | .05b |
| Food intake | 61 (43.9) | 7 (33.3) | 9 (39.1) | 24 (37.5) | 21 (67.7) | .02b |
| Preparation of food | 65 (46.8) | 9 (42.9) | 11 (47.8) | 24 (37.5) | 21 (67.7) | .16 |
| Hazard detection | 73 (52.5) | 7 (33.3) | 14 (60.9) | 31 (48.4) | 21 (67.7) | .05b |
| Work | 28 (20.1) | 4 (19.0) | 4 (17.4) | 12 (18.8) | 8 (25.8) | .58 |
| Social relations | 48 (34.5) | 6 (28.6) | 7 (30.4) | 22 (34.4) | 13 (41.9) | .21 |
| Not answer | 19 (13.7) | 4 (19.0) | 2 (8.7) | 11 (17.2) | 2 (6.5) | NA |
Abbreviation: NA, not applicable.
Adjusted for multiple comparisons between mild hyposmia, moderate hyposmia, severe hyposmia, and anosmia.
Calculated using a χ2 test with a threshold for statistical significance of P ≤ .05.
On univariable logistic regression for epidemiologic and clinical characteristics, olfactory dysfunction was associated with ageusia (OR, 11.14 [95% CI, 4.76-26.07]; P < .001) and inversely associated with headache (OR, 0.41 [95% CI, 0.22-0.76]; P < .001) and sleep disorders (OR, 0.48 [95% CI, 0.26-0.92]; P = .02) (Table 4). On multivariable logistic regression, olfactory dysfunction was significantly associated with ageusia symptoms (OR, 13.24 [95% CI, 5.24-33.47]; P < .001).
Table 4. Association of Olfactory Dysfunction and Severe Olfactory Dysfunction With Clinical Features of the Study Population (N = 219).
| Clinical feature | Olfactory dysfunction (CCCRC score <6) | Severe olfactory dysfunction (CCCRC score <3) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Female | 0.62 (0.30-1.31) | .21 | 0.42 (0.17-1.05) | .06 | 1.20 (0.65-2.24) | .56 | 1.22 (0.61-2.45) | .57 |
| Age (18-59 y) | 1.89 (0.86-4.19) | .11 | 1.89 (0.71-5.05) | .19 | 1.72 (0.71-4.19) | .22 | 1.26 (0.47-3.41) | .64 |
| No hospital admission | 2.05 (0.97-4.38) | .06 | 1.14 (0.46-2.85) | .76 | 2.14 (0.98-4.72) | .05 | 1.34 (0.55-3.30) | .51 |
| Headache | 0.41 (0.22-0.76) | <.001 | 0.56 (0.27-1.22) | .14 | 0.35 (0.20-0.63) | <.001 | 0.36 (0.19-0.71) | .002 |
| Sleep disorder | 0.48 (0.26-0.92) | .02 | 0.64 (0.28-1.50) | .31 | 0.48 (0.26-0.91) | .02 | 0.50 (0.23-1.14) | .09 |
| Depression (BDI score, >20) | 0.58 (0.31-1.13) | .10 | 0.95 (0.38-2.36) | .91 | 0.63 (0.36-1.14) | .13 | 0.80 (0.35-1.87) | .61 |
| Anxiety (BAI score, >20) | 0.65 (0.36-1.21) | .17 | 0.71 (0.31-1.67) | .43 | 0.82 (0.48-1.41) | .48 | 1.04 (0.49-2.22) | .91 |
| Ageusia | 11.14 (4.76-26.07) | <.001 | 13.24 (5.24-33.47) | <.001 | 3.19 (1.83-5.58) | <.001 | 2.82 (1.53-5.21) | <.001 |
| Tingling extremities | 0.96 (0.48-1.95) | .92 | 1.63 (0.65-4.12) | .29 | 1.21 (0.65-2.28) | .54 | 2.23 (1.00-5.00) | .05 |
| Mild cognitive disorder | 0.72 (0.40-1.31) | .28 | 0.85 (0.38-1.90) | .69 | 0.72 (0.42-1.25) | .24 | 0.95 (0.49-1.85) | .88 |
| Tiredness or fatigue | 1.03 (0.57-1.89) | .90 | 1.47 (0.66-3.29) | .34 | 1.03 (0.61-1.78) | .89 | 1.41 (0.72-2.78) | .30 |
| Dyspnea | 0.86 (0.41-1.84) | .70 | 0.49 (0.19-1.31) | .15 | 0.9 (0.46-1.81) | .78 | 0.78 (0.35-1.77) | .56 |
| Time from onset of symptoms (>6 mo) | 1.64 (0.86-3.16) | .13 | 2.11 (0.95-4.68) | .06 | 1.58 (0.86-2.90) | .13 | 1.76 (0.90-3.45) | .09 |
Abbreviations: BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; CCCRC, olfactory test of Connecticut Chemosensory Clinical Research Center; OR, odds ratio.
On univariable analysis for the epidemiologic and clinical characteristics associated with severe olfactory dysfunction (CCCRC score, <3), inverse associations were found for headache (OR, 0.35 [95% CI, 0.20-0.63]; P < .001) and sleep disorders (OR, 0.48 [95% CI, 0.26-0.91]; P = .02); no hospitalization (OR, 2.14 [95% CI, 0.98-4.72]; P = .05) demonstrated a significant association with severe olfactory dysfunction (Table 4). Tingling was associated with severe olfactory dysfunction in multivariable analysis (OR, 2.23 [95% CI, 1.00-5.00]; P = .05), and ageusia was associated with severe olfactory dysfunction in both analyses (univariable: OR, 3.19 [95% CI, 1.83-5.58]; P < .001; multivariable: OR, 2.82 [95% CI, 1.53-5.21]; P < .001).
Discussion
Loss of smell was the most reported neurologic symptom among the 219 patients in this study with long COVID, primarily seen among women, adults, and those not hospitalized during the acute phase of COVID-19. Patients with olfactory dysfunction after COVID-19 may develop severe degrees of olfactory loss (severe hyposmia or anosmia) even 1 year after the onset of symptoms, suggesting the possibility of permanent sequelae. The daily life activities most associated with olfactory dysfunction among patients with anosmia were personal hygiene, food intake, and prevention of accidents. Logistic regression analyses found that ageusia was the only risk factor associated with the occurrence of olfactory dysfunction, whereas headache and sleep disorders showed an inverse association with the occurrence of olfactory dysfunction.
Previous studies found similar results, and olfactory dysfunction was associated with female sex,22,23,24,25 middle age,22,24,25 outpatient clinical course,23,24,25,26,27 and lower probability of being admitted to the hospital owing to COVID-19.26,27 One hypothesis is that a small, focused viral load of SARS-CoV-2 in the upper airways may lead to mild infection and consequently decreases the risk of overloading the host’s immune response and hospitalization.15,25
Most COVID-19–associated olfactory dysfunctions are transient, lasting approximately 2 to 3 weeks.22,27 This finding is consistent with the fact that SARS-CoV-2 has a high affinity for the sustentacular cells of the olfactory epithelium that express angiotensin-converting enzyme 2 (ACE2) and possess substantial capacity for repair and regeneration after damage.28,29,30,31,32 However, epithelial injury secondary to ACE2-mediated entry does not completely explain the inverse association between olfactory dysfunction and disease severity.
A recent report proposed a second route for viral entry mediated by neuropilin 1 (NRP1), an immune cell expressed by regulatory T cells that exerts immunosuppressive effects.33 Neuropilin 1 is abundant in all olfactory cells and can facilitate direct damage to olfactory receptor neurons and, consequently, the olfactory bulb. The authors argued that variability in NRP1 expression by age, race and ethnicity, or sex may explain the differing levels of morbidity of infection and the inverse association between anosmia and COVID-19 severity. A higher expression of NRP1 may lead to a higher risk of olfactory dysfunction but greater activation of regulatory T cells that suppress a cytokine storm.33 Our results support this hypothesis.
Conversely, the absence of ACE2 expression by olfactory sensory neurons has weakened the neurotropic potential of patients with COVID-19, suggesting that olfactory dysfunction is not associated with viral damage to neuronal cells and other areas of the central nervous system.29,34 The pathologic changes in the central nervous system may originate from the hematogenous route and spread through the blood-brain barrier.35,36 These different mechanisms of viral entry may explain the inverse association found between the occurrence of olfactory dysfunction and other central nervous system disorders, such as headache and sleep disorders.
The mild forms of COVID-19 in the acute phase contrast with the persistence and severity of olfactory dysfunction among patients with long COVID.37,38,39 Possible causes of prolonged olfactory dysfunction after COVID-19 include damage to basal cells, continuous inflammation, and chronic SARS-CoV-2 infection in the olfactory epithelium.29 Chronic inflammation could modulate gene expression and switch the function of olfactory epithelium basal cells from neural regeneration to inflammatory signaling and immune cell proliferation.40
In our study, we found a long-term association of olfactory dysfunction with all domains of daily life listed in our questionnaire, especially among patients with anosmia. It is known that patients with chronic olfactory dysfunction have considerable disruption to their daily life37,41,42 and have the highest risk of developing mood disorders, such as anxiety and depression,43,44 and neurodegenerative diseases, such as Parkinson disease and Alzheimer disease.45,46,47
Several studies have used psychological tests to evaluate olfactory dysfunction among patients with COVID-19. The use of objective tests to evaluate alterations in smell is strongly encouraged when compared with subjective assessments based on the patients’ perception.48,49 The Sniffin’ Sticks test is the most commonly used test.16,49,50 Other olfactory sensitivity tests include the University of Pennsylvania Smell Identification Test,51 the Toyota & Takagi Olfactometer,52 the Cross-Cultural Smell Identification Test,53 the Brief Smell Identification Test,54 and the CCCRC test.20 In the present investigation, the entire cohort was evaluated using the CCCRC test, which is used worldwide and has the advantages of low cost and the possibility of large-scale clinical use.55 In addition, a recent version of the CCCRC test has been validated in the Brazilian population.21
To our knowledge, this is the first study conducted in a Brazilian population containing a large number of patients with long COVID, attributing internal validity to our results. These data are important because the prevalence rates of olfactory dysfunction among patients with COVID-19 appear to vary between populations; for example, a recent report found that White individuals are 3 times more likely to develop olfactory dysfunction (54%) than Asian individuals (17.7%).55,56,57
The prevalence of olfactory dysfunction was high (63.5%) in our study; a possible explanation would be the predominance of wild-type SARS-CoV-2 infection in our study population because almost all of the participants reported the onset of symptoms around March, April, and May 2020. A recent study showed that anosmia associated with COVID-19 is more frequent and severe among patients infected with the wild-type virus than among those infected with the Delta variant (B.1.167.2), as well as increasing the likelihood of chronic olfactory dysfunction.58
High recovery rates of persistent olfactory dysfunctions are expected within 1 year8; however, despite our analyses occurring within this period, the group with olfactory dysfunction had long COVID for a significantly longer time than the group with normosmia, and most patients with olfactory dysfunction had severe dysfunction. Long periods of severe olfactory dysfunction are associated with worse diagnosis and risk of permanent sequelae.59,60,61 Other studies have found similar results with a high prevalence of olfactory dysfunction after 6 months39,62 and reinforce the finding that a marked proportion of patients do not recover quickly.
Limitations
This study has some limitations. Dysgeusia was not tested with psychophysical tests, which could mean 2 confounding factors in our analyses: (1) an overestimation of this symptom in our sample, because retronasal smell and true dysgeusia are often confused by patients, and (2) it is not known whether the reported difficulties in food intake, also assessed subjectively, are a consequence of the severity of the olfactory dysfunction, dysgeusia, or both. Because long COVID is characterized by a series of overlapping symptoms, it is possible that some associations with the quality-of-life domains are the result of interactions between the symptoms and not just the olfactory dysfunction in isolation.
In this study, qualitative olfactory disorders, such as parosmia, phantosmia, and cacosmia, were not analyzed. The data are being collected as part of the follow-up research of this cohort and will be published in the future because the presence of these olfactory dysfunctions may be associated with the recovery of sense of smell.63,64
The absence of formal data regarding previous clinical history and the acute phase of COVID-19 is a potential confounding factor, which was minimized by carefully using an anamnesis form and specialized consultation with neurologists and otolaryngologists. Future studies should continue the monitoring of this population, prioritizing interdisciplinary research in clinical, epidemiologic, and basic science, such as genetics and immunology. These data should not be generalized because they were from a single center in 1 region of Brazil. The data, however, serve as a benchmark for further studies.
Conclusions
The results of our investigation reaffirmed that olfactory dysfunction is one of the most important long-term neurologic symptoms of COVID-19, with the highest prevalence among women, adults, and outpatients. We observed in this cohort that patients with olfactory dysfunction may experience persistent severe hyposmia or anosmia more than 1 year from the onset of symptoms, suggesting the possibility of permanent sequelae.
Our results highlight the need to continue monitoring the rate of recovery of olfactory function among individuals with long COVID to evaluate whether it is a chronic or permanent sequela. In addition, clinical trials and longitudinal studies are recommended to verify the effectiveness of potential treatments and the postulated risk for an increase in neurologic sequelae or neurodegenerative disorders in this population.
eFigure. Description of Connecticut Chemosensory Clinical Research Center Test Procedures and Score Calculation
References
- 1.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raveendran AV. Long COVID-19: challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab Syndr. 2021;15(1):145-146. doi: 10.1016/j.dsx.2020.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis. 2022;74(7):1191-1198. doi: 10.1093/cid/ciab611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4(5):e2111417. doi: 10.1001/jamanetworkopen.2021.11417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou SH, Beghi E, Helbok R, et al. ; GCS-NeuroCOVID Consortium and ENERGY Consortium . Global incidence of neurological manifestations among patients hospitalized with COVID-19—a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021;4(5):e2112131. doi: 10.1001/jamanetworkopen.2021.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renaud M, Thibault C, Le Normand F, et al. Clinical outcomes for patients with anosmia 1 year after COVID-19 diagnosis. JAMA Netw Open. 2021;4(6):e2115352. doi: 10.1001/jamanetworkopen.2021.15352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jafar A, Lasso A, Shorr R, Hutton B, Kilty S. Olfactory recovery following infection with COVID-19: a systematic review. PLoS One. 2021;16(11):e0259321. doi: 10.1371/journal.pone.0259321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses. 2014;39(3):185-194. doi: 10.1093/chemse/bjt072 [DOI] [PubMed] [Google Scholar]
- 11.Boesveldt S, Postma EM, Boak D, et al. Anosmia—a clinical review. Chem Senses. 2017;42(7):513-523. doi: 10.1093/chemse/bjx025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkholi SMA, Abdelwahab MK, Abdelhafeez M. Impact of the smell loss on the quality of life and adopted coping strategies in COVID-19 patients. Eur Arch Otorhinolaryngol. 2021;278(9):3307-3314. doi: 10.1007/s00405-020-06575-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller A, Malaspina D. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord. 2013;13(1):8. doi: 10.1186/1472-6815-13-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pence TS, Reiter ER, DiNardo LJ, Costanzo RM. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951-955. doi: 10.1001/jamaoto.2014.1675 [DOI] [PubMed] [Google Scholar]
- 15.Saussez S, Lechien JR, Hopkins C. Anosmia: an evolution of our understanding of its importance in COVID-19 and what questions remain to be answered. Eur Arch Otorhinolaryngol. 2021;278(7):2187-2191. doi: 10.1007/s00405-020-06285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boscutti A, Delvecchio G, Pigoni A, et al. Olfactory and gustatory dysfunctions in SARS-CoV-2 infection: a systematic review. Brain Behav Immun Health. 2021;15:100268. doi: 10.1016/j.bbih.2021.100268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(suppl 1):S31-S34. doi: 10.4103/sja.SJA_543_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Brayne C, Feigin VL, et al. Development of the standards of reporting of neurological disorders (STROND) checklist: a guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Eur J Epidemiol. 2015;30(7):569-576. doi: 10.1007/s10654-015-0034-5 [DOI] [PubMed] [Google Scholar]
- 20.Cain WS, Gent JF, Goodspeed RB, Leonard G. Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope. 1988;98(1):83-88. doi: 10.1288/00005537-198801000-00017 [DOI] [PubMed] [Google Scholar]
- 21.Fenólio GHM, Anselmo-Lima WT, Tomazini GC, et al. Validation of Connecticut olfactory test (CCCRC) adapted to Brazil. Braz J Otorhinolaryngol. Published online November 6, 2020. doi: 10.1016/j.bjorl.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos REA, da Silva MG, do Monte Silva MCB, et al. Onset and duration of symptoms of loss of smell/taste in patients with COVID-19: a systematic review. Am J Otolaryngol. 2021;42(2):102889. doi: 10.1016/j.amjoto.2020.102889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323(20):2089-2090. doi: 10.1001/jama.2020.6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechien JR, Chiesa-Estomba CM, Place S, et al. ; COVID-19 Task Force of YO-IFOS . Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335-344. doi: 10.1111/joim.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 2020;10(7):821-831. doi: 10.1002/alr.22592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boscolo-Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;146(8):729-732. doi: 10.1001/jamaoto.2020.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 2020;35(18):e174. doi: 10.3346/jkms.2020.35.e174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19–associated anosmia. Sci Adv. 2020;6(31):eabc5801. doi: 10.1126/sciadv.abc5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang F, Wang Y. COVID-19 anosmia: high prevalence, plural neuropathogenic mechanisms, and scarce neurotropism of SARS-CoV-2? Viruses. 2021;13(11):2225. doi: 10.3390/v13112225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doty RL. The mechanisms of smell loss after SARS-CoV-2 infection. Lancet Neurol. 2021;20(9):693-695. doi: 10.1016/S1474-4422(21)00202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaira LA, Hopkins C, Sandison A, et al. Olfactory epithelium histopathological findings in long-term coronavirus disease 2019 related anosmia. J Laryngol Otol. 2020;134(12):1123-1127. doi: 10.1017/S0022215120002455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Melo GD, Lazarini F, Levallois S, et al. COVID-19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596):eabf8396. doi: 10.1126/scitranslmed.abf8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins C, Lechien JR, Saussez S. More that ACE2? NRP1 may play a central role in the underlying pathophysiological mechanism of olfactory dysfunction in COVID-19 and its association with enhanced survival. Med Hypotheses. 2021;146:110406. doi: 10.1016/j.mehy.2020.110406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bryche B, St Albin A, Murri S, et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. 2020;89:579-586. doi: 10.1016/j.bbi.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24(2):168-175. doi: 10.1038/s41593-020-00758-5 [DOI] [PubMed] [Google Scholar]
- 36.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290-e299. doi: 10.1016/S2666-5247(20)30144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prem B, Liu DT, Besser G, et al. Long-lasting olfactory dysfunction in COVID-19 patients. Eur Arch Otorhinolaryngol. 2022;279(7):3485-3492. doi: 10.1007/s00405-021-07153-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moraschini V, Reis D, Sacco R, Calasans-Maia MD. Prevalence of anosmia and ageusia symptoms among long-term effects of COVID-19. Oral Dis. 2021. doi: 10.1111/odi.13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boscolo-Rizzo P, Hummel T, Hopkins C, et al. High prevalence of long-term olfactory, gustatory, and chemesthesis dysfunction in post–COVID-19 patients: a matched case-control study with one-year follow-up using a comprehensive psychophysical evaluation. Rhinology. 2021;59(6):517-527. doi: 10.4193/Rhin21.249 [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Reed RR, Lane AP. Chronic inflammation directs an olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell. 2019;25(4):501-513. doi: 10.1016/j.stem.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auinger AB, Besser G, Liu DT, Renner B, Mueller CA. Long-term impact of olfactory dysfunction on daily life. Wien Klin Wochenschr. 2021;133(19-20):1004-1011. doi: 10.1007/s00508-020-01751-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu DT, Prem B, Besser G, Renner B, Mueller CA. Olfactory-related quality of life adjustments in smell loss during the coronavirus-19 pandemic. Am J Rhinol Allergy. 2022;36(2):253-260. doi: 10.1177/19458924211053118 [DOI] [PubMed] [Google Scholar]
- 43.Speth MM, Singer-Cornelius T, Oberle M, Gengler I, Brockmeier SJ, Sedaghat AR. Mood, anxiety and olfactory dysfunction in COVID-19: evidence of central nervous system involvement? Laryngoscope. 2020;130(11):2520-2525. doi: 10.1002/lary.28964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yom-Tov E, Lekkas D, Jacobson NC. Association of COVID19-induced anosmia and ageusia with depression and suicidal ideation. J Affect Disord Rep. 2021;5:100156. doi: 10.1016/j.jadr.2021.100156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graves AB, Bowen JD, Rajaram L, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E ε4 status. Neurology. 1999;53(7):1480-1487. doi: 10.1212/WNL.53.7.1480 [DOI] [PubMed] [Google Scholar]
- 46.Doty RL. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16(6):478-488. doi: 10.1016/S1474-4422(17)30123-0 [DOI] [PubMed] [Google Scholar]
- 47.Xydakis MS, Albers MW, Holbrook EH, et al. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20(9):753-761. doi: 10.1016/S1474-4422(21)00182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42(6):1252-1258. doi: 10.1002/hed.26204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lechien JR, Cabaraux P, Chiesa-Estomba CM, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020;42(7):1583-1590. doi: 10.1002/hed.26279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. “Sniffin’ sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39-52. doi: 10.1093/chemse/22.1.39 [DOI] [PubMed] [Google Scholar]
- 51.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2, pt 1):176-178. doi: 10.1288/00005537-198402000-00004 [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Wei Y, Yu D, Zhang J, Liu Y. Olfactory and gustatory function in healthy adult Chinese subjects. Otolaryngol Head Neck Surg. 2010;143(4):554-560. doi: 10.1016/j.otohns.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 53.Doty RL, Marcus A, Lee WW. Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope. 1996;106(3, pt 1):353-356. doi: 10.1097/00005537-199603000-00021 [DOI] [PubMed] [Google Scholar]
- 54.Menon C, Westervelt HJ, Jahn DR, Dressel JA, O’Bryant SE. Normative performance on the Brief Smell Identification Test (BSIT) in a multi-ethnic bilingual cohort: a Project FRONTIER study. Clin Neuropsychol. 2013;27(6):946-961. doi: 10.1080/13854046.2013.796406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen DT, Rumeau C, Gallet P, Jankowski R. Olfactory exploration: state of the art. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(2):113-118. doi: 10.1016/j.anorl.2015.08.038 [DOI] [PubMed] [Google Scholar]
- 56.Wei G, Gu J, Gu Z, et al. Olfactory dysfunction in patients with coronavirus disease 2019: a review. Front Neurol. 2022;12:783249. doi: 10.3389/fneur.2021.783249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Bartheld CS, Hagen MM, Butowt R. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci. 2020;11(19):2944-2961. doi: 10.1021/acschemneuro.0c00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klimek L, Hagemann J, Hummel T, et al. Olfactory dysfunction is more severe in wild-type SARS-CoV-2 infection than in the Delta variant (B.1.617.2). World Allergy Organ J. 2022;15(6):100653. doi: 10.1016/j.waojou.2022.100653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124(4):826-831. doi: 10.1002/lary.24340 [DOI] [PubMed] [Google Scholar]
- 60.Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132(3):265-269. doi: 10.1001/archotol.132.3.265 [DOI] [PubMed] [Google Scholar]
- 61.London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 2008;63(2):159-166. doi: 10.1002/ana.21293 [DOI] [PubMed] [Google Scholar]
- 62.Lucidi D, Molinari G, Silvestri M, et al. Patient-reported olfactory recovery after SARS-CoV-2 infection: a 6-month follow-up study. Int Forum Allergy Rhinol. 2021;11(8):1249-1252. doi: 10.1002/alr.22775 [DOI] [PubMed] [Google Scholar]
- 63.Bussiere N, Mei J, Levesque-Boissonneault C, et al. Persisting chemosensory impairments in 366 healthcare workers following COVID-19: an 11-month follow-up. Chem Senses. 2022;47:bjac010. doi: 10.1093/chemse/bjac010 [DOI] [PubMed] [Google Scholar]
- 64.Ohla K, Veldhuizen MG, Green T, et al. A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology. Published online April 10, 2022. doi: 10.4193/Rhin21.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Description of Connecticut Chemosensory Clinical Research Center Test Procedures and Score Calculation