Introduction
Cicatricial alopecia describes hair loss wherein inflammation targets and ultimately destroys the hair follicle, resulting in the loss of follicular ostia.1 Though there is no cure, current first-line treatments are aimed at terminating the inflammatory process to prevent further hair loss and alleviate symptoms. These include topical and intralesional corticosteroids, immunosuppressants, and 5-α reductase inhibitors.2 Auxillary therapies including low-dose oral minoxidil can be utilized to increase the thickness of unaffected hair and conceal adjacent alopecic areas.3
Hair regrowth is unexpected during the course of treatment due to the conditions’ permanent destruction of multipotent follicular stem cells.4 Rare cases of hair regrowth in patients with cicatricial alopecia have been sporadically reported.1 The authors present a remarkable case of hair regrowth achieved with oral pioglitazone, topical clobetasol solution, topical minoxidil foam, and low-level laser therapy in a patient with multiple biopsies confirming a diagnosis of lichen planopilaris (LPP).
Case report
A 52-year-old female with a history of anemia, hypothyroidism, and basal cell carcinoma presented to our clinic for evaluation of biopsy-confirmed LPP. She reported significant hair shedding associated with scalp erythema, pruritus, and flaking for over 1 year. She was initially treated with ketoconazole 2% shampoo thrice per week and clobetasol 0.05% solution twice a day for 2 weeks and then to be applied as needed. However, given a lack of improvement after 8 months of this regimen, an initial 3-mm punch biopsy was performed at the site of involvement, revealing a scarring alopecia with polytrichia, perifollicular fibrosis, a perifollicular lymphocytic infiltrate, and largely preserved sebaceous gland lobules. Treatment was subsequently escalated to include hydroxychloroquine 200 mg twice a day, doxycycline 100 mg twice a day, and topical minoxidil 5% foam twice a day. Three months after following this regimen, she reported persistent scalp pain and pruritus, prompting referral to our clinic for further evaluation.
Physical examination revealed an alopecic area in the centroparietal scalp, with trichoscopy showing perifollicular hyperkeratosis and polytrichia. She was instructed to continue all medications and administered 2 cc of intralesional triamcinolone acetonide at 10 mg/cc and 5 cc of platelet-rich plasma. She received 5 total rounds of intralesional triamcinolone injections and 3 total platelet-rich plasma sessions. Four months later, she was found to have significant worsening of her alopecia and persistent scaling and pruritus, prompting initiation of oral prednisone 40 mg tapered over 8 weeks.
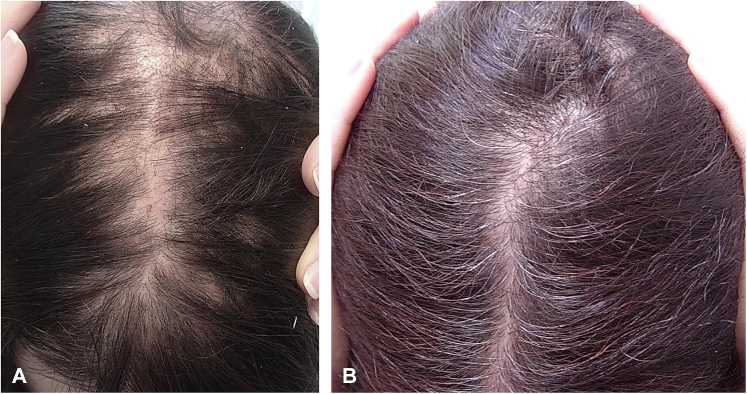
At a 6-month follow-up, a second, 4-mm punch biopsy was performed due to the patient’s unremitting symptoms and lack of response to conventional therapy. Repeat biopsy was notable for polytrichia, perifollicular fibrosis, follicular dropout, a mildly dense lymphocytic infiltrate, and loss of sebaceous glands, confirming the diagnosis of LPP. At this 6-month follow-up, the patient was started on oral pioglitazone 30 mg daily and began using a near monochromatic low-level laser therapy device every other day. Six weeks later, her symptoms persisted with continued hair loss and scalp pruritus, prompting initiation of oral methotrexate 15 mg once weekly with a folic acid 1-mg supplement. Hydroxychloroquine and doxycycline were discontinued due to perceived lack of efficacy. One month later, she was found to have minimal improvement with residual mild itching, prompting discontinuation of methotrexate. After 3 months, she was found to have a remarkable increase in hair density at the previously alopecic scarred area with resolution of scalp pruritus (Fig 1). No signs of active inflammation were observed on trichoscopy.
Fig 1.
Gross images demonstrating hair regrowth in a patient with cicatricial alopecia before (A) and 3 months after treatment with pioglitazone and LLLT (B). A, before treatment. B, 3 months after treatment. LLLT, Low-level light therapy.
Discussion
Though the pathogenesis of cicatricial alopecia remains poorly understood, disease activity is thought to be driven by hormonal and androgenetic factors perpetuating autoinflammatory destruction of the hair follicle.5 Current treatments are aimed at reducing the inflammatory burden of disease; however, the current literature lacks large randomized controlled trials to guide therapeutic decisions. Corticosteroids, immunosuppressants, and 5-α reductase inhibitors are among first-line treatment options for scarring hair loss.2 Additional medications, including pioglitazone, are considered as adjuvant therapies when first-line therapies fail to control disease progression and alleviate symptoms.
Pioglitazone is a peroxisome proliferator-activated receptor gamma agonist, conventionally used as a hypoglycemic agent. However, pioglitazone’s recently uncovered immunomodulating properties, as well as the revelation that decreased peroxisome proliferator-activated receptor gamma is implicated in the development of cicatricial alopecia, have established its role in the management of scarring hair loss.6 Pioglitazone has demonstrated efficacy in reducing patient-reported symptoms and objective signs of inflammation in LPP in oral doses of 15 mg or 30 mg daily.5 A retrospective analysis of 23 patients with LPP revealed that 78% of patients achieved stabilization or substantial improvement with the addition of pioglitazone to their treatment regimen.5 Though pioglitazone carries a black box warning for an increased risk of bladder cancer, the data in diabetic patients are conflicting, and no cases of bladder cancer occurring with pioglitazone use for the treatment of LPP have been reported.7
Low-level light therapy (LLLT) has been widely studied for the management of inflammatory conditions and is approved by the US Food and Drug Administration for the treatment of androgenetic alopecia and musculoskeletal pain. Though its exact mechanism in inducing hair growth is unknown, LLLT is hypothesized to act on mitochondria to alter cell metabolism, increase vasodilation, and modulate inflammation.8 LLLT has shown effectiveness in reducing inflammation and causing some hair regrowth after daily use for 3 months in several patients with LPP.9
Very few reported cases of significant hair regrowth in patients with biopsy-confirmed primary cicatricial alopecia exist. This unique case demonstrates dramatic hair regrowth in the setting of twice biopsy-confirmed cicatricial alopecia. This is the first reported case describing hair regrowth in cicatricial alopecia associated with pioglitazone addition. The authors believe pioglitazone was the main contributor in causing regrowth, as regrowth was seen 4 months after incorporating pioglitazone and prior therapies failed to control disease activity. This case establishes that hair regrowth following treatment for cicatricial alopecia is possible. However, further investigation into the inflammatory process underlying disease pathogenesis is warranted to elucidate the mechanisms by which these treatments work to cause regrowth in scarred areas.4
Conflict of interest
Dr Shapiro is a consultant for Aclaris Therapeutics, Incyte, and Replicel Life Sciences. Drs Shapiro and Lo Sicco have been investigators for Regen Lab and are investigators for Pfizer. Dr Lo Sicco is a consultant for Pfizer.
Footnotes
Authors Karim and Klein are co-first authors.
Drs Lo Sicco and Shapiro are co-senior authors.
Funding sources: None.
IRB approval status: Not applicable.
Patient consent: The patient provided consent for the publication of this case, including consent for clinical and pathological images.
References
- 1.Poliner A.D., Tosti A. Hair regrowth in cicatricial alopecia: a literature review. J Dermatol. 2021;48(8):1113–1128. doi: 10.1111/1346-8138.15902. [DOI] [PubMed] [Google Scholar]
- 2.Svigos K., Yin L., Fried L., Lo Sicco K., Shapiro J. A practical approach to the diagnosis and management of classic lichen planopilaris. Am J Clin Dermatol. 2021;22(5):681–692. doi: 10.1007/s40257-021-00630-7. [DOI] [PubMed] [Google Scholar]
- 3.Vañó-Galván S., Trindade de Carvalho L., Saceda-Corralo D., et al. Oral minoxidil improves background hair thickness in lichen planopilaris. J Am Acad Dermatol. 2021;84(6):1684–1686. doi: 10.1016/j.jaad.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Bolduc C., Sperling L.C., Shapiro J. Primary cicatricial alopecia: lymphocytic primary cicatricial alopecias, including chronic cutaneous lupus erythematosus, lichen planopilaris, frontal fibrosing alopecia, and Graham-Little syndrome. J Am Acad Dermatol. 2016;75(6):1081–1099. doi: 10.1016/j.jaad.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 5.Peterson E.L., Gutierrez D., Brinster N.K., Lo Sicco K.I., Shapiro J. Response of lichen planopilaris to pioglitazone hydrochloride. J Drugs Dermatol. 2019;18(12):1276–1279. [PubMed] [Google Scholar]
- 6.Karnik P., Tekeste Z., McCormick T.S., et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129:1243–1257. doi: 10.1038/jid.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis J.D., Habel L.A., Quesenberry C.P., et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314(3):265–277. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 8.Avci P., Gupta G.K., Clark J., Wikonkal N., Hamblin M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46(2):144–151. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randolph M.J., Salhi W.A., Tosti A. Lichen planopilaris and low-level light therapy: four case reports and review of the literature about low-level light therapy and lichenoid dermatosis. Dermatol Ther (Heidelb) 2020;10(2):311–319. doi: 10.1007/s13555-020-00359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]