Abstract
In this study, 11 hydroxybenzoic acids, 6 hydroxycinnamic acids, 6 flavonoids, and 2 synthetic phenolic antioxidants were evaluated according to their scavenging capacity and structure relationships. The IC50 was calculated for all compounds and the effects of the concentration of antioxidant and the length of the reaction on antioxidant capacity were taken into consideration. Based on the data of tested phenolics some structure-activity relationships were suggested and discussed in detail. Poor correspondence of the results between ABTS+• and DPPH• assays was attained, indicating that the antioxidant properties of each compound differ with regards to the applied method. Nevertheless, it can be argued that the number of electron-donating substituents (-OH and -OCH3) and their configuration has a significant impact on the antioxidant capacity. Undoubtedly, concerns about the reliability of these assays demand further in-depth investigations to give detailed insight into the structure and antioxidant activity relationships.
Keywords: Antioxidant activity, Free radical, DPPH•, ABTS+•, Phenolic compounds, Structure-activity relationship
Antioxidant activity; Free radical; DPPH•; ABTS+•; Phenolic compounds; Structure-activity relationship.
1. Introduction
Phenolic compounds are a vast group of phytochemicals that are naturally occurring in plants, vegetables, and fruits. The main functions of these compounds in plants are defense responses against UV radiation and pathogens; however, they also play a role as structural polymers, attractants, and signaling intermediates (D. Lin et al., 2016). For human health, redox homeostasis has a significant role in the prevention of aging and age-related chronic disorders like cardiovascular and neurodegenerative diseases. Oxidative stress is generally defined as the imbalance between reactive oxygen species (ROS) and antioxidants. Reactive oxygen species (ROS) are radicals that contain oxygen atoms. The members of this group include superoxide (O2•—), peroxide (O22—), singlet oxygen, and hydroxyl radical (•OH). Reactive nitrogen species include nitric oxide (NO−) and peroxynitrite anion (OONO−). Both groups are generated by normal cellular functions.
Excess amounts of ROS lead to oxidative damage to cells as they can attack various human macromolecules like protein, DNA, and lipids which might result in oncogene over-expression, mutagen formation, inflammation, and induction of atherogenic activity, and as a consequence, tissue damage and disease development are seen (Salehi et al., 2020).
Many studies have shown that phenolic compounds have high antioxidant activity (Fernandez-Panchon et al., 2008; Martins et al., 2016; Wojdyło et al., 2007). Antioxidants are stable compounds that react with the radicals and after the reaction, they continue to remain stable, even though that they contain radical electrons. This stability is because of the conjugated double bonds; therefore, radical electrons can be delocalized. As a result, antioxidants prevent damage by scavenging radicals and stopping the chain reactions (Halliwell, 1991). They are effective against reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive chlorine species (RCS) which are associated with some human diseases (Evans and Halliwell, 2001).
Phenolic antioxidants came from various sources of plants and Olive and olive oil, wine, apple vinegar, cumin, ginger, and whole grains serve as the dietary sources of phenolic antioxidants (Ninfali et al., 2005) and include flavonoid compounds, cinnamic acid derivatives, coumarins, tocopherols, and polyfunctional organic acids.
Recently, there has been an increasing interest in the use of phenolics as beneficial substances in the treatment of scurvy, cancer, osteoporosis, diabetes, and cardiovascular diseases (Abdel-Aty et al., 2019; Arts and Hollman, 2005; Barakat et al., 2020). Polyphenols are considered to be effective in preventing the development of coronary heart disease and atherosclerosis. Phenolics have an inhibitory effect on platelet aggregation and have an endothelium-dependent vasorelaxant effect (Cooper et al., 2004). Responsiveness of the cells to polyphenols is via interactions of polyphenols with receptors or enzymes that are involved in the signal transduction process, which may start a series of reactions to modify the redox state of the cells (Moskaug et al., 2005)As antioxidants, polyphenols’ actions are to improve cell survival. However, the effects of polyphenols in the human body are not limited to this. One example is the soy isoflavones interaction with estrogen receptors that modify the endocrine function and prevents bone resorption in postmenopausal women (Morabito et al., 2002).
Structures of phenolics contain benzene rings with hydroxyl substitutes linked to sugar residues. They range from simple conjugate molecules to highly complex polymerized molecules (Morabito et al., 2002). They are classified according to the number of phenol rings and double bonds in the ring system. There are 4 main classes of phenolics known as phenolic acids, lignans, flavonoids, and stilbenes (Spencer et al., 2008).
The mechanisms of action of antioxidants differ from compound to compound (Shahidi, 2000). When evaluating the structure-activity relationship of the phenolic antioxidants, it was understood that substitution at the para position with an ethyl or n-butyl group rather than a methyl group of phenol improves the antioxidant activity (Gordon, 1990). Besides, the presence of multiple hydroxyl groups contribute to antioxidant activity, likewise, the presence of a second hydroxy group at the ortho- or para-position (1,2- dihydroxybenzene derivative) seen in catechol and hydroquinone gives a better scavenging activity (Heijnen et al., 2001). In dihydroxybenzene derivatives, the antioxidant activity is because of the initially produced semiquinoid radical which can be formed into a quinone (Heim et al., 2002). Also, the presence of a carbonyl functional group in the C ring and double bond improves the stability of the flavonoid radical by helping to the delocalization of electrons and thus boosting its activity (Heim et al., 2002). The concentration of an antioxidant generally affects the activity of the antioxidant inversely. Phenolic antioxidants generally lose their activity at high concentrations and act as a pro-oxidant by contributing to the initiation reactions of radicals (Cillard et al., 1980).
Among phenolics, flavonoids and cinnamic acids show great antioxidant activity and act as free radical acceptors (Mehta and Seshadri, 1959). Flavanols act as metal ion chelators at the 3- hydroxy-4-keto group and/or 5-hydroxy-4-keto group (Pratt and Hudson, 1990). In these compounds degree and position of hydroxylation plays a key role in the antioxidant activity of the compound as the ortho-dihydroxylation of the B ring gives antioxidant activity; all flavonoids with 3′,4′-dihydroxy configuration have antioxidant activity (Pratt and Hudson, 1990). Also, the addition of a hydroxy group to the 5th position increases the antioxidant activity as seen in myricetin. Generally, the presence of ortho-dihydroxy groups on one ring and para-dihydroxy groups on the other ring produce powerful antioxidants (Pratt and Hudson, 1990). Glycosylation of flavonoids in the 3 position reduces the antioxidant activity.
For phenolic acids degree of hydroxylation and the number of the hydroxy groups in the molecule is important for their antioxidant activity. In these compounds presence of two or three phenolic hydroxy groups with a carbonyl group in the form of lactone or a chalcone, flavonone or flavone is essential for antioxidant activity (Dziedzic and Hudson, 1983).
There are various methods for the measurement of antioxidant activity. Radical scavenging assays which are a type of chemical assays are methods that are based on either hydrogen atom transfer (HAT) mechanism or single electron transfer (SET) mechanism. ABTS•+ radical cation decolorization assay and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical scavenging assay are methods that are based on electron donation of antioxidants to reduce the radical (Shahidi and Ambigaipalan, 2015).
Since in the literature phenolics demonstrate varying antioxidant efficacy under various experiment conditions, in this study, the antioxidant properties and structure-activity relationships of 25 phenolic compounds from various classes were assessed collectively using the ABTS•+ radical cation decolorization test and the DPPH• free radical scavenging assay under same experiment conditions in order to ensure the consistency of the results.
2. Materials and methods
2.1. Materials
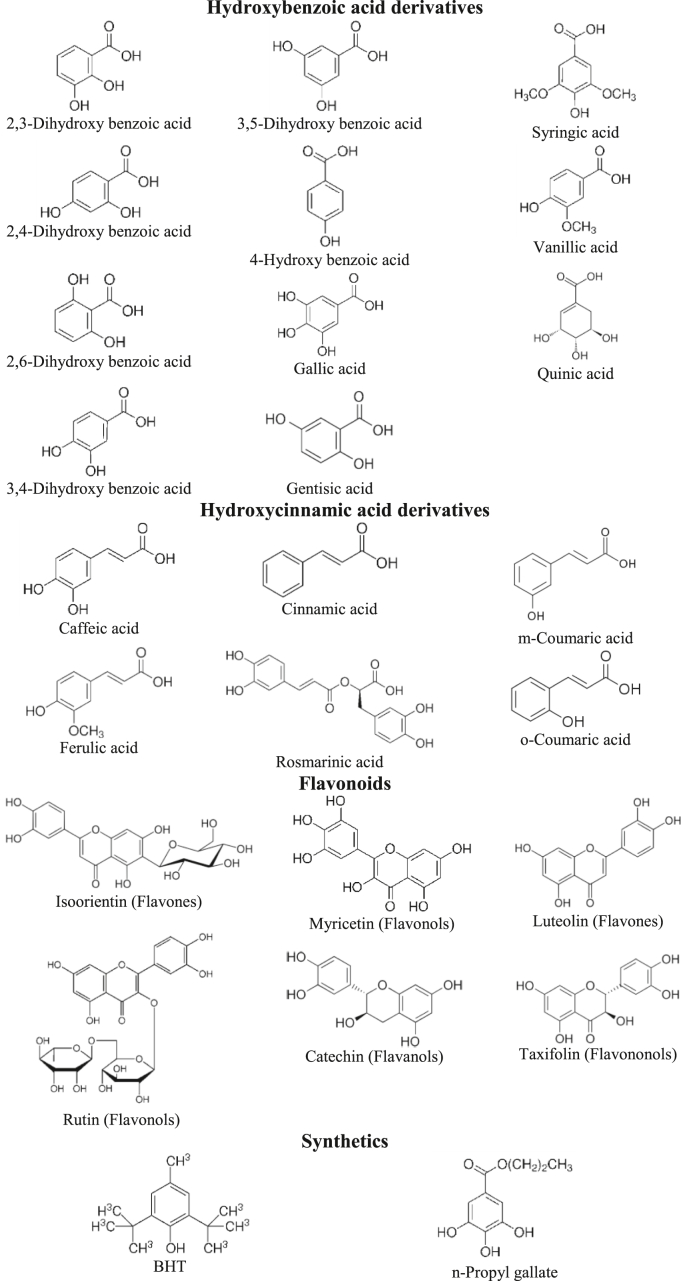
2,3-dihydroxy benzoic acid, 2,4-dihydroxy benzoic acid, 2,6-dihydroxy benzoic acid, 3,4-dihydroxy benzoic acid, 3,5-dihydroxy benzoic acid, 4-hydroxy benzoic acid, butylated hydroxytoluene (BHT), caffeic acid, catechin, cinnamic acid, ferulic acid, gallic acid, gentisic acid, isoorientin, luteolin, m-coumaric acid, myricetin, n-propyl gallate, o-coumaric acid, quinic acid, rosmarinic acid, rutin, syringic acid, taxifolin, and vanillic acid were used as test samples (Figure 1). Analytical grade DPPH•, ABTS+•, all chemicals and solvents were purchased from Sigma-Aldrich.
Figure 1.
Structures of tested phenolics.
2.2. DPPH• free radical scavenging assay
50 μL of sample was added in appropriate concentration for each dilution. 450 μL of 50 mmol L−1 Tris-HCl (pH 7.4) was added and then the well plate slowly shaked with the hand for 10 s to mix the solutions. 1.0 mL of 0.1 mmol L−1 DPPH• solution was added before 30 min incubation period in dark. The absorbance was measured at 517 nm using multimode plate reader. 50 μL of methanol was added instead of sample solution. Standard phenolic compounds were dissolved in methanol in 4 mg/mL concentration as stock solution and 4 different appropriate dilutions were prepared with same solvent. The results are given in mean value after three measurements. IC50 values were calculated by giving the value 50 to Y and depending on incubation time (Eq. 1).
| (1) |
2.3. ABTS+• radical cation decolorization assay
To preparation of ABTS+ stock solution, 36 mg of ABTS+ and 6.6 mg of K2O8S2 was weighed and dissolved in 10 mL of distilled water. Then, the solution was kept in a sealed screw mouth glass bottle in a dark place at room temperature for 16 h. At the end of this period, its absorbance was adjusted between 0.7-0.8 at 734 nm with water. This dilute solution was used in measurements. 50 μL of sample solution was added in appropriate concentration for each dilution. 100 μL of ABTS•+ solution was added and then, shook the microwell plate for 10 s to mix the reaction solution. With 5 min intervals, at 5, 10, 15 min reaction times, the absorbance values were recorded by performing UV spectral measurement at 734 nm using multiplate reader. For blank, 50 μL of methanol was added instead of sample solution. The results are given in mean value after three measurements. IC50 values were calculated by giving the value 50 to Y and depending on incubation time (Eq. 1).
3. Results and discussion
In this study, 25 phenolic compounds (Figure 1) used to investigate the relationship between antiradical activities and structure of phenolics such as benzoic acids, hydroxycinnamic acids and flavonoids. DPPH• and ABTS+• radicals which are often used in the literature were used and IC50 values were calculated for each compound and given in mean value in Table 1.
Table 1.
IC50 values of the tested phenolic compounds in DPPH• and ABTS•+ assays.
| Compounds | IC50 Values (μg/mL) |
|||
|---|---|---|---|---|
| DPPH assay | ABTS•+ assay 5 min | ABTS•+ assay 10 min | ABTS•+ assay 15 min | |
| hydroxybenzoic acids | ||||
| 2,3-dihydroxy benzoic acid | 29.559 | 4.604 | 4.012 | 3.782 |
| 2,4-dihydroxy benzoic acid | nd∗ | 27.553 | 48.442 | 85.856 |
| 2,6-dihydroxy benzoic acid | nc∗∗ | 12.075 | 7.514 | 5.895 |
| 3,4-dihydroxy benzoic acid | 47.524 | 8.655 | 7.314 | 6.943 |
| 3,5-dihydroxy benzoic acid | 10.755 | 2.872 | 2.720 | 2.605 |
| 4-hydroxy benzoic acid | nc | 101.609 | 52.731 | 32.115 |
| gallic acid | 49.913 | 2.260 | 1.811 | 1.572 |
| gentisic acid | 30.901 | 4.063 | 4.070 | 4.058 |
| syringic acid | 202.150 | 5.531 | 5.409 | 5.324 |
| vanillic acid | 351.993 | 3.984 | 3.635 | 3.449 |
| quinic acid | nc | 640.123 | 681.147 | 698.182 |
| hydroxycinnamic acids | ||||
| caffeic acid | 49.382 | 4.707 | 4.571 | 4.455 |
| cinnamic acid | nc | 368.182 | 297.585 | 245.126 |
| m-coumaric acid | 3.328 | 3.569 | 3.349 | 3.221 |
| o-coumaric acid | nc | 5.290 | 5.048 | 4.865 |
| ferulic acid | 217.730 | 2.238 | 4.234 | 4.135 |
| rosmarinic acid | 30.288 | 6.130 | 6.352 | 6.073 |
| Flavonoids | ||||
| isoorientin (flavones) | 191.933 | 5.382 | 5.045 | 4.767 |
| luteolin (flavones) | 52.444 | 4.005 | 3.564 | 1.952 |
| myricetin (flavonols) | 345.848 | 2.660 | 2.511 | 2.378 |
| rutin (flavonols) | 159.824 | 7.571 | 6.939 | 6.617 |
| catechin (flavanols) | 47.138 | 4.002 | 3.583 | 3.419 |
| taxifolin (flavononols) | 231.440 | 6.324 | 4.715 | 3.761 |
| Synthetics | ||||
| BHT | 191.998 | 10.095 | 8.490 | 7.707 |
| n-propyl gallate | 27.602 | 4.077 | 3.895 | 3.767 |
nd, not detected.
nc, not calculated.
3.1. DPPH• assay
Experimental results showed that among various classes of compounds that exhibited antioxidant activity, the scavenging ability of test samples increased with higher concentrations of test samples and indicated the scavenging capability of compounds with certain dose-effect relationships. The incubation time for all test samples was 30 min in the DPPH• assay. Thus, speculation about time-effect relationships was negligible in this study using DPPH• assay.
3.1.1. Hydroxybenzoic acids
Comparing IC50 values of compounds with hydroxybenzoic acid moiety indicated that 2,4-dihydroxybenzoic acid, 2,6-dihydroxybenzoic acid, 4-hydroxybenzoic acid, and quinic acid displayed poor activity in the same concentrations in the DPPH• assay. However, powerful antioxidant activities were represented by other test compounds of this group in the same concentrations. The order of antioxidant activity for these compounds is as follows: 3,5-dihydroxy benzoic acid > gentisic acid >3,4-dihydroxybenzoic acid > gallic acid > syringic acid > vanillic acid (Tables 1 and 2 and Figure 2). Previous studies have suggested that the degree of hydroxylation and hydroxylation positions affect the antioxidant abilities of compounds (Alcalde et al., 2019). The obtained data confirmed this hypothesis. For instance, 4-hydroxybenzoic acid a monohydroxy benzoic acid represented poor activity while 3,5-dihydroxybenzoic acid demonstrated strong activity. Moreover, activity among various dihydroxybenzoic acids with different hydroxylation positions was not the same and variant behavior was observed among compounds. The higher antioxidant activities of compounds seemed to be due to the presence of more than one hydroxyl group as well as hydroxylation in meta positions (3 or 5) with the highest activity seen in 3,5-dihydroxybenzoic acids with both 3 and 5 positions substituted by hydroxyl groups. In contrast, compounds with hydroxyl substituents in ortho (2 and 6) and para positions (4) with no meta position (3, 5) hydroxyl substituents were almost inactive. Additionally, the presence of electron-donating groups like methoxy substituents (-OCH3) in meta positions (3 and 5) seemed to contribute to the antioxidant activities of vanillic acid and syringic acid. However, the antioxidant activities of these compounds were far less than gallic acid which has hydroxyl substituents in meta position (3 and 5) indicating that hydroxyl substituents in meta positions increased antioxidant activity more than electron-donating groups. Comparison between 3,5-dihydroxybenzoic acid and gallic acid (3,4,5-trihydroxy benzoic acid) suggested that an additional hydroxy substituent at para position (4) led to a decrease in antioxidant activity of the gallic acid. The present investigation did not detect any antioxidant activity regarding quinic acid ((3R, 5R)-1,3,4,5-tetrahydroxycyclohexane-1-carboxylic acid). This was because of the absence of an aromatic structure, to permit the stabilization of the unpaired electron by resonance (Uranga et al., 2016).
Table 2.
Equations & R2 values of DPPH• assay and ABTS•+ graph results.
| Compounds | Equations & R2 Values |
|||
|---|---|---|---|---|
| DPPH• | ABTS•+ 5 min | ABTS•+ 10 min | ABTS•+ 15 min | |
| Hydroxybenzoic acids | ||||
| 2,3-dihydroxy benzoic acid | y = 22,533ln(x) - 26,306 | y = 8,9558x +8,7673 | y = 8,6252x + 15,39 | y = 8,4199x +18,153 |
| R2 = 0,8659 | R2 = 0,9008 | R2 = 0,7942 | R2 = 0,7619 | |
| 2,4-dihydroxy benzoic acid | y = 0,0269ln(x) - 0,3024 | y = -0,8579x+73,638 | y = -0,5726x+77,738 | y = -0,3502x + 80,067 |
| R2 = 0,0019 | R2 = 0,3816 | R2 = 0,2306 | R2 = 0,1097 | |
| 2,6-dihydroxy benzoic acid | y = -0,523ln(x) + 4,202 | y = 3,1351x +12,142 | y = 4,5313x +15,951 | y = 5,1916x +19,392 |
| R2 = 0,0764 | R2 = 0,9708 | R2 = 0,9364 | R2 = 0,9052 | |
| 3,4-dihydroxy benzoic acid | y = 27,667ln(x) - 56,829 | y = 5,5105x +2,3052 | y = 6,7069x +0,9442 | y = 7,0178x +1,2695 |
| R2 = 0,9727 | R2 = 0,996 | R2 = 0,9993 | R2 = 0,9985 | |
| 3,5-dihydroxy benzoic acid | y = 7,2191ln(x) - 27,642 | y = 7,3649x +28,846 | y = 7,2007x +30,407 | y = 7,0612x +31,599 |
| R2 = 0,9734 | R2 = 0,7012 | R2 = 0,696 | R2 = 0,6935 | |
| 4-hydroxy benzoic acid | y = 0,717ln(x) - 6,5962 | y = 0,3317x +16,296 | y = 0,4997x + 23,65 | y = 0,6571x +28,897 |
| R2 = 0,5678 | R2 = 0,996 | R2 = 0,9988 | R2 = 0,9949 | |
| gallic acid | y = 16,453ln(x) - 14,336 | y = 6,755x + 34,731 | y = 6,2792x +38,627 | y = 6,0553x +40,479 |
| R2 = 0,8706 | R2 = 0,6933 | R2 = 0,6946 | R2 = 0,6944 | |
| gentisic acid | y = 20,714ln(x) - 21,066 | y = 8,4234x +15,772 | y = 8,4601x +15,565 | y = 8,4678x + 15,63 |
| R2 = 0,867 | R2 = 0,8618 | R2 = 0,8587 | R2 = 0,8534 | |
| syringic acid | y = 15,886ln(x) - 34,339 | y = 8,8906x +0,8238 | y = 9,0153x +1,2277 | y = 9,0618x +1,7499 |
| R2 = 0,9276 | R2 = 0,9983 | R2 = 1 | R2 = 0,9993 | |
| vanillic acid | y = 18,198ln(x) - 56,706 | y = 7,4061x +20,491 | y = 7,4958x +22,747 | y = 7,5456x +23,969 |
| R2 = 0,9922 | R2 = 0,8172 | R2 = 0,8 | R2 = 0,7811 | |
| quinic acid | y = -0,228ln(x) + 3,2284 | y = 0,0762x +1,2226 | y = 0,073x + 0,2762 | y = 0,0716x +0,0101 |
| R2 = 0,0116 | R2 = 0,1631 | R2 = 0,2288 | R2 = 0,2726 | |
| Hydroxycinnamic acids | ||||
| caffeic acid | y = 25,419ln(x) - 49,124 | y = 8,7381x +8,8634 | y = 8,6571x +10,423 | y = 8,5701x +11,812 |
| R2 = 0,9793 | R2 = 0,951 | R2 = 0,9332 | R2 = 0,9193 | |
| cinnamic acid | y = -1,027ln(x) + 6,357 | y = 0,1411x - 1,9505 | y = 0,1755x - 2,2262 | y = 0,2145x - 2,5797 |
| R2 = 0,6459 | R2 = 0,8375 | R2 = 0,8428 | R2 = 0,8617 | |
| m-coumaric acid | y = 0,2398ln(x) - 1,6392 | y = 6,2795x +27,583 | y = 6,5439x +28,081 | y = 6,719x + 28,354 |
| R2 = 0,1882 | R2 = 0,7141 | R2 = 0,7066 | R2 = 0,7056 | |
| o-coumaric acid | y = 6,7012ln(x) - 40,586 | y = 8,8414x +3,2226 | y = 8,6253x + 6,454 | y = 8,4617x + 8,827 |
| R2 = 0,9646 | R2 = 0,9988 | R2 = 0,9957 | R2 = 0,9914 | |
| ferulic acid | y = 17,487ln(x) - 44,137 | y = 6,6475x +35,121 | y = 8,9241x +12,207 | y = 8,7703x +13,727 |
| R2 = 0,957 | R2 = 0,7163 | R2 = 0,6871 | R2 = 0,6832 | |
| rosmarinic acid | y = 19,03ln(x) - 14,907 | y = 8,6155x - 2,8215 | y = 7,4164x + 2,886 | y = 7,3909x +5,1093 |
| R2 = 0,8559 | R2 = 0,9616 | R2 = 0,7473 | R2 = 0,8208 | |
| Flavonoids | ||||
| Isoorientin | y = 13,257ln(x) - 19,694 | y = 8,5707x + 3,867 | y = 8,6168x + 6,527 | y = 8,5531x +9,2233 |
| R2 = 0,8943 | R2 = 0,9947 | R2 = 0,987 | R2 = 0,9649 | |
| Luteolin | y = 21,565ln(x) - 35,392 | y = 7,8483x + 18,56 | y = 7,5396x +23,126 | y = 7,3676x +25,616 |
| R2 = 0,952 | R2 = 0,9276 | R2 = 0,8803 | R2 = 0,8506 | |
| Myricetin | y = 11,564ln(x) - 17,612 | y = 7,1765x +30,905 | y = 7,0466x +32,303 | y = 6,9401x +33,496 |
| R2 = 0,7466 | R2 = 0,7125 | R2 = 0,7078 | R2 = 0,699 | |
| Rutin | y = 12,055ln(x) - 11,168 | y = 5,9994x +4,5775 | y = 6,4475x +5,2555 | y = 6,721x + 5,5234 |
| R2 = 0,6767 | R2 = 0,9841 | R2 = 0,9803 | R2 = 0,9793 | |
| Catechin | y = 22,859ln(x) - 38,078 | y = 8,6177x +15,505 | y = 8,4397x +19,756 | y = 8,3161x +21,566 |
| R2 = 0,9958 | R2 = 0,8063 | R2 = 0,7194 | R2 = 0,6949 | |
| Taxifolin | y = 16,659ln(x) - 40,697 | y = 5,1243x +17,591 | y = 5,9487x + 21,95 | y = 6,3619x +26,069 |
| R2 = 0,8768 | R2 = 1 | R2 = 0,9949 | R2 = 0,9867 | |
| Synthetics | ||||
| BHT | y = 22,141ln(x) - 66,406 | y = 5,011x - 0,588 | y = 5,8966x -0,0665 | y = 6,4687x + 0,1425 |
| R2 = 0,9984 | R2 = 0,9069 | R2 = 0,9165 | R2 = 0,9231 | |
| n-propyl gallate | y = 6,6524ln(x) + 27,928 | y = 8,1591x +16,728 | y = 8,0406x +18,679 | y = 7,9547x + 20,03 |
| R2 = 0,4045 | R2 = 0,8699 | R2 = 0,8568 | R2 = 0,8446 | |
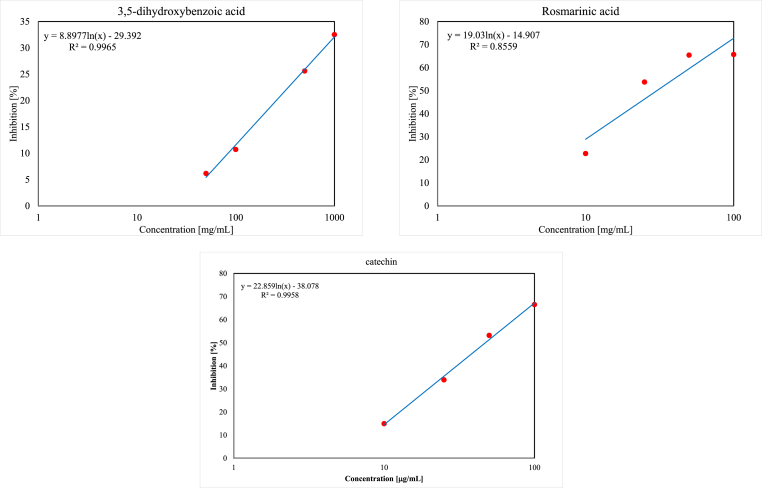
Figure 2.
DPPH• inhibition graphs of most active compounds for each phenolic group.
3.1.2. Hydroxycinnamic acids
Previous studies have uncovered important structural features of hydroxycinnamic acids (HCAs) that seemed to play important roles in the antioxidant activities of these compounds (Barone et al., 2009; Dziedzic and Hudson, 1984; Esteves et al., 2008; Graf, 1992; Siquet et al., 2006). The first one is the presence of hydroxyl substituents (-OH) on the benzene ring that might generate a phenoxy radical intermediate to terminate the free radical chain reaction (Graf, 1992). The number of the hydroxyl substituents on the aromatic ring also influenced the antioxidant activities of HCAs since adjacent hydroxyl groups can stabilize the formed phenoxy radical by electron donation (Dziedzic and Hudson, 1984). The second one was the side chain with an unsaturated ethylene bond that can stabilize the formed phenoxy radical or provide another reaction site for ROS (Barone et al., 2009). Additionally, several investigations have indicated that an increase in the number of hydroxyl substituents led to greater antioxidant activity (Zhu et al., 2006). Moreover; molecules containing ortho-dihydroxy or 4-hydroxy-3-methoxyl groups displayed more antioxidant activity (Cheng et al., 2007). Obtained data from the present investigation showed that cinnamic acid lacks antioxidant capability seemingly due to the absence of any hydroxyl substituent on the aromatic ring. Structurally similar monohydroxy substituted m-coumaric acid and o-coumaric represented dissimilar results. The antioxidant activity for m-coumaric acid was negligible while its isomer o-coumaric acid displayed poor activity. This might be seemingly due to the position of hydroxyl substituent in the aforementioned compounds indicating that species with o-configuration are more active than molecules with m-configuration. Higher antioxidant values associated with rosmarinic acid and caffeic acid compared to previous compounds seemed to be due to the presence of catechol moiety in these compounds. Catechol moiety allows the stabilization of produced phenoxy radical intermediate through intramolecular hydrogen bonding (Razzaghi-Asl et al., 2013). Lower antioxidant activity levels in ferulic acid than catechol moiety bearing molecules might be due to substitution of the 3-hydroxyl group with a methoxy (-OCH3) electron-donating group. The order of activity of these compounds is as follows: rosmarinic acid > caffeic acid > ferulic acid (Tables 1 and 2 and Figure 2).
3.1.3. Flavonoids
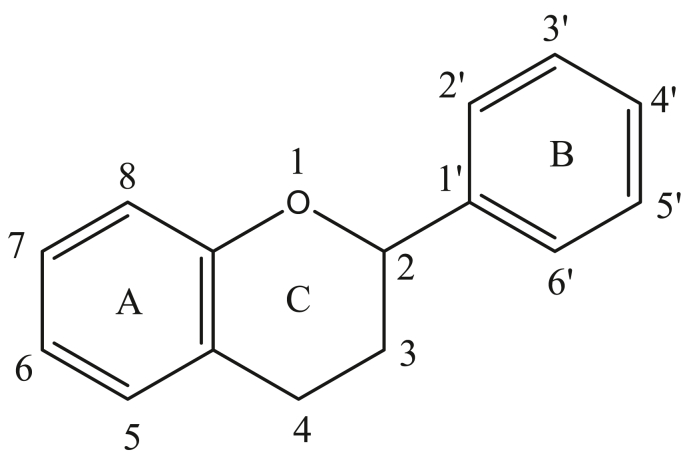
Flavonoids are constructed of a fundamental backbone structure called flavan nucleus which has 3 rings designated as rings A, B, C and is composed of 15 carbon atoms in C6–C3–C6 configuration (Figure 3) (Pietta, 2000).
Figure 3.
Basic flavonoid structure.
Flavonoids have a substituted phenyl ring at position 2 of the C-ring. The classification of flavonoids is according to C-ring structure which can be γ-pyrone for flavonols and flavones, a dihydropyrone for dihydroflavonols and flavanones, and a dihydropyrane for flavanols and within a class type and position of substituents on A and B rings differentiates the individual compounds (Heim et al., 2002; Wojdyło et al., 2007).
Rather than the nucleus flavan itself, the antioxidant activity of flavonoids seemed to rely on the presence and arrangement of substituents around this structure (Heim et al., 2002). Studies indicated that both the arrangement and the number of hydroxyl groups play a determinant role in the scavenging activity (Heim et al., 2002).
Configuration of hydroxyl substituents on B-ring was also of great importance in scavenging ROS and RNS species (Burda and Oleszek, 2001) while the influence of hydroxyl group configuration on A-ring in antioxidant activity seemed to be less significant (Heim et al., 2002). Reports suggested that a hydroxyl group on position 5 of A ring might have an impact on antioxidant activity. Additionally, the presence of a free 3-OH group has been suggested to have a great impact on the free radical scavenging of flavonoids (Burda and Oleszek, 2001). It is speculated that 3-OH formed intramolecular hydrogen bonds with B-ring hydroxyl groups and helped molecular structural planarity. Removal of 3-OH group abolished planarity of structure which played an important part in conjugation and electron delocalization and as a result compromised scavenging ability (Heim et al., 2002). The previous investigations on DPPH• scavenging activity of flavonoids have suggested that the presence of catechol or pyrogallol moiety on the B-ring was essential for the DPPH• radical scavenging activity (Cotelle et al., 1996).
Studies about the influence of methoxy groups for the antioxidant activity of flavonoids have suggested that methoxy groups increase lipophilicity, impose steric obstruction and disturb planarity of structure. The decrease in antioxidant activity due to O-methylation has been reported by previous work (Burda and Oleszek, 2001). Besides, the position of methoxy groups in B-ring seemed to have great influence since alteration of 6′-OH/4′-OMe configuration to 6′-OMe/4′-OH suppressed the ability to scavenge of DPPH• (Mathiesen et al., 1997). Also, 4′-O-methylation resulted in a remarkable decrease in antioxidant capability due to steric obstruction of the 3′4′-catechol structure (Dugas et al., 2000). Likewise, flavones with A-ring ortho-dimethoxy or trimethoxy structures showed less antioxidant activity due to the presence of methoxy groups on A-ring which seemed to inflict a negative effect on antioxidant activity by prevention of formation of the oxidation product malondialdehyde (MDA) in these compounds (Mora et al., 1990). However, a microsomal peroxidation assay reported that O-methylation increases antioxidant activity (Cholbi et al., 1991). Thereby, it has been suggested that the effect of methoxy groups in antioxidant activity differs according to the method, type of radical, and the oxidizable substrate which might be a lipid structure in which lipophilicity is important (Heim et al., 2002).
In general previous investigations supported the theory that although consideration of other structural features was essential, the presence of a 2,3 double bond and a 4-oxo group seemed to enhance antioxidant activities of flavonoids variably, and lacking one or both factors resulted in less effective antioxidants (Heim et al., 2002).
The correlation between the presence or absence of glycosidic moieties and antioxidant activity was sought out by several studies proposing that glycosidic moiety compromised the antioxidant activity of flavonoids and in doing so the position, total number, and structure of glycosidic moiety played a major role (Gao et al., 1999; Hollman et al., 1999; Ratty and Das, 1988). Thereby it was concluded that aglycones were more powerful antioxidants than corresponding glycosides, however, glucose moiety played role in bioavailability and increased absorption of dietary flavonoids in some cases (Heim et al., 2002). Glycoside moiety is usually located in position 3 and 7 of the dietary flavonoids however a 4′-glycoside was more suppressive than 3 or 7 position substitution. Additionally, A-ring sugar substitution diminished the activity more than 3-glycosylation (Mora et al., 1990). The reason behind such effects was considered to lie in i) disturbance of co-planarity of B-ring and ii) possession of free –OH groups, which is required to exert radical scavenging, by sugar moieties (Heim et al., 2002).
According to our findings the order of the tested flavonoids is as follows: catechin > luteolin > rutin > isoorientin > taxifolin > myricetin (Tables 1 and 2 and Figure 2).
The experiments showed the highest antioxidant activity for (±)-catechin (flavan-3-ol). By comparing chemical structure and IC50 values of catechin and taxifolin it can be postulated that higher antioxidant activity of catechin in DPPH• assay was due to the absence of a 4-oxo functional group which is present in taxifolin (flavononol). The 4-oxo functional group in taxifolin seemed to decrease the antioxidant activity since both compounds possess similar structure: (i) catechol moiety in B-ring, (ii) 3,5,7 trihydroxy substitution, and (iii) saturated 2,3-double bond. This decline in activity might be due to electron-withdrawing characteristics of the oxo-functional group (=O). luteolin with a similar structure has an additional unsaturated 2,3-double bond and lacks 3-OH substituent. This configuration has increased the antioxidant potency of luteolin compared to taxifolin and the presence of the 2,3-unsaturated double bond might provide conjugation of the 4-oxo group with the unsaturated bond allowing electron delocalization thereby increasing scavenging capability. Our experiments evidenced lower antioxidant activity for isoorientin (luteolin-6-C-glucoside) corresponding to luteolin. This was possibly due to the presence of glycosidic moiety in isoorientin which decreased the overall scavenging capability as reported by previous studies that sugar moieties suppress activity (Heim et al., 2002; Hopia and Heinonen, 1999). However, an exception to this trend is 3-O-glycoside rutinose disaccharide which did not seem to diminish the antioxidant activity of rutin greatly by its presence as explained in previous experiments (Heim et al., 2002). The least antioxidant activity among the tested flavonoids was observed with myricetin. This might be due to the presence of pyrogallol moiety which has lower antioxidant potential than catechol moiety in B-ring (Furuno et al., 2002). Also, it was reported that antioxidant activity leveled up with an increase in hydroxy groups up to five of –OH while in compounds with six hydroxyl substitutions like myricetin there was a decline in activity (Mikamo et al., 2000).
3.1.4. Synthetic phenolic antioxidants
Synthetic antioxidants are widely used in food and cosmetics. Two major components are BHT and propyl gallate. propyl gallate (PG) is the n-propyl ester of gallic acid. It is thought that antioxidant activity resides in its hydroxyl groups which donate hydrogens to free radicals compromising the radical propagation chain during the oxidation process.
Additionally, the carboxylic group (-COOH) has an electron-withdrawing feature that adversely affects the hydrogen donation ability of the molecules thus it was suggested that esterification neutralizes this negative effect. Moreover, the esterification of gallic acid has provided PG with enhanced lipid solubility in organic media (Garrido et al., 2012).
The results indicated higher antioxidant activity of PG corresponding to gallic acid and BHT (Tables 1 and 2). It can be suggested that this might be due to more –OH groups of PG in number (pyrogallol moiety of PG) compared to BHT which might play important role in hydrogen donation. With regards to gallic acid, changing the carboxylic group of gallic acid to an ester moiety seemed to enhance the antioxidant activity of PG.
BHT was used as a standard antioxidant in this work and showed relatively lower antioxidant activities compared to others. BHT belongs to a class of compounds referred to as hindered phenols (Yehye et al., 2015). The antioxidant activity of BHT resides in its structural features. It contains two tert-butyl groups at 2 and 6-positions and a methyl group on 4-position. These groups have electron-donating characteristics, and it has been evidenced by prior studies that such substitutions at 2,4 and 6-positions contribute to the higher antioxidant activity of phenols. The underlying reason behind such effects is thought to be because of reducing bond dissociation energy (BDE) of the phenolic –OH group and providing stabilization to phenoxyl radical via inductive and hyperconjugation effects. Also, it has been reported that steric hindrance resulted from ortho-position substituents minimize unwanted reactions such as pro-oxidation (E. Klein et al., 2005).
3.2. ABTS+• assay
It has been reported that the rate of radical trapping by antioxidants is necessary to be considered along with the obtained antioxidant capacity to deduce the antioxidant reactivity of compounds and ABTS+• assay only measures the antioxidant capacity and does not evaluate and provide information about neither the antioxidant reactivity nor concurrent inhibition rates (Kajimoto and Kaneto, 2004). Also, the reaction end-time for the ABTS+• reaction is generally considered as 4 or 6 min. The results obtained from the ABTS+• assay are a bit controversial as it is hard to predict the underlying chemistry using this method and the TEAC values obtained for the same antioxidant show differences between different studies (Ilyasov et al., 2020). This might be because of (i) reaction time limitations; meaning the scavenging reaction of ABTS+• doesn’t end at 4 or 6 min endpoints and inhibition of ABTS+• continues after this time by some antioxidants (O. I. Klein et al., 2018; Zheng et al., 2016) and (ii) concentration of some antioxidants play an important role on obtained antioxidant capacities (Ilyasov et al., 2018). Additionally, ABTS+• radical site accessibility and formation of coupling adducts with ABTS+• by a certain group of antioxidants, which have their own exclusive ABTS+• scavenging ability that contributes to the overall antioxidant capacity of the compound, might have an impact on the obtained results (Ilyasov et al., 2020). However, more evidence is required to determine the extent of the impact of these adducts on overall scavenging capacity.
Investigation of results from various studies pointed out that although hydroxyl substituents have an impact on scavenging capacity, the number of these substituents did not seem to always have a connection with scavenging capacity since some substances with a lower number of –OH groups showed higher scavenging capacity and also the configuration of hydroxyl groups had significant effects on the results (Nenadis et al., 2004). Further investigation is required to elucidate the underlying mechanism in ABTS+• radical scavenging as several studies have suggested various possible mechanisms for quenching ABTS+• radical (Ilyasov et al., 2020; Schaich et al., 2015). However, apparently for phenolic compounds, it is estimated that the sequential proton loss electron transfer (SPLET) mechanism stands out to be the most potential mechanism where phenoxide anions are formed from phenolic compounds in water and alcohol and act as electron donors in the SPLET mechanism (Ilyasov et al., 2020).
| ROH → RO− + H+ |
| RO− + ABTS•+ → RO• + ABTS |
| ABTS + H+ → ABTSH+ |
| SPLET Mechanism |
Our findings demonstrated that test samples from various phenolic compounds represented different antioxidant capacities. Also, obtained values from the ABTS•+ assay was not consistent with data from the DPPH• assay. This might be due to substantial differences in these assays such as different reaction media (DPPH• media is more suitable for hydrophobic antioxidants while ABTS+• media applies to both hydrophilic and lipophilic antioxidants), solubility of test samples in corresponding reaction media, and different inherent properties of radicals such as molecular size (Floegel et al., 2011; C. Lin et al., 2014; Miller et al., 1993; Re et al., 1999; Schaich et al., 2015).
The obtained data indicated that the antioxidant capacities of evaluated phenolics are dependent on the concentration of the test samples as higher concentrations were associated with more scavenging capacity. Additionally, it was observed that various compounds showed different time-scavenging capacity relationships. Generally, in most of the compounds (not all) increase in the reaction time/incubation time correlated with higher antioxidant capacity while the antioxidant capacity of some compounds seemed to be time-independent. Few compounds also represented invert time-scavenging capacity relationships.
3.2.1. Hydroxybenzoic acids
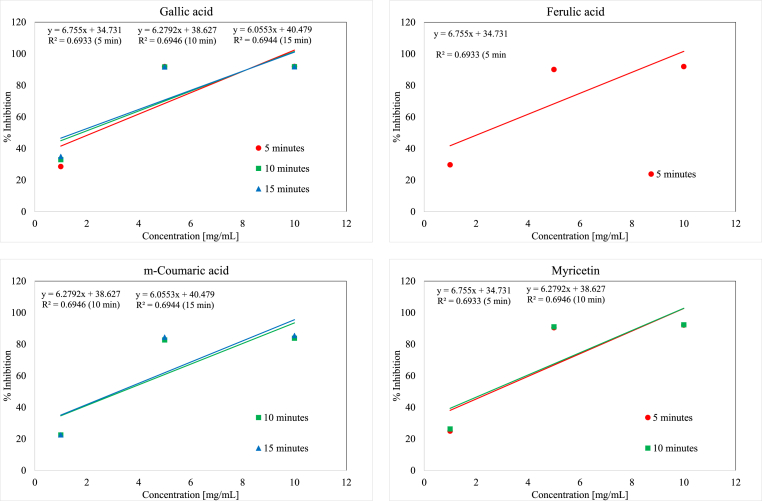
Experimental results represented no antioxidant capability for quinic acid similar to DPPH• assay which might be due to the lack of aromatic structure of this compound. However, other compounds of this group showed relatively acceptable antioxidant capabilities. The order of antioxidant capability of other compounds of this group at 5, 10, 15 min reaction times is as follows (Tables 1 and 2 and Figure 4):
- 5 min Reaction Time
- gallic acid >3,5-dihydroxy benzoic acid > vanillic acid > gentisic acid >2,3-dihydroxy benzoic acid > syringic acid >3,4-dihydroxy benzoic acid >2,6-dihydroxy benzoic acid >2,4-dihydroxy benzoic acid > 4-hydroxy benzoic acid
- 10 min Reaction Time
- gallic acid >3,5-dihydroxy benzoic acid > vanillic acid >2,3-dihydroxy benzoic acid > gentisic acid > syringic acid >3,4-dihydroxy benzoic acid >2,6-dihydroxy benzoic acid >2,4-dihydroxy benzoic acid > 4-hydroxy benzoic acid
- 15 min Reaction Time
- gallic acid >3,5-dihydroxy benzoic acid > vanillic acid >2,3-dihydroxy benzoic acid > gentisic acid > syringic acid >2,6-dihydroxy benzoic acid >3,4-dihydroxy benzoic acid > 4-hydroxy benzoic acid >2,4-dihydroxy benzoic acid
Figure 4.
ABTS+● inhibition graphs of most active compounds for each phenolic group.
From the results, it can be estimated that the number and position of substituents contributed to the scavenging potential of the compounds. Also, the incubation time (reaction time) of the compounds with ABTS+• radical affected the antioxidant capacity of compounds. Most of the compounds showed higher antioxidant capacities at longer reaction times. The results for gentisic acid did not show a great change at different reaction times indicating that the antioxidant capacity of gentisic acid was time independent. Interestingly in the case of 2,4-dihydroxybenzoic acid weaker antioxidant capacities were observed at longer incubation periods that might be due to the pro-oxidation reaction caused by this compound. Additionally, some variations in the order of antioxidant capacities of compounds could be seen at 10- and 15-min reaction times corresponding to data obtained from 5 min reaction time indicating the slow reaction of such antioxidants and the continuation of inhibition of ABTS+• radical by such antioxidants. For instance, 2,6-dihydroxybenzoic acid showed greater antioxidant capacity when compared to gentisic acid at 10- and 15-min reaction times while it was lower at 5 min reaction time. Likewise, the scavenging capacity of 2,6-dihydroxybenzoic and 4-hydroxybenzoic acid were relatively higher than 3,4-dihydroxybenzoic acid and 2,4-dihydroxybenzoic acid at 10- and 15-min reaction times respectively.
From a structural aspect, based on the results it could be suggested that substitutions in 3 and 5 positions played a more prominent role in antioxidant capacity than substitutions in 2,4, and 6 positions. The results didn’t give us a clear idea about the relationship of the number of hydroxyl groups and methoxy groups with antioxidant capacities as contraindicating points were attained. For instance, the highest antioxidant capacity in all reaction times was seen with gallic acid which has a 3,4,5-trihydroxy configuration (3-OH) suggesting that the configuration and number of hydroxyl groups might be determinant. Likewise, 3,5-dihydroxybenzoic acid (2-OH) represented good but slightly lower antioxidant capacities than gallic acid in all reaction times which might be due to lack of a hydroxyl group at 4-position. However, vanillic acid with 3-methoxy 4-hydroxy configuration (1-OH) showed better antioxidant capacities in all reaction times than other dihydroxybenzoic acids and especially the 3,4-dihydroxybenzoic acid (2-OH) that might be due to the presence of methoxy group (-OCH3) in 3 position rather than hydroxyl group, indicating more potency was attained with methoxy groups. In contrast to the latter statement, when gallic acid was compared to syringic acid, conversion of the 3,5-dihydroxy groups to 3,5-dimethoxy group in syringic acid resulted in lower antioxidant capacity indicating a decrease in values due to the presence of methoxy groups rather than hydroxyl groups in aforementioned positions. Additionally, it could be seen that the number of methoxy groups did not correlate to antioxidant capacity when values obtained for syringic acid (2-OCH3) with vanillic acid (1-OCH3) were compared. Comparison of IC50 values of Gentisic acid with 2,5 dihydroxybenzoic acid and 2,3-dihydroxybenzoic acid showed almost similar scavenging capacities which were relatively higher than 2,6-dihydroxybenzoic acid, 2,4-dihydroxy benzoic, and 4-hydroxybenzoic acid. These findings point outed the significance of the configuration of hydroxyl groups as test samples with at least one hydroxyl group at meta position (3,5-position) exhibited higher antioxidant capacities. 4-Hydroxybenzoic acid with one hydroxyl group had the least antioxidant capacity that might be due to the lower number of hydroxyl groups compared to other dihydroxybenzoic acids and the absence of methoxy groups which might be contributed to higher antioxidant capacities of vanillic acid and syringic acid.
3.2.2. Hydroxycinnamic acids
Identical to the DPPH• assay, results attained from the ABTS+• assay demonstrated that the antioxidant capacity of cinnamic acid was null. This might be due to the absence of hydroxyl substituents on the benzene ring. Nevertheless, good antioxidant capacities were displayed for other members of this class. The order of antioxidant capabilities of other compounds of this group at 5,10-, and 15-min reaction times were as follows (Tables 1 and 2 and Figure 4):
- 5 Min Reaction Time
- ferulic acid > m-coumaric acid > caffeic acid > o-coumaric acid > rosmarinic acid
- 10 Min Reaction Time
- m-coumaric acid > ferulic acid > caffeic acid > o-coumaric acid > rosmarinic acid
- 15 Min Reaction Time
- m-coumaric acid > ferulic acid > caffeic acid > o-coumaric acid > rosmarinic acid
Although it was difficult to give a definite opinion about the structure-activity of hydroxycinnamic acids based on ABTS+• assay, attained outcomes from the ABTS+• assay of tested hydroxycinnamic acids supplied us with some estimations about the role of the number and configuration of substituents in the antioxidant capacity of compounds. Clearly, it could be said that the antioxidant capacity of these compounds was concentration dependent as more scavenging ability was seen in higher concentrations. The relationship between the incubation time and antioxidant capacity varied according to the tested subject. caffeic acid, m-coumaric acid, and its isomer o-coumaric acid showed time-dependent antioxidant capacity as increase in incubation time translated to higher scavenging capacities in the aforementioned compounds. rosmarinic acid illustrated a time-independent scavenging ability as with the increasing of incubation time there was almost little change in the IC50 values. Interestingly ferulic acid represented a decline in scavenging capacity as incubation time increased from 5 min to 10 min and if it was increased to 15 min a similar decrease in scavenging capacity was observed but to a slightly lesser degree than scavenging capacity seen at 10 min incubation time. This might be due to the occurrence of side reactions such as the formation of coupling adducts with ABTS+• by ferulic acid or pro-oxidation reaction that was encouraged by the increase of the reaction time.
From structural prospect, it seemed that the contribution of meta-position substituents (3-position) on benzene ring to antioxidant capacity was significant. Much could not be said about the relationship of the number of hydroxyl or methoxy substituents with antioxidant capacity as variant results were displayed regarding the matter. The highest scavenging at the 5 min reaction time belonged to ferulic acid with 3-methoxy and 4-hydroxy configuration. In the longer reaction times (10 and 15 min) this place belonged to m-coumaric acid with a single hydroxyl group on position 3. Interestingly in all reaction times, ferulic acid showed relatively higher antioxidant capacity than caffeic acid with 3,4 dihydroxy substitution. Additionally, m-coumaric acid with meta-substitution had a higher scavenging capacity than the isomer o-coumaric acid with ortho-substitution. Based on this, it was speculated that hydroxyl group substitution on meta-position had a greater impact on scavenging capacity than ortho hydroxyl group substitution. Also, changing the hydroxyl substituent in position 3 to methoxy group in dihydroxy (3,4) compounds led to greater scavenging capacity. No correlation between the number of hydroxyl groups and scavenging capacity could be deduced from the results. Although rosmarinic acid contained 2 catechol moieties, the lowest antioxidant capacity among tested samples was associated with it. This might be due to the large molecular size of rosmarinic acid and limitations in accessing the ABTS+• radical site.
3.2.3. Flavonoids
IC50 values of the tested flavonoids implied that the scavenging capacity depended on the reaction time. All the tested samples showed greater scavenging capacities in longer reaction times. Also, there was a clear dependency of scavenging capacity on the concentration of test subjects demonstrated by higher scavenging capacity in higher concentrations. The order of scavenging capacity at different reaction times was as follows (Tables 1 and 2 and Figure 4):
- 5 Min Reaction Time
- myricetin > catechin ≥ luteolin > isoorientin > taxifolin > rutin
- 10 Min Reaction Time
- myricetin > luteolin > catechin > taxifolin > isoorientin > rutin
- 15 Min Reaction Time
- luteolin > myricetin > catechin > taxifolin > isoorientin > rutin
The variations in the order of the scavenging capacities of the tested flavonoids at different reaction times might be explained by the slow reaction of some compounds with ABTS+• radical. luteolin and taxifolin were compounds that seemed to exhibit such characteristics. At 5 min reaction time, they displayed low scavenging capacity compared to others but as the reaction time increased there was a much greater change in their scavenging capacity than other compounds.
From the structural point of view, better scavenging activity was seen in catechin while comparing it with taxifolin in all reaction times. These two compounds were structurally similar with only a 4-oxo group present in taxifolin. The electron-withdrawing characteristic of 4-oxo might be the reason for the lower scavenging capacity of taxifolin compared to catechin. luteolin showed almost the same scavenging capacity as catechin at 5 min reaction time but was more potent than catechin at longer reaction times which became the compound with the highest scavenging capacity at 15 min reaction time. It also demonstrated better scavenging capacity than taxifolin in all of the reaction times. One explanation for this might be that the compound had a slow reaction with ABTS+•. Additionally, the presence of an additional 2,3-double bond in the structure with no 3-OH group, might allow conjugation and better electron delocalization in presence of the 4-oxo group. isoorientin which was a glycosidic derivative of luteolin had a lower scavenging capacity corresponding to parent compound luteolin. It could be speculated that this might be because of the presence of glycoside moiety in the structure at the 6th position in A ring which suppressed the scavenging ability. In contrast to DPPH• assay data, the results for scavenging capacity of myricetin indicated that it had the highest scavenging capacities at 5- and 10-minute reaction times with only lower than luteolin at 15 min reaction time. It might be explained that these values were observed because of: (i) pyrogallol moiety (3-OH) in B-ring rather than the catechol moiety (2-OH); and (ii) presence of a free –OH group at position 3 in addition to the presence of the important 4-oxo group and 2,3-double bond functional groups. Based on ABTS+• assay, rutin which has a rutinose disaccharide moiety at position 3 was associated with the lowest scavenging capacity among all tested flavonoids. It might be hypothesized that this situation is because of suppression of scavenging capacity by the glycosidic moiety of rutin as well as the large molecular size of rutin which might affect its accessibility to the ABTS+• radical site. These findings were not consistent with the results from the DPPH• assay and further investigation is required to elucidate the chemistry behind these contraindicating findings from both assays.
3.2.4. Synthetic phenolic antioxidants
Between the two tested synthetic phenolic antioxidants, it turned out that propyl gallate had better scavenging capacity than BHT in all the recorded reaction times (Tables 1 and 2). Also, both compounds displayed time-dependent scavenging capacity that increased at longer reaction times that might be due to the slow reaction of these compounds with ABTS+•. About the SAR of these compounds, it was known that BHT is a hindered phenol. Thus, steric accessibility to ABTS+• radical site might affect the scavenging capacity of the compound. Also, Orto-substituted tert-butyl groups might have a lowering effect on (BDE) bond dissociation energy of phenolic –OH group affecting the overall scavenging capacity.
PG which is an ester derivative of gallic acid showed relatively lower antioxidant capacity than the parent compound. It seemed that the esterification of the carboxylic group had led to lower antioxidant capacities in ABTS+• assay opposing its possible effect in scavenging ability of DPPH• radical. This variation might be due to the more hydrophobic nature of the DPPH• reaction medium than the ABTS+• reaction medium and the fact that esters have better solubility in hydrophobic media.
4. Conclusion
By analyzing the results from DPPH• and ABTS+• assays it can be concluded that poor correlation among assays exists. Our investigation had revealed that cinnamic acid and quinic acid are inert regardless of the method. Also, the concentration of samples affects greatly the reactivity of compounds that exhibit antioxidant activity in ABTS+• and DPPH• assays. Additionally, it was found that reaction time has a significant role in determining the scavenging capability of tested phenolics in ABTS+• assay as most of the compounds showed greater values in longer durations. An explanation that could be suggested was the slow reaction of some test samples compared to others or the formation of coupling adducts with ABTS+• radical by these compounds. Poor reactivity of some compounds seen in DPPH• assay such as some of the hydroxybenzoic acid derivatives compared to ABTS+• assay might be explained by the more hydrophobic nature of DPPH• reaction media. However, better structure-activity relationship patterns of phenolic compounds could be formed by the data from DPPH• assay than ABTS+• assay as these patterns also correlate to previous studies. Taken all together current results suggested that in DPPH• assay, (i) the number and configuration of hydroxyl groups had a determinant role in scavenging ability of hydroxybenzoic acids, hydroxycinnamic acids, and flavonoids (ii) glycosidic moieties with exception of rutin generally had an activity suppressing role in flavonoids (iii) presence of both 2,3-double bond and a 4-oxo group or absence of both associated with the presence of a 3-OH group enhanced the activity of flavonoids (iv) the number of –OH groups and esterification was associated with the activity of PG and Orto di-tert butyl group of BHT contribute to its antioxidant activity. Regarding the scavenging capacities in ABTS+• it could be argued that rather than the specific number and configuration of –OH groups, the number and configuration of electron-donating groups (both –OH and -OCH3) as general contributed to antioxidant capacities with more emphasis on the position on substituents. It is important to note that even though ABTS+• and DPPH• assays were used abundantly, the structure-antioxidant activity of phenolics was yet to be defined and this work provided a basis for further elucidation of the chemistry behind antioxidant activities of phenolics. Further investigation of the pharmacokinetic and pharmacodynamic characteristics of these compounds was essential to evaluate their benefits. Further studies on the bioavailability and toxicity of phenolic compounds and their metabolites and their excretion mechanisms in animal models and human subjects played a key role in such assessments. Finally, isolating pure phenolics from extracts using various methods and compounding them with other ingredients to be delivered as dietary supplements may promote health and reduce the risk of various health disorders. To achieve such a goal further assessment of possible interaction of phenolics with excipients and other drugs is crucial.
Declarations
Author contribution statement
Amir Moazzen: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nesrin Öztinen; Ezgi Ak-Sakalli: Contributed reagents, materials, analysis tools or data.
Müberra Koşar: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdel-Aty A.M., Hamed M.B., Salama W.H., Ali M.M., Fahmy A.S., Mohamed S.A. Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: phytochemical screening, antioxidant and cytotoxic properties. Biocatal. Agric. Biotechnol. 2019;20 [Google Scholar]
- Alcalde B., Granados M., Saurina J. Exploring the antioxidant features of polyphenols by spectroscopic and electrochemical methods. Antioxidants. 2019;8(11):523. doi: 10.3390/antiox8110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts I.C.W., Hollman P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81(1 Suppl):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Barakat A.Z., Hamed A.R., Bassuiny R.I., Abdel-Aty A.M., Mohamed S.A. Date palm and saw palmetto seeds functional properties: antioxidant, anti-inflammatory and antimicrobial activities. J. Food Meas. Char. 2020;14(2):1064–1072. [Google Scholar]
- Barone E., Calabrese V., Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology. 2009;10(2):97–108. doi: 10.1007/s10522-008-9160-8. Biogerontology. [DOI] [PubMed] [Google Scholar]
- Burda S., Oleszek W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001;49(6):2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- Cheng J.-C., Dai F., Zhou B., Yang L., Liu Z.-L. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: mechanism and structure–activity relationship. Food Chem. 2007;104(1):132–139. [Google Scholar]
- Cholbi M.R., Paya M., Alcaraz M.J. Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation. Experientia. 1991;47(2):195–199. doi: 10.1007/BF01945426. [DOI] [PubMed] [Google Scholar]
- Cillard J., Cillard P., Cormier M. Effect of experimental factors on the prooxidant behavior of α-tocopherol. JAOCS (J. Am. Oil Chem. Soc.) 1980;57(8):255–261. [Google Scholar]
- Cooper K.A., Chopra M., Thurnham D.I. Wine polyphenols and promotion of cardiac health. Nutr. Res. Rev. 2004;17(1):111–130. doi: 10.1079/NRR200482. [DOI] [PubMed] [Google Scholar]
- Cotelle N., Bernier J.L., Catteau J.P., Pommery J., Wallet J.C., Gaydou E.M. Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med. 1996;20(1):35–43. doi: 10.1016/0891-5849(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Dugas A.J., Castañeda-Acosta J., Bonin G.C., Price K.L., Fischer N.H., Winston G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: structure-activity relationships. J. Nat. Prod. 2000;63(3):327–331. doi: 10.1021/np990352n. [DOI] [PubMed] [Google Scholar]
- Dziedzic S.Z., Hudson B.J.F. Polyhydroxy chalcones and flavanones as antioxidants for edible oils. Food Chem. 1983;12(3):205–212. [Google Scholar]
- Dziedzic S.Z., Hudson B.J.F. Phenolic acids and related compounds as antioxidants for edible oils. Food Chem. 1984;14(1):45–51. [Google Scholar]
- Esteves M., Siquet C., Gaspar A., Rio V., Sousa J.B., Reis S., Marques M.P.M., Borges F. Antioxidant versus cytotoxic properties of hydroxycinnamic acid derivatives - a new paradigm in phenolic research. Arch. Pharmazie. 2008;341(3):164–173. doi: 10.1002/ardp.200700168. [DOI] [PubMed] [Google Scholar]
- Evans P., Halliwell B. Micronutrients: oxidant/antioxidant status. Br. J. Nutr. 2001;85(Suppl 2):S67–74. [PubMed] [Google Scholar]
- Fernandez-Panchon M.S., Villano D., Troncoso A.M., Garcia-Parrilla M.C. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008;48(7):649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- Floegel A., Kim D.-O., Chung S.-J., Koo S.I., Chun O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011;24(7):1043–1048. [Google Scholar]
- Furuno K., Akasako T., Sugihara N. The contribution of the pyrogallol moiety to the superoxide radical scavenging activity of flavonoids. Biol. Pharm. Bull. 2002;25(1):19–23. doi: 10.1248/bpb.25.19. [DOI] [PubMed] [Google Scholar]
- Gao Z., Huang K., Yang X., Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta Gen. Subj. 1999;1472(3):643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Garrido J., Garrido E.M., Borges F. Studies on the food additive propyl gallate: synthesis, structural characterization, and evaluation of the antioxidant activity. J. Chem. Educ. 2012;89(1):130–133. [Google Scholar]
- Gordon M.H. Food Antioxidants. Springer Netherlands; 1990. The mechanism of antioxidant action in vitro; pp. 1–18. [Google Scholar]
- Graf E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992;13(4):435–448. doi: 10.1016/0891-5849(92)90184-i. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am. J. Med. 1991;91(3):S14–S22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Heijnen C.G., Haenen G.R.M., Vekemans J.A.J., Bast A. Peroxynitrite scavenging of flavonoids: structure activity relationship. Environ. Toxicol. Pharmacol. 2001;10(4):199–206. doi: 10.1016/s1382-6689(01)00083-7. [DOI] [PubMed] [Google Scholar]
- Heim K.E., Tagliaferro A.R., Bobilya D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13(Issue 10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Hollman P.C.H., Bijsman M.N.C.P., Van Gameren Y., Cnossen E.P.J., De Vries J.H.M., Katan M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999;31(6):569–573. doi: 10.1080/10715769900301141. [DOI] [PubMed] [Google Scholar]
- Hopia A., Heinonen M. Antioxidant activity of flavonol aglycones and their glycosides in methyl linoleate. JAOCS (J. Am. Oil Chem. Soc.) 1999;76(1):139–144. [Google Scholar]
- Ilyasov I.R., Beloborodov V.L., Selivanova I.A. Three ABTS•+ radical cation-based approaches for the evaluation of antioxidant activity: fast- and slow-reacting antioxidant behavior. Chem. Pap. 2018;72(8):1917–1925. [Google Scholar]
- Ilyasov I.R., Beloborodov V.L., Selivanova I.A., Terekhov R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020;21(3) doi: 10.3390/ijms21031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto Y., Kaneto H. Mitochondrial Pathogenesis. Springer Berlin Heidelberg; 2004. Role of oxidative stress in pancreatic β-cell dysfunction; pp. 168–176. [DOI] [PubMed] [Google Scholar]
- Klein E., Lukes V., Cibulková Z. On the energetics of phenol antioxidants activity. Pet. Coal. 2005;47:33–39. [Google Scholar]
- Klein O.I., Kulikova N.A., Filimonov I.S., Koroleva O.V., Konstantinov A.I. Long-term kinetics study and quantitative characterization of the antioxidant capacities of humic and humic-like substances. J. Soils Sediments. 2018;18(4):1355–1364. [Google Scholar]
- Lin C., Zhu C., Hu M., Wu A., Zerendawa B., Suolangqimei K. Structure-activity relationships of antioxidant activity in vitro about flavonoids isolated from pyrethrum tatsienense. Journal of Intercultural Ethnopharmacology. 2014;3(3):123. doi: 10.5455/jice.20140619030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Xiao M., Zhao J., Li Z., Xing B., Li X., Kong M., Li L., Zhang Q., Liu Y., Chen H., Qin W., Wu H., Chen S. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21(10) doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins N., Barros L., Ferreira I.C.F.R. In vivo antioxidant activity of phenolic compounds: facts and gaps. Trends Food Sci. Technol. 2016;48:1–12. [Google Scholar]
- Mathiesen L., Malterud K.E., Sund R.B. Hydrogen bond formation as basis for radical scavenging activity: a structure-activity study of C-methylated dihydrochalcones from Myrica gale and structurally related acetophenones. Free Radic. Biol. Med. 1997;22(1–2):307–311. doi: 10.1016/s0891-5849(96)00277-8. [DOI] [PubMed] [Google Scholar]
- Mehta A.C., Seshadri T.R. Flavonoids as antioxidants. J. Sci. Ind. Res. B. 1959;18:24–28. [Google Scholar]
- Mikamo E., Okada Y., Semma M., Ito Y., Morimoto T., Nakamura M. Studies on structural-correlation with antioxidant activity of flavonoids (2) Japanese Journal of Food Chemistry and Safety. 2000;7(2):97–101. [Google Scholar]
- Miller N.J., Rice-Evans C., Davies M.J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993;84(4):407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- Mora A., Payá M., Ríos J.L., Alcaraz M.J. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem. Pharmacol. 1990;40(4):793–797. doi: 10.1016/0006-2952(90)90317-e. [DOI] [PubMed] [Google Scholar]
- Morabito N., Crisafulli A., Vergara C., Gaudio A., Lasco A., Frisina N., D’Anna R., Corrado F., Pizzoleo M.A., Cincotta M., Altavilla D., Ientile R., Squadrito F. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J. Bone Miner. Res. Official J. Am. Soci. Bone Mineral Res. 2002;17(10):1904–1912. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- Moskaug J.Ø., Carlsen H., Myhrstad M.C.W., Blomhoff R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 2005;81(1):277S–283S. doi: 10.1093/ajcn/81.1.277S. [DOI] [PubMed] [Google Scholar]
- Nenadis N., Wang L.F., Tsimidou M., Zhang H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS .+ assay. J. Agric. Food Chem. 2004;52(15):4669–4674. doi: 10.1021/jf0400056. [DOI] [PubMed] [Google Scholar]
- Ninfali P., Mea G., Giorgini S., Rocchi M., Bacchiocca M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005;93(2):257–266. doi: 10.1079/bjn20041327. [DOI] [PubMed] [Google Scholar]
- Pietta P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Pratt D.E., Hudson B.J.F. Food Antioxidants. Springer Netherlands; 1990. Natural antioxidants not exploited commercially; pp. 171–191. [Google Scholar]
- Ratty A.K., Das N.P. Effects of flavonoids on nonenzymatic lipid peroxidation: structure-activity relationship. Biochem. Med. Metab. Biol. 1988;39(1):69–79. doi: 10.1016/0885-4505(88)90060-6. [DOI] [PubMed] [Google Scholar]
- Razzaghi-Asl N., Garrido J., Khazraei H., Borges F., Firuzi O. Antioxidant properties of hydroxycinnamic acids: a review of structure- activity relationships. Curr. Med. Chem. 2013;20(36):4436–4450. doi: 10.2174/09298673113209990141. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Salehi B., Azzini E., Zucca P., Maria Varoni E., Anil Kumar N.V., Dini L., Panzarini E., Rajkovic J., Valere Tsouh Fokou P., Peluso I., Prakash Mishra A., Nigam M., El Rayess Y., El Beyrouthy M., Setzer W.N., Polito L., Iriti M., Sureda A., Magdalena Quetglas-Llabrés M., et al. Plant-derived bioactives and oxidative stress-related disorders: a key trend towards healthy aging and longevity promotion. Appl. Sci. 2020;10(3) [Google Scholar]
- Schaich K.M., Tian X., Xie J. Hurdles and pitfalls in measuring antioxidant efficacy: a critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct.Foods. 2015;14:111–125. [Google Scholar]
- Shahidi F. Antioxidants in food and food antioxidants. Nahrung-Food. 2000;44(3):158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects – a review. J. Funct.Foods. 2015;18:820–897. [Google Scholar]
- Siquet C., Paiva-Martins F., Lima J.L.F.C., Reis S., Borges F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acids - a structure-activity relationship study. Free Radic. Res. 2006;40(4):433–442. doi: 10.1080/10715760500540442. [DOI] [PubMed] [Google Scholar]
- Spencer J.P.E., Abd El Mohsen M.M., Minihane A.-M., Mathers J.C. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr. 2008;99(1):12–22. doi: 10.1017/S0007114507798938. [DOI] [PubMed] [Google Scholar]
- Uranga J.G., Podio N.S., Wunderlin D.A., Santiago A.N. Theoretical and experimental study of the antioxidant behaviors of 5-O-caffeoylquinic, quinic and caffeic acids based on electronic and structural properties. ChemistrySelect. 2016;1(13):4113–4120. [Google Scholar]
- Wojdyło A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940–949. [Google Scholar]
- Yehye W.A., Rahman N.A., Ariffin A., Abd Hamid S.B., Alhadi A.A., Kadir F.A., Yaeghoobi M. European Journal of Medicinal Chemistry. Vol. 101. Elsevier Masson SAS; 2015. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): a review; pp. 295–312. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhao M., Xiao C., Zhao Q., Su G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: importance of controlling reaction pH and time. Food Chem. 2016;192:288–294. doi: 10.1016/j.foodchem.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Zhu H., Chen S., Hao S., Zhang Z., Wang W., Yao S. Double roles of hydroxycinnamic acid derivatives in protection against lysozyme oxidation. Biochim. Biophys. Acta Gen. Subj. 2006;1760(12):1810–1818. doi: 10.1016/j.bbagen.2006.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.