Abstract
The Staphylococcus hyicus exfoliative toxin B (SHETB) gene was cloned into pUC118 and expressed in Escherichia coli. The nucleotide sequence of the SHETB gene consists of a coding region of 804 bp specifying a polypeptide of 268 amino acid residues, which included a putative 20-residue signal sequence.
Staphylococcus hyicus is the causative agent of exudative epidermitis (EE) in pigs. Amtsberg (1) suggested that the culture filtrate of S. hyicus contains an exotoxin involved in exfoliative activity. Sato et al. (17) isolated such an exotoxin from the culture filtrate of S. hyicus and designated it SHET.
Exfoliative toxin (ET)-producing strains of Staphylococcus aureus cause staphylococcal scalded skin syndrome in humans (11). The ET has been divided into two serotypes, ETA and ETB (9).
The ET and SHET have some similarities, including their target site (5, 17, 20) and their molecular weight (7, 8, 17, 18), but they only react with homologous antibodies. The production of ETA is controlled by chromosomal DNA, while that of ETB is controlled by plasmid DNA (12, 13, 15). S. hyicus P-23, the SHETB-producing strain, harbors the large plasmid (pKUH-1). The plasmid-eliminated substrain of S. hyicus P-23 has lost its toxic activity. From these findings, it appears that SHETB production is controlled by plasmid DNA (H. Sato, T. Tanabe, T. Watanabe, K. Teruya, A. Ohtake, H. Saito, and N. Maehara, Proc. 14th IPVS Cong. Italy, p. 339, 1996).
In this study, we cloned the SHETB gene (shetb) on plasmid DNA of S. hyicus P-23 in Escherichia coli and determined the nucleotide sequence and the predicted amino acid sequence.
S. hyicus P-23 is a SHETB-producing strain and was isolated from a pig affected with EE (19). E. coli DH5α was used as the host strain in cloning experiments. Bacteria were grown in TY broth (6) for S. hyicus and in Luria-Bertani broth (16) for E. coli. Both bacteria were cultured in a Bio-shaker BR-160LF (Taitec Co., Ltd., Tokyo, Japan) at 37°C with shaking operated at 75 oscillations per min for 18 h. The vector plasmid pUC118 (Takara Shuzo Co. Ltd., Tokyo, Japan) was used in the cloning experiments.
Isolation of plasmid DNA from S. hyicus and E. coli was carried out by a modification of the method described by O'Reilly et al. (12) and Birnboim and Doly (3), respectively. To purify plasmid DNA from S. hyicus and E. coli, the dye-buoyant density centrifugation was performed in a P65AT rotor (Hitachi Koki Co., Ltd., Tokyo) at 45,000 rpm, and supercoiled DNA was separated from residual chromosomal DNA and nicked plasmid DNA.
The large plasmid (pKUH-1) of S. hyicus P-23 was digested to completion with restriction endonucleases EcoRI, BamHI, and HindIII (Nippon Gene Inc., Toyama, Japan), and the resulting fragments were separated by electrophoresis with a 1.0% agarose gel. The transfer of the DNA onto a nylon membrane (Hybond N+; Amersham Pharmacia Biotech Co. Ltd., Uppsala, Sweden) was performed by alkaline blotting with fixation by a UV cross-linker. The nylon membrane was hybridized to a biotin-labelled ET probe (conservative sequence between ETA and ETB genes, 5′-TCTGGATCAGGTATATTTAAT-3′), and the signal was visualized with Lumi-Phos 530 (Nippon Gene) and the ECL Direct labelling and detection system (Amersham Pharmacia), according to the manufacturer's instructions.
The DNA fragments corresponding to those hybridized to the ET probe were isolated from the agarose gels by a freeze-and-thaw method. The DNA fragments pretreated with HindIII and alkaline phosphatase (Nippon Gene) were ligated into pUC118 with DNA ligation kit version 2 (Takara). The recombinant plasmids were transformed into E. coli DH5α. The transformants were screened by blue-white selection.
Each transformant forming the white colonies was cultured at 37°C overnight in Luria-Bertani broth supplemented with 50 μg of ampicillin/ml. After centrifugation (10,000 × g, 20 min) of the above bacterial culture, 30 ml of supernatants was passed through a membrane filter (0.45-μm pore size; Toyo Roshi Inc., Tokyo, Japan). The filtrates were lyophilized, resuspended in 3 ml of 50 mM Tris-HCl (pH 7.5) and used as concentrated culture filtrate (CCF) in the following tests. One gram of the cell pellets was resuspended in 10 ml of Dulbecco's phosphate-buffered saline without CaCl2 or MgCl2 (pH 7.2) and sonicated at 28 kHz (maximum, 20 W) for 5 min with Handy Sonic UR-20P (Tomy Seiko Inc., Tokyo, Japan). After sonication, the cell suspension was centrifuged at 10,000 × g for 20 min, and the supernatants were used as the cell lysates in the following test.
One-day-old conventional White Leghorn chickens were purchased from Kanto Shokkei Co. Ltd., Tokyo, Japan, and used for the detection of SHETB. A portion (0.4 ml) of cell lysates and CCF of transformants was injected subcutaneously into 1-day-old chickens. The production of SHETB was regarded as positive when the Nikolsky sign (peeling off the skin surface was easily caused by slight rubbing with the fingertip) (7, 11) was identifiable within 3 h of injection.
SHETB is a heat-labile toxin and loses its toxic activity when heated at 60°C for 30 min. To confirm the heat stability of recombinant SHETB (r-SHETB) from E. coli transformants, the culture filtrates were heated at 60°C for 15 and 30 min and at 100°C for 20 min. After heat treatment, 0.4 ml of culture filtrates was inoculated subcutaneously into 1-day-old chickens.
Template DNAs for cycle sequencing were obtained by the alkaline method (3) and were purified by RNase A (Sigma) digestion and polyethylene glycol precipitation. DNA sequencing was carried out with the DYEnamic Direct cycle sequencing kit (Amersham Pharmacia) and the 373S DNA Sequencing System (Perkin-Elmer, Norwalk, Conn.) according to the manufacturer's instructions. A computer-assisted sequence analysis was carried out with GENETYX-MAC Version 8, a software package (Software Development Co., Ltd., Tokyo, Japan).
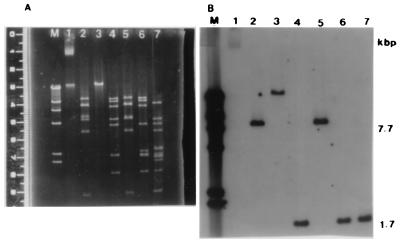
Isolated plasmid DNA from S. hyicus P-23 was digested with EcoRI, BamHI, and HindIII and was separated by agarose gel electrophoresis (Fig. 1A). The size of the plasmid DNA was estimated to be 42 kb, based on the sum of these DNA fragments. We speculated that SHET and ET have a highly conserved region since the activity of SHET is similar to that of ETs. Then the DNA probe (ET probe) for the detection of ET gene was synthesized and used for Southern blot hybridization. In the Southern blot analysis, the DNA probe was hybridized to a 7.7-kb EcoRI fragment, a 7.7-kb EcoRI-BamHI fragment, a 1.7-kb HindIII fragment, a 1.7-kb HindIII-EcoRI fragment, and a 1.7-kb HindIII-BamHI fragment (Fig. 1B). The 1.7-kb HindIII fragment was subsequently used for cloning in E. coli.
FIG. 1.
Southern blot hybridization of ET probe to 42-kb plasmid. (A) Agarose gel electrophoresis of plasmid DNA of S. hyicus P-23. (B) Southern blot analysis of plasmid DNA of S. hyicus P-23. Lane M, λ/HindIII; lane 1, 42-kb plasmid; lane 2, EcoRI digest; lane 3, BamHI digest; lane 4, HindIII digest; lane 5, EcoRI-BamHI digest; lane 6, BamHI-HindIII digest; lane 7, HindIII-EcoRI digest.
The 1.7-kb HindIII fragment of the plasmid was ligated with pUC118. After transformation of the recombinant plasmid in E. coli DH5α, a SHETB-producing clone was detected by inoculation of the CCF and cell lysates into a 1-day-old chicken. Two of the clones resulted in the positive Nikolsky sign within 3 h in the chickens inoculated with the culture filtrates, but the Nikolsky sign was not observed in the chickens inoculated with cell lysates. The recombinant plasmids were isolated from these two clones and were designated pSHETB-1 and pSHETB-2.
The toxic activity of SHETB was stable when the toxin was heated at 60°C for 15 min but lost its toxicity after heating at 60°C for 30 min. Similarly, the Nikolsky sign was not observed for the 1-day-old chicken inoculated with CCF of E. coli transformant (r-SHETB) heated at 60°C for 30 min. These results indicate that r-SHETB from the transformant, as well as SHETB, is a heat-labile toxin.
The inserts included in pSHETB-1 and pSHETB-2 were sequenced. The nucleotide sequence of the SHETB gene is shown in Fig. 2. Only one open reading frame (ORF) that could code for SHETB was identified by computer analysis. The GC content of the SHETB ORF was 34%, which is typical of the staphylococcal genome. However, the GC content of the 150-bp sequence upstream from the start codon was lower (24%), suggesting that this region could serve as a potential binding site for RNA polymerase to initiate transcription. A −35 sequence and a −10 sequence that could serve as potential promoter regions were identified. Furthermore, the probable start codon is preceded 8 bp downstream by the sequence AGATGA, which qualifies as a potential ribosome binding site.
FIG. 2.
Nucleotide sequence of the DNA fragment containing the SHETB gene. The presumptive signal sequence is indicated in bold letters. The presumptive promoter region (−10 and −35) and Shine-Dalgarno ribosome binding region (SD) are indicated by underlining. The ET probe annealing site is indicated by double underlining.
The 804-bp ORF was capable of coding a peptide of 268 amino acid residues with a molecular mass of 29,093 daltons. The deduced amino acid sequence of SHETB was compared with those of ETA and ETB. The SHETB sequence showed higher amino acid identity of 43.5% to ETA and 60.8% to ETB than the ETA-ETB amino acid identity of 42.0%.
The SHET can be divided into two serotypes, SHET produced by the plasmidless strain (SHETA) and that produced by the plasmid-carrying strain (SHETB). Dancer et al. (4) reported that there are three highly conserved regions between ETA and ETB. In our recent study, ET probe hybridized to plasmid DNA of strain P-23 (SHETB producer) (Sato et al., 14th IPVS Cong.). These results suggest that this probe is specific to the highly conserved region between ET and SHETB.
The 1.7-kb HindIII fragment of pKUH-1 from S. hyicus P-23 was hybridized to ET probe by Southern blot analysis. The 1.7-kb HindIII fragment was ligated into pUC118 since the SHETB gene was located in the 1.7-kb HindIII fragment. The culture filtrates of E. coli transformants harboring recombinant plasmids (pSHETB-1 and pSHETB-2) showed the positive Nikolsky sign. However, the cell lysates of such transformants did not cause the Nikolsky sign. The SHETB and r-SHETB have the same heat stability, as both toxins have been observed to lose their toxicity after treatment at 60°C for 30 min. These results suggest that E. coli transformants harboring pSHETB-1 and pSHETB-2 secrete r-SHETB into the culture medium.
The nucleotide sequence of the 1.7-kb HindIII fragment containing the SHETB gene was determined. This fragment contains only one large ORF that can code for SHETB. The length of the ORF is 804 bp, and the molecular weight of SHETB estimated from the deduced amino acid sequence is 29,093. The signal sequence is presumed to consist of 20 amino acid residues, since the molecular weight of mature SHETB is 27,000, and the staphylococcal signal protease is cut at alanine or lysine residues (10, 13, 15). The predicted total number of amino acid residues of the mature SHETB is 248, and its molecular weight is 26,915. This molecular weight is close to the 27,000 of the mature SHETB, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
The SHETB showed 43.5 and 60.8% homology with ETA and ETB, respectively. Although ETA and ETB were produced by the same species of bacteria, the homology between ETA and ETB is merely 42.0%. Dancer et al. (4) reported that the residues of the catalytic triad of staphylococcal V8 protease, His-72, Asp-120, and Ser-195 (ETA numbering), is present in the conserved regions between ETA and ETB. Bailey and Smith (2) reported that diisopropyl phosphorofluoridate is specifically bound to Ser-195 of ETA, the homologue of the active site serine residue of V8 protease. In the report by Prévost et al. (14), the substitution of Ser-195 of ETA by a cysteine residue was shown to lead to the production of biologically inactive protein. In addition, SHETB was shown to possess the catalytic triad and the active serine residue similar to ETA and ETB. These findings suggest that exfoliative toxins including SHETB are serine proteases.
Nucleotide sequence accession number.
The nucleotide sequence of the SHETB gene has been deposited into GenBank under accession no. AB036768.
Acknowledgments
This research was supported by grants-in-aid for scientific research (no. 06660391 and no. 089660372) from the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Amtsberg G. Nachweis von Exofoliation aus-lösenden Substanzen in Kulturen von Staphylococcus hyicus des Schweines und Staphylococcus epidermidis Biotyp 2 des Rindes. Zentbl Vetmed Reihe B. 1979;26:257–272. [PubMed] [Google Scholar]
- 2.Bailey C J, Smith T P. The reactive serine residue of epidermolytic toxin A. Biochem J. 1990;269:535–537. doi: 10.1042/bj2690535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dancer S J, Garratt R, Saldanha J, Jhoti H, Evans R. The epidermolytic toxins are serine proteases. FEBS Lett. 1990;268:129–132. doi: 10.1016/0014-5793(90)80990-z. [DOI] [PubMed] [Google Scholar]
- 5.Elias P M, Fritsch P, Tappeiner G, Mittermayer H, Wolff K. Experimental staphylococcal toxic necrolysis (TEN) in adult humans and mice. J Lab Clin Med. 1975;84:414–424. [PubMed] [Google Scholar]
- 6.Kapral F A, Miller M M. Product of Staphylococcus aureus responsible for the scalded-skin syndrome. Infect Immun. 1971;4:541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo I, Sakurai S, Sarai Y. Purification of exfoliatin produced by Staphylococcus aureus of bacteriophage group 2 and its physicochemical properties. Infect Immun. 1973;8:156–164. doi: 10.1128/iai.8.2.156-164.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondo I, Sakurai S, Sarai Y. New type of exfoliatin obtained from staphylococcal strains, belonging to phage groups other than group 2, isolated from patients with impetigo and Ritter's disease. Infect Immun. 1974;10:851–861. doi: 10.1128/iai.10.4.851-861.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo I, Sakurai S, Sarai Y, Futaki S. Two serotypes of exfoliatin and their distribution in staphylococcal strains isolated from patients with scalded skin syndrome. J Clin Microbiol. 1975;1:397–400. doi: 10.1128/jcm.1.5.397-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C Y, Schumidt J J, Johnson-Winegar A D, Spero L, Iandolo J. Sequence determination and comparison of the exfoliative toxin A and toxin B gene from Staphylococcus aureus. J Bacteriol. 1987;169:3904–3909. doi: 10.1128/jb.169.9.3904-3909.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melish M E, Glasgow L A. The staphylococcal scalded skin syndrome: development of an experimental model. N Engl J Med. 1970;282:1114–1119. doi: 10.1056/NEJM197005142822002. [DOI] [PubMed] [Google Scholar]
- 12.O'Reilly M, Dougan G, Foster J, Arbuthnott J P. Plasmid in epidermolytic strain of Staphylococcus aureus. J Gen Microbiol. 1981;124:99–107. doi: 10.1099/00221287-124-1-99. [DOI] [PubMed] [Google Scholar]
- 13.O'Toole P W, Foster T J. Nucleotide sequence of the epidermolytic toxin A gene of Staphylococcus aureus. J Bacteriol. 1987;169:3910–3915. doi: 10.1128/jb.169.9.3910-3915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prévost G, Rifai S, Chaix M L, Piémont Y. Functional evidence that the Ser-195 residue of staphylococcal exfoliative toxin A is essential for biological activity. Infect Immun. 1991;59:3337–3339. doi: 10.1128/iai.59.9.3337-3339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai S, Suzuki H, Kondo I. DNA sequencing of the eta gene coding for staphylococcal exfoliative toxin serotype A. J Gen Microbiol. 1988;134:711–717. doi: 10.1099/00221287-134-3-711. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Sato H, Tanabe T, Kuramoto M, Tanaka K, Hashimoto T, Saito H. Isolation of exfoliative toxin from Staphylococcus hyicus subsp. hyicus and its exfoliative activity in the piglet. Vet Microbiol. 1991;27:263–275. doi: 10.1016/0378-1135(91)90153-7. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe T, Sato H, Kuramoto M, Saito H. Purification of exfoliative toxin produced by Staphylococcus hyicus and its antigenicity. Infect Immun. 1993;61:2973–2977. doi: 10.1128/iai.61.7.2973-2977.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanabe T, Sato H, Sato H, Watanabe K, Hirano M, Hirose K, Kurokawa S, Nakano K, Saito H, Maehara N. Correlation between occurrence of exudative epidermitis and exfoliative toxin-producing ability of Staphylococcus hyicus. Vet Microbiol. 1996;48:9–17. doi: 10.1016/0378-1135(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 20.Tanabe T, Sato H, Ueda K, Chihara H, Watanabe T, Nakano K, Saito H, Maehara N. Possible receptor for exfoliative toxin produced by Staphylococcus hyicus and Staphylococcus aureus. Infect Immun. 1995;63:1591–1594. doi: 10.1128/iai.63.4.1591-1594.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]