Abstract
Proper management of patients affected by genetic disorders causing life-threatening arrhythmias is important for several reasons, including even societal ones, given the predominantly young age of those affected. Incorrect management often has dire consequences, ranging from unnecessary psychologic damage for the patients whose life becomes too limited by the fear of sudden death to equally avoidable tragedies when the entire armamentarium of effective therapies is not fully utilized. In this review, we focus primarily on long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT) and deal specifically with the clinical impact of the most commonly used cardiac sympathetic denervation (CSD), namely left cardiac sympathetic denervation (LCSD). The two of us have used LCSD in the management of our patients with either LQTS or CPVT for a very long time and have been involved in ∼500 such interventions. It is on the basis of this personal and direct experience that we wish to share our views with clinical cardiologists and electrophysiologists, adult and paediatric, and with genetic cardiologists. We will begin by reviewing the history and rationale underlying sympathetic denervation therapy and will continue with a disease-specific intensification of therapy, and then with a discussion on how the impressive efficacy of LCSD should translate into guideline-directed therapy in both current and future guidelines, in order to upgrade the quality of care in the era of precision medicine.
Keywords: Cardiac sympathetic denervation, Catecholaminergic polymorphic ventricular tachycardia, Genetic disorders, Left cardiac sympathetic denervation, Long QT syndrome, Sudden cardiac death
Graphical Abstract
Graphical Abstract.
How recommendations for cardiac sympathetic denervation in patients with either long QT syndrome or catecholaminergic polymorphic ventricular tachycardia would look like if they were written by the two of us. LQTS, long QT syndrome; CPVT, catecholaminergic polymorphic ventricular tachycardia.
Introduction
Cardiac arrhythmias of genetic origin are often deadly.1 Their management and prevention are among the potentially most rewarding challenges for paediatric and adult cardiologists and electrophysiologists, and for genetic cardiologists, because—at variance with those representing the inexorable culmination of advanced structural cardiac damage—they do not represent a self-defeating objective.2
All too often the therapeutic choice oscillates, dangerously for the patient, between an antiarrhythmic drug (mostly beta-blockers, βBs) and the implantable cardioverter defibrillator (ICD). The quality of life and clinical efficacy seem to be at the two extremes, unable to coexist within one therapeutic approach. However, this is a short-sighted view. Here, we will highlight and discuss a third approach that combines efficacy of treatment and quality of life: namely, cardiac sympathetic denervation (CSD). The two of us have used extensively, and for a long time,3,4 left cardiac sympathetic denervation (LCSD) in the management of our patients with life-threatening arrhythmias of genetic origin, primarily long QT syndrome (LQTS), and catecholaminergic polymorphic ventricular tachycardia (CPVT). Thus, we can knowledgeably examine the contribution that LCSD, and occasionally, bilateral CSD, can offer to the management of patients with genetic arrhythmogenic disorders.
We will review the history and rationale underlying sympathetic denervation therapy. We will analyse the data available for LCSD not only for channelopathies, chiefly LQTS and CPVT, but also for other arrhythmogenic conditions as well. We will consider when and how to integrate denervation therapy into disease-specific and genotype-guided intensification of therapy. Finally, we will discuss the impact that the efficacy of this intervention should have on the decisions that clinical cardiologists and electrophysiologists must make when facing a patient who either is not adequately protected by either pharmacological or device therapy, or is not acceptably tolerating those therapies in terms of quality of life.
Background
History
The details on the introduction of LCSD in the clinic and of its unforeseen evolution are available.5–7 In 1916, Jonnesco performed the first LCSD in a patient with intractable angina pectoris accompanied by cardiac arrhythmias and unexpectedly observed that both the attacks of angina and the arrhythmias disappeared after surgery.8 For many years and until the advent of βBs, LCSD remained as an effective therapy for angina. It was only in the 1960s that first Estes and Izlar9 and then Zipes et al.10 successfully used bilateral CSD (stellate ganglion plus seven thoracic ganglia!) in two patients with intractable ventricular tachycardia (VT), but no one followed. The game changer took place in the early 1970s when Moss and McDonald11 and then Schwartz and Malliani12 started to use LCSD for their patients with LQTS who were refractory to pharmacotherapy.
Despite the clear therapeutic success of these pioneering interventions (both patients remained free of cardiac events for more than 45 years), one of us (P.J.S.) remained the lone standard bearer of LCSD in the setting of genetic arrhythmias. In 2005, the second of us (M.J.A.) joined forces and started to use the thoracoscopic approach to provide LCSD therapy for his LQTS patients.4 This approach, far less complex than the retro-pleural approach,13 paved the way to minimally invasive surgical cardiac denervation therapy being performed in many different centres. Currently, LCSD is an integral part of the management strategy for both LQTS and CPVT. Meanwhile, in the early 2000s, Shivkumar revived the Estes–Zipes idea and began to use, very successfully, bilateral CSD for intractable VT in patients with structural heart disease such as dilated cardiomyopathies and ischaemic heart disease.14,15
Rationale
As the rationale underlying the clinical use of LCSD has been described in the past,3,4 here we will just summarize its main mechanisms of action with the appropriate references for the interested reader. With one exception, all the consequences of LCSD (Table 1) derive from the fact that the centrally mediated sympathetic activation can no longer lead to its normal physiologic response, i.e. the release of norepinephrine (NE) upon the ventricular myocardium from the quantitatively dominant left-sided nerves. The localized neural release of NE by the sympathetic terminals, at variance with the rather uniform effect resulting from the blood-borne, adrenal medulla-derived epinephrine, increases the heterogeneity of repolarization and thereby increases the probability of a ventricular arrhythmia by reentry.16,17
Table 1.
Effects of left cardiac sympathetic denervation
| Physio- and pathophysiological parameters | Effect |
|---|---|
| Release of norepinephrine at nerve endings16,17 | Decrease |
| Arrhythmias associated with myocardial ischaemia20 | Decrease |
| Ventricular refractory period22 | Increase |
| Ventricular fibrillation threshold18 | Increase |
| Myocardial reactive hyperaemia21 | Increase |
| Cardiac performance during exercise26 | Unaffected |
Left cardiac sympathetic denervation increases the ventricular fibrillation (VF) threshold, making it more difficult for a heart to fibrillate.18 Probably, this is its single most important effect and it affects much more the onset of VF than a short run of torsades-de-pointes (TdP) VT, the signature arrhythmia of LQTS.19 Thus, following LCSD, one can expect a reduction of the occurrence of VF greater than that of otherwise self-terminating arrhythmias (e.g. syncope).
The other effects include a reduction in ischaemia-related arrhythmias20 and an increased capability of the coronary bed to dilate:21 two factors important especially for patients with ischaemic cardiomyopathy. Other antiarrhythmic effects include the prolongation of ventricular refractoriness,22 which also reduces the probability of a reentrant arrhythmia16,17 and the reflex increase in cardiac vagal efferent activity23 with its well-known antiarrhythmic effect.24 On the safety side, it is important to remember that, due to the compensatory effect of right cardiac sympathetic nerves—which is in part reflexly mediated25—neither heart rate nor cardiac contractility decrease after LCSD.26,27 It is self-evident that the compensation by right cardiac nerves is lost with bilateral CSD. Another clinically relevant point, which often escapes clinicians, is that LCSD represents a pre-ganglionic denervation and, as the synapses are removed and as they do not regenerate, no reinnervation is possible. Similarly, LCSD—at variance with post-ganglionic denervation—is not accompanied by post-denervation supersensitivity,28,29 which could have a dangerous proarrhythmic effect.
In contrast to many antiarrhythmic therapies, the precise mechanisms of action of LCSD have been dissected carefully and understood.6 This should be reassuring for both doctors and patients.
Long QT syndrome
Here, we will address the straightforward and the potentially controversial aspects of LCSD in the clinical management of LQTS. To avoid misinterpretations, it seems fair to remind that the two of us have recommended and overseen surgical denervation therapy for nearly 400 patients with LQTS over several decades, a number greater than the total performed worldwide by other investigators.30,31 Thus, please understand that after so many years and so many patients, the time has come for unambiguous statements. In this regard, what we are doing could well be regarded as a Consensus Statement of two, or as our own Recommendations (Graphical Abstract).
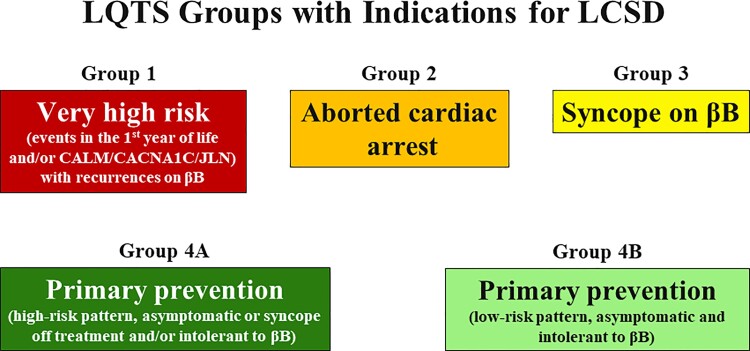
A preliminary and important point is our recent realization that the probability of success for LCSD varies according to specific subgroups and that it would be naïve to continue to look at the results without considering the clinical/genetic features of the individual patients.31,32Figure 1 outlines the five different scenarios for which LCSD could be considered. Group 1 includes patients regarded as at very high risk because of severe genotypes (e.g. calmodulin-mediated LQTS/CVPT) with recurrences while on the preferred βBs nadolol or propranolol, who require both an ICD and LCSD, the ICD as a safety net and LCSD to decrease as much as possible the appropriate shocks. Group 2 includes patients with an aborted cardiac arrest (ACA) either off or on treatment; in the first case we recommend βBs + ICD, in the second ICD + LCSD as they are already on βBs. Group 3, probably the most important in terms of numbers, are patients who have a syncopal episode while taking βBs; for them, we recommend LCSD with several potential developments, as outlined in Dusi et al.31 Group 4A refers to patients regarded as at increased risk even though they are either still asymptomatic or have had syncope off therapy and do not tolerate βBs; in this case, we consider a primary prevention LCSD. Group 4B includes asymptomatic patients who appear to be at relatively low risk and are either intolerant to βBs or have expressed clear preference for a one-time surgery instead of a life-long therapy with βBs, after having been duly informed on the potentially different degree of protection.
Figure 1.
Groups of patients for whom left cardiac sympathetic denervation could be indicated. The level of risk decreases progressively from Group 1 to Group 4B (from red to light green). The rate of success varies within these groups according to Dusi et al.31 and in some cases an implantable cardioverter defibrillator may become necessary. For Groups 4A and 4B, left cardiac sympathetic denervation is recommended as the primary prevention because these patients are still asymptomatic, but their electrocardiographic pattern suggests a higher or lower risk. See text for details. βB, beta-blocker; CALM, calmodulin; JLN, Jervell and Lange-Nielsen; LCSD, left cardiac sympathetic denervation; LQTS, long QT syndrome.
Updated management of LQTS mandates that cardiologists understand the gene-specific risk and the genotype/phenotype features that define more severe disease requiring treatment intensification,33,34 including gene-specific therapy.35,36 This reflects the growing role of precision medicine.37
Having said that, as a general approach, we will now discuss selected aspects related to LCSD.
Extent of denervation
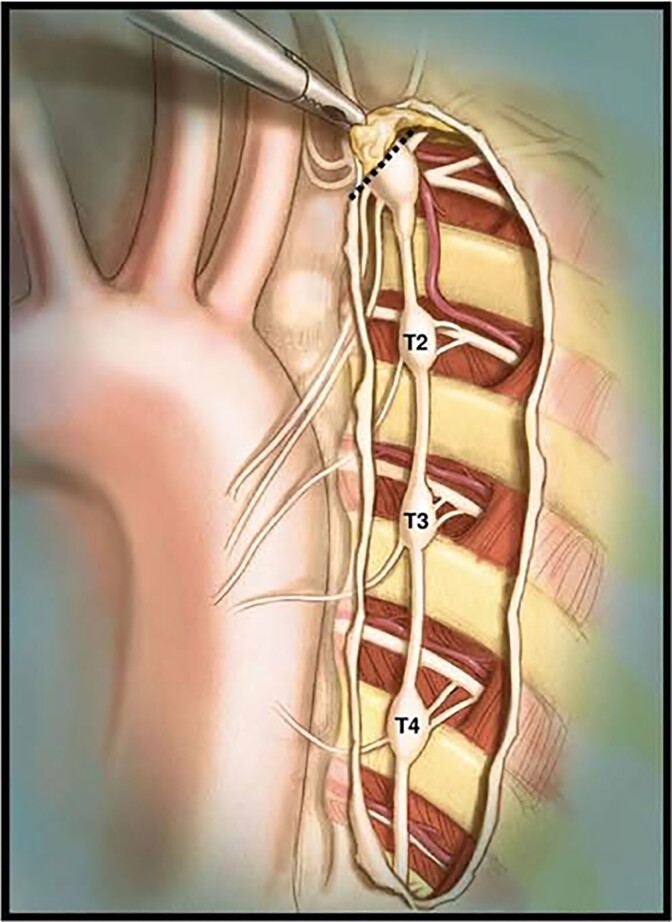
The logical extent of surgery is dictated by anatomy and physiology, and it must include the lower half of the stellate ganglion (i.e. T1) together with the thoracic ganglia from T2 to and including T4 (Figure 2). The same approach is used at UCLA by Shivkumar’s group, the only other one in the world having a reasonably large experience with CSD.38 In our previous reports, with respect to either LQTS or CPVT, incomplete denervation was associated regularly with a higher degree of failures.39–42 Thereby, we regard as medically unacceptable and ethically disquieting the recent attempts to ‘simplify’ surgery by either leaving behind both stellate ganglia altogether or T4.43–45 In other words, T2–T3 or T3–T4/T2–T5 resection does not constitute LCSD and should be considered essentially ‘sham’ surgery. Ethical Committees worldwide should not authorise ‘experimental surgery’ in humans when the evidence for the correct and effective procedure is well established.
Figure 2.
An anatomical drawing of the left cardiac sympathetic chain after exposure through the pleura that is resected during video-assisted thoracic left cardiac sympathetic denervation. The stellate ganglion is located under the superior edge of the incision. The dashed line indicates the resection of the lower half of the left stellate ganglion occurring just above the major lower branches, to minimize the risk of the Horner syndrome. The lower section should take place below T4. Prior to performing the section, lidocaine should be applied on the sympathetic chain. (From Collura et al.4 with permission.)
Clinical efficacy
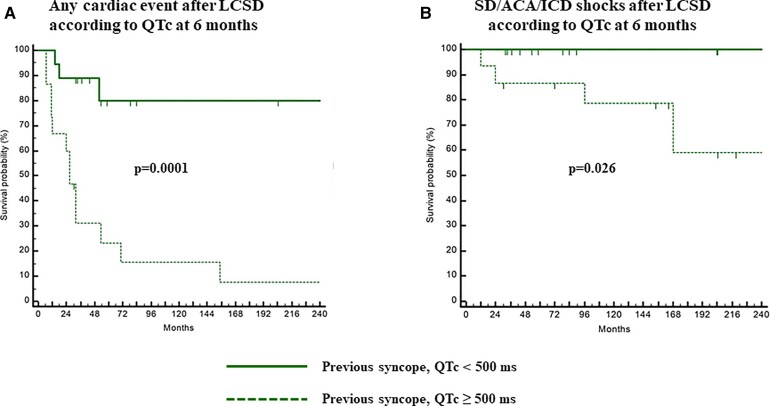
The efficacy of LCSD is excellent, as shown by our reports,30,31,39,40,46 but, like most therapies, does not provide 100% protection. As mentioned above, the clinical presentation offers insights on the probability of success31 and on the possible need for adjunct measures such as ICD, atrial pacing, initiating other medications like mexiletine, or proceeding to right-sided cardiac sympathetic denervation (RCSD). Especially important is the predictive role of QTc following LCSD. The most recent data31 indicate that whether or not after LCSD, the QTc remains above or below 500 ms makes a difference (Figure 3) and that up to half of the patients with a pre-LCSD QTc >500 ms will shorten it by a mean of 60 ms.
Figure 3.
On treatment Kaplan–Meier curves of cumulative survival to any cardiac event (A) and to a sudden death/aborted cardiac arrest/implantable cardioverter defibrillator shocks (B) after left cardiac sympathetic denervation in LQTS patients with previous syncope/implantable cardioverter defibrillator shocks according to post-left cardiac sympathetic denervation QTc <500 or ≥500 ms. The patients who at 6 months post-left cardiac sympathetic denervation have a QTc <500 ms are at a significantly lower risk for all cardiac events and especially for sudden death/aborted cardiac arrest/implantable cardioverter defibrillator shocks. (Modified from Dusi et al.31 with permission.) SD, sudden death; ACA, aborted cardiac arrest; ICD, implantable cardioverter defibrillator; LCSD, left cardiac sympathetic denervation.
Clinical cardiologists should also consider that a consequence of the anti-fibrillatory effect of LCSD is that its efficacy is greater in preventing the deterioration from TdP to VF (which causes cardiac arrest or death) than the onset of a transient episode of TdP (which leads just to syncope). This matters when one has to decide what to do for a patient with syncope on βB therapy.
Bilateral cardiac sympathetic denervation
The addition of RCSD following LCSD, thus leading to a complete bilateral CSD, can be a useful treatment, when LCSD appears insufficient. We have used it since the mid-1980s in a few cases but with overall rewarding albeit anecdotal results.31,39,40 Anecdotal because we have seldom needed to proceed to the RCSD to complete a bilateral CSD in patients with either LQTS or CVPT. In fact, in our joint experience, of the >450 denervations performed to date for either LQTS or CVPT, <20 RCSD followed LCSD. At Mayo Clinic, the approach is to do a re-do LCSD if the surgery was done elsewhere for the patient with a post-LCSD breakthrough cardiac event rather than go straight to the right side.42 In doing so, we have found either an untouched left stellate ganglion, a bifid stellate, or the distal T3–T4 sympathetic chain. Accordingly, it is important to avoid misunderstandings: the potential additional value of RCSD does not legitimize at all the performance of a bilateral CSD at outset,44,45 without having first assessed whether LCSD is sufficient. There should be caution before depriving, without proven necessity, the patients of the contribution of right-sided cardiac nerves to heart rate control (where they dominate)47 and to ventricular contractility.26
Right-sided cardiac sympathetic denervation is indicated as an intermediate step before considering ICD implant when LCSD does not provide sufficient protection, and when the patient continues to receive appropriate ICD shocks following LCSD. When, despite LCSD, patients with premature ventricular beats continue to ‘feel them’ and develop anxiety leading to TdP, the performance of RCSD interrupts this traumatizing feedback loop, thanks to the interruption of the cardiac sympathetic afferents which are activated by the ventricular mechanoreceptors.48
Left cardiac sympathetic denervation monotherapy
Already in the 2004 worldwide report on LCSD40 in 147 patients, 17 (12%) were treated with LCSD as monotherapy and 82% of them became completely asymptomatic and had a mean QTc shortening of 75 ms. It was since the early days that we knew that some patients could not be treated with βBs, mostly because of severe asthma. The proportion of LQTS patients treated with LCSD monotherapy has significantly increased, nowadays mostly due to intolerance to βBs, 31%30 at Mayo Clinic, and 10% in Milan.31 Understandably, the majority of these patients (75%) was asymptomatic. Dusi et al.31 reported LCSD monotherapy because of βBs intolerance in 12 (10%) patients but, interestingly, eight had previous syncope or previous ACA and over a meaningful follow-up of 18 ± 12 years, there were only two patients with syncopal episodes (both with previous ACA).
On the basis of these data, it is now possible to make informed statements about LCSD monotherapy. The ideal therapy for LQTS should always include βBs. However, it is our opinion that in the presence of clear contraindications or true intolerance to βBs, there is now sufficient evidence to allow the patients, including symptomatic ones, to continue with LCSD monotherapy. As the level of protection could be related to the degree of QTc shortening, we advise a stricter follow-up for these patients.
Catecholaminergic polymorphic ventricular tachycardia
The main problem in the management of CPVT is that it is perhaps the only cardiac disease in which the ICD itself may contribute to not only morbidity but also the patient’s very own mortality.1,49–52 Indeed, the pain and fear triggered by ICD shocks, appropriate and inappropriate alike, can precipitate a severe and ultimately fatal electrical storm.49–52 The most recent analysis of the effect of ICD implants in patients with CPVT has concluded that ICD use should be limited as much as possible, favouring LCSD.53
Left cardiac sympathetic denervation was used for the first time in CPVT patients in 200854 and then an extensive single institution experience from Mayo Clinic was reported in 2012.55 This was followed by a multicentre study in 63 patients in 201541 and by several other cases.51 The proportion of patients with major cardiac events despite optimal medical therapy was reduced from 100 to 32% after LCSD, and among the 29 patients with a pre-denervation ICD, the rate of shocks dropped by 93% from 3.6 to 0.6 shocks per person per year (P < 0.001).41 Left cardiac sympathetic denervation is an effective anti-fibrillatory intervention for patients with CPVT. The conclusion of that study was that whenever syncope occurs despite optimal medical therapy, LCSD could be considered the next step rather than an ICD and could complement ICDs in patients with recurrent shocks. This view was fully endorsed by another large multicentre study51 and can now be regarded as the expert opinion.
Whenever βBs appear as not adequately protective in CPVT patients, there should be no hesitation whatsoever in proceeding with LCSD. This is a problematic population in which an arrhythmia breakthrough can happen with just a single missed dose of medication, and for which LCSD provides another layer of protection should this occur. For CPVT patients with a sentinel event of sudden cardiac arrest prior to diagnosis, we endorse ‘triple therapy’ with nadolol, flecainide, and LCSD (Mayo Clinic) or nadolol, LCSD, and an ICD (Milan) as reasonable treatment strategies.
A randomized clinical trial for long QT syndrome or catecholaminergic polymorphic ventricular tachycardia
From time to time someone questions what we call ‘optimal medical treatment’ for LQTS and CPVT, as described above, saying that ‘there has never been a randomized clinical trial (RCT). Yes, there has never been one and hopefully never will be, given that it would be neither ethical nor feasible. As the current treatment options for both diseases are most effective and as for the most severe cases the ICDs represent an effective addition and way out, it would not be ethically acceptable to randomize some patients to a treatment of unproven efficacy. And not even the use of ICDs as a ‘safety net’ would be justifiable, when they are not absolutely necessary, because once implanted the risk of shocks should always be minimized. Finally, it is difficult to imagine how parents would accept to have their child affected by LQTS or CPVT randomized to a treatment of uncertain efficacy which could result in either sudden death or unnecessary ICD shocks. There were no RCTs for penicillin.
Other monogenic genetic disorders besides long QT syndrome and catecholaminergic polymorphic ventricular tachycardia
It is premature to extend the confidence in LCSD’s therapeutic efficacy beyond LQTS and CPVT at this time. Although we and others have reported LCSD in patients with hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy,56 and even a patient with a classic congenital heart malformation of d-transposition of the great arteries and late onset VT,55,57,58 the potential utilization of LCSD in these conditions requires a clear elucidation that the arrhythmic event was triggered by sympathetic activation. If so, and if these disease-associated arrhythmias persisted while already on guideline-directed therapies (GDTs), then LCSD can be considered. At this time, evidence for adequate protection by CSD for HCM and ACM is still insufficient.
Post-left cardiac sympathetic denervation sequelae and complications
Post-LCSD, patients will experience a drier and warmer left hand as the consequence of the interruption of the sympathetic fibres innervating sweat glands. Rarely, patients will develop a transient harlequin appearance of the face after an aerobic workout or emotional excitement. Concerns about a full Horner syndrome have been exaggerated substantially. A permanent minor left ptosis, that approximates the eyelid asymmetry seen in about 10% of all humans, occurs in about 3–5% of patients. In most patients, a modest ptosis can be seen within the first days after LCSD that gradually resolves over the ensuing months. A major ptosis, requiring an aesthetic surgical correction, occurs in no more than 1% of patients.13
The most disturbing consequence of LCSD is neuropathic pain, which was practically absent with the previously used retro-pleural approach13 and is probably due to the fact that with the thoracoscopic approach, it is more likely that the surgeon will ‘pull’ the sympathetic chain before cutting it. Approximately 30% of our patients have some level of post-LCSD neuropathic pain which is transient and spontaneously resolves within the first few months after surgery. The administration of low-dose gabapentin starting 24 h before surgery may be a reasonable consideration especially in the phenotypic subset at greatest risk for this side effect (females, age 20–40 years, with prior pain sensitization conditions).
Quality of life
Even though the primary reason to perform LCSD is to reduce the risk for life-threatening arrhythmias, the physicians’ choice of treatment should always consider the impact on the quality of life59 of their patients, especially when they are young. Antiel et al.60 assessed in 100 patients with LQTS and CPVT whether LCSD had an impact on their quality of life. The vast majority (92%) of patients and families alike were satisfied with their surgery and would recommend it to other patients.60
Left cardiac sympathetic denervation improves the quality of life especially among LQTS and CPVT patients with an ICD.61 Among 233 LQTS patients with a transvenous ICD, 25% had major adverse events within 5 years after implant.62 The effect of LCSD on the number of ICD shocks is impressive, as quantitatively reported in two studies. In five patients the mean yearly rate of shocks per patient dropped post-LCSD by 95% from 29.3 to 3.3 shocks (P = 0.02)40 and in seven patients it was reduced by >97% from 17 to 0.5 during a mean follow-up of 6 years,31 thereby providing some a meaningful therapeutic defence against to the profound post-traumatic stress disorder that can come from the ICD shocks. It had been hoped that the use of a subcutaneous ICD for LQTS and CPVT would have avoided catheter/endovascular-related complications. However, several reasons discourage its use: the potential need for pacing, the inability to prolong detection times, and the probability of more painful shocks.
Although there are a few occasions where we fully support consideration for a prophylactic ICD in asymptomatic patients, before recommending an ICD on the basis of the clinical presentation, it is critical to assess and reassess their risk of a sentinel event once anti-fibrillatory therapies (Bβs + LCSD) and QT shortening (mexiletine) are put in place.
Left cardiac sympathetic denervation, guidelines, and guideline-directed therapy
Historically, guidelines have not endorsed denervation therapy in general or LCSD in particular with greater than a Class II recommendation. Further, these cardiac society-based, expert opinion-derived guidelines and consensus statements relegated the LCSD as a treatment consideration AFTER the ICD. However, this appropriately changed with the most recent 2017 guidelines from the American Heart Association (AHA), American College of Cardiology (ACC), and Heart Rhythm Society (HRS).63 After analysing the extensive, published reports with LCSD in LQTS and CVPT, AHA/ACC/HRS GDT now includes a Class I recommendation for the LCSD as (i) a therapeutic modality for treatment intensification for the patient who has had a disease-associated breakthrough cardiac event including either symptoms while on pharmacotherapy or VF-terminating shocks among those with an ICD and (ii) a bona fide treatment alternative in those who do not tolerate βB therapy or have contraindications to βB therapy.
Importantly, the AHA/ACC/HRS guidelines struck equipoise in each situation as it endorsed that the clinician could consider modification of drug therapy, LCSD, or device therapy with an ICD as equally reasonable options for treatment intensification rather than directing/mandating that the ICD is second in line and that the LCSD can only be considered AFTER the ICD has been installed. This enlightened approach is counteracting the rapid reflex towards an ICD disquietingly seen in North America and Europe.62,64 Along the same lines, but even more forcefully, is the 2021 PACES Expert Consensus statement just published.65 Whether or not the upcoming European guidelines will strike a similar level of equipoise for the LCSD in GDT for both LQTS and CPVT remains to be determined.
A very recent study66 on 3035 patients enrolled in the US portion of the International LQTS Registry67 reported that those with ICDs had a lower risk of death and concluded by supporting ICD implantation in LQTS patients with ACA and with syncope on βBs, and suggesting the same even for those with syncope off βBs. Data from registries have significant limitations because, while reflecting a ‘real-world’ scenario, with a non-uniform management strategies the outcomes are at great variance with those observed in highly experienced centres managing daily patients with LQTS on fully personalized, optimized GDTs.68 It is our view that most patients with LQTS and CPVT do NOT need and should NOT receive an ICD.
It is fair to remember that the Task Force of the European Society of Cardiology on the legal implications of medical guidelines69 has stated that those who generate them should be respected for their expertise which, for the indications in favour or against CSD, should be highly specific.
Meanwhile, we share what would constitute ‘recommendations’ related to the potential use of CSD in the management of either LQTS or CPVT (Graphical Abstract) if it were up to us to decide.
Right to be informed
For the LQTS and CPVT patients not fully protected by βBs, the availability of both LCSD and ICDs makes it imperative that patients and families know about LCSD and the additional protection it can afford with fewer adverse events compared with ICD. The right of the patients is matched by the responsibility of the physicians to provide adequate and fair information, at risk of medico-legal consequences.70
Conclusions
Our conclusions are the logical consequence of having witnessed, presented, and discussed the impressive clinical impact of LCSD for different subgroups of patients affected by LQTS or CPVT. Our views of what should be done are expressed in what we have unabashedly defined as ‘our own recommendations’, which should be viewed not so much as a criticism of what has been done so far but as a constructive proposal to make future guidelines more representative of what our experience has taught us and should teach others.
Finally, we cannot hide our dismay to note how so many cardiologists around the world, even from some of the most advanced countries, are ready to implant ICDs in a large number of LQTS/CPVT patients but seem unable to offer them the alternative of LCSD. Just because an ICD can be implanted almost anywhere is not a compelling justification to keep families from being fully informed of the GDTs available to them.
Acknowledgements
The authors are grateful to Pinuccia De Tomasi, BS, for expert editorial support and to Veronica Dusi, MD, PhD, for constructive suggestions.
Contributor Information
Peter J. Schwartz, Center for Cardiac Arrhythmias of Genetic Origin and Laboratory of Cardiovascular Genetics, Istituto Auxologico Italiano, IRCCS, Via Pier Lombardo, 22, 20135 Milan, Italy
Michael J. Ackerman, Department of Cardiovascular Medicine, Division of Heart Rhythm Services (Windland Smith Rice Genetic Heart Rhythm Clinic), Mayo Clinic, Rochester, MN, USA Department of Pediatric and Adolescent Medicine, Division of Pediatric Cardiology, Mayo Clinic, Rochester, MN, USA; Department of Molecular Pharmacology & Experimental Therapeutics (Windland Smith Rice Sudden Death Genomics Laboratory), Mayo Clinic, Rochester, MN, USA.
Funding
This work was partially supported by the Fondation Leducq grant 18CVD05 ‘Towards Precision Medicine with Human iPSCs for Cardiac Channelopathies’ to P.J.S. and the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program to M.J.A.
Conflict of interest: P.J.S. is a consultant with UpToDate. M.J.A. is a consultant with Abbott, ARMGO Pharma, Boston Scientific, Bristol Myers Squibb, Daiichi Sankyo, Invitae, LQT Therapeutics, Medtronic, and UpToDate. M.J.A. and Mayo Clinic have a potential equity/royalty relationship with AliveCor, Anumana, and Pfizer.
References
- 1. Schwartz PJ, Ackerman MJ, Antzelevitch C, Bezzina CR, Borggrefe M, Cuneo BF, et al. Inherited cardiac arrhythmias. Nat Rev Dis Primers 2020;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lown B. Sudden cardiac death: the major challenge confronting contemporary cardiology. Am J Cardiol 1979;43:313–328. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz PJ. 1970–2020: 50 years of research on the long QT syndrome—from almost zero knowledge to precision medicine. Eur Heart J 2021;42:1063–1072. [DOI] [PubMed] [Google Scholar]
- 4. Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm 2009;6:752–759. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz PJ. Cutting nerves and saving lives. Heart Rhythm 2009;6:760–763. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz PJ. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat Rev Cardiol 2014;11:346–353. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz PJ, De Ferrari GM, Pugliese L. Cardiac sympathetic denervation 100 years later: Jonnesco would have never believed it. Int J Cardiol 2017;237:25–28. [DOI] [PubMed] [Google Scholar]
- 8. Jonnesco T. Traitement chirurgical de l’angine de poitrine par la résection du sympathique cervico-thoracique. Presse Med 1921;20:221–230. [Google Scholar]
- 9. Estes EH Jr, Izlar HR Jr. Recurrent ventricular tachycardia. A case successfully treated by bilateral cardiac sympathectomy. Am J Med 1961;31:493–497. [DOI] [PubMed] [Google Scholar]
- 10. Zipes DP, Festoff B, Schaal SF, Cox C, Sealy WC, Wallace AG. Treatment of ventricular arrhythmia by permanent atrial pacemaker and cardiac sympathectomy. Ann Intern Med 1968;68:591–597. [DOI] [PubMed] [Google Scholar]
- 11. Moss AJ, McDonald J. Unilateral cervicothoracic sympathetic ganglionectomy for the treatment of long QT interval syndrome. N Engl J Med 1971;285:903–904. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz PJ, Malliani A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J 1975;89:45–50. [DOI] [PubMed] [Google Scholar]
- 13. Odero A, Bozzani A, De Ferrari GM, Schwartz PJ. Left cardiac sympathetic denervation for the prevention of life-threatening arrhythmias: the surgical supraclavicular approach to cervico-thoracic sympathectomy. Heart Rhythm 2010;7:1161–1165. [DOI] [PubMed] [Google Scholar]
- 14. Bourke T, Vaseghi M, Michowitz Y, Sankhla V, Shah M, Swapna N, et al. Neuraxial modulation for refractory ventricular arrhythmias: value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 2010;121:2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen P-S, Esler M, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 2016;594:3911–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han J, Moe GK. Nonuniform recovery of excitability in ventricular muscle. Circ Res 1964;14:44–60. [DOI] [PubMed] [Google Scholar]
- 17. Han J, Garciadejalon P, Moe GK. Adrenergic effects on ventricular vulnerability. Circ Res 1964;14:516–524. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz PJ, Snebold NG, Brown AM. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am J Cardiol 1976;37:1034–1040. [DOI] [PubMed] [Google Scholar]
- 19. Viskin S. Long QT syndromes and torsade de pointes. Lancet 1999;354:1625–1633. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz PJ, Stone HL, Brown AM. Effects of unilateral stellate ganglion blockade on the arrhythmias associated with coronary occlusion. Am Heart J 1976;92:589–599. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz PJ, Stone HL. Tonic influence of the sympathetic nervous system on myocardial reactive hyperemia and on coronary blood flow distribution in dogs. Circ Res 1977;41:51–58. [DOI] [PubMed] [Google Scholar]
- 22. Schwartz PJ, Verrier RL, Lown B. Effect of stellectomy and vagotomy on ventricular refractoriness in dogs. Circ Res 1977;40:536–540. [DOI] [PubMed] [Google Scholar]
- 23. Cerati D, Schwartz PJ. Single cardiac vagal fibers activity, acute myocardial ischemia, and risk for sudden death. Circ Res 1991;69:1389–1401. [DOI] [PubMed] [Google Scholar]
- 24. Vanoli E, De Ferrari GM, Stramba-Badiale M Jr, Hull S, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991;68:1471–1481. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz PJ. The answers to questions surrounding left cardiac sympathetic denervation are in the library, covered by dust. Heart Rhythm 2020;17:1646–1648. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz PJ, Stone HL. Effects of unilateral stellectomy upon cardiac performance during exercise in dogs. Circ Res 1979;44:637–645. [DOI] [PubMed] [Google Scholar]
- 27. Anderson HN, Bos JM, Rohatgi RK, Ackerman MJ. The effect of left cardiac sympathetic denervation on exercise in patients with long QT syndrome. JACC Clin Electrophysiol 2019;5:1084–1090. [DOI] [PubMed] [Google Scholar]
- 28. Cannon WB. A law of denervation. Am J Med Sci 1939;198:737–750. [Google Scholar]
- 29. Schwartz PJ, Stone HL. Left stellectomy and denervation supersensitivity in conscious dogs. Am J Cardiol 1982;49:1185–1190. [DOI] [PubMed] [Google Scholar]
- 30. Niaz T, Bos JM, Sorensen KB, Moir C, Ackerman MJ. Left cardiac sympathetic denervation monotherapy in patients with congenital long QT syndrome. Circ Arrhythm Electrophysiol 2020;13:e008830. [DOI] [PubMed] [Google Scholar]
- 31. Dusi V, Pugliese L, De Ferrari GM, Odero A, Crotti L, Dagradi F, et al. Left cardiac sympathetic denervation for the long QT syndrome: 50 years’ experience provides guidance for management. JACC Clin Electrophysiol 2022;8:281–294. [DOI] [PubMed] [Google Scholar]
- 32. Bos JM, Bos KM, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation in long QT syndrome: analysis of therapeutic nonresponders. Circ Arrhythm Electrphysiol 2013;6:705–711. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz PJ, Moreno C, Kotta MC, Pedrazzini M, Crotti L, Dagradi F, et al. KCNQ1-p.A341 V and neighboring mutations: the role of location and IKs regulation in the arrhythmic risk of Long QT Syndrome Type 1. Eur Heart J 2021;42:4743–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz PJ, Crotti L, George AL Jr. Modifier genes for sudden cardiac death. Eur Heart J 2018;39:3925–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantù F, Towbin JA, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation 1995;92:3381–3386. [DOI] [PubMed] [Google Scholar]
- 36. Bos JM, Crotti L, Rohatgi RK, Castelletti S, Dagradi F, Schwartz PJ, et al. Mexiletine shortens the QT interval in patients with potassium channel-mediated Type 2 long QT syndrome. Circ Arrhythm Electrophysiol 2019;12:e007280. [DOI] [PubMed] [Google Scholar]
- 37. Gnecchi M, Sala L, Schwartz PJ. Precision medicine and cardiac channelopathies: when dreams meet reality. Eur Heart J 2021;42:1661–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dusi V, Gornbein J, Do DH, Sorg JM, Khakpour H, Krokhaleva Y, et al. Arrhythmic risk profile and outcomes of patients undergoing cardiac sympathetic denervation for recurrent monomorphic ventricular tachycardia after ablation. J Am Heart Assoc 2021;10:e018371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome: a worldwide report. Circulation 1991;84:503–511. [DOI] [PubMed] [Google Scholar]
- 40. Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long QT syndrome. Circulation 2004;109:1826–1833. [DOI] [PubMed] [Google Scholar]
- 41. De Ferrari GM, Dusi V, Spazzolini C, Bos JM, Abrams DJ, Berul CI, et al. Clinical management of catecholaminergic polymorphic ventricular tachycardia: the role of left cardiac sympathetic denervation. Circulation 2015;131:2185–2193. [DOI] [PubMed] [Google Scholar]
- 42. Bos J, Sorensen KB, Moir C, Ackerman MJ. Re-do left cardiac sympathetic denervation (LCSD) following breakthrough cardiac events in long QT syndrome (LQTS) and catecholaminergic polymorphic ventricular tachycardia (CPVT). Eur Heart J 2020;41:749. [Google Scholar]
- 43. Cauti FM, Rossi P, Bianchi S, Bruno K, Iaia L, Rossi C, et al. Outcome of a modified sympathicotomy for cardiac neuromodulation of untreatable ventricular tachycardia. JACC Clin Electrophysiol 2021;7:442–449. [DOI] [PubMed] [Google Scholar]
- 44. Akkuş M, Seyrek Y, Kafalı HC, Ergül Y. Bilateral cardiac sympathetic denervation in children with long-QT syndrome and catecholaminergic polymorphic ventricular tachycardia. J Electrocardiol 2020;61:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ergül Y, Tunca Şahin G, Kafalı HC, Öztürk E, Özgür S, Haydin S, et al. Clinical and genetic characteristics and course of congenital long QT syndrome in children: a nine-year single-center experience. Anatol J Cardiol 2021;25:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rohatgi RK, Sugrue A, Bos JM, Cannon BC, Asirvatham SJ, Moir C, et al. Contemporary outcomes in patients with long QT syndrome. J Am Coll Cardiol 2017;70:453–462. [DOI] [PubMed] [Google Scholar]
- 47. Randall WC, Rohse WG. The augmentor action of the sympathetic cardiac nerves. Circ Res 1956;4:470–475. [DOI] [PubMed] [Google Scholar]
- 48. Malliani A, Recordati G, Schwartz PJ. Nervous activity of afferent cardiac sympathetic fibres with atrial and ventricular endings. J Physiol 1973;229:457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyake CY, Webster G, Czosek RJ, Kantoch MJ, Dubin AM, Avasarala K, et al. Efficacy of implantable cardioverter defibrillators in young patients with catecholaminergic polymorphic ventricular tachycardia: success depends on substrate. Circ Arrhythm Electrophysiol 2013;6:579–587. [DOI] [PubMed] [Google Scholar]
- 50. Roses-Noguer F, Jarman JW, Clague JR, Till J. Outcomes of defibrillator therapy in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2014;11:58–66. [DOI] [PubMed] [Google Scholar]
- 51. Roston TM, Jones K, Hawkins NM, Bos JM, Schwartz PJ, Perry F, et al. Implantable cardioverter–defibrillator use in catecholaminergic polymorphic ventricular tachycardia: a systematic review. Heart Rhythm 2018;15:1791–1799. [DOI] [PubMed] [Google Scholar]
- 52. Pizzale S, Gollob MH, Gow R, Birnie DH. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:1319–1321. [DOI] [PubMed] [Google Scholar]
- 53. van der Werf C, Lieve KV, Bos JM, Lane CM, Denjoy I, Roses-Noguer F, et al. Implantable cardioverter–defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J 2019;40:2953–2961. [DOI] [PubMed] [Google Scholar]
- 54. Wilde AAM, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med 2008;358:2024–2029. [DOI] [PubMed] [Google Scholar]
- 55. Coleman MA, Bos JM, Johnson JN, Owen HJ, Deschamps C, Moir C, et al. Videoscopic left cardiac sympathetic denervation for patients with recurrent ventricular fibrillation/malignant ventricular arrhythmia syndromes besides long QT syndrome. Circ Arrhythm Electrophysiol 2012;5:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Assis FR, Krishnan A, Zhou X, James CA, Murray B, Tichnell C, et al. Cardiac sympathectomy for refractory ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 2019;16:1003–1010. [DOI] [PubMed] [Google Scholar]
- 57. Johnson JN, Harris KM, Moir C, Lau YR, Ackerman MJ. Left cardiac sympathetic denervation in a pediatric patient with hypertrophic cardiomyopathy and recurrent ventricular fibrillation. Heart Rhythm 2011;8:1591–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bonura ED, Moir C, Ackerman MJ, Wackel P. Left cardiac sympathetic denervation for recurrent ventricular tachyarrhythmias in children with congenital heart disease. HeartRhythm Case Rep 2019;5:392–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schwartz PJ. When the risk is sudden death, does quality of life matter? Heart Rhythm 2016;13:70–71. [DOI] [PubMed] [Google Scholar]
- 60. Antiel RM, Bos JM, Joyce DD, Owen HJ, Roskos PL, Moir C, et al. Quality of life after videoscopic left cardiac sympathetic denervation in patients with potentially life-threatening cardiac channelopathies/cardiomyopathies. Heart Rhythm 2016;13:62–69. [DOI] [PubMed] [Google Scholar]
- 61. Waddell-Smith KE, Ertresvaag KN, Li J, Chaudhuri K, Crawford JR, Hamill JK, et al. Physical and psychological consequences of left cardiac sympathetic denervation in long-QT syndrome and catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 2015;8:1151–1158. [DOI] [PubMed] [Google Scholar]
- 62. Schwartz PJ, Spazzolini C, Priori SG, Crotti L, Vicentini A, Landolina M, et al. Who are the long-QT syndrome patients who receive an implantable cardioverter defibrillator and what happens to them? Data from the European long-QT syndrome implantable cardioverter-defibrillator (LQTS ICD) Registry. Circulation 2010;122:1272–1282. [DOI] [PubMed] [Google Scholar]
- 63. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017;2018:e272–e391. [DOI] [PubMed] [Google Scholar]
- 64. Horner JM, Kinoshita M, Webster TL, Haglund CM, Friedman PA, Ackerman MJ. Implantable cardioverter defibrillator therapy for congenital long QT syndrome: a single-center experience. Heart Rhythm 2010;7:1616–1622. [DOI] [PubMed] [Google Scholar]
- 65. Shah MJ, Silka MJ, Avari Silva JN, Balaji S, Beach CM, Benjamin MN, et al. 2021 PACES Expert Consensus Statement on the indications and management of cardiovascular implantable electronic devices in pediatric patients. Heart Rhythm 2021;18:1888–1924. [DOI] [PubMed] [Google Scholar]
- 66. Wang M, Peterson DR, Rosero S, McNitt S, Rich DQ, Seplaki CL, et al. Effectiveness of implantable cardioverter-defibrillators to reduce mortality in patients with long QT syndrome. J Am Coll Cardiol 2021;78:2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moss AJ, Schwartz PJ. 25th anniversary of the International Long-QT Syndrome Registry: an ongoing quest to uncover the secrets of long-QT syndrome. Circulation 2005;111:1199–1201. [DOI] [PubMed] [Google Scholar]
- 68. Schwartz PJ, Ackerman MJ.. A commentary on: Effectiveness of implantable cardioverter-defibrillators to reduce mortality in patients with long QT syndrome. PracticeUpdate website. https://www.practiceupdate.com/content/effectiveness-of-icds-to-reduce-mortality-in-patients-with-long-qt-syndrome/127203/65/2/1 (1 December 2021 data last accessed).
- 69. Schwartz PJ, Breithardt G, Howard AJ, Julian DG, Rehnqvist Ahlberg N. The legal implications of medical guidelines. A Task Force of the European Society of Cardiology. Eur Heart J 1999;20:1152–1157. [DOI] [PubMed] [Google Scholar]
- 70. Schwartz PJ. Efficacy of left cardiac sympathetic denervation has an unforeseen side effect: medicolegal complications. Heart Rhythm 2010;7:1330–1332. [DOI] [PubMed] [Google Scholar]