Key Points
Question
In patients with advanced heart failure, what is the difference in the composite end point of survival to transplant, recovery, or left ventricular assist device (LVAD) support free of debilitating stroke or reoperation to replace the pump 5 years following implantation of a fully magnetically levitated centrifugal-flow vs axial-flow LVAD?
Findings
This observational 5-year follow-up study included 477 of 536 surviving patients from the MOMENTUM 3 randomized trial at 2 years. In a per-protocol analysis of patients receiving a fully magnetically levitated vs axial-flow LVAD, the composite outcome occurred in 54.0% vs 29.7% of patients (hazard ratio, 0.55) and overall survival in 58.4% vs 43.7% of patients (hazard ratio, 0.72). Results of both comparisons were statistically significant.
Meaning
Among patients with advanced heart failure, receipt of a fully magnetically levitated centrifugal-flow LVAD vs axial-flow LVAD was associated with a better composite outcome and higher likelihood of overall survival at 5 years.
Abstract
Importance
Although durable left ventricular assist device (LVAD) therapy has emerged as an important treatment option for patients with advanced heart failure refractory to pharmacological support, outcomes, including survival, beyond 2 years remain poorly characterized.
Objective
To report the composite end point of survival to transplant, recovery, or LVAD support free of debilitating stroke (Modified Rankin Scale score >3) or reoperation to replace the pump 5 years after the implant in participants who received the fully magnetically levitated centrifugal-flow HeartMate 3 or axial-flow HeartMate II LVAD in the MOMENTUM 3 randomized trial and were still receiving LVAD therapy at the 2-year follow-up.
Design, Setting, and Participants
This observational study was a 5-year follow-up of the MOMENTUM 3 trial, conducted in 69 US centers, that demonstrated superiority of the centrifugal-flow LVAD to the axial-flow pump with respect to survival to transplant, recovery, or LVAD support free of debilitating stroke or reoperation to replace the pump at 2 years. A total of 295 patients were enrolled between June 2019 to April 2021 in the extended-phase study, with 5-year follow-up completed in September 2021.
Exposures
Of 1020 patients in the investigational device exemption per-protocol population, 536 were still receiving LVAD support at 2 years, of whom 289 received the centrifugal-flow pump and 247 received the axial-flow pump.
Main Outcomes and Measures
There were 10 end points evaluated at 5 years in the per-protocol population, including a composite of survival to transplant, recovery, or LVAD support free of debilitating stroke or reoperation to replace the pump between the centrifugal-flow and axial-flow pump groups and overall survival between the 2 groups.
Results
A total of 477 patients (295 enrolled and 182 provided limited data) of 536 patients still receiving LVAD support at 2 years contributed to the extended-phase analysis (median age, 62 y; 86 [18%] women). The 5-year Kaplan-Meier estimate of survival to transplant, recovery, or LVAD support free of debilitating stroke or reoperation to replace the pump in the centrifugal-flow vs axial-flow group was 54.0% vs 29.7% (hazard ratio, 0.55 [95% CI, 0.45-0.67]; P < .001). Overall Kaplan-Meier survival was 58.4% in the centrifugal-flow group vs 43.7% in the axial-flow group (hazard ratio, 0.72 [95% CI, 0.58-0.89]; P = .003). Serious adverse events of stroke, bleeding, and pump thrombosis were less frequent in the centrifugal-flow pump group.
Conclusions and Relevance
In this observational follow-up study of patients from the MOMENTUM 3 randomized trial, per-protocol analyses found that receipt of a fully magnetically levitated centrifugal-flow LVAD vs axial-flow LVAD was associated with a better composite outcome and higher likelihood of overall survival at 5 years. These findings support the use of the fully magnetically levitated LVAD.
Trial Registration
ClinicalTrials.gov Identifier: NCT02224755 and NCT03982979
This observational follow-up study of patients from the MOMENTUM 3 randomized trial compares outcomes and overall survival in individuals who received a fully magnetically levitated centrifugal-flow vs an axial-flow left ventricular assist device.
Introduction
Despite benefits of pharmacological therapy for chronic heart failure, some patients exhibit refractoriness to therapy. Patients with refractory heart failure have worse quality of life, inability to tolerate drug therapy, and poor prognosis.1,2 Advanced heart failure may be fatal without use of a left ventricular assist device (LVAD), cardiac transplant, or palliative inotropic support. Among available LVADs, the centrifugal-flow HeartMate 3 has been engineered with wide blood flow pathways, friction-free movement using fully magnetically levitated technology, and intrinsic pulsatility to reduce shear stress and stasis of blood, while the Heartmate II is a continuous axial-flow pump that requires thoracoabdominal placement. Although LVAD therapy has gained support, clinical outcomes and life prolongation beyond 2 years remain poorly characterized.3 As a consequence, referrals for LVAD implantation may occur late and with worse end-organ function.
The Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) randomized clinical trial demonstrated that the fully magnetically levitated centrifugal-flow LVAD, compared with the axial-flow pump, resulted in better outcomes driven by greater hemocompatibility 2 years after the implant.4 However, the overall survival difference between the treatment group at 2 years did not reach statistical significance (hazard ratio [HR], 0.88 [95% CI, 0.67-1.16]).4 The US Food and Drug Administration approved the centrifugal-flow pump for lifelong use (destination therapy) in advanced heart failure in 2018, conditional on an extended evaluation to 5 years of follow-up among those still receiving LVAD support in the trial.
The purpose of this study was to report the composite end point of survival to transplant, recovery, or LVAD support free of debilitating stroke (Modified Rankin Scale score >3) or reoperation to replace the pump 5 years after the implant in participants who received the centrifugal-flow pump or axial-flow pump in the investigational device exemption (IDE) trial and were still receiving LVAD support at the 2-year follow-up.
Methods
Study Design
The IDE trial was designed to compare the safety and effectiveness of the centrifugal-flow pump with the axial-flow pump in patients with advanced heart failure who were refractory to pharmacological support through 2 years after the implant and was conducted at 69 centers in the US (NCT02224755). Both LVADs are manufactured by Abbott. Patients were included irrespective of the intended goal of therapy of either bridge to transplant or destination therapy. As shown in Figure 1, patients were randomized to the centrifugal-flow pump or axial-flow pump group (intent-to-treat population). Between September 2014 and August 2016, a total of 515 patients in the centrifugal-flow pump group and 505 in the axial-flow pump group underwent implantation of their assigned study device (per-protocol population). The trial was originally designed to conclude after 2 years of follow-up, which occurred in 2018. There were 536 patients (289 in the centrifugal-flow pump group and 247 in the axial-flow pump group) still receiving LVAD support at the completion of the 2-year follow-up in the pivotal trial. The original trial design, results, and trial protocol have been previously published.4,5
Figure 1. Flow of Patients in a Trial of 5-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices .

The investigational device exemption trial followed up patients for 2 years and the postapproval extended-phase study followed up patients still receiving left ventricular assist device (LVAD) therapy up to 5 years.
aSpecific inclusion and exclusion criteria were not documented at screening.
bPatients were randomized in a 1:1 ratio and randomization was stratified by study center and blocked to maintain the 1:1 ratio over time.
cIncarceration, nonadherence, suicide attempt/depression, or cancer diagnosis.
dA total of 178 patients with magnetically levitated centrifugal-flow pumps and 117 with axial-flow pumps were consented then enrolled in the extended-phase study protocol. Limited 5-year outcome data were obtained in 63 patients in the centrifugal-flow pump group and 87 in the axial-flow pump group who were not successfully enrolled in the extended-phase study. There were 17 patients in the centrifugal-flow pump group and 15 in the axial-flow pump group who did not provide consent for extended follow-up and were considered to be alive on the date of refusal and then censored in the analysis.
The US Food and Drug Administration approval for the centrifugal-flow pump long-term indication was granted in October 2018. As a condition of approval, an observational extended follow-up study of patients still receiving LVAD support in the pivotal trial from 2 to 5 years after the implant was mandated. This postapproval extended-phase study was conducted under a unique protocol.
The protocol for the postapproval extended-phase study was separate from that prepared for the original trial and required participating centers to complete the start-up process as a de-novo study, obtain institutional review board approval, and obtain consent from all patients still receiving LVAD therapy beyond 2 years (Supplement 1). The study was conducted at the same sites that participated in the pivotal (IDE) trial. Centers determined their patients’ eligibility for the extended-phase protocol based on medical records at the site. If a patient died before providing consent (and after 2-year follow-up), centers could provide the date and cause of death as part of institutional review board approval. Patients receiving LVAD support who provided consent to the extended-phase follow-up were followed up until 5 years after the implant or a prespecified outcome (death, transplant, explant/permanent deactivation, or withdrawal), whichever occurred first. Patients who underwent a device exchange to a pump other than their study-assigned LVAD were withdrawn. Data were collected from study visits performed at 3, 4, and 5 years after the implant. In addition, there were some centers that declined to participate in the extended-phase study protocol; however, institutional review board approval was obtained for the centers to provide limited outcome data within 5 years after the implant for their patients (death, transplant, explant, still receiving LVAD therapy at 5 years, and outcome dates).
End Points
There were 10 end points assessed in the per-protocol population. The composite end point was survival to transplant, recovery, or LVAD support free of debilitating stroke (Modified Rankin Scale score >3) or reoperation to replace the pump 5 years after the implant. Other end points included patient outcomes (transplant, explant/permanent deactivation, or withdrawal) and survival and frequency and incidence of serious adverse events (bleeding [including gastrointestinal bleeding], major infection, hemolysis, device thrombosis and device malfunction, and neurological dysfunction [including stroke]). Functional status as assessed by New York Heart Association (NYHA) class and 6-minute walk distance (6MWD) was also evaluated.
Study Conduct and Analysis Plan Modifications
Due to the gap created by ending the pivotal trial and starting the extended-phase study, data beyond 2 years could not be collected in some patients (eg, their site declined to provide any extended follow-up data, they were unable to be reached to complete consent, they transferred care to a nonparticipating center) (Figure 1). A total of 295 patients were successfully consented and enrolled under the extended-phase study protocol from June 2019 to April 2021 (eTable 1 in Supplement 2), and adverse events and functional status assessments could be collected only in these patients. The final 5-year follow-up visit was completed in September 2021. During the study follow-up, the COVID-19 pandemic created difficulties with obtaining NYHA class and 6MWD. Details regarding study conduct are provided in Supplement 1.
The operational challenges of the extended-phase study reduced the investigators’ ability to assess the original intended primary end point of the study with completeness, which was a composite requiring data on the serious adverse event of stroke. Due to the limitations imposed by missing data, the trial publication and presentation committee determined that overall survival should serve as the modified principal study end point because it was least influenced by the operational challenges and offered the most completeness in data collection. Additional post hoc end points were specified, including an analysis in patients who received an LVAD for destination therapy intent and cause-specific mortality with deaths categorized into those due to hemocompatibility-related events (stroke, bleeding, and device thrombosis), heart failure (including right ventricular failure), infection, and all other causes. All deaths occurring during the 2-year follow-up of the pivotal trial were adjudicated by a clinical events committee (98 deaths in the centrifugal-flow pump group and 103 in the axial-flow pump group). Deaths in patients who successfully consented for extended follow-up were also adjudicated by the clinical events committee (7 in the centrifugal-flow pump group and 9 in the axial-flow pump group). Subgroup analyses were performed for categories based on age, sex, race and ethnicity, intended goal of therapy (bridge to transplant or destination therapy), and severity of illness (based on Interagency Registry for Mechanically Assisted Circulatory Support [INTERMACS] profile) at baseline). Race and ethnicity were collected in the trial to inform on the treatment effect across different subgroups and were self-reported based on predefined categories of Asian, Black, Native Hawaiian or Pacific Islander, and White race and an open category for all other.
Statistical Methods
Continuous variables are presented as mean (SD) or median (IQR) and categorical variables as counts and percentages. The sample size for the extended phase included patients from the IDE trial still receiving LVAD support at 2 years who then contributed any data beyond 2 years for at least 1 end point analysis.
The composite end point of survival to transplant, recovery, or LVAD support free of debilitating stroke or reoperation to replace the pump was evaluated with the Kaplan-Meier method and Cox proportional hazards modeling. Proportional hazards assumptions were confirmed by creating and testing the significance of time-dependent covariates. Events constituting failure of the composite end point included death, debilitating stroke, and reoperation. Withdrawals were also considered failure events; however, in the extended phase this only applied to patients who were successfully consented for extended follow-up. Serious adverse events during the extended phase were not collected in all patients contributing to the analysis cohort, and it was assumed that these patients had no debilitating stroke after 2 years.
Survival rates were estimated with the Kaplan-Meier method and HRs with 95% CIs were calculated with Cox proportional hazards modeling. Patients who underwent heart transplant, device removal/deactivation, or withdrawal were censored at the time of the outcome. The Fine-Gray model was used to evaluate mortality risk while accounting for the competing risk of heart transplant and to evaluate cause-specific mortality as a post hoc analysis. To assess the treatment effect on survival across different subgroups (based on age, sex, race and ethnicity, intended goal of therapy, and INTERMACS profile at baseline), an interaction term between the subgroup covariate and the treatment group was included in the Cox proportional hazards models.
Because outcome data in the extended-phase study were not able to be collected in all patients who were receiving LVAD support at 2 years, the robustness of the observed survival rates in the extended-phase study was evaluated in a post hoc analysis. Missing outcome data from the extended-phase study were filled in with additional data (occurrence of death, transplant, device explant, including dates of these outcomes, within 5 years after implant) from the sponsor’s device tracking database as permissible by state regulations. No data from patients refusing consent for the extended-phase study were used. A tipping point analysis using δ-adjusted bootstrap-based multiple imputation was implemented to test the assumption of noninformative censoring for the patients remaining withdrawn after inclusion of the supplemental device tracking data, excluding those undergoing exchange to a non–study-assigned device. Thirty rounds of imputation were performed and the δ for centrifugal-flow pump was increased by 1 unit each time until the treatment effect in favor of centrifugal-flow pump was no longer significant. In the axial-flow pump group, the mortality hazard was assumed to be the same between withdrawn vs patients who were not withdrawn.
Rates for serious adverse events occurring from 0 to 5 years are presented in events per patient-year and compared between treatment groups with rate ratios and 95% CIs calculated using Poisson regression. Only patients who consented for extended follow-up contributed serious adverse events after 2 years (178 in the centrifugal-flow pump group and 117 in the axial-flow pump group).
Two-sided P values <.05 were considered significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. All statistical analyses were performed with SAS, version 9.4 (SAS Institute).
Results
Patient Population
In the IDE trial’s per-protocol population of 1020 patients, 515 patients received the centrifugal-flow LVAD and 505 patients received the axial-flow LVAD (Figure 1). A total of 289 patients in the centrifugal-flow pump group and 247 in the axial-flow pump group were still receiving LVAD support at 2 years. Due to the gap created by ending the pivotal trial and starting the extended-phase study, there were 59 patients for whom no data beyond 2 years were able to be collected. The remaining 477 patients had data beyond 2 years available and constitute the sample size for the extended phase. Of these patients, 295 patients were successfully consented and enrolled under the extended-phase study protocol. In the remaining 182 patients, limited 5-year outcome data were obtained in 150 patients and 32 patients who did not provide consent for extended follow-up were considered to be alive on the date of refusal and then censored, and no additional data were retrieved from these patients.
The baseline characteristics for the treatment group in the per-protocol population are shown in the Table and are largely similar between the groups. There were more Black patients and fewer White patients in the centrifugal-flow pump group compared with the axial-flow pump group. Baseline demographics were also similar between treatment groups in the destination therapy population. Patients in the centrifugal-flow pump group had a higher pulmonary vascular resistance and more mitral regurgitation of any severity compared with patients in the axial-flow pump group, but they were less likely to be receiving diuretics (eTable 2 in Supplement 2). Demographic information is also provided in eTable 3 in Supplement 2 for the 477 patients who constitute the sample size for the extended phase.
Table. Baseline Characteristics in a Study of 5-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices .
| Characteristic | No. (%) | |
|---|---|---|
| Magnetically levitated centrifugal-flow pump (n = 515) | Axial-flow pump (n = 505) | |
| Age, median (IQR), y | 62 (52-68) | 63 (54-69) |
| Men | 410 (79.6) | 413 (81.8) |
| Women | 105 (20.4) | 92 (18.2) |
| Race and ethnicitya | ||
| Asian | 8 (1.6) | 3 (0.6) |
| Black | 145 (28.2) | 119 (23.6) |
| Otherb | 21 (4.1) | 17 (3.4) |
| Native Hawaiian or Pacific Islander | 0 | 4 (0.8) |
| White | 341 (66.2) | 362 (71.7) |
| Hispanic ethnicity | 28 (5.4) [n = 514] | 27 (5.3) |
| Body surface area, mean (SD), m2 | 2.07 (0.27) | 2.08 (0.28) |
| BMI, median (IQR) | 28.4 (24.6-33.0) | 27.9 (24.2-32.1) |
| Ischemic cause of heart failure | 216 (41.9) | 236 (46.7) |
| Medical history | ||
| Diabetes | 233 (45.2) | 221 (43.8) |
| Atrial fibrillation | 215 (41.7) | 235 (46.5) |
| Coronary artery bypass | 102 (19.8) | 111 (22.0) |
| Stroke | 50 (9.7) | 56 (11.1) |
| Valve replacement/repair | 36 (7.0) | 30 (5.9) |
| Cardiac function and pressures, median (IQR)c | ||
| Mean arterial pressure, mm Hg | 80.1 (10.8) | 78.9 (10.0) [n = 503] |
| Pulmonary-capillary wedge pressure, mm Hg | 23 (17-29) [n = 503] | 23 (17-29) [n = 495] |
| Cardiac index, L/min/m2 | 1.90 (1.60-2.24) [n = 511] | 1.89 (1.60-2.20) [n = 503] |
| Pulmonary vascular resistance, Wood units | 2.84 (1.90-4.10) [n = 498] | 2.74 (1.90-3.88) [n = 493] |
| Right atrial pressure, mm Hg | 10 (6-15) [n = 504] | 9 (5-15) [n = 495] |
| Laboratory values, median (IQR)d | ||
| Serum sodium, mmol/L | 136 (133-138) | 136 (133-138) |
| Serum creatinine, mg/dL | 1.29 (1.01-1.61) | 1.30 (1.06-1.60) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 58 (43-75) | 56 (43-72) |
| Intended use of device | ||
| Destination therapy | 317 (61.6) | 307 (60.8) |
| Bridge to transplant | 112 (21.7) | 120 (23.8) |
| Bridge to candidacy for transplant | 86 (16.7) | 78 (15.4) |
| INTERMACS profilee | n = 514 | n = 501 |
| 1-2 | 167 (32.5) | 159 (31.7) |
| 3 | 272 (52.9) | 251 (50.1) |
| 4-7 | 75 (14.6) | 91 (18.2) |
| Valvular insufficiencyf | n = 513 | |
| Aortic | 152 (29.6) | 147 (29.1) |
| Moderate or severe | 11 (2.1) | 7 (1.4) |
| Mitral | 464 (90.4) | 439 (86.9) |
| Moderate or severe | 216 (42.1) | 228 (45.1) |
| Tricuspid | 431 (84.0) | 419 (83.0) |
| Moderate or severe | 158 (30.8) | 127 (25.1) |
| Concomitant medications | ||
| Inotrope | 444 (86.2) | 417 (82.6) |
| Diuretic | 435 (84.5) | 460 (91.1) |
| β-blocker | 284 (55.1) | 269 (53.3) |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor antagonist | 158 (30.7) | 171 (33.9) |
| Intraaortic balloon pump | 64 (12.4) | 75 (14.9) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Race and ethnicity were self-reported according to the predefined categories shown.
Includes self-reported descriptors such as Hispanic, Latino, Mexican, Caribbean-American, Middle Eastern, Multi-racial, and Native American.
Cardiac function and pressures were evaluated by invasive hemodynamic assessment. Reference values were as follows: mean arterial pressure, 70-105 mm Hg; pulmonary-capillary wedge pressure, 6-12 mm Hg; cardiac index, 2.2-4 L/min/m2; pulmonary vascular resistance, ≤3 Wood units; right atrial pressure, 0-5 mm Hg.
Laboratory reference values were as follows: serum sodium, 135-145 mmol/L; serum creatinine, 0.8-1.3 mg/dL; estimated glomerular filtration rate, >60 mL/min/1.73 m2.
Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles range from 1 to 7; a profile of 1 represents the most severe illness and a profile of 7 represents the least severe illness. Profiles range from 1 to 3 in patients requiring inotropic therapy (ranging from unstable to stable) and 4 to 7 in those without inotropic therapy but with symptoms including heart failure at rest and advanced New York Heart Association class III.
Moderate and severe valvular insufficiency were determined by the site investigators based on echocardiography.
Composite End Point
The composite of 5-year survival to transplant, recovery, or LVAD support free of debilitating stroke or reoperation to replace the pump occurred in 336 of 515 patients (65.2%) in the centrifugal-flow group vs 240 of 505 (47.5%) in the axial-flow group. The Kaplan-Meier estimates of event-free survival at 5 years were 54.0% in the centrifugal-flow group vs 29.7% in the axial-flow group (HR, 0.55 [95% CI, 0.45-0.67]; P < .001) (Figure 2A). The first event type leading to failure of the composite end point is shown in eTable 4 in Supplement 2. Censoring of patients who refused consent for extended follow-up at 2 years (instead of the date of refusal) did not significantly change the 5-year event-free survival rates.
Figure 2. Composite End Point and Overall Survival in a Study of 5-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices (LVADs).
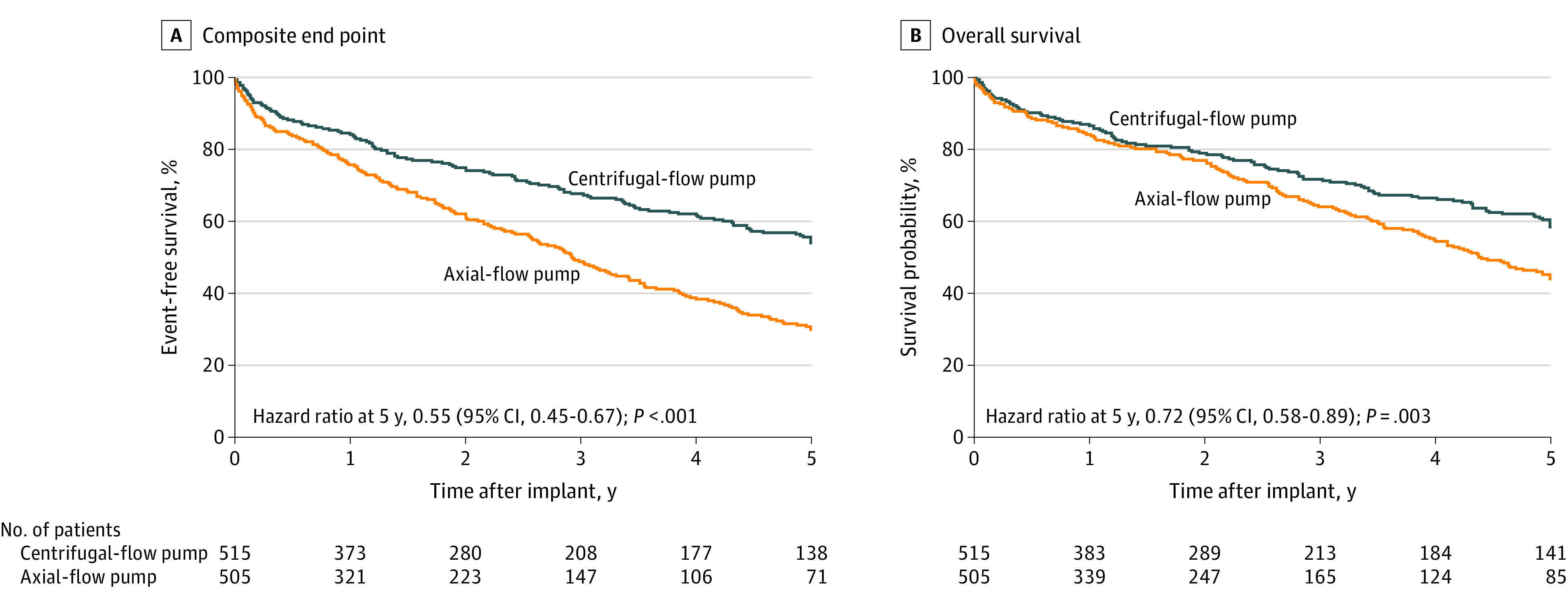
A, Comparison of survival to transplant, recovery, or LVAD support free of debilitating stroke (Modified Rankin Scale score >3) or reoperation to replace the pump 5 years after the implant. The 5-year event-free survival was 54.0% (95% CI, 48.5%-59.1%) in the magnetically levitated centrifugal-flow pump group and 29.7% (95% CI, 24.7%-34.9%) in the axial-flow pump group. Patients receiving transplants for reason other than device malfunction are censored at time of transplant assuming there was no prior pump replacement or debilitating stroke. B, Five-year survival was 58.4% (95% CI, 52.8%-63.6%) in the centrifugal-flow pump group and 43.7% (95% CI, 37.6%-49.6%) in the axial-flow pump group. Patients who received transplants were censored at time of transplant. The median (IQR) LVAD support duration was 2.01 (0.94-5.0) years for the centrifugal-flow pump group and 1.86 (0.75-3.98) years for the axial-flow pump group. In the extended phase after 2 years, a total of 477 patients contributed data to these analyses.
Overall Survival
During the 5-year follow-up period, 156 of 515 patients (30.3%) in the centrifugal-flow pump group and 184 of 505 (36.4%) in the axial-flow pump group died. At 5 years, the Kaplan-Meier overall survival rates were 58.4% in centrifugal-flow pump group and 43.7% in axial-flow pump group (HR, 0.72 [95% CI, 0.58-0.89]; P = .003) (Figure 2B). In a post hoc analysis, there were consistent findings in the destination therapy–specific subgroup, with a 5-year Kaplan-Meier survival rate of 54.8% in the centrifugal-flow pump group compared with 39.4% in the axial-flow pump group (HR, 0.70 [95% CI, 0.55-0.90]; P = .005) (eFigure 1 in Supplement 2). Censoring of patients who refused consent for extended follow-up at 2 years instead of the date of refusal did not significantly change the 5-year survival rates.
Serious Adverse Events
Serious adverse event rates from 0 to 5 years are presented in Figure 3 and eTable 5 in Supplement 2. Rates for hemocompatibility-related adverse events were significantly lower in the centrifugal-flow group compared with the axial-flow pump group (device thrombosis: 0.010 vs 0.108 events/patient-years; stroke: 0.050 vs 0.136 events/patient-years; bleeding: 0.430 vs 0.765 events/patient-years). Infection, cardiac arrhythmias, and right ventricular failure were similar between the groups. Serious adverse event rates during the extended phase from 2 to 5 years are shown in eTable 6 in Supplement 2.
Figure 3. Serious Adverse Events in a Study of 5-Year Outcomes in Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices .
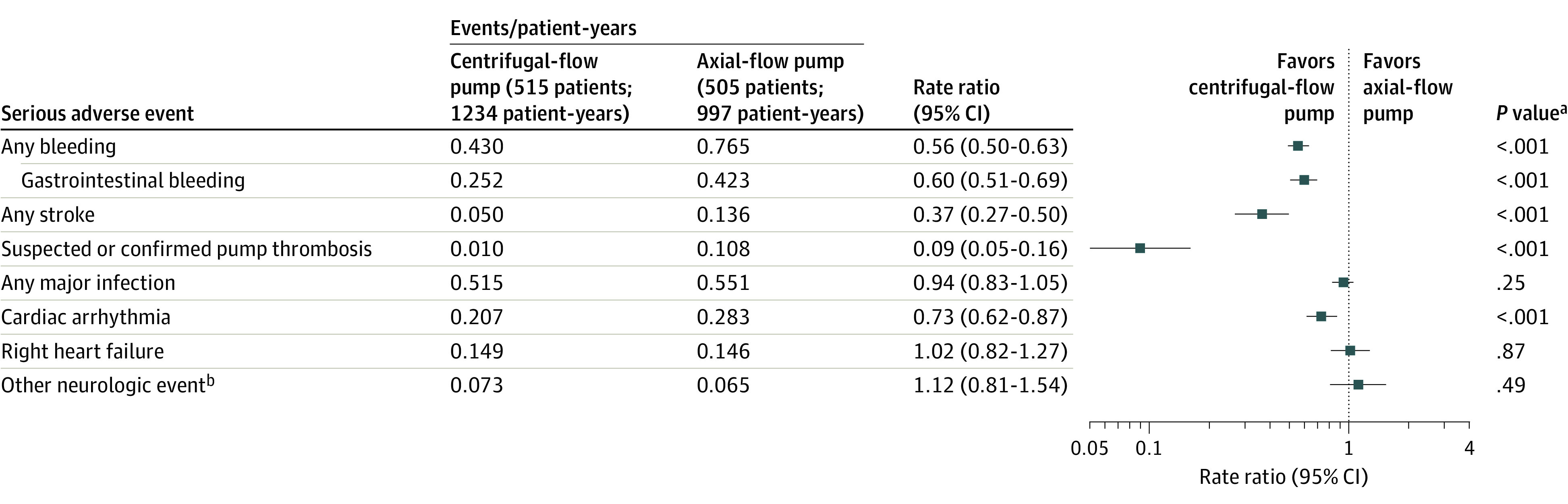
Serious adverse events are defined as those causing death or congenital abnormality or birth defect or a life-threatening illness or injury that results in permanent disability, requires hospitalization, or prolongs a hospitalization and/or requires intervention to prevent permanent injury or damage. Serious adverse events occurring after 2 years were collected in 178 patients in the magnetically levitated centrifugal-flow pump group and 117 patients in the axial-flow pump group. Other secondary end points are shown in eTable 6 and 7 in Supplement.
aRate ratios and P value from Poisson regression.
bIncludes encephalopathy, seizure, transient ischemic attack, and other neurological events other than stroke.
Functional Status
Although NYHA class was collected in only 178 patients and 6MWD in only 89 patients at 5 years, the available data showed similar results between the treatment groups. At 5 years, 74 of 109 patients (67.9%) in the centrifugal-flow pump group and 48 of 69 (69.6%) in the axial-flow pump group were in NYHA class I or II (eTable 7 in Supplement 2).
Post Hoc End Points and Analyses
Cause-Specific Mortality
The causes of death are shown in eTable 8 in Supplement 2. In the axial-flow pump group, there were 54 deaths due to hemocompatibility-related adverse events, which were the leading causes of death. In the centrifugal-flow pump group, the risk of hemocompatibility-related mortality was significantly lower than in the axial-flow pump group (3.9% vs 10.7%; HR, 0.33 [95% CI, 0.20-0.55]; P < .001), resulting from reduced strokes and device thrombosis events (Figure 4). Heart failure was the next leading cause of death in both groups, followed by infection. The risk of death due to these causes were similar between treatment groups.
Figure 4. Cause-Specific Mortality 5 Years After Implant in a Study of Patients With Fully Magnetically Levitated vs Axial-Flow Left Ventricular Assist Devices .

Comparison of deaths due to hemocompatibility-related events, heart failure, infection, and other causes. In the extended phase after 2 years, a total of 477 patients contributed data to the analysis.
aDifference shown for magnetically levitated centrifugal-flow pump minus axial-flow pump values.
bHazard ratios and P values were calculated from the Fine-Gray model.
cOther causes are provided in eTable 8 in Supplement 2. The most common other causes included ventricular arrhythmia or cardiac arrest, trauma, hepatic or kidney failure, respiratory failure, and driveline or power cable disconnection.
Competing Outcomes
The outcomes occurring in the centrifugal-flow pump and axial-flow pump groups during the 5-year study are shown in eFigure 2 in Supplement 2. At the completion of the 5-year follow-up, 141 of 515 patients (27.4%) in the centrifugal-flow pump group and 85 of 505 (16.8%) in the axial-flow pump group remained receiving LVAD support. Of all 1020 patients, 322 (31.6%) underwent heart transplant. Survival in the presence of the competing risk of transplant was evaluated with the Fine-Gray model, and the mortality risk continued to remain significantly lower with the centrifugal-flow pump compared with the axial-flow pump (HR, 0.78 [95% CI, 0.63-0.97]; P = .023).
In the subgroup of patients in whom the intended goal for therapy was bridge to transplant or bridge to candidacy for transplant, the transplant rates were 102 of 198 patients (51.5%) in the centrifugal-flow group and 110 of 198 (55.6%) in the axial-flow pump group (eFigure 3 in Supplement 2). Of these 396 patients, 85 (21.5%) died before receiving a transplant. In addition, a portion of patients in whom the initial goal for therapy was destination therapy (54 of 317 [17.0%] in the centrifugal-flow pump group and 56 of 307 [18.2%] in the axial-flow pump group) were eventually put on a transplant list and received a transplant (eFigure 4 in Supplement 2). Because some patients in whom the intended goal was destination therapy ultimately received a transplant, survival was reevaluated in the destination therapy subgroup excluding patients who received a transplant (eFigure 5 in Supplement 2). In this subgroup of patients who underwent destination therapy, a significant survival benefit with the centrifugal-flow pump was demonstrated (HR, 0.70 [95% CI, 0.55-0.89]; P = .004).
Subgroup Analysis of Survival
No significant interaction between the groups was observed for the prespecified subgroups of age, sex, race and ethnicity, intended goal of pump support (bridge to transplant or destination therapy), or INTERMACS profile with regard to overall survival (eFigure 6 in Supplement 2). Thus, the treatment effect with respect to survival was consistent across different subgroups.
Tipping Point Sensitivity Analysis
The tipping point δ was between 3 and 4, which indicated that the mortality hazard of the patients in the centrifugal-flow pump group that were withdrawn would have to be at least 3-fold greater than those who were not withdrawn to abolish the centrifugal-flow pump survival benefit compared with the axial-flow pump.
Discussion
In this observational follow-up study of patients from the IDE trial, per-protocol analyses found that implantation of a fully magnetically levitated LVAD vs axial-flow LVAD was associated with a better composite outcome and higher likelihood of overall survival at 5 years. These findings support the use of the fully magnetically levitated LVAD.
Unlike the pivotal 2-year analysis, there was higher likelihood of overall survival in patients who received the centrifugal-flow pump compared with the axial-flow pump at this 5-year time point. The statistically significant difference in survival was attributable to fewer deaths due to hemocompatibility-related adverse events, including stroke, bleeding, and pump thrombosis. The median survival in patients in the centrifugal-flow pump group with advanced heart failure exceeded 5 years, including the subgroup who received an LVAD with a therapeutic intent of destination therapy and were deemed ineligible for cardiac transplant at the time of enrollment in the trial.
Engineering advances have introduced technologically improved LVADs in the past 2 decades with a gain in 2-year survival.3,6,7 The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial investigated an early-generation LVAD (HeartMate vented electric device, Thoratec) compared with optimal medical therapy and demonstrated a 23% survival with the LVAD at 2 years.8 This represented a marked improvement over that expected in a cohort of transplant-ineligible patients with advanced heart failure (8% survival at 2-year in the medical therapy group). A randomized clinical trial of a continuous-flow pump compared with the older-generation pulsatile-flow LVAD used in the REMATCH trial demonstrated a significant improvement in survival of 58% at 2 years.9 Another centrifugal-flow pump that did not require an abdominal surgical pocket and could be implanted directly in the thorax, the Heartware HVAD (Medtronic) was deemed to be noninferior to the axial-flow pump with a similar survival at 2 years.10 However, these devices have had hemocompatibility-related adverse events, with a dominance of pump thrombosis in the axial-flow pump group and stroke events in the HVAD group.11,12 The centrifugal-flow pump was engineered to reduce hemocompatibility-related adverse events by increasing the blood flow pathways and developing an intrinsic pulsatility in the pump while using a frictionless fully magnetically levitated rotor system.13,14,15
Until this trial, registry analyses such as INTERMACS have provided the best look at 5-year survival, but their analyses have been dominated by devices other than the centrifugal-flow pump. Estimates from those analyses indicated an overall survival of 48.2% in the current era, but lower, at 40.6%, in the destination therapy subgroup at 5 years.16,17 These survival estimates are concordant with the observed survival in the axial-flow pump group in the current trial.
In the current trial, adverse events and deaths due to heart failure, followed by infection, remained dominant with the centrifugal-flow pump LVAD. There continues to be concerns about hemodynamic-related adverse events (heart failure) and morbidity of device-related infection.18,19,20 Clinical algorithms that utilize invasive hemodynamic assessments in concert with cardiac and device-based imaging may be required to discriminate the associated causes of heart failure and to target therapy toward the primary underlying deficit.17 These may include speed adjustments to facilitate left ventricular unloading, amelioration of aortic regurgitation (which can result in recirculation), or assessing for defects such as obstruction of the outflow graft. Dealing with driveline-related infections may require engineering of fully internalized power sources through transcutaneous energy transfer or other techniques.3
Limitations
This study has several limitations. First, as a result of the operational challenges in this trial due to the gap between completion of the pivotal trial, the need to initiate a separate study protocol, and challenges encountered due to the unanticipated occurrence of the COVID-19 pandemic, there was a high percentage of missing data for the originally intended primary end point and serious adverse events. This required modifying the principal end point analysis to focus on survival, which provided the most complete information in this study. However, the rates of nonfatal events in end points should be interpreted carefully due to the magnitude and imbalance of missingness of values between the treatment groups. Second, data to define the accompanying quality of life experienced during device therapy were not collected, making cost-effectiveness assessment difficult.21 Third, some researchers may consider the clinical trial population as different from those in the clinical setting. However, a previous investigation that examined this issue concluded that most exclusion criteria used in LVAD clinical trials did not afford a substantially greater risk to patients in the clinical setting. The outcomes in the axial-flow pump LVAD group were similar to those reported in INTERMACS.22 Fourth, not all initial centers chose to continue the extended 5-year follow-up. However, the sponsor device tracking database was used to reduce the dropout rates in a sensitivity analysis.
Conclusions
In this observational follow-up study of patients from the IDE trial, per-protocol analyses found that receipt of a fully magnetically levitated LVAD vs axial-flow LVAD was associated a better composite outcome and higher likelihood of overall survival at 5 years. These findings support the use of the fully magnetically levitated LVAD.
Protocol
Study Devices
Study Organization And Conduct
eFigures
eTables
Data sharing statement
References
- 1.Greene SJ, Fonarow GC, Butler J. Risk profiles in heart failure: baseline, residual, worsening, and advanced heart failure risk. Circ Heart Fail. 2020;13(6):e007132. doi: 10.1161/CIRCHEARTFAILURE.120.007132 [DOI] [PubMed] [Google Scholar]
- 2.Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(11):1505-1535. doi: 10.1002/ejhf.1236 [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR, Gustafsson F. Left ventricular assist devices at the crossroad of innovation in advanced heart failure. J Card Fail. 2021;27(11):1291-1294. doi: 10.1016/j.cardfail.2021.06.003 [DOI] [PubMed] [Google Scholar]
- 4.Mehra MR, Uriel N, Naka Y, et al. ; MOMENTUM 3 Investigators . A fully magnetically levitated left ventricular assist device: final report. N Engl J Med. 2019;380(17):1618-1627. doi: 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 5.Heatley G, Sood P, Goldstein D, et al. ; MOMENTUM 3 Investigators . Clinical trial design and rationale of the Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant. 2016;35(4):528-536. doi: 10.1016/j.healun.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 6.Sidhu K, Lam PH, Mehra MR. Evolving trends in mechanical circulatory support: clinical development of a fully magnetically levitated durable ventricular assist device. Trends Cardiovasc Med. 2020;30(4):223-229. doi: 10.1016/j.tcm.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 7.Jakus N, Brugts JJ, Claggett B, et al. ; PCHF-VAD registry . Improved survival of left ventricular assist device carriers in Europe according to implantation eras: results from the PCHF-VAD registry. Eur J Heart Fail. 2022;24(7):1305-1315. doi: 10.1002/ejhf.2526 [DOI] [PubMed] [Google Scholar]
- 8.Rose EA, Gelijns AC, Moskowitz AJ, et al. ; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group . Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435-1443. doi: 10.1056/NEJMoa012175 [DOI] [PubMed] [Google Scholar]
- 9.Slaughter MS, Rogers JG, Milano CA, et al. ; HeartMate II Investigators . Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241-2251. doi: 10.1056/NEJMoa0909938 [DOI] [PubMed] [Google Scholar]
- 10.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376(5):451-460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 11.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33-40. doi: 10.1056/NEJMoa1313385 [DOI] [PubMed] [Google Scholar]
- 12.Milano CA, Rogers JG, Tatooles AJ, et al. ; ENDURANCE Investigators . HVAD: the ENDURANCE supplemental trial. JACC Heart Fail. 2018;6(9):792-802. doi: 10.1016/j.jchf.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 13.Mehra MR, Naka Y, Uriel N, et al. ; MOMENTUM 3 Investigators . A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017;376(5):440-450. doi: 10.1056/NEJMoa1610426 [DOI] [PubMed] [Google Scholar]
- 14.Mehra MR. The burden of haemocompatibility with left ventricular assist systems: a complex weave. Eur Heart J. 2019;40(8):673-677. doi: 10.1093/eurheartj/ehx036 [DOI] [PubMed] [Google Scholar]
- 15.Uriel N, Colombo PC, Cleveland JC, et al. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135(21):2003-2012. doi: 10.1161/CIRCULATIONAHA.117.028303 [DOI] [PubMed] [Google Scholar]
- 16.Shah P, Yuzefpolskaya M, Hickey GW, et al. Twelfth interagency registry for mechanically assisted circulatory support report: readmissions after left ventricular assist device. Ann Thorac Surg. 2022;113(3):722-737. doi: 10.1016/j.athoracsur.2021.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varshney AS, DeFilippis EM, Cowger JA, Netuka I, Pinney SP, Givertz MM. Trends and outcomes of left ventricular assist device therapy: JACC focus seminar. J Am Coll Cardiol. 2022;79(11):1092-1107. doi: 10.1016/j.jacc.2022.01.017 [DOI] [PubMed] [Google Scholar]
- 18.Patel CB, Blue L, Cagliostro B, et al. Left ventricular assist systems and infection-related outcomes: a comprehensive analysis of the MOMENTUM 3 trial. J Heart Lung Transplant. 2020;39(8):774-781. doi: 10.1016/j.healun.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 19.Vidula H, Takeda K, Estep JD, et al. Hospitalization patterns and impact of a magnetically-levitated left ventricular assist device in the MOMENTUM 3 trial. JACC Heart Fail. 2022;10(7):470-481. doi: 10.1016/j.jchf.2022.03.007 [DOI] [PubMed] [Google Scholar]
- 20.Mehra MR, Salerno C, Cleveland JC, et al. Healthcare resource use and cost implications in the MOMENTUM 3 long-term outcome study. Circulation. 2018;138(18):1923-1934. doi: 10.1161/CIRCULATIONAHA.118.035722 [DOI] [PubMed] [Google Scholar]
- 21.Lim HS, Shaw S, Carter AW, Jayawardana S, Mossialos E, Mehra MR. A clinical and cost-effectiveness analysis of the HeartMate 3 left ventricular assist device for transplant-ineligible patients: a United Kingdom perspective. J Heart Lung Transplant. 2022;41(2):174-186. doi: 10.1016/j.healun.2021.11.014 [DOI] [PubMed] [Google Scholar]
- 22.Mezzacappa C, Ravindra NG, Caraballo C, et al. Clinical implications of differences between real world and clinical trial usage of left ventricular assist devices for end stage heart failure. PLoS One. 2020;15(12):e0242928. doi: 10.1371/journal.pone.0242928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol
Study Devices
Study Organization And Conduct
eFigures
eTables
Data sharing statement


