Abstract
We have used Escherichia coli alkaline phosphatase to show the interplay among the characteristics of two amino-terminal domains in the preprotein (the signal peptide and the early mature region), the efficiency with which this protein is transported, and its requirement for SecB to accomplish the transport process. The results suggest that although alkaline phosphatase does not normally require SecB for transport, it is inherently able to utilize SecB, and it does so when its ability to interface with the transport machinery is compromised.
SecB is a molecular chaperone which is required for the efficient transport of a subset of proteins in Escherichia coli. A tetramer of identical 16-kDa subunits, it functions by binding some nascent secretory proteins as they emerge from the ribosome in the cytosol. SecB is thought to interact with the mature region of the preprotein (16, 24, 27) to prevent its premature folding and to target the preprotein to the membrane in a transport-competent state (4, 14). The SecB-preprotein complex binds SecA at the gateway of translocation sites, and this interaction is thought to facilitate the transfer of the preprotein to SecA with the subsequent release of SecB (10, 14). SecA, in turn, initiates the passage of the preprotein through the translocon via its associated ATPase activity (3, 4).
The rules which govern a requirement for SecB assistance in protein transport are unknown. A variety of outer membrane-bound and periplasmic proteins require SecB for efficient transport (e.g., OmpA, OmpF, PhoE, MBP) while others do not (e.g., PhoA, RBP, β-lactamase). Moreover, SecB exhibits a very broad substrate specificity involving either charged or hydrophobic regions of preproteins (19, 22), suggesting that SecB utilization is not dictated by a requirement for specific high-affinity binding sites. Nor does the mere presence of SecB-binding sites determine whether a preprotein is inherently SecB dependent; modifications in SecB-independent proteins can lead to SecB utilization, indicating that at least some proteins are endowed with the ability to employ SecB whether they need to or not (17, 18). Furthermore, no consensus sequence or region consistent among proteins has been found responsible for conferring SecB dependence (1, 12, 30). One model suggests that the requirement for SecB depends on the partitioning of the preprotein between transport-productive and aggregation-prone, nonproductive pathways (6, 13); while a functional signal peptide does not directly bind SecB (24), it functions to impede the folding of the preprotein so that SecB can bind (6, 23), while a defective signal peptide does not. This model has been questioned, however, because SecB can also bind fully folded proteins (26) and the rate of SecB-ligand association can be much faster than the rate of folding of SecB-independent proteins (9).
Alkaline phosphatase is a normally SecB-independent protein with which we have made a series of small well-defined adjustments to show its incremental conversion to a SecB-dependent protein. The results indicate that SecB plays a fundamental role in enhancing the efficiency with which the amino-terminal domain of the preprotein engages the transport pathway.
Discrete changes in the early mature region of alkaline phosphatase gradually shift the equilibrium toward SecB utilization.
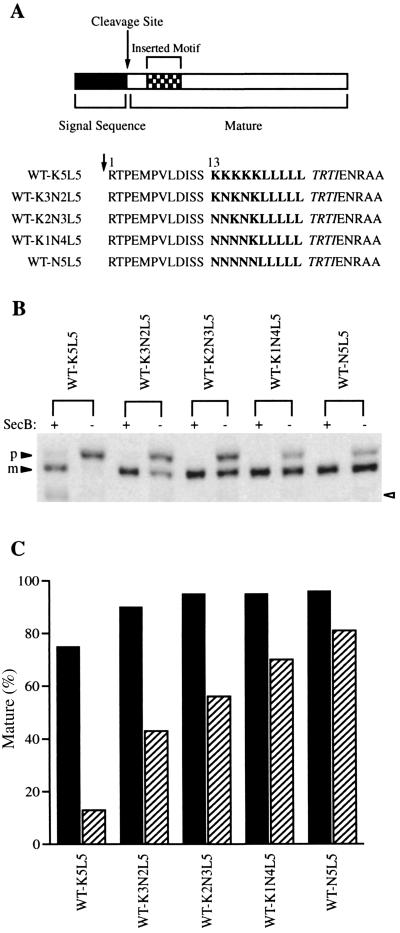
We have previously shown that a 10-residue motif, including a cluster of basic amino acids in the mature region of alkaline phosphatase, confers SecB dependence and that the impact of the basic motif parallels the accessibility of its location within the protein to the secretion apparatus (18). In order to determine the extent to which the basic residues are specifically responsible for the SecB dependence, a series of mutant alkaline phosphatases were generated in which the lysines were successively replaced with asparagines within the motif inserted at residue 13 of the mature region of the preprotein (Fig. 1A). secB+ and secB null cells were grown in Luria-Bertani medium and then transferred to MOPS (morpholinepropanesulfonic acid) medium lacking phosphate to induce expression of the mutant alkaline phosphatases as described previously (18). The cells were then pulse labeled with [35S]methionine (30 s) followed by incubation with excess cold methionine for an additional 30 s to ensure the progression of protein synthesis. Alkaline phosphatase was immunoprecipitated and the relative ratio of the precursor to mature forms was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and quantified with a phosphorimager (18). The ratio of precursor to mature forms of alkaline phosphatase provides a quantifiable earmark of the relative extent to which each mutant accomplishes protein transport in the presence and absence of SecB. In every case, when the mature form of the protein was generated it was localized in the periplasm (data not shown). As shown in Fig. 1B and C, the presence of the basic motif results in little or no accumulation of precursor when expressed in the presence of SecB. However, in the absence of SecB there is a direct correlation between the number of basic residues and the accumulation of the precursor form of the protein. Although these mutants are processed in the presence of SecB with transport kinetics comparable to those of the wild-type SecB-dependent protein, MBP (18), transport of mutants with more lysine residues is more sensitive to the loss of SecB.
FIG. 1.
SecB dependence of mutant alkaline phosphatases carrying different numbers of basic residues in the early mature region and expressed in secB+ and secB null cells. (A) The amino acid sequences of the inserted motifs and the flanking regions. Alkaline phosphatase is shown diagrammatically with the signal sequence region darkly shaded, the mature region is shown as an open bar, and the signal peptide cleavage site is marked by an arrow. The sequences inserted at the thirteenth residue of the mature protein (checkered) and the flanking sequences are shown. The inserted sequences are shown in boldface, and the amino acids generated during the cloning procedure are shown in italics. (B) Precursor processing of the mutant alkaline phosphatases expressed in secB+ (AW1043) and secB null (CK1953) cells. The positions of the precursor (p) and mature (m) forms of alkaline phosphatase are indicated. Strain CK1953 has a chromosomal copy of the alkaline phosphatase gene, the product of which is indicated with the open arrowhead. (C) Quantification of the extent of processing of the mutant alkaline phosphatases from the experiment depicted in panel B. Darkly shaded bars represent the extent of processing in the secB+ strain, and hatched bars represent the processing in the secB null strain.
It is not clear why the basic motif inhibits the transport process in the absence of SecB. It is not likely that the ability of the preprotein to interact with SecB has changed; rather, the efficiency with which the preprotein interacts with the translocon may be reduced. Intriguingly, previous studies have shown that the sec-independent membrane insertion of M13 procoat can tolerate several positively charged residues following the signal peptide while a sec-dependent procoat mutant is very sensitive to positive charges (20). Using leader peptidase, it was demonstrated that a string of basic residues impaired translocation efficiency when positioned proximal to the uncleaved signal peptide but not when positioned outside the “export initiation domain” (2). Consistent with the notion that the first several residues of the mature region, in addition to the signal peptide, must be poised for productive interactions with the translocon, we have found that a second, cleavable signal peptide located within this domain, but not beyond, is recognized and processed (18).
Titration of signal peptide hydrophobicity directly parallels a requirement for SecB in the transport process.
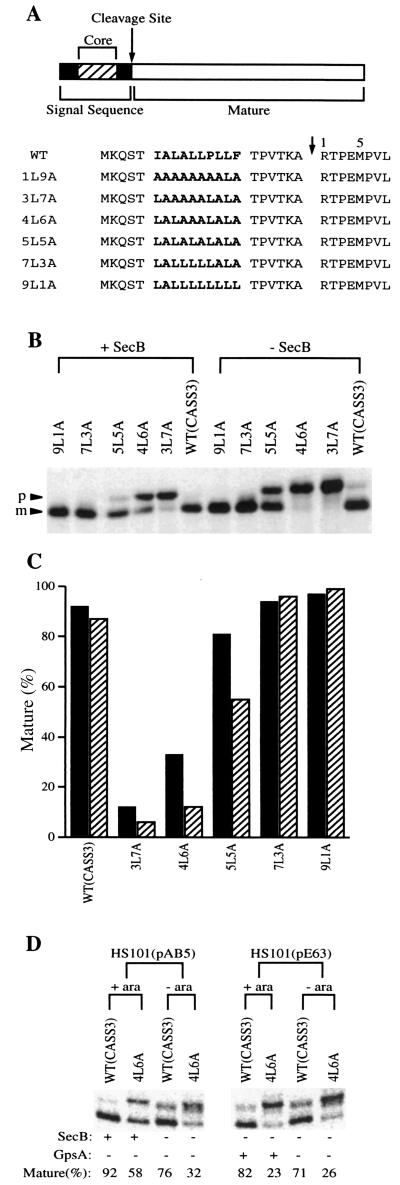
We considered the possibility that SecB rescues transport of alkaline phosphatase with the basic motif by overcoming an unfavorable or inefficient interaction with the transport machinery. This model suggests that alkaline phosphatase with signal sequence defects which are known to reduce the interaction of the preprotein with components of the transport pathway should be SecB dependent. To examine this possibility, we employed a series of alkaline phosphatase signal peptide mutants in which the core region is replaced with various combinations of leucine and alanine residues (Fig. 2A). Earlier studies have demonstrated a clear correlation between the hydrophobicity of these signal peptides, the efficiency of in vivo transport (7, 15), and cross-linking with P48 and with SecA (28) in vitro and with binding to SecA in reconstituted systems (21, 29).
FIG. 2.
SecB dependence of mutant alkaline phosphatases carrying signal peptides of different hydrophobicity and expressed in secB+ and secB null cells. (A) The amino acid sequences of the signal peptide region of the preproteins. Alkaline phosphatase is shown diagrammatically with the signal peptide core region represented by the hatched area within the darkly shaded signal peptide, the mature region is represented by an open bar, and the signal peptide cleavage site is marked by an arrow. The sequences of the signal peptides are given below with the hydrophobic core region shown in boldface. (B) Precursor processing of the mutant alkaline phosphatases expressed in secB+ (AW1043) and secB null (CK1953) cells. The positions of the precursor (p) and mature (m) forms of alkaline phosphatase are indicated. Expression of a chromosomal copy of the phoA gene was minimized by 40 mM phosphate. (C) Quantification of the extent of processing of the mutant alkaline phosphatases from the experiment depicted in panel B. Darkly shaded bars represent the extent of processing in the secB+ strain, and hatched bars represent the processing in the secB null strain. (D) A chloramphenicol-resistant plasmid carrying the wild type (WT) or 4L6A mutant was introduced into strain HS101(pAB5) or HS101(pE63). SecB or GpsA expression was induced by 0.2% arabinose. Alkaline phosphatase was expressed as shown in panel B.
secB+ and secB null cells transformed with the mutant alkaline phosphatases under the control of the lac promoter were pulse labeled with [35S]methionine for 40 s in the presence of 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and chased with excess unlabeled methionine for 40 s. Samples were immunoprecipitated and analyzed for the extent of precursor processing as described above. Indeed, as shown in Fig. 2B and C, those alkaline phosphatase mutants with less-hydrophobic signal sequences (5L5A, 4L6A, and 3L7A) exhibit a more pronounced dependence on SecB than those with more- hydrophobic signal sequences (7L3A and 9L1A). These results show that precursor proteins with relatively less-hydrophobic signal sequences, which do not effectively interact with P48 and SecA, rely on SecB for transport whereas ones that have relatively more-hydrophobic signal sequences can be efficiently transported even without SecB.
To verify that these results are a direct reflection of a requirement for SecB, two additional points were considered. First, the secB null strain, CK1953, exhibits reduced expression of the gpsA gene (25). Therefore, precursor accumulation in this strain could be due to reduced levels of GpsA rather than the absence of SecB. To address this issue, we also used the SecB null strain, HS101(MC4100 ara+ zhe::Tn10 malTc secB8) carrying the plasmid pE63 in which gpsA expression is under the control of the araB promoter (25). Using pulse-chase analysis as above, overexpression of GpsA in the presence of 0.2% arabinose did not relieve the precursor accumulation of 4L6A (Fig. 2D). Secondly, precursor accumulation should be sensitive to enhanced SecB levels. In a parallel experiment in which SecB is overexpressed from plasmid pAB5, a further improvement in 4L6A processing is observed (Fig. 2D) relative to that when SecB is expressed in single copy (Fig. 2B). These data demonstrate that SecB is responsible for the phenotype observed.
Signal peptides optimized for transport override the requirement for SecB conferred by the basic motif.
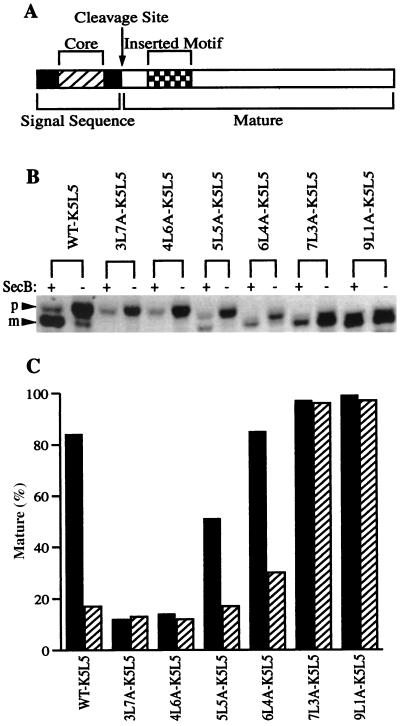
If SecB rescues transport of alkaline phosphatase with the basic motif by overcoming an unfavorable or inefficient interaction with the transport machinery, then this unfavorable interaction should be stabilized by enhancing the degree of the preprotein interaction by other means. This possibility was tested using the series of alkaline phosphatase mutants which correspond to the signal peptide mutants described above but which also carry the basic motif in the early mature region of the protein (Fig. 3A). Again, secB+ and secB null cells expressing these mutant alkaline phosphatases were pulse labeled with [35S]methionine and analyzed for the extent of precursor processing as described for Fig. 1. As shown in Fig. 3B and C for the preprotein carrying the basic motif, as the hydrophobicity of the signal peptide increases the dependence on SecB decreases. For preproteins with the 3L7A and 4L6A signal peptides, no transport is observed. Interestingly, transport of alkaline phosphatase carrying the basic motif with the 5L5A signal peptide is severely restricted relative to that with the same signal peptide in the absence of the motif, pointing to the interplay between these two regions in achieving transport efficiency. In contrast, alkaline phosphatases with the 6L4A, 7L3A, and 9L1A signal peptides are transported efficiently irrespective of the presence of the basic motif, with only the 6L4A mutant requiring SecB assistance. The most hydrophobic signal peptides apparently ameliorate the problem caused by the basic motif, and SecB is no longer required for efficient entry into the transport pathway. At the other extreme, however, SecB cannot rescue those preproteins with very weak signal peptides. It seems reasonable that a preprotein must have an affinity for the transport pathway that at least meets some threshold level in order for SecB to be effective. This provides a mechanism for excluding cytoplasmic proteins from SecB-mediated entrance into the transport pathway.
FIG. 3.
SecB dependence of mutant alkaline phosphatases with signal peptides of different hydrophobicity and with the basic motif (K5L5) in the early mature region. (A) Alkaline phosphatase is shown diagrammatically as in Fig. 1A and 2A. The hydrophobic core region (hatched) of the signal peptide was replaced with various hydrophobic sequences while maintaining the K5L5 motif (checkered) in the early mature region. (B) Precursor processing of the mutant alkaline phosphatases expressed in secB+ (AW1043) and secB mutant (CK1953) cells. The positions of the precursor (p) and mature (m) forms of alkaline phosphatase are indicated. (C) Quantification of the extent of processing of the mutant alkaline phosphatases from the experiment depicted in panel B. Darkly shaded bars represent the extent of processing in the secB+ strain and hatched bars represent processing in the secB mutant strain.
These results also show that small, incremental changes in two different regions of the preprotein result in a corresponding incremental change in sensitivity to loss of SecB. For the series of mutant preproteins, there is a continuum between the extremes of SecB-independent and -dependent transport which suggests the possibility that SecB-dependent and -independent pathways are not mutually exclusive. Rather, a protein such as alkaline phosphatase is inherently able to utilize SecB and may well do so to some extent under normal conditions. Indeed, sites corresponding to SecB recognition sites have recently been identified within the mature region of wild-type alkaline phosphatase (19). By making the preprotein less than ideal for entry into the secretion pathway, we have simply shifted the equilibrium toward reliance on SecB participation. This is consistent with the apparent conversion to SecB dependence observed with signal sequence-less mutants in previous studies (5, 11).
Previous studies have shown that a functional signal peptide, but not a nonfunctional one, retards the folding of MBP (23). It has, therefore, been suggested that functional signal peptides may play a fundamental role in promoting partitioning of a preprotein into a slow folding pathway and thus SecB utilization (6). This implies that preproteins with functional signal peptides should exhibit more reliance on SecB than those with less-efficent signal peptides. To the contrary, in the context of this study, the trend is toward greater reliance on SecB as the signal peptide is rendered less efficient. This suggests that the extent to which the signal peptide influences the folding of the mature domain may not be a primary factor in SecB dependence.
In this study, we have used unnatural sequences in the signal peptide and early mature regions of alkaline phosphatase for elucidating the mechanisms by which proteins are distinguished for SecB-dependent versus -independent transport. The extremes of the mutant sequences employed are not common in nature. However, there is significant variation among natural preproteins within the domains studied. For those proteins with signal peptides and/or mature regions that are somewhat less than ideal, SecB may provide a mechanism for accelerating critical interactions with the transport machinery.
Traditionally, proteins have been empirically defined as SecB-independent because they are transported rather efficiently in the absence of SecB. However, this does not necessarily mean that SecB plays no role in the transport of these proteins under normal conditions. Our results are consistent with a model of protein transport in which all preproteins which utilize the general Sec pathway can utilize SecB. However, if the preprotein interacts very well with the transport machinery, loss of SecB will simply not have an observable impact on its transport.
Acknowledgments
We thank Sharyn Rusch and Alexander Miller for critically reading the manuscript, Carol Kumamoto for providing the SecB null strain CK1953, and Hajime Tokuda for providing strains HS101(pAB5) and HS101(pE63).
This work was supported by NIH grant GM 37639 (to D.A.K.) and NATO collaborative research grant CRG 960684 (to D.A.K. and J.L.).
REFERENCES
- 1.Altman E, Emr S D, Kumamoto C A. The presence of both the signal sequence and a region of mature LamB protein is required for the interaction of LamB with the export factor SecB. J Biol Chem. 1990;265:18154–18168. [PubMed] [Google Scholar]
- 2.Andersson H, von Heijne G. A 30-residues-long “export initiation domain” adjacent to the signal sequence is critical for protein translocation across the inner membrane of Escherichia coli. Proc Natl Acad Sci USA. 1991;88:9751–9754. doi: 10.1073/pnas.88.21.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brundage L, Hendrick J P, Schiebel E, Driessen A J M, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 4.Collier D N, Bankaitis V A, Weiss J B, Bassford P J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988;53:273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- 5.Derman A I, Puziss J W, Bassford P J, Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993;12:879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond D L, Randall L L. Kinetic partitioning. J Biol Chem. 1997;272:28994–28998. doi: 10.1074/jbc.272.46.28994. [DOI] [PubMed] [Google Scholar]
- 7.Doud S K, Chou M M, Kendall D A. Titration of protein transport activity by incremental changes in signal peptide hydrophobicity. Biochemistry. 1993;32:1251–1256. doi: 10.1021/bi00056a008. [DOI] [PubMed] [Google Scholar]
- 8.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 9.Fekkes P, den Blaauwen T, Driessen A J M. Diffusion-limited interaction between unfolded polypeptides and the Escherichia coli chaperone SecB. Biochemistry. 1995;34:10078–10085. doi: 10.1021/bi00031a032. [DOI] [PubMed] [Google Scholar]
- 10.Fekkes P, van der Does C, Driessen A J M. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 1997;16:6105–6113. doi: 10.1093/emboj/16.20.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flower A M, Doebele R C, Silhavy T J. PrlA and PrlG suppressors reduce the requirement for signal sequence recognition. J Bacteriol. 1994;176:5607–5614. doi: 10.1128/jb.176.18.5607-5614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gannon P M, Li P, Kumamoto C A. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989;171:813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy S J S, Randall L L. A kinetic partitioning model of selective binding of nonnative proteins by the bacterial chaperone SecB. Science. 1991;251:439–443. doi: 10.1126/science.1989077. [DOI] [PubMed] [Google Scholar]
- 14.Hartl F U, Lecker S, Schiebel E, Hendrick J P, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 15.Izard J W, Rusch S L, Kendall D A. The amino-terminal charge and core region hydrophobicity interdependently contribute to the function of signal sequences. J Biol Chem. 1996;271:21579–21582. doi: 10.1074/jbc.271.35.21579. [DOI] [PubMed] [Google Scholar]
- 16.Khisty V J, Munske G R, Randall L L. Mapping of the binding frame for the chaperone SecB within a natural ligand, galactose-binding protein. J Biol Chem. 1995;270:25920–25927. doi: 10.1074/jbc.270.43.25920. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Lee Y, Kim C, Park C. Involvement of SecB, a chaperone, in the export of ribose-binding protein. J Bacteriol. 1992;174:5219–5227. doi: 10.1128/jb.174.16.5219-5227.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Kendall D A. Identification of a sequence motif that confers SecB dependence on a SecB-independent secretory protein in vivo. J Bacteriol. 1998;180:1396–1401. doi: 10.1128/jb.180.6.1396-1401.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoblauch N T M, Rüdiger S, Schönfeld H-J, Driessen A J M, Schneider-Mergener J, Bukau B. Substrate specificity of the SecB chaperone. J Biol Chem. 1999;274:34219–34225. doi: 10.1074/jbc.274.48.34219. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn A, Zhu H-Y, Dalbey R E. Efficient translocation of positively charged residues of M13 procoat protein across the membrane excludes electrophoresis as the primary force for membrane insertion. EMBO J. 1990;9:2385–2389. doi: 10.1002/j.1460-2075.1990.tb07413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller A, Wang L, Kendall D A. Synthetic signal peptides specifically recognize SecA and stimulate ATPase activity in the absence of preprotein. J Biol Chem. 1998;273:11409–11412. doi: 10.1074/jbc.273.19.11409. [DOI] [PubMed] [Google Scholar]
- 22.Randall L L. Peptide binding by chaperone SecB: implications for recognition of nonnative structure. Science. 1992;257:241–245. doi: 10.1126/science.1631545. [DOI] [PubMed] [Google Scholar]
- 23.Randall L L, Hardy S J S. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986;46:921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- 24.Randall L L, Topping T B, Hardy S J S. No specific recognition of leader peptide by SecB, a chaperone involved in protein export. Science. 1990;248:860–863. doi: 10.1126/science.2188362. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu H, Nishiyama K, Tokuda H. Expression of gpsA encoding biosynthetic sn-glycerol 3-phosphate dehydrogenase suppresses both the LB− phenotype of a secB null mutant and the cold-sensitive phenotype of a secG null mutant. Mol Microbiol. 1997;26:1013–1021. doi: 10.1046/j.1365-2958.1997.6392003.x. [DOI] [PubMed] [Google Scholar]
- 26.Stenberg G, Fersht A R. Folding of barnase in the presence of the molecular chaperone SecB. J Mol Biol. 1997;274:268–275. doi: 10.1006/jmbi.1997.1398. [DOI] [PubMed] [Google Scholar]
- 27.Topping T B, Randall L L. Determination of the binding frame within a physiological ligand for the chaperone SecB. Protein Sci. 1994;3:730–736. doi: 10.1002/pro.5560030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valent Q A, Scotti P A, High S, de Gier J W L, von Heijne G, Lentzen G, Wintermeyer W, Oudega B, Luirink J. The Escherichia coli SRP and SecB targeting pathways converge at the translocon. EMBO J. 1998;17:2504–2512. doi: 10.1093/emboj/17.9.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Miller A, Kendall D A. Signal peptide determinants of SecA binding and stimulation of ATPase activity. J Biol Chem. 2000;275:10154–10159. doi: 10.1074/jbc.275.14.10154. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Hayashi S, Wu H C. Synthesis and export of the outer membrane lipoprotein in Escherichia coli mutants defective in generalized protein export. J Bacteriol. 1988;170:4001–4007. doi: 10.1128/jb.170.9.4001-4007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]