Abstract
Dengue is an arboviral infection endemic in tropical countries. Neurological sequelae to dengue infection are not uncommon, and psychiatric manifestations are increasingly reported. This narrative review aims to present the varied manifestations, postulated mechanisms, and the available treatment options for psychiatric morbidity associated with dengue. The evidence available from eight observational studies is summarized in this review. Depression and anxiety are noted to be prevalent during both the acute and convalescent stages of the infection. The presence of encephalopathy and other neurological conditions is not a prerequisite for developing psychiatric disorders. However, treatment options to manage such psychiatric manifestations were not specified in the observational studies. Anecdotal evidence from case reports is outlined. Special attention is paid to the role of epigenetic modifications following dengue infections and the role of histone deacetylase inhibitors in the management. DNA methylation inhibitors such as valproic acid play a significant role in reversing stress-, viral-, or drug-induced epigenetic modifications.
Keywords: Dengue, psychiatry, depression, valproate, histone deacetylase, quetiapine
Dengue is a viral illness caused by a single-stranded ribonucleic acid (RNA) virus belonging to the Flaviviridae family with four different dengue serotypes (DENV 1–4). It is transmitted by infected Aedes mosquitoes. It is a global epidemic with an incidence of around 96 million apparent infections per year 1 and a significant social and economic burden.2,3 India alone contributes more than one-third of such apparent clinical infections. 1 Dengue is endemic in tropical countries, and the incidence of the infection has been progressively increasing through the last two decades.4,5 The illness usually has an abrupt onset, with a course characterized by fever, critical, and convalescence phases. 5 The World Health Organization classifies the illness into two groups: uncomplicated and severe dengue. 6 In the Indian context, severe dengue has been estimated to be 35% of all dengue cases, with a mortality rate of 2% to 3%. 7 All four serotypes are prevalent in India. 8 The critical period marks the essential phase of the severity of the illness that may ultimately lead to hepatic, cardiac, and neurological consequences. To date, there are no approved antiviral drugs available to treat dengue, and the management is mostly symptomatic and supportive. 6
Dengue’s neurological involvement is increasingly recognized but not well understood. DENV-2 and DENV-3 serotypes are reported to be associated with neurological manifestations. 9 Although the studies on incidence rates of neurological sequelae secondary to dengue infection are limited in the Indian context, 10 neurological manifestations were reported in 7% to 10% of the infected adults11,12 and 28% of the infected children. 13 The prevalence of dengue encephalopathy has been estimated to range from 0.5% to 6% in patients with dengue hemorrhagic fever. 14 Postulated mechanisms include vasculitis with fluid extravasations, brain parenchymal edema, metabolic derangements, vascular hypoperfusion, autoimmune cross-reactivity, and, importantly, direct neurotoxic effects of the dengue virus.5,9,15 Dengue infection leads to a wide range of manifestations from headache, fatigue, motor weakness, Guillain–Barre syndrome, seizures, meningitis, and encephalopathy to coma.7,8 Patients with dengue infection and its associated complications are reported to present with psychiatric disorders such as mood disorders, anxiety disorders, personality changes, and rapid cognitive decline. This narrative review focuses on the various psychiatric manifestations of dengue infection and its sequelae, possible mechanisms, and the management options to address the psychiatric symptoms. A note is also added on the effects of histone deacetylase (HDAC) enzyme with respect to the psychiatric sequelae and the novel HDAC inhibitors that are conceptualized to be neuroprotective.
Search Strategy
The authors (DD and VS) searched PubMed and EBSCO independently with the following search terms: “Dengue,” “Psychiatry,” “Psychosis,” “Mania,” “Catatonia,” “Schizophrenia,” “Depression,” “Bipolar,” “Anxiety,” “Obsessive-compulsive disorder,” “psychology,” “Neuropsychiatry,” “Dementia,” and “Histone deacetylase.” We identified 226 manuscripts. The search was restricted neither to languages nor time. Manuscripts (case reports, case series, and observational studies) describing the psychiatric manifestations, in all the age groups, during the acute and convalescent phases of dengue viral infection, and their management were included for the review. The outcome of the search strategy is depicted in Figure 1. The total number of unique manuscripts identified for the final review was 27. This included 18 case reports, one case series, and eight observational studies. A detailed review of the eight observational studies16–23 (five cross-sectional, two case-control, and one cohort study) is provided below and summarized in Table 1.
Figure 1. Outcome of the Literature Review.
Table 1.
Psychiatric Manifestations in Dengue Infection
| Study/Country/Type | Manifestations | Mechanisms | Management | Remarks |
| Gill et al.
16
(Pakistan) Prospective study n = 119 |
During the acute phase, almost 80% to 90% had anxiety symptoms (thanatophobia), and around 60% had depressive symptoms. The symptoms subsided soon: at recovery, only 10% had anxiety and 5% had depressive symptoms. At three months’ follow-up, 5% had persistent symptoms. | Authors suggested that the development of depression in 5% of patients as an aftermath of dengue may be secondary to the individuals’ personality proneness. | Reassurance reportedly alleviated anxiety in most patients. Only 20% of the
diagnosed people were given benzodiazepines for anxiety. Around 5% were treated
with antidepressants (medication details not provided). |
Prospective evaluation provided valuable insights into the self-limiting nature of most anxiety and depressive symptoms. |
| Hashmi et al.
17
(Pakistan) Cross-sectional study n = 531 patients |
62.2% met criteria for depression, and 59% met criteria for
anxiety. Women had more severe anxiety and depression. |
Authors postulated the possibility of inflammatory cytokines released by the infection to have played a role in psychiatric morbidity. | Psychiatric treatment provided to such patients had not been discussed in the manuscript. | HAD scale was used. Psychiatric morbidity correlated negatively with the platelet count. |
| Khan et al.
18
(Pakistan) Cross-sectional study n = 97 |
Among acutely ill dengue patients, around 82% had depression, and 66% had anxiety features. | Attributable mechanisms were not discussed. | Treatment provided to the patients not described. | HAD scale was used. |
| Mushtaq and Zahir
19
(Pakistan) Cross-sectional n = 200 |
Depression, anxiety, and stress scores correlated negatively with self-efficacy scores. | Severity of dengue infection correlated with depression and anxiety scores. | Treatment options not discussed. | DASS-21 scale was used. Self-efficacy was measured by GSES. |
| Gunathilaka et al.
20
(Sri Lanka) Case-control study n = 53 in each arm |
Delayed depression, anxiety, and stress symptoms were assessed in patients who
had confirmed dengue 6–24 months ago vs. age-matched controls without
dengue. Dengue group had significantly more depressive, anxiety, and stress symptoms. |
Clinical and subclinical viral encephalitis was postulated as the possible mechanism. Around 13% of the dengue group have had encephalopathy during the acute illness. | Treatment provided to the participants diagnosed with depression is not discussed in the report. | DASS-21 scale was used. Depression determined by psychiatrists based on DSM-5
criteria was more in the dengue group, 15.1% vs. 7.5%; however, this was not
statistically significant. The mean lowest platelet count value in the study group was 62,000 cells/µL, which correlated positively with depressive scores. |
| Uvais and Moideen
21
(India) Descriptive study n = 14 |
Out of 253 dengue-infected patients, only 5% (n = 14) received
any psychotropic medications. However, only 2% (n = 4) were
referred for psychiatric consultation. Around 1% (n = 2) was diagnosed with depressive reaction. |
Discussed the possibilities of neurotropism, capillary leakage, and release of pro-inflammatory cytokines leading to the neuropsychiatric manifestation. | Commonly prescribed psychotropics were clonazepam and quetiapine. Sertraline and amitriptyline were given for one patient each. | A retrospective descriptive study about psychotropic use in patients admitted with dengue infection. |
| Herbuela et al.
23
(Philippines) Case-control study Cases n = 255 Controls n = 260 |
In the pediatric population (age 8–17), during the acute phase of dengue
infection, 34.2% with dengue fever had anxiety symptoms compared to 16% of
controls. Around 13.3% had borderline or clinical depression compared to 3.5% in controls. Additionally, 26% reported irritability, visual hallucinations, agitation, and aggressiveness during the onset of infection. |
Age and initial few days of hospitalization associated with anxiety
symptoms. The presence of myalgia and arthralgia with a family history of dengue fever predicted depressive symptoms. Anxiety and depressive symptoms were postulated to be secondary to raised inflammatory markers during acute infection. |
Treatment provided to children with anxiety and depressive symptoms during acute dengue infection was not discussed in the report. | Screening of psychiatric morbidities was done using the RCADS-25
scale. Specific dengue serotypes were not assessed. |
| Psychiatric morbidity may lead to worsening of dengue spread in endemic regions | ||||
| Caixeta et al.
22
(Brazil) Cross-sectional |
Compulsive hoarding | Hoarding of trash leads to mosquito proliferation, thereby increasing the risk of dengue spread. | Not applicable. | Interesting to note that psychiatric morbidity such as hoarding might potentially worsen the spread of dengue in an endemic population. |
DASS, depression anxiety stress scale; DSM, diagnostic statistical manual; GSES, general self-efficacy scale; HAD, hospital anxiety and depression scale; RCADS-25, revised child anxiety and depression scale-25 items.
Psychiatric Manifestations
Most reported patients are from Asian countries or involved travelers returning from dengue-endemic regions. Depressive disorders were the most common psychiatric presentations. During the acute phase, most (60% to 90%) patients had both anxiety and depression symptoms,16,17 and syndromal depression was prevalent in 5% to 15% of patients in convalescence. 20 High rates (80% to 90%) of anxiety symptoms (thanatophobia) noted during the acute phase subsided during convalescence, and only 5% had persistent symptoms at three months’ follow-up.16,18 In another study, 62% and 59% met the criteria for depression and anxiety during the acute phase. Women had more severe depressive symptoms than men. 17 Severity of depression, anxiety, and stress correlated negatively with the self-efficacy scores during acute infection. 19 A case-control study involving pediatric population observed the prevalence of depression (13.3%) and anxiety symptoms (34.2%) during acute dengue infection to be significantly higher than matched controls. 23 Further, around 25% of the admitted children exhibited agitation, aggression, irritability, and visual hallucinations. Delayed psychiatric manifestations of dengue infection were reported to be predominantly depression and anxiety. At 6–24 months’ follow-up after dengue infection, the rates of depression were 15%. 20 There are multiple case reports of manic presentation,24–31 acute polymorphic psychosis,32–39 prolonged depression, 40 and catatonia29,41 and one on rapid cognitive decline. 42 However, the evidence for a psychotic, manic, or catatonic presentation from observational studies is lacking. It is also interesting to note that psychiatric presentations such as compulsive hoarding lead to the accumulation of trash, thereby endangering the proliferation of the Aedes mosquito and the increased spread of dengue. Such a possible relationship is observed in a cross-sectional study from Brazil. 22 Only a few studies used standard diagnostic criteria and structured psychopathology and cognition rating scales to quantify the presentation.17,18,25,26,29 Most studies had little/no information on follow-up details, and retrospective studies had suggested predominant anxiety and depressive symptoms as chronic consequences. However, the persistence of these symptoms and the need for prolonged treatment for secondary psychiatric manifestations are not specifically described in the available studies.16,20
Postulated Mechanisms
Although there was an argument that psychiatric presentations such as mania were mere coincidental findings during dengue fever,43,44 with a rising incidence of dengue infections and growing reports of such presentations in the existing literature, it is essential to review the probable postulated mechanisms underlying psychiatric morbidity in dengue. Available observational or longitudinal studies did not elicit the etiopathogenetic mechanisms underlying psychiatric manifestations. The mechanisms postulated are largely from case reports/series and hence are anecdotal.
Interestingly, only a few reported patients with psychiatric symptoms had additional neurological manifestations such as confusion, seizures, cognitive decline, delirium, and gait disturbances.36,39,42 Cerebrospinal fluid analysis showed increased protein levels and lymphocyte cells in two reports.36,39 But, the analysis was considered normal in the other two reports.27,42 Brain imaging studies such as computed tomography25–27,34,35,41 and magnetic resonance imaging32,33,36,38 in most reports were normal. Metabolic derangements, including elevated liver enzymes,24,25 electrolyte abnormalities, 27 vasculitis with capillary leak, and fluid extravasations, were postulated. 5 Platelet counts ranged from 20,000 to 2,00,000 cells/µL,24,35 and there were inconsistent correlations between platelet count and psychiatric morbidity.17,20 Only four studies reported the dengue virus serotypes. DENV-2 was identified in two reports,38,39 while DENV-1 40 and DENV-4 39 were associated in one report each. Direct neuronal invasion by the dengue virus was doubted for long but has been reported to be possible.26,28,45 Recently, the role of secondary immune activation and epigenetic modifications in dengue-related neuropsychiatric complications has been increasingly studied. 5 Encephalopathy alone was not the prerequisite to psychiatric presentations, and in fact, most patients exhibited clear sensorium during manic/psychotic breakdown, making a case for other etiological possibilities. 34 Further systematic studies are necessary in identifying the underlying mechanisms better.
Management Options
Management options for the primary dengue infection are beyond the scope of the current review, and detailed reviews on the topic are available.46–48 The supportive management for dengue fever primarily involves anti-inflammatory agents and corticosteroids. But, studies on psychiatric manifestations either completely neglected to report the management details of primary dengue infection17–20,23,38 or did not clearly specify the agents and the doses used.16,21 The literature exploring psychiatric manifestations in relation to these agents used to treat dengue is more important given the well-known association of some of them in precipitating behavioral and mood symptoms.
Only limited information is available about the management of secondary anxiety and depression symptoms. Most observational studies that aimed at understanding acute and delayed psychiatric morbidity in dengue patients did not report the treatment provided for the identified patients.17,19,20 Successful management of agoraphobic symptoms with oral sertraline (dose not available) 39 and prolonged depression with duloxetine (dose 40 mg/day) 40 was reported. Mania in dengue patients was managed with antipsychotic/benzodiazepines or a combination of mood stabilizers and antipsychotic/benzodiazepines.24,26–29 For psychotic presentations, a good response was achieved with oral antipsychotic agents, mostly in low doses.32–37,39 Catatonic presentation responded well to intravenous lorazepam 4 mg/day, which was tapered and stopped over four weeks. 41
It was noted that clonazepam and low-dose quetiapine were started for dengue patients with psychiatric comorbidity without an appropriate specialist referral. Only less than 2% of the patients with psychiatric morbidity were usually referred for formal assessment. 21 There is a lack of evidence on the preference of any specific psychotropics in managing psychiatric symptoms, and the chosen treatment seems to be in line with their conventional use in general psychiatric practice.
Histone Deacetylase (HDAC) Enzyme and Epigenetic Modifications
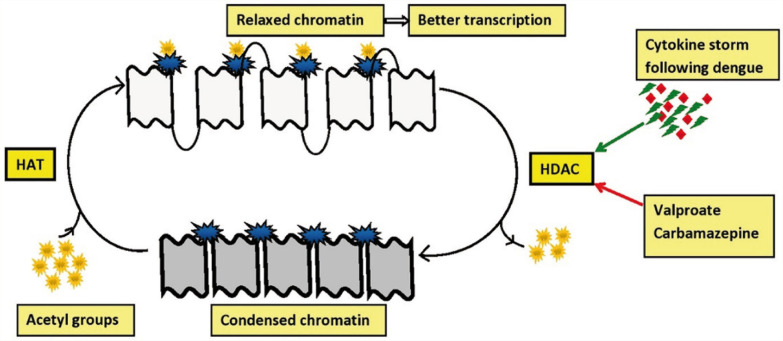
Histone proteins are implicated in organizing the DNA structure. Acetylation of histone proteins favors the binding of relevant transcription factors to the DNA. 49 Histone deacetylase (HDAC) removes histone acetylation and condense the chromatin, leading to a slowing of gene transcription.50,51 After the recruitment of HDAC by factors such as DNA methyltransferase (DNMT), the silencing of promoter genes is ensured through DNA methylation. 52 The inactivation of genes with increased HDAC and DNMT activity has been implicated in the manifestation of psychiatric conditions. 53
HDAC enzymes are classified into two families, four groups, and 18 isoforms (HDAC 1–11 and silent information regulator-2 related proteins 1–7).49,54 Virus-mediated gene transduction was shown to result in altered HDAC activity, impairing neuroplasticity and cognition. 55 Dengue infection, especially hemorrhagic fever, was reported to result in a “cytokine storm” characterized by elevated levels of circulating cytokines and chemokines. 5 Cytokine storm following dengue infection may lead to oxidative stress, which promotes the HDAC activity.56,57 (Inflammatory mechanisms were postulated in the etiopathogenesis of bipolar disorder in animal models. 58 HDAC overexpression in adult mouse hippocampus was reported to result in decreased prepulse inhibition. This aberration was described in both human and animal models of schizophrenia.59,60 Such epigenetic modifications play a crucial role in understanding the etiopathogenesis of most psychiatric disorders.)
Role of HDAC Inhibitors in Treatment
Numerous preclinical studies reported the antimanic effects of HDAC inhibitors. Valproate is a potent inhibitor of HDAC and has been used as a mood stabilizer for decades.49,61 Carbamazepine and topiramate are also reported to inhibit HDAC to some extent, while other antiepileptics such as levetiracetam, phenobarbitone, or gabapentin did not inhibit HDAC.62–64 The ability of lithium to inhibit HDAC has not been elucidated. 65 However, lithium plays a role in gene transcription regulation through glycogen synthase kinase-3 inhibition. 66
Interestingly, chronic exposure to second-generation antipsychotic drugs has been reported to result in overexpression of HDAC2 and thereby proposed to result in cognitive dysfunction.55,67 Quetiapine was shown to reduce DNMT, a close associate of HDAC, in the hippocampus and nucleus accumbens, thereby reducing DNA methylation in animal models and also promoting hippocampal neurogenesis.63,68,69 The HDAC/DNMT inhibitors reactivate the suppressed genes, which ultimately results in clinical improvement 53 (see Figure 2).
Figure 2. Role of HDAC Inhibitors in Relaxing the Chromatin.
HDAC, histone deacetylase enzyme; HAT, histone acetyltransferase enzyme.
Inhibition of Class I and Class III HDACs was reported to promote antidepressant action of fluoxetine, while additional inhibition of Class II HDACs was required to promote anti-anxiety action of the molecule. 70 In animal models, high-frequency repetitive transcranial magnetic stimulation was reported to promote histone acetylation and gene expression. 71 Selective HDAC2 isoform inhibitors were reported to ameliorate cognitive deficits in animal models. 72
In our review, generally, the prognosis of psychiatric morbidity following dengue was good. Seven of the reviewed reports utilized any one agent (valproate, quetiapine, or carbamazepine) with HDAC/DNMT inhibition.24–29,31,34 These reports suggested an early and favorable outcome. However, reports utilizing other agents without HDAC/DNMT inhibition also suggested favorable outcomes. Clinicians are recommended to follow appropriate precautions while prescribing HDAC inhibitors such as valproate and carbamazepine to women in the childbearing age group because of high teratogenicity and other side effects. The role of HDAC inhibitors in treatment is limited, owing to the lack of specificity for selective HDAC isoforms and severe side effects such as teratogenicity, cytotoxicity, polycystic ovarian syndrome, etc.73,74
Conclusion and Future Directions
The dengue epidemic is on the rise for two decades. Neurological and especially psychiatric manifestations are increasingly reported in acute as well as convalescent phases of dengue infection. Frank psychiatric manifestations without neurological consequences are also reported, with mood disorders being the commonest. A cytokine storm unleashed by dengue infection potentially leads to significant neuropsychiatric manifestations. Proposed etiopathogenesis involves epigenetic mechanisms such as overexpression of HDAC enzymes. Mood stabilizers such as valproate and second-generation antipsychotics such as quetiapine and clozapine show inhibition of DNA methylation. These agents provide clinical scope and utility to manage post-dengue psychiatric manifestations. However, the specificity of HDAC class and isoforms associated with psychiatric symptoms needs further delineation. The development of HDAC isoform-specific inhibitors might improve the clinical application, with reduced side effects. The authors consider that the following steps are essential in improving the understanding of psychiatric manifestations and management of dengue infection.
Documentation of phenomenological psychiatric presentations.
Delineating neurological consequences (seizures, encephalitis, confusion, etc.).
Reporting the identified dengue serotype involved and other relevant immunological investigations.
Reporting the details of treatment provided and the response.
Following up for longitudinal consequences.
Systematic studies to evaluate the association and effectiveness of psychotropic agents that have properties of modulating specific epigenetic factors in dengue-related psychiatric disorders.
Despite reporting a significant proportion of dengue infections, longitudinal studies describing the neuropsychiatric manifestations and its management are a few from India. Multidisciplinary research is the need of the hour. Clinical suspicion regarding comorbid depression and anxiety, with an appropriate referral for psychiatric consultation, would ultimately result in better outcomes. Longitudinal studies are sparse and, whenever available, are with further limitations such as neuropsychiatric manifestations being assessed without standardized tools, inadequate attention paid to postulating possible mechanisms, and describing the treatment options used. The development and availability of vaccines to prevent dengue infection are in their early stages. Systematically designed prospective studies with the suggested steps might elucidate the pathogenesis of dengue-related psychiatric consequences and probably shed light on novel treatment options.
Acknowledgments
This work is supported by the Department of Science and Technology (Government of India) Research Grant (DST/SJF/LSA-02/2014-15) to GV.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature, 2013; 496: 504–507. DOI: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis, 2016; 16: 712–723. DOI: 10.1016/s1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard DS, Undurraga EA, Halasa YA, et al. The global economic burden of dengue: A systematic analysis. Lancet Infect Dis, 2016; 16: 935–941. DOI: 10.1016/s1473-3099(16)00146-8. [DOI] [PubMed] [Google Scholar]
- Shepard DS, Undurraga EA, and Halasa YA. Economic and disease burden of dengue in Southeast Asia. PLoS Negl Trop Dis, 2013; 7: e2055. DOI: 10.1371/journal.pntd.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG and Harris E. Dengue. Lancet, 2015; 385: 453–465. DOI: 10.1016/s0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization Dengue guidelines for diagnosis, treatment, prevention and control: New edition. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- Agrawal VK, Prusty BSK, Reddy CS, et al. Clinical profile and predictors of Severe Dengue disease: A study from South India. Caspian J Intern Med, 2018; 9: 334–340. DOI: 10.22088/cjim.9.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Srivastava S, Jain A, et al. Dengue in India. Indian J Med Res, 2012; 136: 373. [PMC free article] [PubMed] [Google Scholar]
- Li GH, Ning ZJ, Liu YM, et al. Neurological manifestations of dengue infection. Front Cell Infect Microbiol, 2017; 7: 449. DOI: 10.3389/fcimb.2017.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Sharma P, Garg RK, et al. Neurological complications of dengue fever: Experience from a tertiary center of north India. Ann Indian Acad Neurol, 2011; 14: 272–278. DOI: 10.4103/0972-2327.91946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeraja M, Iakshmi V, Teja VD, et al. Unusual and rare manifestations of dengue during a dengue outbreak in a tertiary care hospital in South India. Arch Virol, 2014; 159: 1567–1573. 2014/February/11. DOI: 10.1007/s00705-014-2010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu R, Verma R, Jain A, et al. Neurologic complications in dengue virus infection: A prospective cohort study. Neurology, 2014; 83: 1601–1609. 2014/September/26. DOI: 10.1212/wnl.0000000000000935. [DOI] [PubMed] [Google Scholar]
- Sil A, Biswas T, Samanta M, et al. Neurological manifestations in children with dengue fever: An Indian perspective. Trop Doct, 2017; 47: 145–149. 2016/December/04. DOI: 10.1177/0049475516679788. [DOI] [PubMed] [Google Scholar]
- Varatharaj A. Encephalitis in the clinical spectrum of dengue infection. Neurol India, 2010; 58: 585–591. DOI: 10.4103/0028-3886.68655. [DOI] [PubMed] [Google Scholar]
- Kumar R, Tripathi S, Tambe J, et al. Dengue encephalopathy in children in Northern India: Clinical features and comparison with non dengue. J Neurol Sci, 2008; 269 41–48. [DOI] [PubMed] [Google Scholar]
- Gill KU, Ahmad W, and Irfan M. A clinical study to see the psychological effects of dengue fever. Pak J Med Health Sci, 2011; 5 101–104. [Google Scholar]
- Hashmi AM, Butt Z, Idrees Z, et al. Anxiety and depression symptoms in patients with dengue fever and their correlation with symptom severity. Int J Psychiatry Med, 2012; 44: 199–210. DOI: 10.2190/PM.44.3.b. [DOI] [PubMed] [Google Scholar]
- Khan MA, Ahmad M, Mir S, et al. Anxiety and depression in patients of dengue fever. Rawal Med J, 2012; 37 239–242. [Google Scholar]
- Mushtaq M and Zahir M. Depression, anxiety, stress and their effect upon the self-efficacy in dengue patients. J Postgrad Med Inst, 2016; 30 62–65. [Google Scholar]
- Gunathilaka N, Chandradasa M, Champika L, et al. Delayed anxiety and depressive morbidity among dengue patients in a multi-ethnic urban setting: First report from Sri Lanka. Int J Ment Health Syst, 2018; 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvais NA and Moideen S. Psychiatric morbidity among patients admitted with dengue fever. Ankara Med J, 2018; 18(2): 215–218. [Google Scholar]
- Caixeta L, Azevedo PVB, Caixeta M, et al. Psychiatry disorders and dengue: Is there a relationship? Arq Neuropsiquiatr, 2011; 69 920–923. [DOI] [PubMed] [Google Scholar]
- Herbuela V, de Guzman FS, Sobrepeña GD, et al. Depressive and anxiety symptoms among pediatric in-patients with dengue fever: A case-control study. Int J Environ Res Public Health, 2019; 17 (1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendhekar D, Aggarwal P, and Aggarwal A. Classical mania associated with dengue infection. Indian J Med Sci, 2006; 60(3): 115–116. [PubMed] [Google Scholar]
- Jhanjee A, Bhatia MS, and Srivastava S. Mania in dengue fever. Ind Psychiatry J, 2011; 20: 56–57. DOI: 10.4103/0972-6748.98418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S and Mishra N. Late onset mania in dengue fever. Immunol Infect Dis, 2014; 2 1–3. [Google Scholar]
- Saifuddin TM, Ismail AF, and Harun NA. Late onset mania post dengue fever in an elderly patient: A case report. Malays J Psychiatry, 2018; 27(1): 37–40. [Google Scholar]
- Krishnan L, Subramoniam V, Kazhungil F, et al. Post dengue mania: A case series. Indian J Psychiatry, 2019; 61: 100–101. DOI: 10.4103/psychiatry.IndianJPsychiatry_311_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakaran D, Tholasappa V, Lhamu T, et al. Treating post-dengue mania: Is role of valproate and quetiapine related to histone deacetylase inhibition? Bipolar Disord, 2020; 22(5): 543–545. [DOI] [PubMed] [Google Scholar]
- Harder J, Sharma S, and Gitlin D. Secondary mania as a possible neuropsychiatric complication of dengue fever. Psychosomatics, 2014; 55: 512–516. DOI: 10.1016/j.psym.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Pariwatcharakul P and Srifuengfung M. Clonidine and tizanidine for management of bipolar disorder due to dengue encephalopathy: A case report. Psychosomatics, 2020; 61: 727–731. DOI: 10.1016/j.psym.2020.08.001. [DOI] [PubMed] [Google Scholar]
- Kar S. Post dengue psychosis. Indian J Biol Psychiatry, 2013; 1 58–59. [Google Scholar]
- Bhatnagar R and Prasad P. Acute psychosis in a child with severe dengue. J Nepal Paediatr Soc, 2017; 37 273–275. [Google Scholar]
- Abdullah M and Bakar M. A case of psychotic disorder due to dengue fever. ASEAN J Psychiatry, 2017; 18 119–122. [Google Scholar]
- Chaudhury S, Jagtap B, and Ghosh DK. Psychosis in dengue fever. Med J Dr DY Patil Univ, 2017; 10: 202. [Google Scholar]
- Baldaçara L, Ferreira JR, Filho LCPS, et al. Behavior disorder after encephalitis caused by dengue. J Neuropsychiatry Clin Neurosci, 2013; 25: E44. [DOI] [PubMed] [Google Scholar]
- Blum JA, Pfeifer S, and Hatz CF. Psychiatric manifestations as the leading symptom in an expatriate with dengue fever. Infection, 2010; 38: 341–343. DOI: 10.1007/s15010-010-0029-9. [DOI] [PubMed] [Google Scholar]
- Rapp C, Debord T, Imbert P, et al. [A psychiatric form of dengue after a visit to Djibouti]. Presse Med, 2002; 31: 1704. [PubMed] [Google Scholar]
- Rittmannsberger H, Foff C, Doppler S, et al. [Psychiatric manifestation of a dengue-encephalopathy]. Wien Klin Wochenschr, 2010; 122 Suppl 3: 87–90. DOI: 10.1007/s00508-010-1460-8. [DOI] [PubMed] [Google Scholar]
- Hitani A, Yamaya W, To M, et al. A case of dengue fever and subsequent long-lasting depression accompanied by alopecia in a Japanese traveler returning from Bali, Indonesia. Kansenshogaku Zasshi, 2015; 89 279–282. [DOI] [PubMed] [Google Scholar]
- Aggarwal A and Nimber JS. Dengue fever-associated catatonia. J Neuropsychiatry Clin Neurosci, 2015; 27: e66–e67. [DOI] [PubMed] [Google Scholar]
- Mohammed AP, Koraddi A, Prabhu A, et al. Rapidly progressive dementia with seizures: A post-dengue complication. Trop Doct, 2019; 50(1): 81–83. [DOI] [PubMed] [Google Scholar]
- Wiwanitkit S and Wiwanitkit V. Psychological manifestation in dengue: Did it really exist? Indian J Psychol Med, 2013; 35: 222. DOI: 10.4103/0253-7176.116268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwanitkit V. Neuropsychiatric complication of dengue. Psychosomatics, 2014; 55: 417. DOI: 10.1016/j.psym.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Miagostovich MP, Ramos R, Nicol A, et al. Retrospective study on dengue fatal cases. Clin Neuropathol, 1997; 16 204–208. [PubMed] [Google Scholar]
- Hermann LL, Gupta SB, Manoff SB, et al. Advances in the understanding, management, and prevention of dengue. J Clin Virol, 2015; 64: 153–159. DOI: 10.1016/j.jcv.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Khetarpal N and Khanna I. Dengue fever: Causes, complications, and vaccine strategies. J Immunol Res, 2016; 2016: 6803098. DOI: 10.1155/2016/6803098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S, Oliva-Ramírez J, Vázquez-Villegas P, et al. Dengue fever: A worldwide threat an overview of the infection process, environmental factors for a global outbreak, diagnostic platforms and vaccine developments. Curr Top Med Chem, 2018; 18: 1531–1549. DOI: 10.2174/1568026618666181105130000. [DOI] [PubMed] [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, et al. The potential use of histone deacetylase inhibitors in the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry, 2016; 64: 320–324. DOI: 10.1016/j.pnpbp.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Lopes-Borges J, Valvassori SS, Varela RB, et al. Histone deacetylase inhibitors reverse manic-like behaviors and protect the rat brain from energetic metabolic alterations induced by ouabain. Pharmacol Biochem Behav, 2015; 128: 89–95. DOI: 10.1016/j.pbb.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Hull EE, Montgomery MR, and Leyva KJ. HDAC inhibitors as epigenetic regulators of the immune system: Impacts on cancer therapy and inflammatory diseases. Biomed Res Int, 2016; 2016. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzenani MK, Zade AE, Ming Y, et al. Genomic DNA hypomethylation by histone deacetylase inhibition implicates DNMT1 nuclear dynamics. Mol Cell Biol, 2011; 31: 4119–4128. DOI: 10.1128/mcb.01304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsli-Ceppioglu S. Epigenetic mechanisms in psychiatric diseases and epigenetic therapy. Drug Dev Res, 2016; 77: 407–413. DOI: 10.1002/ddr.21340. [DOI] [PubMed] [Google Scholar]
- Schmauss C. The roles of class I histone deacetylases (HDACs) in memory, learning, and executive cognitive functions: A review. Neurosci Biobehav Rev, 2017; 83: 63–71. DOI: 10.1016/j.neubiorev.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Kurita M, Holloway T, Garcia-Bea A, et al. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci, 2012; 15: 1245–1254. DOI: 10.1038/nn.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AB, Muthuraman KR, Mariappan V, et al. Oxidative stress response in the pathogenesis of dengue virus virulence, disease prognosis and therapeutics: An update. Arch Virol, 2019; 164: 2895–2908. DOI: 10.1007/s00705-019-04406-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang D, Zhang X, et al. Hypoxia-induced down-regulation of neprilysin by histone modification in mouse primary cortical and hippocampal neurons. PLoS One, 2011; 6: e19229. DOI: 10.1371/journal.pone.0019229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvassori SS, Resende WR, Varela RB, et al. The effects of histone deacetylase inhibition on the levels of cerebral cytokines in an animal model of mania induced by dextroamphetamine. Mol Neurobiol, 2018; 55: 1430–1439. DOI: 10.1007/s12035-017-0384-y. [DOI] [PubMed] [Google Scholar]
- Bahari-Javan S, Maddalena A, Kerimoglu C, et al. HDAC1 regulates fear extinction in mice. J Neurosci, 2012; 32 5062–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RP, Grayson DR, and Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: Analysis of the National Brain Databank microarray collection. Schizophr Res, 2008; 98 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem, 2001; 276: 36734–36741. DOI: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Eyal S, Yagen B, Sobol E, et al. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia, 2004; 45 737–744. [DOI] [PubMed] [Google Scholar]
- Houtepen LC, van Bergen AH, Vinkers CH, et al. DNA methylation signatures of mood stabilizers and antipsychotics in bipolar disorder. Epigenomics, 2016; 8 197–208. [DOI] [PubMed] [Google Scholar]
- Beutler AS, Li S, Nicol R, et al. Carbamazepine is an inhibitor of histone deacetylases. Life Sci, 2005; 76: 3107–3115. DOI: 10.1016/j.lfs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Stertz L, Fries GR, Aguiar BW, et al. Histone deacetylase activity and brain-derived neurotrophic factor (BDNF) levels in a pharmacological model of mania. Braz J Psychiatry, 2014; 36: 39–46. DOI: 10.1590/1516-4446-2013-1094. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Wang Z, Hunsberger JG, et al. Therapeutic potential of mood stabilizers lithium and valproic acid: Beyond bipolar disorder. Pharmacol Rev, 2013; 65: 105–142. DOI: 10.1124/pr.111.005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi D, de la Fuente Revenga M, Kezunovic N, et al. Antipsychotic-induced Hdac2 transcription via NF-kB leads to synaptic and cognitive side effects. Nat Neurosci, 2017; 20: 1247–1259. DOI: 10.1038/nn.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignacio ZM, Reus GZ, Abelaira HM, et al. Quetiapine treatment reverses depressive-like behavior and reduces DNA methyltransferase activity induced by maternal deprivation. Behav Brain Res, 2017; 320: 225–232. DOI: 10.1016/j.bbr.2016.11.044. [DOI] [PubMed] [Google Scholar]
- Luo C, Xu H, and Li X-M. Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res, 2005; 1063 32–39. [DOI] [PubMed] [Google Scholar]
- Schmauss C. An HDAC-dependent epigenetic mechanism that enhances the efficacy of the antidepressant drug fluoxetine. Sci Rep, 2015; 5: 8171. DOI: 10.1038/srep08171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etievant A, Manta S, Latapy C, et al. Repetitive transcranial magnetic stimulation induces long-lasting changes in protein expression and histone acetylation. Sci Rep, 2015; 5: 16873. DOI: 10.1038/srep16873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FF, Zhang YL, Fass DM, et al. Kinetically selective inhibitors of histone deacetylase 2 (HDAC2) as cognition enhancers. Chem Sci, 2015; 6: 804–815. DOI: 10.1039/C4SC02130D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Ibrahim L, and Zarate CA Jr. Histone deacetylases and mood disorders: Epigenetic programming in gene-environment interactions. CNS Neurosci Ther, 2011; 17: 699–704. DOI: 10.1111/j.1755-5949.2010.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peedicayil J. Epigenetic approaches for bipolar disorder drug discovery. Expert Opin Drug Discov, 2014; 9 917–930. [DOI] [PubMed] [Google Scholar]