Summary
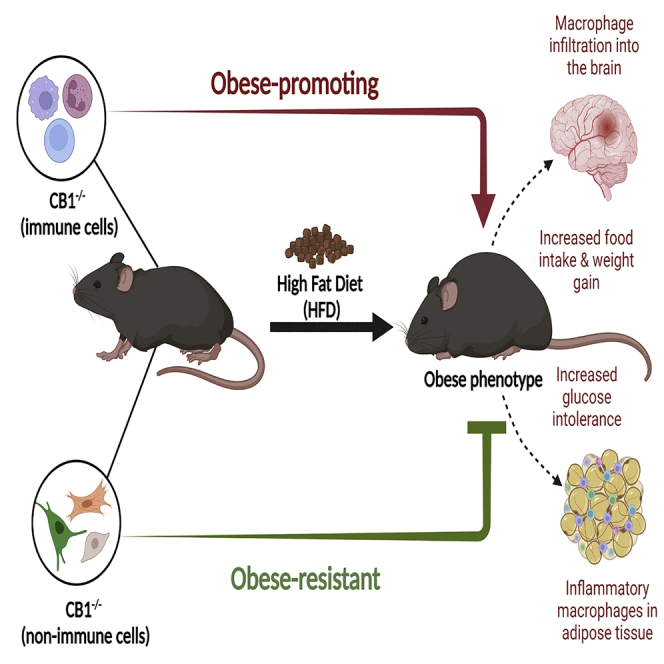
While blockade of cannabinoid receptor 1 (CB1) has been shown to attenuate diet-induced obesity (DIO), its relative role in different cell types has not been tested. The current study investigated the role of CB1 in immune vs non-immune cells during DIO by generating radiation-induced bone marrow chimeric mice that expressed functional CB1 in all cells except the immune cells or expressed CB1 only in immune cells. CB1−/− recipient hosts were resistant to DIO, indicating that CB1 in non-immune cells is necessary for induction of DIO. Interestingly, chimeras with CB1−/− in immune cells showed exacerbation in DIO combined with infiltration of bone-marrow-derived macrophages to the brain and visceral adipose tissue, elevated food intake, and increased glucose intolerance. These results demonstrate the opposing role of CB1 in hematopoietic versus non-hematopoietic cells during DIO and suggests that targeting immune CB1 receptors provides a new pathway to ameliorate obesity and related metabolic disorders.
Subject areas: Immunology, Hematology, Human metabolism
Graphical abstract
Highlights
-
•
Cannabinoid Receptor 1 (CB1), and not CB2, regulates diet-induced obesity (DIO)
-
•
CB1 deficiency in non-immune cell types promotes DIO resistance
-
•
CB1 deficiency in immune cells exacerbates DIO disease phenotype
-
•
CB1 activation in immune cells is a potential therapeutic target for DIO attenuation
Immunology; Hematology; Human metabolism
Introduction
The obesity pandemic is experiencing perpetual growth and robustly contributes to the global health burden due to many related complications that are triggered by chronic low-grade inflammation and consequent metabolic dysfunction (Haslam and James, 2005). The endocannabinoid system (ECS) is a biological system that regulates a variety of obesity-related processes including appetite, inflammation, and metabolism (Cota et al., 2003; Di Marzo et al., 2001; Mehrpouya-Bahrami et al., 2019; Miranda et al., 2019; Ruiz de Azua et al., 2017). The system is comprised of cannabinoid receptors (CB) and their endogenous lipophilic ligands called endocannabinoids. The two main cannabinoid receptors, CB1 and CB2, are G-protein coupled receptors that are expressed in various anatomical locations. CB1 expression is highly localized to the central nervous system where it is well known to channel the psychotropic effects of delta-9-tetrahydrocannabinol (THC). CB1 is additionally expressed in peripheral metabolic tissues such as the liver, adipose tissue, muscle, gastrointestinal (GI) tract, and in immune cells (Laprairie et al., 2012). Previous studies have shown that activation of CB1 can lead to suppression of inflammation (Gutierrez et al., 2007; Hegde et al., 2008; Sido et al., 2015). CB2 is largely expressed in the immune cells and activation of CB2 has been associated with attenuation of inflammatory pathways including pain and cytokine release (Lombard et al., 2007; Singh et al., 2012; Tomar et al., 2015; Turcotte et al., 2016). Importantly, obesity is characterized by energy imbalance, chronic low-grade inflammation, and chronic ECS activation. Therefore, the ECS provides a mechanistic link between inflammation and metabolism during obesity that is not fully understood.
In the early 2000s, it was discovered that activation of CB1 promotes development of obesity (Cota et al., 2003; Di Marzo et al., 2001). Blockade of CB1 reduced food intake in rodents and treatment of diet-induced obese (DIO) mice with the CB1 antagonist SR141716A led to amelioration of both obesity and metabolic impairments (Cota et al., 2003; Ravinet Trillou et al., 2003). Furthermore, CB1 knockout mice were shown to be resistant to development of high-fat diet (HFD)-induced obesity and insulin resistance (Ravinet Trillou et al., 2004). Indeed, pharmacological blockade or genetic ablation of CB1 lessened appetitive behaviors; however, additional appetite-independent metabolic benefits were observed indicating an increase in energy expenditure when CB1 signaling is impaired (Ravinet Trillou et al., 2003, 2004). Thus, CB1 became an attractive target for initiating clinical trials to treat obesity. Unfortunately, due to adverse neuropsychiatric effects, SR141716A, also known as rimonabant, a CB1 antagonist, was removed from the market. Despite this, research in the last decade has further tried to characterize the mechanisms through which CB1 blockade improves metabolic disorder with the hope of identifying pathways that can potentially be targeted. Beneficial mechanisms of systemic CB1 antagonism beyond appetite regulation include decreasing obesity-associated inflammation through microRNA regulation of adipose tissue macrophages (ATM), increased brown adipose tissue thermogenesis, and increased insulin-dependent glucose utilization (Bajzer et al., 2011; Boon et al., 2014; Mehrpouya-Bahrami et al., 2019; Miranda et al., 2019). Adipocyte-specific CB1 knockout also increases energy expenditure and promotes anti-inflammatory M2 ATM polarization, which highlights the importance of peripheral CB1 receptors in metabolic regulation (Ruiz de Azua et al., 2017). In addition, recent evidence shows that peripherally restricted CB1 antagonists can be used to treat obesity without central side effects, albeit, their anti-obesity effects are slightly less than that of central-acting CB1 antagonists (Dong et al., 2018; Han et al., 2018). CB2 is the main cannabinoid receptor expressed on cells of the immune system and has been shown to have anti-inflammatory properties (Lombard et al., 2007; Robinson et al., 2015; Singh et al., 2012). However, previous studies on the role of CB2 in metabolism and obesity-induced inflammation have led to conflicting results (Agudo et al., 2010; Deveaux et al., 2009; Verty et al., 2015). Thus, CB2 plays a complex role in metabolic processes, which may be diet-dependent or involve interaction with CB1. Thus, overall, there is general consensus that CB1, but not CB2, plays a critical role in the regulation of obesity.
While CB1 has been well characterized to play a critical role in the regulation of DIO, because CB1 is expressed in the CNS as well as in the immune cells, it is not clear if activation of CB1 in immune vs non-immune cells plays any differential role. To address this, in the current study, we developed bone-marrow-derived chimeric mice lacking CB1 in either immune or non-immune cells, to study the effect on DIO. Interestingly, using bone marrow chimeras, we noted that CB1 deletion in immune cells led to exacerbation in DIO combined with increased recruitment of bone-marrow-derived macrophages to the CNS, accelerated weight gain, elevated food intake, increased glucose intolerance, and more inflammatory macrophages in visceral adipose tissue. In contrast, CB1 deficiency in non-immune cells of the chimeric mice caused attenuation of DIO. Together, our study demonstrates the contrasting effects of CB1 expression on immune vs non-immune cells and identifies CB1 on immune cells as a potential target for obesity.
Results
CB1 but not CB2 regulates DIO development and associated inflammation
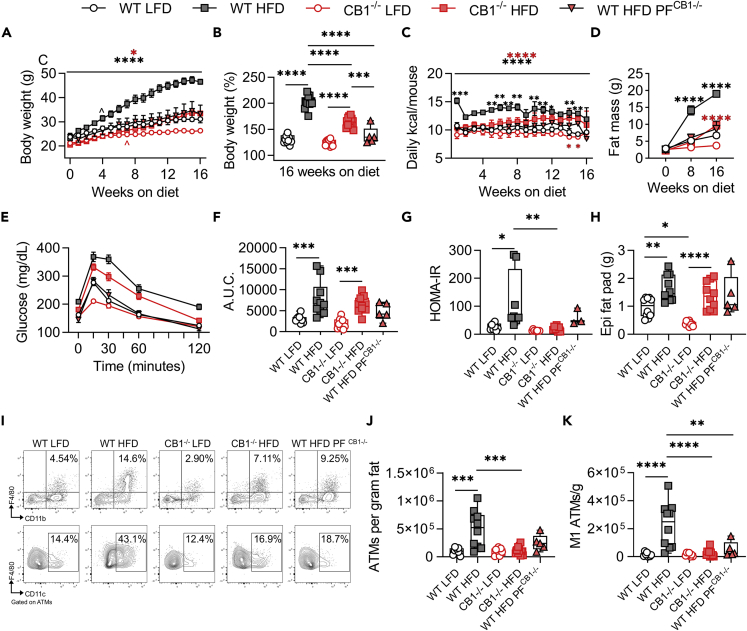
To address the role of cannabinoid receptors in DIO-induced inflammation, WT C57BL6 mice, constitutive CB1−/−, and constitutive CB2−/− mice were fed 60% HFD or 10% LFD for 16 weeks. Consistent with previous reports (Ravinet Trillou et al., 2004), CB1−/− mice were resistant to HFD-induced weight gain, polyphagia, and adiposity (Figures 1A–1D). However, CB1−/− mice developed HFD-induced hyperglycemia during glucose tolerance test and maintained insulin sensitivity with low Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (Figures 1E–1G). The anti-obesity effects of CB1 deletion were largely due to reduced food intake, as pair-fed controls (WT HFDPF CB1−/−) displayed similarity to CB1−/− HFD mice (Figures 1A–1G). It should be noted that while WT pair-fed mice consumed the same amount of calories as the CB1−/− mice, once-daily feeding may have promoted additional anti-obesity benefits due to intermittent fasting, as shown in other reports (Li et al., 2017).
Figure 1.
Constitutive CB1 knockout reduces HFD-induced obesity and adipose tissue inflammation
6- to 8-week-old male mice were fed LFD or HFD for 16 weeks prior to metabolic and inflammatory evaluation.
(A) Body weight growth curves during 16 weeks of diet.
(B) Endpoint body weight expressed as percent of starting weight.
(C) Weekly means of daily calorie intake per mouse.
(D) DEXA fat mass at 0, 8, and 16 weeks on diet.
(E) Oral GTT performed during the 16th week of diet.
(F) GTT area under the curve (A.U.C.).
(G) HOMA-IR insulin resistance index after 16 weeks on diet.
(H) Epididymal fat pad wet weight.
(I) Representative flow cytometry contour plots of epididymal CD11b+F4/80+ ATMs and ATM-gated CD11c+ M1 ATMs.
(J) Quantification of ATMs per gram of epididymal fat.
(K) Quantification of M1 ATMs per gram of epididymal fat. Data are mean ± SEM or box and whisker plots with individual points representing biological replicates. N = 10 mice/group, except N = 5 for WT HFDPF CB1−/-. For C, N = 2 cages except N = 1 for WT HFDPF CB1−/-. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 by one- or two-way ANOVA with Tukey post-hoc tests.
Inflammation associated with obesity promotes development of adipose tissue insulin resistance and is largely driven by expansion of inflammatory CD11c+ ATMs (Li et al., 2010; Lumeng et al., 2007; Nakajima et al., 2016). Therefore, we performed flow cytometry of stromal vascular fractions of epididymal (Epi) fat after 16 weeks of LFD or HFD feeding. While CB1−/− mice experienced HFD-induced fat pad expansion (Figure 1H), they did not have HFD-induced infiltration of CD11b+F4/80+ ATMs and inflammatory CD11c+ M1 ATMs (Figures 1I and 1J). These data confirmed that CB1 signaling regulates DIO development and adipose tissue inflammation.
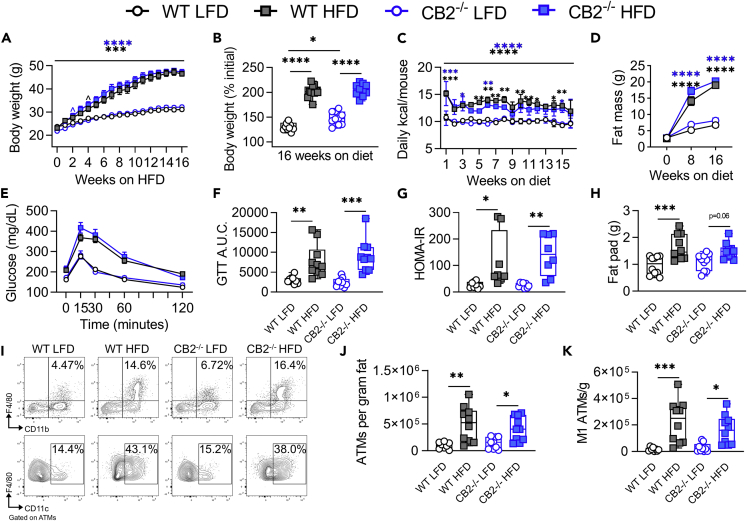
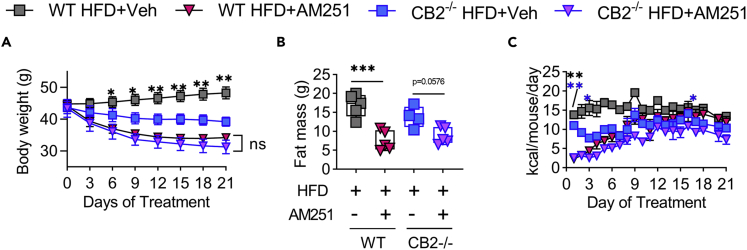
We also evaluated DIO development and inflammation in CB2−/− mice. Previous publications have shown conflicting results on the role of CB2 inhibition in DIO (Agudo et al., 2010; Alshaarawy et al., 2019; Deveaux et al., 2009; Schmitz et al., 2016). In our hands, we observed no significant changes in HFD-induced DIO development or adipose tissue inflammation in CB2−/− versus WT mice (Figures 2A–2K). Furthermore, treatment of 12-week HFD-fed DIO WT or CB2−/− mice with the CB1 antagonist AM251 resulted in weight loss and reduced food intake that was independent of CB2 (Figures 3A–3C). Thus, we concluded that CB1, but not CB2, may be the primary cannabinoid receptor regulating DIO.
Figure 2.
CB2 knockout or inhibition does not alter HFD-induced obesity or inflammation
For A–K, 6- to 8-weeks-old WT or constitutive CB2 knockout mice were fed LFD or HFD for 16 weeks.
(A) Body weight growth curves during 16 weeks of diet.
(B) Endpoint body weight expressed as percent of starting weight.
(C) Weekly means of daily calorie intake per mouse.
(D) DEXA fat mass at 0, 8, and 16 weeks on diet.
(E) Oral GTT performed during the 16th week of diet.
(F) GTT area under the curve (A.U.C.).
(G) HOMA-IR insulin resistance index after 16 weeks on diet.
(H) Epididymal fat pad wet weight.
(I) Representative flow cytometry contour plots of epididymal CD11b+F4/80+ ATMs and ATM-gated CD11c+ M1 ATMs.
(J) Quantification of ATMs per gram of epididymal fat.
(K) Quantification of M1 ATMs per gram of epididymal fat. Data are mean ± SEM or box and whisker plots with individual points representing biological replicates. For A-K, N = 10 mice/group except N = 2 cages for C. For L-R, N = 5 mice/group except N = 2 cages for N. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 by one- or two-way ANOVA with Tukey post-hoc tests.
Figure 3.
CB1 antagonist DIO intervention promotes CB2-independent weight loss and reduced food intake
Male C57BL/6 WT and CB2−/− mice were fed 60% HFD for 12 weeks to induce DIO and then treated with 10 mg/kg body weight of AM251 daily for 3 weeks.
(A) Body weight during the 3-week DIO intervention.
(B) DEXA fat mass post 3-week intervention.
(C) Means of daily calorie intake per mouse during the 3-week intervention.
Data are mean ± SEM or box and whisker plots with individual points representing biological replicates. For A-B, N = 5 mice/group except N = 4 mice/group for CB2−/− HFD + Veh. For C, N = 2 cages of mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, by one- or two-way ANOVA with Tukey post-hoc tests.
CB1 deficiency in immune cells potentiates DIO
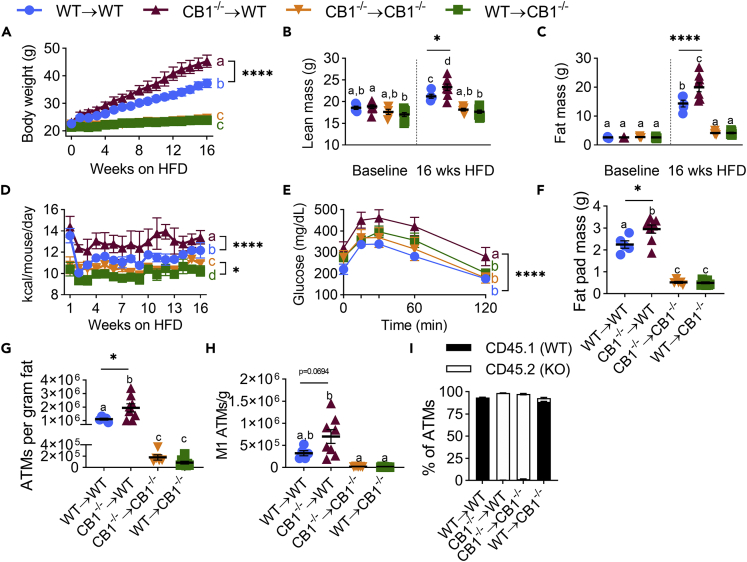
Because CB1 is expressed both on immune and non-immune cells, we next tested whether the deficiency of CB1 on expression of immune vs non-immune cells imparts the resistance to DIO. To that end, we generated radiation-induced bone marrow chimeric mice that expressed functional CB1 on all cells in the body except hematopoietic cells (CB1−/−→WT), or only in hematopoietic cells (WT→CB1−/−). To that end, B6 CD45.1 (CB1 WT) and CB1−/− (CB1 KO expressing CD45.2) were lethally irradiated and adoptively transferred with viable donor bone marrow cells. WT and CB1−/− hosts receiving autologous bone marrow were used as controls (WT→WT or CB1−/−→CB1−/−, respectively). After recovery, mice were fed HFD for 16 weeks. We confirmed chimerism by staining splenocytes for CD45 and CD45.1/CD45.2 alleles (Figure S1). The data showed that CB1−/− →CB1−/− mice or WT→CB1−/− chimeras were resistant to HFD-induced weight gain and adiposity (Figures 4A–4C and S2). The resistance of WT→CB1−/− chimeras to DIO indicated that CB1 deficiency in non-immune cells was necessary for induction of resistance to HFD-induced adiposity. Interestingly, mice deleted for CB1 only in immune cells (CB1−/−→WT) had accelerated weight gain, higher lean mass, higher fat mass than WT controls, increased adipocyte size in epididymal fat pads, and increased leptin levels, suggesting that CB1 deficiency in immune cells leads to exacerbation of DIO (Figures 4A–4C and S2). Weekly monitoring also showed CB1−/−→WT and CB1−/−→CB1−/− had greater food intake than mice containing WT immune cells (Figure 4D). Furthermore, CB1−/−→WT mice displayed elevated glucose intolerance when analyzed by glucose tolerance test (Figure 4E).
Figure 4.
CB1 knockout in hematopoietic cells worsens HFD-induced obesity and adipose tissue inflammation
Irradiated B6 CD45.1 (CB1 WT) and CB1−/− (CB1 KO) male mice were adoptively transferred with donor bone marrow to generate chimeric mice. Reconstituted mice were fed HFD for 16 weeks.
(A) Body weight growth curves during 16 weeks of diet.
(B) DEXA lean mass at 0 and 16 weeks on diet.
(C) DEXA fat mass at 0 and 16 weeks on diet.
(D) Weekly means of daily calorie intake per mouse.
(E) Oral GTT performed during the 16th week of diet.
(F) Epididymal fat pad wet weight.
(G) Quantification of ATMs per gram of epididymal fat.
(H) Quantification of M1 ATMs per gram of epididymal fat.
(I) Percent of ATMs expressing CD45.1 or CD45.2. Data are mean ± SEM with individual points representing biological replicates. N = 8 mice/chimeric group or 5 mice/syngeneic control group. For D, N = 2 cages. ∗p < 0.05, ∗∗∗∗p < 0.0001 by one-way ANOVA with Tukey post-hoc tests. p < 0.05 if alphabetical characters differ between groups. See also Figures S1–S3.
In addition to a worsened obese phenotype, CB1−/−→WT had increased ATM-mediated inflammation, with higher fat pad mass, and more ATMs per gram of fat than WT mice (Figures 4F–4G and S3). Abundance of CD11c+ M1 ATMs also trended higher in these mice (Figure S3 and 4H). Still, CB1−/−→CB1−/− and WT→CB1−/−, were resistant to HFD-induced ATM inflammation, likely due to their decreased obese phenotype (Figures 4F–4H). CD45.1/CD45.2 staining of ATMs confirmed they were infiltrating bone-marrow-derived cells (Figure 4I).
CB1 receptors on immune cells regulate myeloid neuroinflammation and food intake
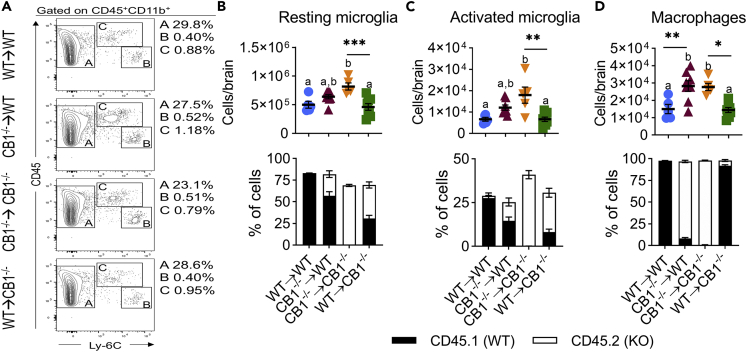
Together our data showed associations between HFD-induced weight gain, adipose tissue inflammation, and glucose intolerance. However, CB1−/−→WT and CB1−/−→CB1−/− also had elevated food intake versus their respective host control. To identify a potential mechanism of immune CB1 in regulation of food intake, we next performed flow cytometry of brain mononuclear cells (MNC) for microglia and macrophages because inflammatory microgliosis and bone-marrow-derived myeloid recruitment to the hypothalamus promotes diet-induced hyperphagia and weight gain (Valdearcos et al., 2017). We identified three populations of myeloid cells in brain MNCs, which were defined as CD45+CD11b+Ly-6C− resting microglia, CD45loCD11b+Ly-6Chi activated microglia, and CD45hiCD11b+Ly-6C+ infiltrating macrophages (Figure 5A). Quantification of these populations per brain and CD45.1/CD45.2 staining showed that CB1−/−→CB1−/− had elevated abundance of all three populations (Figures 5B–5D). Furthermore, CB1−/−→WT had increased infiltration of bone-marrow-derived macrophages (Figure 5D). Microglia numbers trended higher in these mice but were not significant, likely due to presence of irradiation-resistant WT host-derived microglia (Figures 5B and 5C).
Figure 5.
Immune CB1 deletion promotes myeloid neuroinflammation
Chimeric mice were generated as described in Figure 4 legend. After 16 weeks of HFD, brain mononuclear cells were analyzed by flow cytometry.
(A) Representative flow cytometry contour plots of CD45+CD11b+ myeloid immune cell populations. A- CD45+Ly-6C− resting microglia, B- CD45loLy-6Chi activated microglia, C- CD45hiLy-6C+ infiltrating macrophages.
(B) Resting microglia numbers per brain and percentage of resting microglia expressing CD45.1 or CD45.2 alleles.
(C) Activated microglia numbers per brain and percentage of resting microglia expressing CD45.1 or CD45.2 alleles.
(D) Macrophage numbers per brain and percentage of resting microglia expressing CD45.1 or CD45.2 alleles. Data are mean ± SEM with individual points representing biological replicates. N = 8 mice/chimeric group or 5 mice/syngeneic control group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, by one-way ANOVA with Tukey post-hoc tests. p < 0.05 if alphabetical characters differ between groups.
Discussion
The anti-obesity and anti-inflammatory effects of CB1 blockade have been well documented (Mehrpouya-Bahrami et al., 2017, 2019; Miranda et al., 2019; Ravinet Trillou et al., 2003, 2004). However, because CB1 is widely expressed in the body, whether blocking CB1 expression on immune or non-immune cells plays a role in attenuating DIO has not been investigated in the past. This is critical because the CB1 receptor antagonist (Rimonabant), while shown to be effective in inducing weight loss and improving metabolic syndrome, had to be withdrawn from clinical trials due to the emergence of significant side effects, notably severe mood disorders (Christensen et al., 2007; Ravinet Trillou et al., 2003).
In the current study, we therefore investigated the role played by CB1 expressed on immune vs non-immune cells in DIO by using mice deficient in CB1 (CB1−/−) as well as by creating chimeric mice that selectively lacked CB1 in immune vs non-immune cells. Our data showed that CB1 deficiency in both immune and non-immune cells plays a critical role in regulating HFD-induced obesity albeit in opposite ways. We found that CB1 deficiency in immune cells (CB1−/−→WT) promotes adipose tissue inflammation, neuroinflammation, and exacerbation of DIO whereas, CB1 deficiency in non-immune cells (WT→CB1−/−) attenuated DIO by preventing adipose tissue inflammation and potentially regulating gut microbiota. The unique role of CB1 on immune cells is exciting and suggests that endocannabinoid-mediated activation of CB1 on immune cells may reduce adipose tissue inflammation and DIO. Our studies on CB1 deficiency in non-immune cells (WT→CB1−/−) are consistent with data from previous studies which showed that CB1-deficient mice (CB1−/−) as well as conditional deletion of CB1 in adipocytes and neuronal cells promotes resistance to DIO (Cardinal et al., 2015; Quarta et al., 2010; Ravinet Trillou et al., 2004; Ruiz de Azua et al., 2017).
We have also shown 4 weeks treatment of DIO mice with the CB1 antagonists SR141716A and AM251 promotes rapid and sustained weight loss associated with decreased adipose tissue inflammation, but the immune-mediated mechanisms were not clear (De Souza et al., 2005; Mehrpouya-Bahrami et al., 2019; Miranda et al., 2019). Here, we demonstrate chimeric mice lacking CB1 only in hematopoietic cells (CB1−/−→WT) surprisingly promoted inflammation and worsened obesity. Therefore, anti-inflammatory effects of CB1 antagonists observed in our previous studies were likely a consequence of reduced fat mass mediated by CB1 blocking in non-immune cells.
Interestingly, we also found absence of CB1 in immune cells (CB1−/−→WT) promoted incidence of food intake. Therefore, we investigated encephalitogenic myeloid cells because HFDs activate hypothalamic inflammation, which in turn contributes to energy imbalance and impaired insulin signaling (Hegde et al., 2010; Morari et al., 2014). We found that CB1 deficiency in immune cells activated microgliosis and infiltrating macrophages in the brain. Together, our data suggest that non-functional CB1 signaling in microglia and encephalitogenic macrophages contributes to neuroinflammation and consequently increased incidence of food intake, which could be indicative of changes in appetite. Another possible explanation could be that non-functional CB1 signaling in encephalitogenic myeloid cells increases endocannabinoid signaling through neuronal CB1 receptors to increase food intake. Future studies targeting microglial and macrophage CB1 receptors could shed light on therapeutic potential of selective immune-acting CB1 agonists for treating obesity by reducing desire for food intake, or perhaps even appetite. Our studies also suggest that selective activation of CB1 on immune cells may attenuate DIO though suppression of tissue inflammation. There is significant evidence to support this since cannabinoid receptor activation can induce highly immunosuppressive MDSCs and Tregs (Becker et al., 2020; Hegde et al., 2013; Mohammed et al., 2020; Wu et al., 2020). While not entirely deciphered currently, several proposed direct and indirect mechanisms have been explored to explain how CB1 influences the incidence and activation of macrophage phenotypes. In a report by Tian et al., researchers found during liver inflammation that CB1 affected M1/M2 polarization of bone-marrow-derived monocytes/macrophages via two independent signaling pathways linked to G alpha i/o (G(α)i/o) membrane proteins (Tian et al., 2017). Lou et al. reported that M1 macrophage induction after inhibition of CB1 was controlled through TLR-4 and NF-κB/p65 signaling in a mouse model of multiple sclerosis (Lou et al., 2018). In our previous reports, we showed how microRNA alterations affect macrophage polarization during CB1 inhibition, more specifically by promoting anti-inflammatory M2 phenotype (Mehrpouya-Bahrami et al., 2019). Based on these reports, M1 macrophage phenotype may be greatly dependent on CB1 activation. Thus, in the absence of these various signaling events (e.g. CB1 antagonism), the incidence and accumulation of M1 types would be diminished, and most likely an anti-inflammatory M2 phenotype may be favored. An anti-inflammatory response could lead to decreased incidence of neuroinflammation resulting in reduced appetite, the consequence being decreased consumption, and a net negative energy balance leading to overall weight loss.
Studies on the role of CB2 in the regulation of DIO have resulted in conflicting reports. For example, using JWH-133 which acts as a selective agonist for CB2, it was shown that activation of CB2 leads to reduced body weight gain, relieved glucose tolerance, and enhanced insulin sensitivity in a mouse model of obesity (Rossi et al., 2016; Williams et al., 2009). Also, CB2 blockade with AM630 increased the inflammatory adipokine release and fat storage and reduced browning (Williams et al., 2009). In contrast, in another study, JWH-133 was found to increase adipose tissue inflammation in mice on HFD (Cluny et al., 2015). In this study, mice lacking CB2 showed decreased resistance to HFD-induced insulin and hepatic steatosis (Cluny et al., 2015). In the current study, we observed no significant changes in HFD-induced DIO development or adipose tissue inflammation in CB2−/− mice. We also found that HFD-fed CB2−/− mice when treated with the CB1 antagonist AM251 showed weight loss and reduced food intake thereby demonstrating that this response was independent of CB2. While the reasons for discrepancies in the role of CB2 in DIO remain unclear, our studies using CB2−/− mice suggested that CB1, but not CB2, may be the primary cannabinoid receptor regulating DIO.
In the current report using chimeric CB1-deficient mice, we examined macrophage infiltration and activation mainly in the adipose tissue and whole brains as an expansion of our previous reports studying the role of CB1 antagonism as it relates to obesity (Mehrpouya-Bahrami et al., 2019; Miranda et al., 2019). However, there are other organ systems and combined factors which greatly influence obesity phenotype and have shown to be influenced by CB activation or antagonism which needs to be explored further. An example would be the liver and hepatic system, an organ and system known to be greatly involved in several metabolic disorder states, including obesity (Mallat and Lotersztajn, 2006). In a more recent report, it was shown that a partial CB1 agonist/antagonist (RTI1092769) with limited activity in the brain was able to reduce weight gain and improve glucose levels in HFD obese mice while also improving hepatic-associated factors such as triglyceride and biomarkers associated with nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) (Khan et al., 2021). Other researchers linked CB1 antagonism in a DIO mouse model to attenuation of obese phenotype and hepatic steatosis through complex molecular mechanisms involving p53, peroxisome proliferator-activated receptor-alpha (PPAR α), miRNA, and sirtuin 1 (SIRT1) (Azar et al., 2020). To that end, it would be worth exploring how selective CB1 deficiency in immune and non-immune cells affects components of the liver response as it relates to attenuation or exacerbation of the DIO phenotype.
Another entity that influences obesity phenotype is the community of trillions of microbes within the GI tract, also known as the gut microbiota (Cani, 2014; Zhao, 2013). It is becoming increasingly evident that environmental factors such as diet, exercise, stress, pharmaceuticals, and the like can alter the gut microbiota and impact host health (Dopkins et al., 2018). During obesity, altered microbiota composition leads to microbial dysbiosis that impairs health by contributing to gut inflammation, intestinal permeability, and increased energy harvest (Turnbaugh et al., 2006). A recent study from our laboratory demonstrated that blockade of CB1 signaling attenuates obesity and is associated with a shift in the gut microbiome composition that is coupled with decreased intestinal permeability, increased microbial short chain fatty acid production, and dampened adipose tissue macrophage (ATM)-mediated inflammation (Mehrpouya-Bahrami et al., 2017). Others have also demonstrated relationships between CB1 activation, intestinal permeability, decreased gut motility, and lipopolysaccharide (LPS)-regulated adipogenesis (Alhamoruni et al., 2010; Izzo et al., 2001; Muccioli et al., 2010). Given the findings in the current report, mainly the cell-type-specific responses to obesity susceptibility governed by CB1 expression, future studies examining the gut microbiome alterations in these chimeric mouse models should be explored.
In conclusion, our study identifies hematopoietic CB1 as a novel therapeutic target for DIO. Combinatorial analysis of constitutive knockouts, chimeric mice, and pharmacological interventions identified non-hematopoietic CB1 receptors as initiators of HFD-induced weight gain, but hematopoietic CB1 receptors as restrictors of DIO. Of note, chronic administration of the psychoactive CB1 agonist THC in DIO mice reduces HFD-induced polyphagia, weight gain, and adiposity (Clark et al., 2018). Additionally, chronic cannabis users have reduced obesity rates (Sido et al., 2016). Moreover, numerous studies from our laboratory have shown anti-inflammatory effects of THC (Mohammed et al., 2020; Sido et al., 2016). Thus, chronic CB1 agonism may reduce inflammation contributing to reduced food intake and obesity. Therefore, activating immune-specific CB1 receptors may constitute a novel anti-inflammatory therapeutic to lessen DIO and circumvent adverse neuropsychiatric and psychotropic side effects of CB1 antagonist or neuronal CB1 agonist treatments, respectively.
Limitations of the study
In the current report, we made the exciting finding that transplantation of CB1−/− immune cells into irradiated WT host recipients led to an exacerbated DIO phenotype and CB1−/− recipient hosts seemed resistant to DIO induction, thus highlighting the complex cell-specific role CB1 plays during this disease state. However, the irradiation technique does not completely ablate the recipient host immune cells, so there is the possibility that a small number of immune cells, especially radioresistant macrophages survived, which could have impact on the results. Future studies using conditional CB1 knockout mice with even more cell-type specificity (e.g. cre-lox technique) need to be performed to expand upon the results reported herein. Indeed, another limitation of our study was isolating immune cells from entire brain instead of only the hypothalamus. However, obesity is known to induce hypothalamic inflammation, thus differences we observed are likely to be representative of hypothalamic immune cells, though future studies evaluating immune cell presence in particular subsections of the brain should be performed.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| TruStain FcX™ anti-mouse CD16/32 (Clone 93) | Biolegend | Cat#101329; RRID: AB_1574973 |
| APC/Cy7 anti-mouse CD45 (Clone 30-F11) | Biolegend | Cat#103115; RRID: AB_312980 |
| PE anti-mouse CD45.1 (Clone A20) | Biolegend | Cat#110707; RRID: AB_313496 |
| FITC anti-mouse CD45.1 (Clone 104) | Biolegend | Cat#109805; RRID: AB_313442 |
| Alexa Fluor® 700 anti-mouse/human CD11b (Clone M1/70) | Biolegend | Cat#101222; RRID: AB_493705 |
| Brilliant Violet 421™ anti-mouse F4/80 (Clone BM8) | Biolegend | Cat#123137; RRID: AB_2563102 |
| Brilliant Violet 605™ anti-mouse CD11c (Clone N418) | Biolegend | Cat#117334; RRID: AB_2562415 |
| Brilliant Violet 605™ anti-mouse Ly-6C Antibody (Clone HK1.4) | Biolegend | Cat#128035; RRID: AB_2562352 |
| Chemicals, peptides, and recombinant proteins | ||
| AM 251 (chemical name: N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) | Tocris Bioscience | Cat#1117 |
| Tween 80 | Sigma-Aldrich | Cat#9005-65-6 |
| D-(+)-Glucose | Sigma-Aldrich | Cat#50-99-7 |
| 0.5M EDTA | Sigma-Aldrich | Cat#150-38-9 |
| Critical commercial assays | ||
| Insulin Mouse ELISA Kit | Invitrogen | Cat#EMINS |
| Adipose Tissue Dissociation Kit, mouse and rat | Miltenyi Biotec | Cat#130-105-808 |
| Qubit dsDNA HS assay | ThermoFisher Scientific | Cat#Q32854 |
| Neural Tissue Dissociation Kit (P) | Miltenyi Biotec | Cat#130-092-628 |
| Experimental models: Organisms/strains | ||
| C57BL/6J mice | The Jackson Laboratory | Cat#000664; RRID: IMSR_JAX:000,664 |
| B6.SJL-Ptprca Pepcb/BoyJ mice (common name: B6 CD45.1) | The Jackson Laboratory | Cat#002014; RRID:IMSR_JAX:002014 |
| CB1-/- mice | Gifted from Dr. James Pickel (NIH National Institute of Mental Health Transgenic Core Facility, Bethesda, MD, USA); Zimmer et al., 1999 | N/A |
| B6.129P2-Cnr2tm1Dgen/J mice (common name: CB2R KO; also known as CB2−/−) | The Jackson Laboratory | Cat# 005,786; RRID:IMSR_JAX:005,786 |
| C57BL/6J DIO mice (HFD) | The Jackson Laboratory | Cat#380050; RRID:IMSR_JAX:380,050 |
| C57BL/6J DIO Control mice (LFD) | The Jackson Laboratory | Cat# 380,056; RRID:IMSR_JAX:380,056 |
| Software and algorithms | ||
| Graphpad Prism Software | https://www.graphpad.com/ | N/A |
| FlowJo v10 | https://www.flowjo.com/ | N/A |
| Other | ||
| High fat diet (HFD) with 60% kcal fat | Research Diets, Inc. | Cat#D12492 |
| Low fat diet (LFD) with 10% kcal fat | Research Diets, Inc. | Cat#D12450J |
Resource availability
Lead contact
Further information and requests for resources should contact Dr. Prakash Nagarkatti (Prakash@mailbox.sc.edu).
Materials availability
The current study did not generate any new reagents.
Method details
Experimental model and subject details
Six-to 8-week-old male C57BL/6J mice (JAX 000664), B6.SJL-Ptprca Pepcb/BoyJ (common name: B6 CD45.1; JAX 002014), and 18-week-old male C57BL/6J mice fed either 60% kcal high-fat-diet (HFD, D12492, Research Diets) (JAX 380050), or 10% kcal low-fat diet (LFD, D12450J, Research Diets) (JAX 380056) where indicated, were obtained from The Jackson Laboratory, and housed in a specific-pathogen-free (SPF) facility. CB1−/− constitutive knockout mice were gifted from Dr. James Pickel (NIH National Institute of Mental Health Transgenic Core Facility, Bethesda, MD, USA) (Zimmer et al., 1999). CB2−/− constitutive knockout mice (B6.129P2-Cnr2tm1Dgen/J) were obtained from The Jackson Laboratory (JAX 005786). CB1−/− and CB2−/− mice were bred in-house, and colonies maintained at the University of South Carolina School of Medicine animal facility. All mice used in the current study, including constitutive CB−/− knockout strains, were on a C57BL/6J mouse background. Mice were housed 3–5 mice per cage according to treatment group. In all experiments, each treatment group consisted of mice from multiple litters and cages. In some instances, mice were singly housed due to fighting. Pair feeding was performed by measuring the weight of HFD consumed each day, then administering that same weight of food to the Pair-fed (PF) group on the following day. At the conclusion of each study, mice were euthanized by overdose isoflurane inhalation. All experiments were performed in accordance with ethical standards approved by the University of South Carolina Institutional Animal Care and Use Committee (IACUC).
Cannabinoid receptor agonist/antagonist administration
For DIO intervention studies, 12-week HFD-fed obese mice were stratified into treatment groups by equivalent mean DEXA fat mass, then treated with CB1 antagonist AM251 (10 mg/kg), daily for 3-weeks. AM251 was purchased from Tocris (Tocris 1117), suspended in 0.1% Tween 80, and administered by oral gavage. Experimental groups not receiving drug treatments were administered Vehicle (Veh) gavages.
Bone marrow transplantation
Six- to eight-week-old male CB1 WT (B6.SJL-Ptprca Pepcb/BoyJ mice, commonly known as B6 CD45.1) or CB1−/− (expressing CD45.2) mice, both on a C57BL/6 background, were lethally irradiated with a single dose of 900 cGy from a Cesium irradiator, then adoptively transferred intravenously with CB1 WT or CB1−/− donor bone marrow cells. After 4 weeks of recovery, the mice were fed 60% HFD for 16 weeks.
Analytical procedures
Body composition was measured by dual-energy x-ray absorptiometry (DEXA, LUNAR PIXImus) scanning as previously described (Miranda et al., 2018). Histological evaluation was performed on isolated epididymal fat pads, sectioned, stained with hematoxylin and eosin, and imaged using a Swift M3603 series microscope and moto camera bundle (VWR).Body weight was monitored using an electronic gram scale with precision ± 0.1g. For glucose tolerance tests, mice were fasted for 5h, then fasting glucose was measured, immediately followed by oral gavage of 2 g/kg lean mass glucose (Sigma G7528). Blood glucose was measured 15, 30, 60 and 120min after glucose gavage by applying approximately 5uL tail-tip blood to a glucose test strip in a glucometer (Contour Next, Bayer). To calculate a HOMA-IR, mice were fasted for 5h then blood glucose was measured from the tail-tip. Immediately following, mice were euthanized by overdose isoflurane inhalation and ∼500uL hepatic portal vein-derived blood was collected via exposure of the abdominal cavity after euthanasia, insertion of a 1mL syringe into the portal vein below the liver, and collected blood was gently mixed with 15uL 0.5M EDTA as described elsewhere (Becker et al., 2020). To isolate plasma, blood was centrifuged at 5000 RPM, for 5 min, at 4°C and the top layer was collected and stored at −20°C for further analysis. Insulin concentrations were measured in fasted plasma by ELISA (Invitrogen, EMINS). HOMA-IR index was calculated by the following equation [HOMA-IR= (fasting glucose x fasting insulin)/22.5] (Matthews et al., 1985). Leptin concentrations were measured in fasted plasma by ELISA (Abcam).
Adipose tissue dissociation
Epididymal fat pads were excised, weighted, and dissociated using MACS adipose tissue dissociation kit and a gentleMACS dissociator according to manufacturer protocol (130-105-808, Miltenyi Biotec).
Isolation of spleen single-cell suspensions
Spleens were excised from euthanized mice and disrupted in 5mL FACS buffer utilizing a Stomacher80 machine (Seward). Homogenates were filtered through a 70um nylon mesh, pelleted, RBC-lysed, and washed in FACS buffer prior to flow cytometry staining.
Isolation of brain mononuclear cells
Euthanized mice were perfused with 10mL heparinized PBS then brains were harvested and dissociated using MACS neural tissue dissociation kit (P) and a gentleMACS dissociator according to manufacturer protocol (130-092-628, Miltenyi Biotec). The isolated cells were then RBC-lysed and spun through a 33% Percoll gradient (2000 RPM, 15m, 25°C) twice to remove myelin and other cellular debris. The isolated mononuclear cells (MNCs) were then washed in FACS buffer, counted, and plated 1 × 106 cells per well of a 12-well cell culture plate in complete RPMI-1640 medium containing 10% heat-inactivated FBS, 1% penicillin/streptomycin, 2 mM L-glutamine, 10 mM HEPES buffer, and 0.0002% β-mercaptoethanol. The MNCs were cultured for 24h to allow recovery of surface markers, then gently collected by scraping for flow cytometry staining.
Flow cytometry
For surface staining, cells were incubated with FcR-Blocker (TruStain FcX™ anti-mouse CD16/32; clone 93) for 10m followed by appropriate fluorochrome-conjugated antibodies for 30 min (CD45: clone 30-F11, CD45.1: clone A20, CD45.2: clone 104, CD11b: clone M1/70, F4/80: clone BM8, CD11c: clone N418; Ly-6C: clone HK1.4). Stained cells were washed with FACS buffer then analyzed on a BD FACSCelesta flow cytometer. Data were analyzed and visualized with FlowJo v10.
Statistical analysis
Statistical analyses were performed with GraphPad Prism Version 8.1.1 for Mac (GraphPad Software). Data presented are mean (±SEM) or box and whisker plots with individual points representing biological replicates. One-way ANOVA was used for multiple group analyses and two-way ANOVA was used for significance across multiple variables. Post hoc corrections were performed by Tukey and Sidak’s multiple comparisons tests. A p value <0.05 was considered statistically significant.
Acknowledgments
This work was supported in parts by NIH grants P01AT003961, P20GM103641, R01ES030144, R01AI129788, R01AI160896, and R01AI123947.
Author contributions
P.S.N., M.N., and K.M.: Designed the study; K.M., W.B., N.D., O.A., Y.Z., and P.B. performed the experiments; K.M. and J.Z.: Analyzed the data; K.M.: Wrote the draft manuscript; M.N. and P.S.N.: Edited the manuscript; M.N. and P.S.N.: Secured the funding. All authors reviewed the manuscript and approved the final version of the manuscript.
Declaration of interests
The authors declare no conflicts of interest.
Published: September 16, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104994.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Agudo J., Martin M., Roca C., Molas M., Bura A.S., Zimmer A., Bosch F., Maldonado R. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia. 2010;53:2629–2640. doi: 10.1007/s00125-010-1894-6. [DOI] [PubMed] [Google Scholar]
- Alhamoruni A., Lee A.C., Wright K.L., Larvin M., O'Sullivan S.E. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J. Pharmacol. Exp. Ther. 2010;335:92–102. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- Alshaarawy O., Kurjan E., Truong N., Olson L.K. Diet-induced obesity in cannabinoid-2 receptor knockout mice and cannabinoid receptor 1/2 double-knockout mice. Obesity. 2019;27:454–461. doi: 10.1002/oby.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar S., Udi S., Drori A., Hadar R., Nemirovski A., Vemuri K.V., Miller M., Sherill-Rofe D., Arad Y., Gur-Wahnon D., et al. Reversal of diet-induced hepatic steatosis by peripheral CB1 receptor blockade in mice is p53/miRNA-22/SIRT1/PPARalpha dependent. Mol. Metab. 2020;42:101087. doi: 10.1016/j.molmet.2020.101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajzer M., Olivieri M., Haas M.K., Pfluger P.T., Magrisso I.J., Foster M.T., Tschöp M.H., Krawczewski-Carhuatanta K.A., Cota D., Obici S. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia. 2011;54:3121–3131. doi: 10.1007/s00125-011-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W., Alrafas H.R., Wilson K., Miranda K., Culpepper C., Chatzistamou I., Cai G., Nagarkatti M., Nagarkatti P.S. Activation of cannabinoid receptor 2 prevents colitis-associated colon cancer through myeloid cell de-activation upstream of IL-22 production. iScience. 2020;23:101504. doi: 10.1016/j.isci.2020.101504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon M.R., Kooijman S., van Dam A.D., Pelgrom L.R., Berbée J.F.P., Visseren C.A.R., van Aggele R.C., van den Hoek A.M., Sips H.C.M., Lombès M., et al. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J. 2014;28:5361–5375. doi: 10.1096/fj.13-247643. [DOI] [PubMed] [Google Scholar]
- Cani P.D. Metabolism in 2013: the gut microbiota manages host metabolism. Nat. Rev. Endocrinol. 2014;10:74–76. doi: 10.1038/nrendo.2013.240. [DOI] [PubMed] [Google Scholar]
- Cardinal P., Bellocchio L., Guzmán-Quevedo O., André C., Clark S., Elie M., Leste-Lasserre T., Gonzales D., Cannich A., Marsicano G., Cota D. Cannabinoid type 1 (CB1) receptors on Sim1-expressing neurons regulate energy expenditure in male mice. Endocrinology. 2015;156:411–418. doi: 10.1210/en.2014-1437. [DOI] [PubMed] [Google Scholar]
- Christensen R., Kristensen P.K., Bartels E.M., Bliddal H., Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Clark T.M., Jones J.M., Hall A.G., Tabner S.A., Kmiec R.L. Theoretical explanation for reduced body mass index and obesity rates in Cannabis users. Cannabis Cannabinoid Res. 2018;3:259–271. doi: 10.1089/can.2018.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny N.L., Keenan C.M., Reimer R.A., Le Foll B., Sharkey K.A. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with delta9-tetrahydrocannabinol. PLoS One. 2015;10:e0144270. doi: 10.1371/journal.pone.0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D., Marsicano G., Tschöp M., Grübler Y., Flachskamm C., Schubert M., Auer D., Yassouridis A., Thöne-Reineke C., Ortmann S., et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C., Saad M.J.A., Velloso L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Deveaux V., Cadoudal T., Ichigotani Y., Teixeira-Clerc F., Louvet A., Manin S., Nhieu J.T.V., Belot M.P., Zimmer A., Even P., et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS One. 2009;4:e5844. doi: 10.1371/journal.pone.0005844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z., Fezza F., Miura G.I., Palmiter R.D., Sugiura T., Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Dong Z., Gong H., Chen Y., Wu H., Wu J., Deng Y., Song X. LH-21, A peripheral cannabinoid receptor 1 antagonist, exerts favorable metabolic modulation including antihypertensive effect in KKAy mice by regulating inflammatory cytokines and adipokines on adipose tissue. Front. Endocrinol. 2018;9:167. doi: 10.3389/fendo.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopkins N., Nagarkatti P.S., Nagarkatti M. The role of gut microbiome and associated metabolome in the regulation of neuroinflammation in multiple sclerosis and its implications in attenuating chronic inflammation in other inflammatory and autoimmune disorders. Immunology. 2018;154:178–185. doi: 10.1111/imm.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez T., Farthing J.N., Zvonok A.M., Makriyannis A., Hohmann A.G. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br. J. Pharmacol. 2007;150:153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.H., Shin H., Rho J.G., Kim J.E., Son D.H., Yoon J., Lee Y.J., Park J.H., Song B.J., Choi C.S., et al. Peripheral cannabinoid 1 receptor blockade mitigates adipose tissue inflammation via NLRP3 inflammasome in mouse models of obesity. Diabetes Obes. Metab. 2018;20:2179–2189. doi: 10.1111/dom.13350. [DOI] [PubMed] [Google Scholar]
- Haslam D.W., James W.P.T. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- Hegde V.L., Hegde S., Cravatt B.F., Hofseth L.J., Nagarkatti M., Nagarkatti P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: involvement of regulatory T cells. Mol. Pharmacol. 2008;74:20–33. doi: 10.1124/mol.108.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde V.L., Nagarkatti M., Nagarkatti P.S. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur. J. Immunol. 2010;40:3358–3371. doi: 10.1002/eji.201040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde V.L., Tomar S., Jackson A., Rao R., Yang X., Singh U.P., Singh N.P., Nagarkatti P.S., Nagarkatti M. Distinct microRNA expression profile and targeted biological pathways in functional myeloid-derived suppressor cells induced by Delta9-tetrahydrocannabinol in vivo: regulation of CCAAT/enhancer-binding protein alpha by microRNA-690. J. Biol. Chem. 2013;288:36810–36826. doi: 10.1074/jbc.M113.503037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A.A., Fezza F., Capasso R., Bisogno T., Pinto L., Iuvone T., Esposito G., Mascolo N., Di Marzo V., Capasso F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br. J. Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Laudermilk L., Ware J., Rosa T., Mathews K., Gay E., Amato G., Maitra R. Peripherally selective CB1 receptor antagonist improves symptoms of metabolic syndrome in mice. ACS Pharmacol. Transl. Sci. 2021;4:757–764. doi: 10.1021/acsptsci.0c00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie R.B., Kelly M.E.M., Denovan-Wright E.M. The dynamic nature of type 1 cannabinoid receptor (CB(1) ) gene transcription. Br. J. Pharmacol. 2012;167:1583–1595. doi: 10.1111/j.1476-5381.2012.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Xie C., Lu S., Nichols R.G., Tian Y., Li L., Patel D., Ma Y., Brocker C.N., Yan T., et al. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab. 2017;26:672–685.e4. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Lu M., Nguyen M.T.A., Bae E.J., Chapman J., Feng D., Hawkins M., Pessin J.E., Sears D.D., Nguyen A.K., et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J. Biol. Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard C., Nagarkatti M., Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clin. Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Z.Y., Cheng J., Wang X.R., Zhao Y.F., Gan J., Zhou G.Y., Liu Z.G., Xiao B.G. The inhibition of CB1 receptor accelerates the onset and development of EAE possibly by regulating microglia/macrophages polarization. J. Neuroimmunol. 2018;317:37–44. doi: 10.1016/j.jneuroim.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat A., Lotersztajn S. Endocannabinoids as novel mediators of liver diseases. J. Endocrinol. Invest. 2006;29:58–65. [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Mehrpouya-Bahrami P., Chitrala K.N., Ganewatta M.S., Tang C., Murphy E.A., Enos R.T., Velazquez K.T., McCellan J., Nagarkatti M., Nagarkatti P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017;7:15645. doi: 10.1038/s41598-017-15154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrpouya-Bahrami P., Miranda K., Singh N.P., Zumbrun E.E., Nagarkatti M., Nagarkatti P.S. Role of microRNA in CB1 antagonist-mediated regulation of adipose tissue macrophage polarization and chemotaxis during diet-induced obesity. J. Biol. Chem. 2019;294:7669–7681. doi: 10.1074/jbc.RA118.005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K., Mehrpouya-Bahrami P., Nagarkatti P.S., Nagarkatti M. Cannabinoid receptor 1 blockade attenuates obesity and adipose tissue type 1 inflammation through miR-30e-5p regulation of delta-like-4 in macrophages and consequently downregulation of Th1 cells. Front. Immunol. 2019;10:1049. doi: 10.3389/fimmu.2019.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K., Yang X., Bam M., Murphy E.A., Nagarkatti P.S., Nagarkatti M. MicroRNA-30 modulates metabolic inflammation by regulating Notch signaling in adipose tissue macrophages. Int. J. Obes. 2018;42:1140–1150. doi: 10.1038/s41366-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A., Alghetaa H., Sultan M., Singh N.P., Nagarkatti P., Nagarkatti M. Administration of delta9-tetrahydrocannabinol (THC) post-staphylococcal enterotoxin B exposure protects mice from acute respiratory distress syndrome and toxicity. Front. Pharmacol. 2020;11:893. doi: 10.3389/fphar.2020.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morari J., Anhe G.F., Nascimento L.F., de Moura R.F., Razolli D., Solon C., Guadagnini D., Souza G., Mattos A.H., Tobar N., et al. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes. 2014;63:3770–3784. doi: 10.2337/db13-1495. [DOI] [PubMed] [Google Scholar]
- Muccioli G.G., Naslain D., Bäckhed F., Reigstad C.S., Lambert D.M., Delzenne N.M., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Koh V., Kua L.F., So J., Davide L., Lim K.S., Petersen S.H., Yong W.P., Shabbir A., Kono K. Accumulation of CD11c+CD163+ adipose tissue macrophages through upregulation of intracellular 11beta-HSD1 in human obesity. J. Immunol. 2016;197:3735–3745. doi: 10.4049/jimmunol.1600895. [DOI] [PubMed] [Google Scholar]
- Quarta C., Bellocchio L., Mancini G., Mazza R., Cervino C., Braulke L.J., Fekete C., Latorre R., Nanni C., Bucci M., et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11:273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C., Arnone M., Delgorge C., Gonalons N., Keane P., Maffrand J.P., Soubrie P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- Ravinet Trillou C., Delgorge C., Menet C., Arnone M., Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. Relat. Metab. Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- Robinson R.H., Meissler J.J., Fan X., Yu D., Adler M.W., Eisenstein T.K. A CB2-selective cannabinoid suppresses T-cell activities and increases Tregs and IL-10. J. Neuroimmune Pharmacol. 2015;10:318–332. doi: 10.1007/s11481-015-9611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Bellini G., Luongo L., Manzo I., Tolone S., Tortora C., Bernardo M.E., Grandone A., Conforti A., Docimo L., et al. Cannabinoid receptor 2 as antiobesity target: inflammation, fat storage, and browning modulation. J. Clin. Endocrinol. Metab. 2016;101:3469–3478. doi: 10.1210/jc.2015-4381. [DOI] [PubMed] [Google Scholar]
- Ruiz de Azua I., Mancini G., Srivastava R.K., Rey A.A., Cardinal P., Tedesco L., Zingaretti C.M., Sassmann A., Quarta C., Schwitter C., et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Invest. 2017;127:4148–4162. doi: 10.1172/JCI83626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz K., Mangels N., Häussler A., Ferreirós N., Fleming I., Tegeder I. Pro-inflammatory obesity in aged cannabinoid-2 receptor-deficient mice. Int. J. Obes. 2016;40:366–379. doi: 10.1038/ijo.2015.169. [DOI] [PubMed] [Google Scholar]
- Sido J.M., Jackson A.R., Nagarkatti P.S., Nagarkatti M. Marijuana-derived Delta-9-tetrahydrocannabinol suppresses Th1/Th17 cell-mediated delayed-type hypersensitivity through microRNA regulation. J. Mol. Med. 2016;94:1039–1051. doi: 10.1007/s00109-016-1404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sido J.M., Nagarkatti P.S., Nagarkatti M. Role of endocannabinoid activation of peripheral CB1 receptors in the regulation of autoimmune disease. Int. Rev. Immunol. 2015;34:403–414. doi: 10.3109/08830185.2014.921165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U.P., Singh N.P., Singh B., Price R.L., Nagarkatti M., Nagarkatti P.S. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol. Appl. Pharmacol. 2012;258:256–267. doi: 10.1016/j.taap.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Li W., Yang L., Chang N., Fan X., Ji X., Xie J., Yang L., Li L. Cannabinoid receptor 1 participates in liver inflammation by promoting M1 macrophage polarization via RhoA/NF-kappaB p65 and ERK1/2 pathways, respectively, in mouse liver fibrogenesis. Front. Immunol. 2017;8:1214. doi: 10.3389/fimmu.2017.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar S., Zumbrun E.E., Nagarkatti M., Nagarkatti P.S. Protective role of cannabinoid receptor 2 activation in galactosamine/lipopolysaccharide-induced acute liver failure through regulation of macrophage polarization and microRNAs. J. Pharmacol. Exp. Ther. 2015;353:369–379. doi: 10.1124/jpet.114.220368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte C., Blanchet M.R., Laviolette M., Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016;73:4449–4470. doi: 10.1007/s00018-016-2300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Valdearcos M., Douglass J.D., Robblee M.M., Dorfman M.D., Stifler D.R., Bennett M.L., Gerritse I., Fasnacht R., Barres B.A., Thaler J.P., Koliwad S.K. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017;26:185–197.e3. doi: 10.1016/j.cmet.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty A.N.A., Stefanidis A., McAinch A.J., Hryciw D.H., Oldfield B. Anti-obesity effect of the CB2 receptor agonist JWH-015 in diet-induced obese mice. PLoS One. 2015;10:e0140592. doi: 10.1371/journal.pone.0140592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.F., Sontag S.J., Schnell T., Leya J. Non-cardiac chest pain: the long-term natural history and comparison with gastroesophageal reflux disease. Am. J. Gastroenterol. 2009;104:2145–2152. doi: 10.1038/ajg.2009.279. [DOI] [PubMed] [Google Scholar]
- Wu Q., Ma Y., Liu Y., Wang N., Zhao X., Wen D. CB2R agonist JWH-133 attenuates chronic inflammation by restraining M1 macrophage polarization via Nrf2/HO-1 pathway in diet-induced obese mice. Life Sci. 2020;260:118424. doi: 10.1016/j.lfs.2020.118424. [DOI] [PubMed] [Google Scholar]
- Zhao L. The gut microbiota and obesity: from correlation to causality. Nat. Rev. Microbiol. 2013;11:639–647. doi: 10.1038/nrmicro3089. [DOI] [PubMed] [Google Scholar]
- Zimmer A., Zimmer A.M., Hohmann A.G., Herkenham M., Bonner T.I. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.