Graphical abstract
Keywords: Red-G dye, Alpaca wool, Hydrodynamic cavitation, Advanced oxidation processes
Abstract
Red-G dye is one of the main dyes used in the textile industry to dye alpaca wool. Therefore, considering the large volume of processed wool in Perú, the development of efficient technologies for its removal is a present scientific issue. In this study, an integrated system based on hydrodynamic cavitation (HC) and photo-Fenton process was evaluated to remove the Red-G dye. Using a hybrid cavitation device (venturi + orifice plate), the effect of pH was evaluated, achieving 21 % of removal at pH 2 which was more than 80 % higher compared to pH 4 and 6. The effect of temperature was also evaluated in HC-system at pH 2, where percentage of dye degradation increased at lower temperatures (around 20 °C). Then, 50.7 % of dye was removed under optimized condition of HC-assisted Fenton process (FeSO4:H2O2 of 1:30), that value was improved strongly by UV-light incorporation in the HC-system, increasing to 99 % removal efficiency with respect to HC-assisted Fenton process and reducing the time to 15 min. Finally, the developed cavitation device in combination with photo-Fenton process removed efficiently the dye and thus could be considered an interesting option for application to real wastewater.
1. Introduction
Textile sector is one of the heaviest consumers of water and chemicals. Consequently, it discharges an immoderate polluted effluent [1]. There is a significant challenge in effluent treatment from textile industry since, it has a complex composition of chemicals and dyes used in the diverse stages of textile process, producing effluents with elevated load of contaminants which are difficult to remove [1], [2]. Textile wastewaters are mostly toxic, they could contain carcinogenic and mutagenic compounds and have low biodegradability rate, therefore, it is imperative to remove contaminants from these effluents before release them. In addition, the dissolved solids present in the wastewater cause an increase in the concentration of soluble salts, therefore, the water cannot be recycled industrially and much less reused as water for irrigation or domestic use. Additionally, textile wastewater also contains heavy metals that are toxic for aquatic biodiversity and for humans as well [2]. The physicochemical characteristics and composition of the textile wastewater depend on different factors such as the stage of the process from which it was released, the country, the machinery used for its management, and the season due to demands of the market for certain types of clothing for each period of the year [3].
In the world, there are more than 100 thousand commercial dyes and approximately 15 % of the dye annual production is discharged into body waters without an appropriated treatment harming the environment and society health. It is understood that textile industry is still an important economic support for some developing countries, where supply of fresh water is an important issue; therefore, it is imperative to treat textile wastewater in order to preserve unpolluted water resources which are priceless for economic and social development [4].
In Perú, the population of alpacas is 3 million 590 thousand according to the CENAGRO statistics in 2012, they are produced in places of high altitude where agriculture production is very difficult and the level of poverty is high. Due to its incredible features such as softness and luxurious, the demand for clothing of high-quality alpaca wool has been increasing which is beneficial for those poor regions [5].
Several treatment methods have been reported for removing pollutants from effluents, such as advanced oxidation processes (AOPs), which correspond to the physical and chemical mechanisms where free radicals with high oxidative capacity are produced; for that reason, they are an attractive alternative to treat industrial effluents loaded with organic contaminants [6]. AOPs include processes such as Fenton oxidation, hydrogen peroxide (H2O2), ozonation (O3), photocatalysis, ultraviolet (UV), and cavitation [7], [8].
Other AOPs like O3 has a strong oxidation potential because it is broken in oxygen atoms before it is dissolved in the wastewater behaving as an oxidant agent, thus it is better to introduce the O3 flux in the system cavitation where occurs the high rate of mass transference or turbulence [9]. Utilization of O3 mixed with an HC system can act synergically, promoting intensification of pollutants degradation requiring less O3 which is reflected in reduction of operation and energy costs. It is important to mention that if elevated quantities of O3 is used, it could damage the environment and the human health [10].
Hydrodynamic cavitation (CH) corresponds to the formation, growth and violent collapse of micro-nano bubbles generated by devices such as an orifice plate and venturi tubes. The violent collapse of cavities results in the release of large amounts of energy, hot spots (temperature = 1000–15,000 K; pressures = 500–5000 bar), intense turbulence along with liquid circulation currents, and oxidant radicals, which synergically can degrade recalcitrant contaminants or break the organic matter of any type of effluent [10]. The production of the cavitation phenomenon is generated in a reactor, operating with different devices such as plates with one or more holes, also, a venturi tube can be used while its convergent and divergent section making the pressure drop in the fluid, as well, as advanced rotational HC reactors with different configurations, and vortex diode-based cavitation devices of diverse designs. Likewise, ultrasonic devices can be employed [6].
In the present study, a cavitation device composed by an orifice plate with quadratic configuration and a venturi-type section is designed to apply it into a cavitation system for removing Red-G dye used in alpaca wool dyeing, enhancing other AOPs such as Fenton and UV.
2. Material and methods
2.1. Chemicals
The Red G dye (C32H21CrN10O11S·2Na, CAS No: 70209-87-9) was supplied by a local textile company in Arequipa Perú. Hydrogen peroxide at 35 %, Iron (II) sulfate, Sulfuric acid at 95–97 % and Sodium hydroxide were obtained from Merck & Co., Inc and used without further treatment. The aqueous solutions were prepared using distilled water. In the Fig. 1 is observed the chemical structure of Red-G dye.
Fig. 1.

Chemical structure of Red-G dye according to IUPAC.
2.2. Hydrodynamic cavitation (HC) system
The hydrodynamic cavitation system for Red-G dye removal was constructed in stainless steel (Fig. 2). As observed in Fig. 2A, the system has a centrifugal pump (1.4 CV) to force the passage of solution through the orifice plate, generating cavitation. The cavitation device is composed of an orifice plate with quadratic configuration (Fig. 2B) and a venturi-type section (Fig. 2C). The selection of the dimension of the device, mainly the orifice plate, was selected by computational fluid dynamics (CFD) analysis, which resulted in the highest fraction of steam generated at 4 bar of inlet pressure in the cavitation device (data not shown).
Fig. 2.
Hydrodynamic cavitation system. A) schematic representation of HC-system composed by electric panel (1), UV-light generator (2), pump (3), UV-light chamber AQUA system (4), Cavitation device (5), manometer (6), valvule (7), recirculation tank (8); B) orifice plate with quadratic hole; C) cavitation device, dimensions are in millimeter.
2.3. Red-G dye removal under different pH and temperatures
The Red-G dye removal by hydrodynamic cavitation was evaluated. Firstly, the effect of pH (2, 4, 6, 8) was evaluated, using 5 L of solution containing 30 ppm of dye, the pH of the solutions was adjusted using sulfuric acid and sodium hydroxide, both 2 M. Experiments were performed at 4 bar of inlet pressure and 30 °C for 30 min. In the second step, the effect of temperature on the Red-G removal was evaluated at 20, 30, 40 and 50 °C, in same condition of dye concentration, pressure and time described in the first step. During the process, samples were obtained for respective analysis by spectrophotometry at 494 nm, and the respective value was converted to concentration using a standard curve.
2.4. Optimization of Red-G dye removal using experimental design
Experiments were carried out using a Box-Behnken experimental design in order to evaluate the effects of variables such as concentration of hydrogen peroxide (100–240 ppm), Iron (II) sulfate (2.5–7.5 ppm) and dye (20–50 ppm) in the hydrodynamic cavitation process, considering as response variable the Red-G dye removal (%). The studied variables were selected based on the previously evaluated condition for other types of dyes [8], [9]. All experiments were carried out using 5 L of solution at pH 2, the inlet pressure in the cavitation device was 4.5 bar and the temperature 25 ± 3 °C which was controlled by recirculating cold water through a stainless-steel coil. The process was performed for 30 min, and samples were collected periodically to analyze the dye concentration by spectrophotometry.
Design-Expert software 12.0 (stat-Ease, Inc., USA) was used to compose and evaluate an empirical model to describe the response variable (Red-G dye removal) as a function of H2O2, FeSO4 and Red-G dye concentrations. Process optimization was carried out using the numerical optimization feature of the software, based on desirability function. The maximization of Red-G dye removal (%) was considered as a goal for optimization process.
2.5. Red-G dye removal under UV-light
The effect of UV-light incidence on the Red-G dye degradation was evaluated aiming to improve the degradation of Red-G dye in HC-assisted Fenton process. An AQUA system was used as UV-light generator (0.70 A max. operating S410RL-HO 45 W, lamp at 100 V), coupled to an HC-system as shown in Fig. 2. The experiment was performed during 15 min at inlet pressure of 4.5 bar, 25 °C of temperature, and Fenton process condition previously optimized. Samples were obtained periodically for respective dye analysis.
Finally, the effect of concentration of dye was evaluated at 20, 35 and 50 ppm under stablished HC-assisted Fenton + UV method. The obtained results were fit to first order kinetic aiming to evaluate the degradation velocity at 25 °C. Besides, spectrophotometer analysis of dyes, HPLC was used aiming to monitoring the degraded compounds produced in the process. A RP18 (124x4 mm, 5 µm) column at wavelength of 494 nm and 30 °C oven temperature was used. The mobile phase was methanol: water (phosphoric acid 0.1 M/ sodium hexasulphonate 0.05 M 30/70) at 1.5 mL/min, and 30 µL of sample was injected.
3. Results and discussion
3.1. Effect of pH and temperature on Red-G dye degradation in HC-process
The effect of pH on Red-G dye removal was evaluated and results are shown in Fig. 3. As observed in Fig. 3A, percentage of dye degradation at pH 4 and 6 are similar, however, at pH 2, is 84 % higher; moreover, after 30 min, 22 % of degradation was achieved respect to initial concentration. Therefore, hydrodynamic cavitation in acidic conditions favors the generation of •OH radical also offering higher oxidation potential and the lower rate of recombination of •OH radicals, with a significant degradation of Red-G dye, which can be associated to the oxidation of the dye by the hydroxyl radical and shockwaves generated during the violent collapse of microbubbles [11], [12]. The cavitation device developed achieved unidimensional cavitation number (Cv) of 0.11, value between 0.05 and 0.15 considered as optimum for several applications [13]. Moreover, the acid condition stimulates a molecular state of dye increasing the hydrophobicity, thus, they tend to stay at the liquid–gas interface where the hydroxyl radicals are present in much higher concentrations so as to facilitate direct attack and bring out the necessary changes [14]. The effect of acid condition on dyes degradation under HC-process was previously reported, indeed, for other dyes, e.g., dye-methylene blue (MB) at pH 2 under HC combined with Na2S2O8 or O3 [15].
Fig. 3.
Influence of pH (A) and temperature (B) on Red-G dye removal in HC-system.
The effect of temperature was also evaluated in HC-system at pH 2. As shown in Fig. 3B, percentage of dye degradation increased at lower temperatures. For Red-G dye, rise in temperature affects solution properties like increment in the vapor pressure, resulting in more cavitation bubbles which accelerate dye degradation; however, further increase in temperature reduces pressure loss caused by wall friction since higher temperatures reduce solution viscosity, which means lower intensity of collapsing cavities causing a decrease in degradation percentage [16]. Therefore, probably the activity is higher when the dye is more insoluble. For that reason, in anionic dyes, the activity is higher at pH 2 where the compound is in the zwitterionic form. In this way, increasing the temperature, the solubility of Red G increase, decreasing thus the degradation. Finally, low temperatures (around 20 °C) were also reported as efficient for degradation of methyl orange (MO) [17] and brilliant cresyl blue [18]. Therefore, aiming to develop the process near to ambient conditions, 25 °C was selected for subsequent steps.
3.2. Hydrodynamic cavitation treatment and optimization
The influence of important variables on Red-G dye degradation using hydrodynamic cavitation-assisted Fenton process was evaluated and the results are presented in Table 1. As expected, the higher dye removal was observed at high concentration of Fe (II) and H2O2 with low dye concentration, in that condition 47 % of dye was removed (run 4). Fenton reagent is extensively used for degradation of various compounds like dyes, pharmaceutical compounds and wastewater, due to effective generation of hydroxyl radicals (•OH) which is dissociated from H2O2 molecules by reaction with ferrous ions (Fe2+) [19]. Moreover, in HC •OH and •H radicals are generated from water; in addition, they can react with oxygen dissolved in water to produce •O2−, which can also contribute to Red-G dye degradation. The lowest dye degradation achieved could be associated to low concentration of Fenton reagents used, since higher concentrations were used in other studies, e.g., for real industrial wastewater (pH of effluent: 2; H2O2:4.8 g/L; H2O2/Fe2+: 3) [20].
Table 1.
Red-G dye removal in hydrodynamic cavitation-assisted Fenton process after 30 min of treatment.
| Run | Concentration (ppm) |
Dye removal (%) | ||
|---|---|---|---|---|
| Hydrogen peroxide | Iron (II) sulfate | Red-G dye | ||
| 1 | 240 | 7.5 | 35 | 43 |
| 2 | 170 | 2.5 | 20 | 25 |
| 3 | 170 | 5.0 | 35 | 30 |
| 4 | 170 | 7.5 | 20 | 47 |
| 5 | 100 | 5.0 | 20 | 28 |
| 6 | 170 | 2.5 | 50 | 41 |
| 7 | 100 | 5.0 | 50 | 34 |
| 8 | 240 | 5.0 | 20 | 43 |
| 9 | 170 | 7.5 | 50 | 31 |
| 10 | 170 | 5.0 | 35 | 32 |
| 11 | 240 | 5.0 | 50 | 41 |
| 12 | 240 | 2.5 | 35 | 32 |
| 13 | 100 | 7.5 | 35 | 32 |
| 14 | 100 | 2.5 | 35 | 28 |
| 15 | 170 | 5.0 | 35 | 30 |
A quadratic empirical model (Eq. (1)) was composed and an ANOVA test was performed, with the results presented in Table 2. As shown in the table, the model (Eq. (1)) has quite high R-squared value of 0.96 and was significant at 95 % of confidence level. It was also confirmed by p-value of the model (<0.05), F-value (24.03) and non-significant lack of Fit test (p-value > 0.05) at confidence level of 95 %. The most influential variables for Red-G dye removal in a hydrodynamic cavitation-assisted Fenton process correspond to H2O2 and FeSO4.
Table 2.
ANOVA for quadratic model composed for Red-G dye removal using HC-assisted Fenton process.
| Source | Sum of squares | df | Mean square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 629.49 | 7 | 89.93 | 24.03 | 0.0002* |
| A- H2O2 | 183.27 | 1 | 183.27 | 48.98 | 0.0002* |
| B- FeSO4 | 87.52 | 1 | 87.52 | 23.39 | 0.0019* |
| C- Red-G | 1.34 | 1 | 1.34 | 0.3572 | 0.5689 |
| AB | 12.96 | 1 | 12.96 | 3.46 | 0.105 |
| AC | 21.86 | 1 | 21.86 | 5.84 | 0.0463* |
| BC | 267.65 | 1 | 267.65 | 71.53 | < 0.0001* |
| C2 | 54.9 | 1 | 54.9 | 14.67 | 0.0065* |
| Residual | 26.19 | 7 | 3.74 | ||
| Lack of Fit | 24.87 | 5 | 4.97 | 7.49 | 0.1219 |
| Pure Error | 1.33 | 2 | 0.6637 | ||
| Cor Total | 655.68 | 14 |
*Significant at 95% of confidential level.
The equation models the behavior of the experimental design factors predicting Red-G removal percentage for given levels. As observed, H2O2 and FeSO4 concentrations are the independent variables which have more impact by comparing their coefficients.
| (1) |
Where: Y is the response variable “Red-G dye removal”. Moreover, X1, X2 and X3 correspond to actual values of H2O2, FeSO4 and Red-G dye concentration, respectively.
The interaction of two variables is shown in contour plots (Fig. 4A, B, C). As observed in Fig. 4A, dye removal is more than 40 % as H2O2 and FeSO4 concentrations are increased, driving to enhance dye removal when dye concentration is reduced, and H2O2 concentration is high (Fig. 4B). The same phenomenon is observed in Fig. 4C where H2O2 concentration is increased while dye concentration is low improving the dye removal rate to more than 45 % in 30 min.
Fig. 4.
Contour plots (A, C, E) for Red-G dye removal in Fenton process-assisted by hydrodynamic cavitation. A) Interaction of variables A and B. B) Interaction of variables A and C. C) Interaction of variables: B and C. In each case, the third variable was kept at its center point value.
The variables were optimized aiming to maximize the Red-G dye removal. A validation experiment was also performed at the optimized conditions, which were H2O2 (227.2 ppm), FeSO4 (6.9 ppm), dye concentration (20.1 ppm). In this case, dye removal 50.73 ± 4.36 % (average ± 95 % confidence level interval) was predicted by the quadratic model, which was confirmed experimentally, with a value of 51.21 ± 4.85 % (average ± standard deviation). In the optimized condition, additional experiment aiming to evaluate the time for complete degradation of Red-G dye was carried out, achieving 77, 93 and 99 % in 60, 90 and 115 min, respectively. The removal was higher than 30 % of discoloration reported in HC-process at 5 bar in 120 min or higher than 90 % achieved in HC + H2O2 (244 ppm) [21]. Similar result was also reported for acid violet 7 dye, achieving more than 90 % of dye in 120 min under optimized HC-assisted Fenton process (pH of 3, Inlet pressure of 4 bar, initial dye concentration of 20 ppm, 10 ppm loading of Fe2+ and 50 ppm of H2O2 loading) [22].
3.3. Effect of UV-light under Photo-Fenton process-assisted by HC
The effect of UV-light incidence on degradation of Red-G dye under optimized HC-assisted Photo-Fenton were evaluated and results are shown in Fig. 5. As observed, the incorporation of UV-light in the process improves greatly the dye removal in short time, achieving 58, 81 and 86 % more than HC+ Fenton process, HC + UV and HC alone, respectively. The enhanced degradation of dye in this study can be attributed to the increase in the generation of •OH radicals through photolysis of H2O2 and reduction of Fe3+ ions under UV light [23]. Moreover, the amount required of H2O2 in photo-Fenton is the half that in Fenton alone, once the light irradiation produce the reduction of Fe (III) to Fe (II), in the normal Fenton, this process is carried out using one mol of H2O2. The obtained result is in agreement with previously reported for mixed dye (Methylene blue, Methyl orange and Rhodamine-B) removal, achieving 98 % of decolorization in 20 min using HC+ photo-Fenton (1:30 M ratio of FeSO4:H2O2) [24]. In addition, the synergy effect of HC with photolytic process was previously reported for mixed dye [24] and diclofenac sodium [25].
Fig. 5.
Comparison of Red-G dye removal percentage by different hydrodynamic cavitation-assisted advanced oxidation processes.
Finally, the developed process has the potential to application at large scale for dyes and textile wastewater treatment once the cavitation technology has been evaluated by other authors at pilot-scale, e.g., for decolorization of industrial inks and printing ink wastewater [26], bisphenols and other contaminants of emerging concern [27]. Moreover, Cavimax (https://www.cavimax.co.uk/), E-PIC S.R.L (https://www.epic-srl.com/), Arisdyne system® (https://www.arisdyne.com/) and others companies around the world are developing cavitation system for several applications at pilot and industrial scale, maybe one of the most relevant characteristics is the energy efficiency, simplicity to construction and the low cost.
3.4. Effect of Red-G dye concentration on the kinetic of the reaction
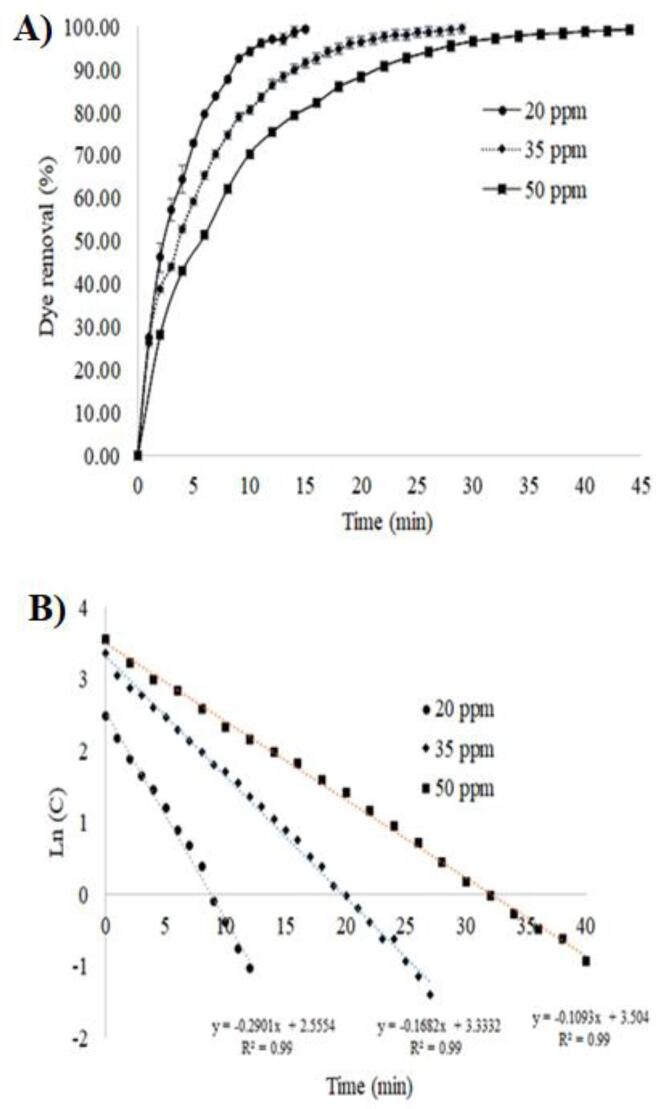
The effect of Red-G dye concentration under HC-assisted photo-Fenton process on dye degradation was evaluated and the results are presented in Fig. 6A. It has been observed that the extent of degradation of Red-G dye is inversely proportional to initial concentration; thus, increasing the concentration from 20 to 35 and 50 ppm around 10 % and 20 % was decreased, respectively. At high concentration of dye, the consumption of •OH radicals will be more, hence the degradation would be also high but in terms of percentage may be low due to higher loading of concentration. Same trend was reported increasing the concentration of compounds, e.g., rhodamine-6G [28], acid Red-18 [29], imidacloprid [30] and methyl parathion [31].
Fig. 6.
A) Comparison of Red-G dye removal percentage and B) Kinetics of Red-G dye degradation considering three different dye concentrations.
The first-order kinetic was considered for the correlation and a rate constant was calculated for each of initial Red-G dye concentration, the Eq. (2) is used for this kinetic. The plotted graph confirmed that the degradation of Red-G dye in HC-process is first-order kinetics (Fig. 6B). Increasing the initial concentration from 20 to 35 and 50 ppm rate constant were decreased from 2.9 × 10−1 to 1.6 × 10−1 and 1.1 × 10−1 min−1, respectively.
| (2) |
Finally, HPLC analysis was performed aiming to evaluate the degradation of Red-G dye in other small compounds (Fig. 7). Reduction in peak height was detected; moreover, HPLC elution profile of pure Red-G dye showed retention time at 28 min. As observed in the Figure, the peak is observed only in sample obtained at 5 min of the process; at 10 and 15 min that peak is not observed, which could be due to low concentration. The oxidation produces shorter hydrophilic compounds which are attributed to the peaks at shorter times. Finally, to find out the exact identity of the broken-down compounds is necessary to carried out GC–MS analysis.
Fig. 7.
HPLC analysis of Red-G dye at different times of HC-assisted photo-Fenton process.
4. Conclusion
Hydrodynamic cavitation-based process has been evaluated for dye removal. The designed device was successfully used for degradation of Red-G, which was strongly improved in combination with photo-Fenton process, reducing significantly the time to 15 min. Therefore, the developed process could be considered as potential method for real wastewater generated in alpaca wool processing industry. But, aiming to determinate the degradation products will be necessary HPLC-MS, once that using only HPLC was not possible to identify.
CRediT authorship contribution statement
Miguel A.D. Flores Alarcón: Visualization, Investigation, Data curation. Rafaela Y. Arenas Jarro: Visualization, Investigation. Muhammad Ajaz Ahmed: Data curation, Writing – original draft. Kiara A. García Bustos: Investigation, Validation. David A. Pacheco Tanaka: Methodology, Writing – review & editing. Ruly Terán Hilares: Conceptualization, Methodology, Software, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the Universidad Católica de Santa María (UCSM)—Grant N° 28048-R-2021.
Data availability
Data will be made available on request.
References
- 1.Sahu O., Singh N. The Impact and Prospects of Green Chemistry for Textile Technology. Woodhead Publishing; 2019. Significance of bioadsorption process on textile industry wastewater; pp. 367–416. [DOI] [Google Scholar]
- 2.P. Senthil Kumar, A. Saravanan, Sustainable wastewater treatments in textile sector, in: Sustainable Fibres and Textiles, Woodhead Publishing, 2017: pp. 323–346. https://doi.org/10.1016/B978-0-08-102041-8.00011-1.
- 3.Yaseen D.A., Scholz M. Textile dye wastewater characteristics and constituents of synthetic effluents: a critical review. Int. J. Environ. Sci. Technol. 2019;16(2):1193–1226. [Google Scholar]
- 4.Qin J., Sun H., Zhang S., Yi L., Ruan Y., Wang S., Zhang Z., Wang J., Fang D. Investigation on the by-pass line orifice plate assisted hydrodynamic cavitation (B-PLOPA HC) degradation of basic fuchsin (BF) in wastewater. Sep. Purif. Technol. 2022;287 doi: 10.1016/j.seppur.2022.120501. [DOI] [Google Scholar]
- 5.Machaca V.M., Pizarro F.C., Ocsa V.P., Bustinza Choque V.A., Quispe Coaquira J.E., Machaca R.M., Cano Fuentes V.R., Arias Huamani K.D. Technological properties of the fibre of white Huacaya alpacas (Vicugna pacos) in the Community of Chapimarca, Apurimac - Peru. Revista de Investigaciones Veterinarias Del Peru. 2021;32 doi: 10.15381/RIVEP.V32I4.20928. [DOI] [Google Scholar]
- 6.Agarkoti C., Thanekar P.D., Gogate P.R. Cavitation based treatment of industrial wastewater: a critical review focusing on mechanisms, design aspects, operating conditions and application to real effluents. J. Environ. Manage. 2021;300 doi: 10.1016/J.JENVMAN.2021.113786. [DOI] [PubMed] [Google Scholar]
- 7.Dixit D., Thanekar P., Bhandari V.M. Degradation of API pollutants using hydrodynamic cavitation and process intensification. Chem. Eng. Process. - Process Intensification. 2022;172:108799. [Google Scholar]
- 8.Bethi B., Sonawane S.H., Bhanvase B.A., Sonawane S.S. Textile industry wastewater treatment by cavitation combined with fenton and ceramic nanofiltration membrane. Chem. Eng. Process. - Process Intensification. 2021;168 doi: 10.1016/J.CEP.2021.108540. [DOI] [Google Scholar]
- 9.Wang J., Wang J., Yuan R., Liu J., Yin Z., He T., Wang M., Ma F., Zhou B., Chen H. Degradation of acid red 73 wastewater by hydrodynamic cavitation combined with ozone and its mechanism. Environ. Res. 2022;210 doi: 10.1016/J.ENVRES.2022.112954. [DOI] [PubMed] [Google Scholar]
- 10.Wang B., Su H., Zhang B. Hydrodynamic cavitation as a promising route for wastewater treatment – A review. Chem. Eng. J. 2021;412 doi: 10.1016/J.CEJ.2021.128685. [DOI] [Google Scholar]
- 11.Dular M., Griessler-Bulc T., Gutierrez-Aguirre I., Heath E., Kosjek T., Krivograd Klemenčič A., Oder M., Petkovšek M., Rački N., Ravnikar M., Šarc A., Širok B., Zupanc M., Žitnik M., Kompare B. Use of hydrodynamic cavitation in (waste)water treatment. Ultrason. Sonochem. 2016;29:577–588. doi: 10.1016/J.ULTSONCH.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Thanekar P., Gogate P. Application of hydrodynamic cavitation reactors for treatment of wastewater containing organic pollutants: intensification using hybrid approaches. Fluids. 2018;3:98. doi: 10.3390/FLUIDS3040098. [DOI] [Google Scholar]
- 13.Bhat A.P., Gogate P.R. Cavitation-based pre-treatment of wastewater and waste sludge for improvement in the performance of biological processes: a review. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/J.JECE.2020.104743. [DOI] [Google Scholar]
- 14.Das S., Bhat A.P., Gogate P.R. Degradation of dyes using hydrodynamic cavitation: process overview and cost estimation. J. Water Process Eng. 2021;42 doi: 10.1016/J.JWPE.2021.102126. [DOI] [Google Scholar]
- 15.Wang B., Wang T., Su H. A dye-methylene blue (MB)-degraded by hydrodynamic cavitation (HC) and combined with other oxidants. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/J.JECE.2022.107877. [DOI] [Google Scholar]
- 16.Malade L.V., Deshannavar U.B. Decolorisation of reactive red 120 by hydrodynamic cavitation. Mater. Today: Proc. 2018;5(9):18400–18409. [Google Scholar]
- 17.Innocenzi V., Prisciandaro M., Centofanti M., Vegliò F. Comparison of performances of hydrodynamic cavitation in combined treatments based on hybrid induced advanced Fenton process for degradation of azo-dyes. J. Environ. Chem. Eng. 2019;7 doi: 10.1016/J.JECE.2019.103171. [DOI] [Google Scholar]
- 18.Cako E., Gunasekaran K.D., Cheshmeh Soltani R.D., Boczkaj G. Ultrafast degradation of brilliant cresyl blue under hydrodynamic cavitation based advanced oxidation processes (AOPs) Water Resour. Ind. 2020;24 doi: 10.1016/J.WRI.2020.100134. [DOI] [Google Scholar]
- 19.Qin J., Ruan Y., Yi L., Sun H., Qi Q., Zhao L., Xiong Y., Wang J., Fang D. Tetracycline (TC) degradation via hydrodynamic cavitation (HC) combined Fenton’s reagent: optimizing geometric and operation parameters. Chem. Eng. Process. - Process Intensification. 2022;172 doi: 10.1016/J.CEP.2022.108801. [DOI] [Google Scholar]
- 20.Gujar S.K., Gogate P.R., Kanthale P., Pandey R., Thakre S., Agrawal M. Combined oxidation processes based on ultrasound, hydrodynamic cavitation and chemical oxidants for treatment of real industrial wastewater from cellulosic fiber manufacturing sector. Sep. Purif. Technol. 2021;257 doi: 10.1016/J.SEPPUR.2020.117888. [DOI] [Google Scholar]
- 21.Gogate P.R., Bhosale G.S. Comparison of effectiveness of acoustic and hydrodynamic cavitation in combined treatment schemes for degradation of dye wastewaters. Chem. Eng. Process.: Process Intensification. 2013;71:59–69. doi: 10.1016/J.CEP.2013.03.001. [DOI] [Google Scholar]
- 22.N.j. L., Gogate P.R., Pandit A.B. Treatment of acid violet 7 dye containing effluent using the hybrid approach based on hydrodynamic cavitation. Process Safety Environ. Protection. 2021;153:178–191. [Google Scholar]
- 23.Peternel I.T., Koprivanac N., Božić A.M.L., Kušić H.M. Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J. Hazard. Mater. 2007;148:477–484. doi: 10.1016/J.JHAZMAT.2007.02.072. [DOI] [PubMed] [Google Scholar]
- 24.Kumar M.S., Sonawane S.H., Bhanvase B.A., Bethi B. Treatment of ternary dye wastewater by hydrodynamic cavitation combined with other advanced oxidation processes (AOP’s) J. Water Process Eng. 2018;23:250–256. doi: 10.1016/J.JWPE.2018.04.004. [DOI] [Google Scholar]
- 25.Salehi M., Hashemipour H., Mirzaee M. Experimental study of influencing factors and kinetics in catalytic removal of methylene blue with TiO2 nanopowder. Am. J. Environ. Eng. 2012;2:1–7. doi: 10.5923/J.AJEE.20120201.01. [DOI] [Google Scholar]
- 26.Zampeta C., Bertaki K., Triantaphyllidou I.E., Frontistis Z., Koutsoukos P.G., Vayenas D.V. Pilot-scale hybrid system combining hydrodynamic cavitation and sedimentation for the decolorization of industrial inks and printing ink wastewater. J. Environ. Manage. 2022;302 doi: 10.1016/J.JENVMAN.2021.114108. [DOI] [PubMed] [Google Scholar]
- 27.Kovačič A., Škufca D., Zupanc M., Gostiša J., Bizjan B., Krištofelc N., Dolenc M.S., Heath E. The removal of bisphenols and other contaminants of emerging concern by hydrodynamic cavitation: From lab-scale to pilot-scale. Sci. Total Environ. 2020;743 doi: 10.1016/J.SCITOTENV.2020.140724. [DOI] [PubMed] [Google Scholar]
- 28.Rajoriya S., Bargole S., Saharan V.K. Degradation of a cationic dye (Rhodamine 6G) using hydrodynamic cavitation coupled with other oxidative agents: reaction mechanism and pathway. Ultrason. Sonochem. 2017;34:183–194. doi: 10.1016/J.ULTSONCH.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Dhanke P.B., Wagh S.M. Intensification of the degradation of Acid RED-18 using hydrodynamic cavitation. Emerg. Contaminants. 2020;6:20–32. doi: 10.1016/J.EMCON.2019.12.001. [DOI] [Google Scholar]
- 30.Patil P.N., Bote S.D., Gogate P.R. Degradation of imidacloprid using combined advanced oxidation processes based on hydrodynamic cavitation. Ultrason. Sonochem. 2014;21:1770–1777. doi: 10.1016/J.ULTSONCH.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 31.Patil P.N., Gogate P.R. Degradation of methyl parathion using hydrodynamic cavitation: effect of operating parameters and intensification using additives. Sep. Purif. Technol. 2012;95:172–179. doi: 10.1016/J.SEPPUR.2012.04.019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.