Abstract
SprE regulates ςS levels in response to nutrient availability by promoting ClpXP-mediated degradation. Paradoxically, we observe that SprE is similarly regulated, accumulating preferentially upon starvation. This regulation of SprE levels is ςS dependent, altering SprE synthesis at the level of translation. Thus, we demonstrate that SprE and ςS function within a regulatory feedback loop.
The Escherichia coli starvation response is largely dependent on the activity of the alternative primary sigma factor ςS, encoded by the rpoS gene (5, 6, 9). Since the physiological adaptations of E. coli growing under starvation conditions are quite dramatic and require a major shift in gene expression (7), the commitment to initiate the starvation response is tightly regulated. Under conditions of nutrient sufficiency, ςS is rapidly degraded by the ClpXP protease (13, 17). However, once nutrients become limiting for growth, degradation ceases and there is a dramatic increase in ςS levels. This regulation of ςS stability in response to nutrient availability is dependent on the two-component response regulator SprE, also termed RssB, which promotes ClpXP-mediated degradation of ςS (10, 12). SprE specifically promotes ςS degradation without influencing the degradation of other ClpXP substrates (18). More recently, SprE has been shown to physically bind ςS in vitro (1), and through this interaction SprE promotes the specific degradation of ςS by ClpXP.
What remains unclear is the molecular nature of the signal(s) that regulates SprE activity in response to nutrient availability. Based on homology with other response regulators, it is likely that SprE activity is modulated by phosphorylation at the conserved aspartic acid residue D58 within the N-terminal receiver domain of SprE. Consistent with this hypothesis, it was observed in vitro that phosphorylated SprE was more efficient at binding ςS than unphosphorylated SprE (1). Thus far, acetyl phosphate is the only reported source of phosphate for SprE (2). The Δ(ackA pta) mutant, which does not synthesize acetyl phosphate, has approximately 2.5-fold higher levels of ςS than the wild type during exponential growth (2). However, increased stabilization of ςS in response to starvation in the Δ(ackA pta) mutants indicates that there is still significant regulation of SprE activity in the absence of acetyl phosphate (2).
A constitutive allele of sprE, sprE19::cam, which results from insertion of a Tncam element 22 bp upstream of the sprE open reading frame, has been described (12). This constitutive allele, which alters the expression level of sprE, promotes degradation of ςS irrespective of growth phase and any phosphorylation signal(s) that may regulate SprE activity. This suggested to us that there might be important growth phase regulation of sprE expression, which is overcome by the sprE19::cam allele. Experiments reported here directly test the hypothesis that SprE levels are responsive to the bacterial growth phase.
SprE levels are growth phase regulated in a ςS-dependent manner.
Strains used in this study are listed in Table 1. To better understand the mechanism(s) behind growth phase regulation of SprE activity, we tested whether SprE levels varied in a growth phase-dependent manner with the idea that decreased levels during stationary phase could account in part for the decreased SprE activity observed. Therefore, we assayed SprE levels throughout the growth curve by Western blot analysis (Fig. 1a). In contrast to our expectation, we observed that SprE levels were minimal during exponential growth and increased dramatically as bacteria entered into stationary phase. In fact, we were unable to reliably detect SprE during mid-exponential phase because protein levels were so low. SprE levels were approximately threefold higher in the gain-of-function sprE19::cam mutant than in the wild type during both exponential (data not shown) and stationary phases (Fig. 1b). However, SprE levels in the sprE19::cam mutant still exhibited greater than 10-fold induction under starvation conditions (data not shown), suggesting that growth phase regulation was independent of sprE transcription.
TABLE 1.
Bacterial strains
| Strain | Genotype |
|---|---|
| MC4100a | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flb5301 ptsF25 deoC1 thiA1 |
| KEG423 | MC4100 sprE::Tn10 |
| KEG424 | MC4100 sprE19::cam |
| KEG425 | MC4100 rpoS::kan |
| KEG426 | MC4100 clpP::cam clpX::kan |
| KEG428 | KEG425 clpP::cam clpX::kan |
| KEG452 | MC4100 λsprE112′-lacZ+ |
| KEG453 | KEG425 λsprE112′-lacZ+ |
| KEG500 | MC4100 pKEG4 |
| KEG501 | KEG425 pKEG4 |
| KEG504 | MC4100 λsprE984′-′lacZ |
| KEG505 | KEG425 λsprE984′-′lacZ |
| KEG512 | MC4100 λsprE114′-′lacZ |
| KEG513 | KEG425 λsprE114′-′lacZ |
See reference 3.
FIG. 1.
Growth phase regulation of SprE as determined by Western blot analysis. Arrows, SprE and maltose-binding protein (MBP; internal loading control). Each strain was grown in LB broth (14) at 37°C with aeration, and 1-ml samples were taken at the indicated A600. Cells were pelleted and resuspended in a volume (in milliliters) of loading buffer (14) equal to A600/5. The resulting whole-cell lysate was used for sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (8), followed by Western blot analysis (16) with anti-SprE polyclonal antiserum used at a dilution of 1 μl per ml of blocking solution. Horseradish peroxidase-linked goat anti-rabbit secondary antibody (Amersham) was used at a dilution of 1 μl per 8 ml of blocking solution. The membrane was subsequently stripped and reprobed with anti-MBP polyclonal antiserum used at a dilution of 1 μl per 5 ml of blocking solution. Protein band intensities were analyzed with ImageQuant, version 5.0. (A) MC4100 (wild-type) growth curve with KEG423 (sprE::Tn10) as a negative control for SprE; (B) MC4100 and the indicated mutant derivatives grown to stationary phase at an A600 of ∼2.0.
We thought it possible that SprE was degraded concomitantly with ςS in vivo, thereby accounting for the growth phase expression pattern we observed. To test this, we assayed SprE levels by Western blotting in both rpoS and clpXP null backgrounds. If the decreased amount of SprE observed during exponential growth was dependent on ςS degradation, we would expect an increased amount of SprE in the absence of ςS or ClpXP. As observed with the wild type, however, SprE is nearly undetectable during exponential growth in the clpXP mutant (data not shown), which constitutively accumulates ςS. In addition, the clpXP null mutation did not significantly alter stationary-phase levels of SprE (Fig. 1b).
In contrast, we observed a significant decrease in SprE levels during stationary phase in the rpoS null mutant (Fig. 1b). This decreased level of SprE was equivalent to that observed during exponential growth in the wild type, conditions in which ςS activity was diminished through rapid ClpXP-mediated degradation. Additionally, the decreased SprE observed in the rpoS null mutant was not reversed in an rpoS clpXP triple mutant, demonstrating that this rpoS-dependent decrease did not result from ClpXP-mediated degradation (Fig. 1b).
The above results demonstrated that SprE was growth phase regulated such that upon starvation protein levels increased dramatically. While ςS was necessary for the observed stationary-phase accumulation of SprE, ςS alone was insufficient to increase SprE levels during exponential growth, as revealed by the clpXP null mutant. This suggested that an additional factor(s), induced upon starvation, acted in concert with ςS to mediate growth phase regulation of SprE.
The sprE112′-lacZ+ transcription fusion is not regulated by ςS.
Since SprE levels varied throughout the growth curve in a ςS-dependent manner, we constructed an sprE112′-lacZ+ transcription fusion (Fig. 2) to test whether this was the result of transcriptional regulation. This seemed an unlikely mechanism, as noted above, since the constitutive sprE19::cam allele was also subject to growth phase regulation. However, we wanted to test this more directly since little was known about sprE transcription. The pKEG3 fusion construct was recombined with λRZ5, and the recombinant λsprE112′-lacZ+ phage was lysogenized into MC4100 at the att site.
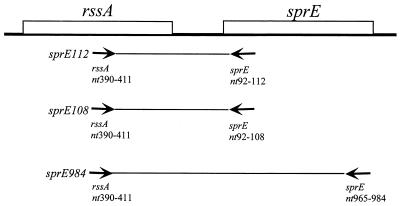
FIG. 2.
Construction of sprE fusions. The putative regulatory sequences required for sprE regulation were amplified by PCR from the MC4100 chromosome, with Taq polymerase (United States Biochemical Corp.) and the primers rssA390 (CTTGCTATTCGCGCATCATGC) and sprE112 (CCACCAGTACCGTTGTCG). The resulting PCR fragment was polished with Pfu polymerase (Stratagene) and blunt end ligated into SmaI-digested pRS415 (15) with T4 ligase (New England Biolabs). The resulting plasmid, pKEG3, contained a sprE112′-lacZ+ transcription fusion. The pKEG3 construct was recombined in vivo with λRZ5 (11), and the recombinant Lac+ Ampr phage was integrated at the att site in MC4100. The sprE114 PCR fragment was amplified using primers rssA390 and sprE114 (TCCCCCGGGAGCCGCCAGTACCGTTGTCG), followed by SmaI digestion and Pfu polymerase polishing. The sprE984 PCR fragment was amplified using primers rssA390 and sprE1014 (CGTTTGCTCATTCTGC), followed by AccI digestion (New England Biolabs) and mung bean nuclease polishing (New England Biolabs). These blunt-ended PCR products were then ligated into the SmaI-digested pRS414 vector (15). The resulting fusions were recombined into the chromosome as described above. nt, nucleotides.
The expression level of λsprE112′-lacZ+ was determined in a β-galactosidase assay during both exponential and stationary phases in rpoS+ and rpoS null backgrounds. We observed no alteration in λsprE112′-lacZ+ expression upon introduction of the rpoS null allele in comparison to the wild type in either Luria-Bertani (LB) media (Table 2) or M63 minimal media with 0.2% glucose (data not shown). Importantly, sprE transcription did increase 25-fold upon starvation, although in a manner independent of ςS activity. While these results demonstrated significant growth phase regulation of sprE transcription, they further supported the conclusion that SprE protein levels increased upon starvation by a ςS-dependent mechanism that functions posttranscriptionally.
TABLE 2.
β-Galactosidase activity of sprE′-lacZ fusions
| Fusion | β-Galactosidase activitya of culture with indicated rpoS background at:
|
|||
|---|---|---|---|---|
| Exponential phase
|
Stationary phase
|
|||
| rpoS+ | rpoS null | rpoS+ | rpoS null | |
| sprE112′-lacZ+ | 6.5 ± 2 | 5.2 ± 1 | 164 ± 26 | 203 ± 16 |
| sprE114′-′lacZ | 0.5 ± 0.2 | 0.2 ± 0.1 | 16 ± 1 | 4.6 ± 0.6 |
| sprE984′-′lacZ | 1.2 ± 0.3 | 0.4 ± 0.2 | 23 ± 3 | 4.5 ± 0.3 |
These data, in Miller units, reflect the averages ± standard deviations of four separate cultures and were independently reproduced at least three times. The cultures were grown in LB media at 37°C. Stationary-phase cultures were harvested after 24 h of growth at an A600 of ∼2.5. Exponential-phase cultures were derived from a 1:100 subculture of the stationary-phase samples and were harvested once they reached an A600 of ∼0.3.
SprE is subject to posttranscriptional ςS-dependent growth phase regulation.
The above results indicated that SprE levels were regulated posttranscriptionally by ςS, at the level of either translation or protein stability. To test this directly, we constructed a set of protein fusions between the open reading frame of sprE and that of lacZ. The sprE114′-′lacZ translation fusion is analogous to the previous sprE112′-lacZ+ transcription fusion, with an additional 2 bp of the sprE open reading frame to allow an in-frame fusion with lacZ (Fig. 2). The sprE984′-′lacZ translation fusion contains nearly the entire open reading frame of sprE, the intent being to include all potential cis-acting regulatory sites (Fig. 2). The resulting pKEG5 and pKEG6 fusion constructs were recombined with λRZ5 and introduced into the chromosome at the att site.
Expression of both translation fusions was determined by a β-galactosidase assay from cultures grown in LB media during exponential and stationary phases in an rpoS+ and rpoS null background (Table 2). Stationary-phase activities of both SprE114′-′LacZ and SprE984′-′LacZ were highly dependent on ςS, such that the wild type possessed approximately fivefold greater activity than the rpoS null mutant (Table 2). In contrast, there was no significant difference between the wild type and the rpoS null mutant during exponential growth (Table 2).
Analogous ςS-dependent growth phase regulation of each translation fusion was observed in M63 minimal media containing 0.2% glucose (data not shown). In fact, the difference in levels of λsprE114′-′lacZ and λsprE984′-′lacZ expression was significant enough to distinguish the wild type, which could form single colonies on M63 minimal 0.2% lactose agar, from the rpoS null mutant, which was unable to grow on the same medium.
These data demonstrate that ςS is necessary for promoting high levels of SprE114′-′LacZ and SprE984′-′LacZ (Table 2) during stationary phase. Since the λsprE114′-′lacZ translation fusion, but not the λsprE112′-′lacZ+ transcription fusion, is sensitive to ςS-dependent growth phase regulation, we conclude that SprE levels are posttranscriptionally regulated by ςS. However, since the rpoS null mutant allows nearly 10-fold induction of SprE114′-′LacZ and SprE984′-′LacZ upon starvation, there is also a ςS-independent means of increasing levels of SprE during stationary phase. This likely reflects regulation of sprE transcription, since the λsprE112′-lacZ+ fusion is strongly induced in a ςS-independent manner. Interestingly, our observation that ςS is not sufficient to induce high levels of SprE during exponential growth in the clpXP null mutant may reflect a requirement for increased transcription of sprE.
SprE translation is regulated by ςS.
Based on the Lac phenotypes described in the previous section, it was clear that at least some of the ςS-dependent cis-acting sites were present in the λsprE984′-′lacZ protein fusion. As discussed above, it appeared likely that ςS-dependent regulation was mediated through effects on either posttranscriptional synthesis of SprE or SprE protein stability. We assayed SprE984′-′LacZ in LB media throughout the growth curve by Western blot analysis and obtained results analogous to those shown for native SprE (data not shown). However, SprE984′-′LacZ and native SprE, expressed from the chromosome in M63 minimal media with 0.2% glucose, were undetectable by [S35]methionine incorporation and immunoprecipitation with our antibodies (data not shown). For this reason, we probed the mechanism behind ςS-dependent regulation of SprE984′-′LacZ with strains containing the medium-copy-number plasmid pKEG4. We assayed SprE984′-′LacZ levels throughout the growth curve in rpoS+ and rpoS null strains by Western blotting and found that the fusion protein expressed from pKEG4 was regulated in a ςS-dependent manner (data not shown). Therefore, the information necessary for ςS-dependent regulation of SprE was present in this protein fusion and functioned within the plasmid construct.
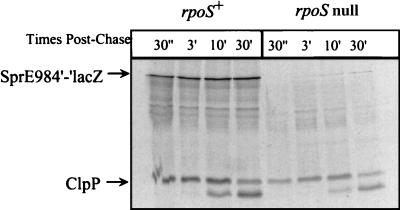
In order to distinguish between regulation of SprE at the levels of translation and protein stability, we performed a pulse-chase analysis of SprE984′-′LacZ during stationary phase (Fig. 3). SprE984′-′LacZ synthesized 3 min postchase was stable for at least 30 min in the wild-type strain. The same degree of SprE984′-′LacZ stability was observed in the rpoS null mutant; however, the overall amount of protein synthesized was decreased significantly (Fig. 3). SprE984′-′LacZ was also stable for up to 30 min during exponential growth in the wild type (data not shown). Thus, once SprE984′-′LacZ had been synthesized, it was quite stable regardless of growth phase or the rpoS allele present. This clearly demonstrates that ςS promotes synthesis of SprE during stationary phase.
FIG. 3.
Growth phase regulation of SprE984′-′LacZ synthesis. Stationary-phase synthesis and stability of SprE984′-′LacZ were determined by pulse-chase analysis (16) performed on stationary-phase (A600 = 1.5) cultures of KEG500 (MC4100 pKEG4) and KEG501 (KEG500 rpoS::kan). The strains were grown in M63 minimal media containing 0.2% glucose plus 25 μg of ampicillin/liter at 37°C with aeration. Three milliliters of cell culture was labeled with 100 μCi of [35S]methionine/ml for 2 min, followed by addition of 3 ml of minimal medium containing 0.2% glucose plus 0.8% cold methionine. One-milliliter samples were taken at the indicated times postchase, and total protein was precipitated with trichloroacetic acid. The labeled whole-cell lysate was added to 1 ml of immunoprecipitation buffer containing 4 μl of LacZ antiserum and 1 μl of ClpP antiserum. The immunoprecipitated proteins were pelleted and resuspended in 40 μl of protein sample buffer. Subsequently, the sample was boiled, and 12 μl was analyzed by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and autoradiography (4).
Conclusions.
Our results demonstrate ςS-dependent posttranscriptional regulation of SprE synthesis during the growth cycle. At present, the mechanism of this growth phase-dependent regulation of SprE remains unknown. However, both SprE′-′LacZ protein fusions were similarly regulated by ςS, suggesting that all the information necessary for this growth phase regulation is present in the shorter SprE114′-′LacZ fusion. Since ςS regulates promoter recognition and transcription initiation of core RNA polymerase, it likely alters SprE translation indirectly through the regulated expression of a small regulatory RNA or an RNA-binding protein. While ςS is required to regulate SprE translation, it is not sufficient when overexpressed during exponential growth, so another factor, whose concentration also responds to nutrient availability, must be involved. As noted above, we suspect that this unknown factor acts at the level of transcription. The precise nature of the starvation signal that promotes SprE translation is also unclear. We observe translational regulation of SprE in both rich LB broth and M63 minimal medium with glucose, but whether it occurs under other growth conditions remains to be determined.
Interestingly, SprE levels are quite low during logarithmic growth; however, they are clearly sufficient to promote ClpXP-mediated ςS degradation. In contrast, SprE accumulates during stationary phase but is not competent to promote ςS degradation. Thus, the regulation of SprE levels is secondary to the growth phase regulation of SprE activity with regard to ClpXP-mediated degradation of ςS. However, our observation that a mechanism for increasing the intracellular pool of SprE under starvation conditions exists suggests that this accumulation could be important for a rapid transition from stationary phase to exponential growth once nutrients become available by providing a large pool of SprE receptive to activating signals. This regulation could also provide a feedback mechanism for reducing ςS levels during exponential growth after transient induction by stresses such as heat or osmotic shock, which are known to lead to elevated levels of ςS (5).
Alternatively, SprE could function to regulate the stability of additional target proteins in a growth phase-dependent manner. In this way, the absolute amount of SprE could play a regulatory role through differential affinity for the various target proteins. The maintenance of an appropriate amount of SprE appears to be quite important for E. coli, since overexpression of this protein results in dramatic growth defects and loss of viability (our unpublished observation). This decreased viability is not relieved by loss of ςS, consistent with the idea that there might be other regulatory targets of SprE with important physiological roles during growth.
Acknowledgments
We are indebted to Weihong Hsing for her contribution to generating polyclonal antibodies to SprE. S. Gottesman has kindly provided us with ClpP polyclonal antibodies. We thank N. Ruiz and T. Raivio for critical reading of the manuscript, and many thanks go to S. DiRenzo for help with preparation of the manuscript.
T.J.S. was supported by a grant from the NIGMS (GM35791).
REFERENCES
- 1.Becker G, Klauck E, Hengge-Aronis R. Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc Natl Acad Sci USA. 1999;96:6439–6444. doi: 10.1073/pnas.96.11.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouche S, Klauck E, Fischer D, Lucassen M, Jung K, Hengge-Aronis R. Regulation of RssB-dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator-controlled process. Mol Microbiol. 1998;27:787–795. doi: 10.1046/j.1365-2958.1998.00725.x. [DOI] [PubMed] [Google Scholar]
- 3.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage λ and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlin J. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979;98:132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- 5.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 1497–1512. [Google Scholar]
- 6.Huisman G, Kolter R. Regulation of gene expression at the onset of stationary phase in Escherichia coli. In: Piggot P, Moran J C P, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C.: American Society for Microbiology; 1994. pp. 21–40. [Google Scholar]
- 7.Huisman G W, Siegle D A, Zambrano M M, Kolter R. Morphological and physical changes during stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: American Society for Microbiology; 1996. pp. 1672–1682. [Google Scholar]
- 8.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 10.Muffler A, Fischer D, Altuvia S, Storz G, Hengge-Aronis R. The response regulator RssB controls stability of the ςS subunit of RNA polymerase in Escherichia coli. EMBO J. 1996;15:1333–1339. [PMC free article] [PubMed] [Google Scholar]
- 11.Ostrow K S, Silhavy T J, Garrett S. cis-acting sites required for osmoregulation of ompF expression in Escherichia coli K-12. J Bacteriol. 1986;168:1165–1171. doi: 10.1128/jb.168.3.1165-1171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pratt L A, Silhavy T J. The response regulator SprE controls the stability of RpoS. Proc Natl Acad Sci USA. 1996;93:2488–2492. doi: 10.1073/pnas.93.6.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schweder T, Lee K H, Lomovskaya O, Matin A. Regulation of Escherichia coli starvation sigma factor (ςS) by ClpXP protease. J Bacteriol. 1996;178:470–476. doi: 10.1128/jb.178.2.470-476.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 15.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 16.Snyder W B, Silhavy T J. Enhanced export of β-galactosidase fusion proteins in prlF mutants is Lon dependent. J Bacteriol. 1992;174:5661–5668. doi: 10.1128/jb.174.17.5661-5668.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zgurskaya H I, Keyhan M, Matin A. The sigmaS level in starving Escherichia coli cells increases solely as a result of its increased stability, despite decreased synthesis. Mol Microbiol. 1997;24:643–651. doi: 10.1046/j.1365-2958.1997.3961742.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Gottesman S. Regulation of proteolysis of the stationary-phase sigma factor RpoS. J Bacteriol. 1998;180:1154–1158. doi: 10.1128/jb.180.5.1154-1158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]