Abstract
Background: Pharmacologic and non-pharmacologic treatments for stroke are essential but can be costly or harmful, whereas probiotics are a promising alternative. This scoping review aimed to synthesize the in vitro and in vivo evidence of probiotics on stroke-related neurological, biochemical, and histochemical outcomes. Methods: A systematic review was conducted in PubMed, Embase, and Cochrane Central Register of Clinical Trials (CENTRAL) up to 7 May 2021. Titles and abstracts were screened and assessed by two independent reviewers. The initial screening criteria aimed to include studies using probiotics, prebiotics, and symbiotics both in vitro and in vivo for the prevention and/or treatment of stroke. Results: Of 6293 articles, 4990 passed the initial screen after excluding duplication articles, of which 36 theme-related full texts were assessed and 13 were included in this review. No in vitro studies passed the criteria to be included in this review. Probiotics can ameliorate neurological deficits and show their anti-inflammation and anti-oxidative properties. Decreased loss of cerebral volume and inhibition of neuronal apoptosis were revealed in histopathological evidence. Conclusions: There are potential cognitive benefits of probiotic supplementation, especially among animal models, on decreasing cerebral volume, increasing neurological score, and decreasing the inflammatory response. However, further investigation is needed to validate these conclusions in various populations.
Keywords: probiotic, stroke, gut microbiome, meta-analysis
1. Introduction
Stroke is a cerebrovascular disease that has been known to be a significant burden in low- to middle-income countries and to be declining in high-income countries. In 2019, stroke was identified as the second leading cause of disability-adjusted life years (DALYs) worldwide [1]. Numerous rehabilitation programs and interventions that require multimodality teams, both pharmacologic and nonpharmacologic, have been conducted. However, the outcomes have not met the needs of the burden of this disease [2]. Several monoaminergic drugs have been investigated for use in stroke, including serotonergic and dopaminergic drugs [3], but these drugs may cause a variety of side effects, including sexual dysfunction, weight gain, insomnia, or somnolence in selective serotonin reuptake inhibitors (SSRIs), and gastrointestinal side effects, motor disturbances, and others in dopaminergic drugs [4,5]. These potential side effects may have a significant impact on the patient’s quality of life if taken for an extended period. Stroke patients require interventions with lower costs and fewer side effects, such as probiotics. Probiotics are live microorganisms found in a variety of foods that may benefit a variety of human organ systems and conditions, including the immune system, dysbiosis, and others [6]. A recent study discovered that the gut microbiota–brain axis, which is involved in multiple systems, including the endocrine, neurological, and digestive systems, had a direct effect on these systems and may be used cautiously in certain psychiatric diseases such as depression [7]. The gut microbiota–brain axis was also discussed in a study involving stroke and the microbiota, in which it was found that altering the gut microbiota could affect stroke outcome by impacting both neuroendocrine and metabolic pathways including the serotoninergic system, γ-aminobutyric acid (GABA), plasma cortisol and catecholamine [8,9].
Moreover, previous data found that microbiota could impact the central nervous system by using the vagus nerve [10] and local signals including histamine, acetylcholine, melatonin, nitric oxide and hydrogen oxide, which can affect enteroendocrine cells. The microbiome has been known to play an essential part in microglial maturation and activation which can change behavior in studied samples [11]. Furthermore, mice given different foods present different physical activity, memory and anxiety behavior, which shows the impact microbiota have via bottom-up pathways [9,12,13].
These pathways are further investigated in other studies looking at the use of probiotics to alter the gut microbiota–brain axis, which found that probiotic use could improve the outcome of stroke model disease [14]. To our knowledge, few systematic reviews have been conducted on this topic and additional investigation is required. Therefore, the aim of this systematic review is to further investigate the outcome of probiotic use for the treatment of in vitro and in vivo study models of stroke disease and see if it could serve as part of an effective, low-cost treatment plan in some stroke patients.
2. Materials and Methods
2.1. Registration of Protocol
This study was guided by the recommendation of the Preferred Reporting Items of Systematic reviews and Meta-Analyses extension for Scoping Review (PRISMA-ScR) statement.
2.2. Data Sources and Search Strategy
We used three databases including PubMed, Embase, and Cochrane Central Register of Clinical Trials (CENTRAL) due to their coverage of international peer review articles to search for publications in the English language up to 7 May 2021. The terms probiotics, prebiotics, and symbiotics were used in combination with ischemic stroke, cerebrovascular disorders, and brain ischemia as the keywords for a systematic literature search along with any synonyms. The details of the search terms are presented in the Supplementary Materials. In addition, the reference lists of included articles were searched, as well as relevant citations from other journals via Google Scholar.
2.3. Study Selection
In this systematic scoping review, we worked with an information specialist to design an appropriate search strategy to identify original peer-reviewed articles that look at the use of probiotics, symbiotics, or prebiotics as a form of prevention or treatment for stroke. We defined that the population of each study could be conducted either in vitro (using components of an organism that have been isolated from their usual biological surroundings, ex. microorganisms, cells, or biological molecules) or in vivo (living organisms, ex. animals). The interventions were probiotics, symbiotics, or prebiotics compared with placebo. Probiotics were further categorized into food and non-food based. Food-based probiotics ranged from ginseng and yam gruel to fermented soybeans, which have been traditionally used in Chinese traditional medicine [15]. Non-food based probiotics were composed of a variety of probiotics ranging from single-strain bacteria, such as Clostridium butyricum or lactobacillus, to a mixture of probiotics. Clostridium butyricum has been known to be a probiotic commonly used and studied in Asia with an effect on modulating immune processes and inflammatory processes in the intestinal tract [16]. Article screening for eligible studies that correlated with our inclusion and exclusion criteria was conducted by two independent reviewers (C.Sam. and T.N.). The independent reviewers were selected for their experience in probiotics, stroke, and systematic review methodology. Discrepancies between the two reviewers were resolved by consensus.
2.4. Data Extraction
Data extraction was done by two independent reviewers (C.S. and C.Sam.) for a published summary of probiotic effects in stroke animals and in vitro studies. The following data were extracted: study characteristics (authors, year of publication, study type, journal name, contact information, country, and funding); intervention characteristics (probiotics, symbiotics, and prebiotics; food-based vs non-food based; the number of case and control); and outcome characteristics (neurological outcome, biochemical profile, dysbiosis index, and histopathology). We included all associated text, tables, and figures for extraction. We excluded non-original articles (review articles, protocols, letters, comments, and guidelines); non-human studies; unpublished data or non-peer-reviewed data; and studies published in languages other than English.
2.5. Data Synthesis and Analysis
The primary outcomes included in the systematic review are neurological outcome, biochemical profile, dysbiosis index, and histopathology among in vitro and in vivo study models.
2.6. Patient and Public Involvement
There was no patient or public involvement in the study. However, the results of the studies included in this review (both in vivo and in vitro) could promote further research on this topic.
3. Results
3.1. Study Characteristics
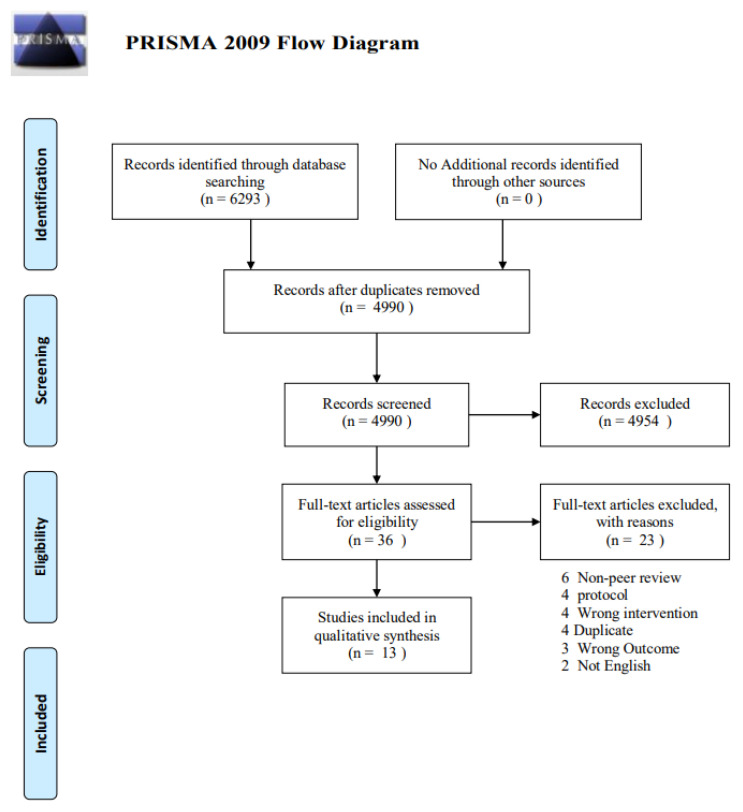
The database search identified 6293 potential records. After removing duplicates, 4990 titles passed the initial screen, and 36 theme-related abstracts were selected as further full-text articles to be assessed for eligibility (Figure 1). A total of 23 articles were excluded for the following: 6 non-peer-reviewed, 4 duplicates, 4 protocols, 4 wrong interventions using something other than probiotics/prebiotics and symbiotics as an intervention, 3 wrong outcomes i.e., using outcomes other than neurological outcomes such as gastrointestinal symptoms, and 2 non-English languages. Thirteen studies were eligible for the data extraction and data synthesis.
Figure 1.
PRISMA flow diagram.
The 13 included studies investigated probiotic interventions, neurological outcomes, biochemical changes, and/or histopathological findings. All studies were animal studies with eight rats (61.5%), four mice (30.8%), and one gerbil (7.7%) as a study model. The study period was from 2004 to 2020. To the best of our knowledge, in vitro studies that correlate with our criteria in all of the databases were not found. The studies took place in Northern America, Europe, and Asia. There were no studies from Australia, Africa, and Southern America.
3.2. Summary of Probiotic Impact in Stroke-Induced Animal
There were multiple parameters used to evaluate the effect of probiotics in the animal model. Of the 13 included articles, both neurological outcomes and biochemical profiles were measured in 11 articles (84.6%). Similarly, histopathology was measured in 11 out of 13 studies (84.6%) (Table 1).
Table 1.
The characteristics of the articles in which probiotics are the intervention in stroke animal models.
| Author | Country | Type of Intervention | Animal Type | Number of Case | Number of Control | Measure Outcome | Result of Outcome | |
|---|---|---|---|---|---|---|---|---|
| Non-food based | Food-based | |||||||
| Akhoundzadeh, 2018 [14] | Iran | Combination of 4 viable probiotic bacteria strains, namely Bifidobacterium breve, Lactobacillus casei, Lactobacillus bulgaricus (Lactobacillus delbrueckii subsp. bulgaricus), and Lactobacillus acidophilus | Mice | 5 | 5 mice received saline, 5 mice were sham operated | Neurological outcome | Could not improve neurological function | |
| Histopathology (infarct size) | Reduced infarct size 52% | |||||||
| Biochemical markers | Decreased the malondialdehyde content and TNF alpha level | |||||||
| Bae, 2004 [17] | South Korea | Red ginseng and fermented red ginseng | Rat | Ginseng 5, red ginseng 5, fermented red ginseng 5 | 10 | Histopathology (infarction area, volume) | Fermented red ginseng treated group showed reduction of the infarction area in all regions and total infarction volume | |
| Chen R, 2019 [18] | United States | Puerariae Lobatae Radix(PLR) + Chuanxiong Rhizoma(CXR) | Rat | Not found | Not found | Neurological function score | Repaired neurological impairment | |
| Histopathology (body weight gain, cerebral infarction area) | Reduced the cerebral infarction | |||||||
| Biochemical level (serum level of LDL/HDL/TG/T CHO/ blood viscosity/fibrinogen level/ platelet aggregation rate) | Reversed the dyslipidemiaReduced the blood viscosity and thrombotic risk | |||||||
| Li, 2018 [19] | United States | Panax Notoginsenoside extract(PNE) | Germ free rat | Not found | Not found | Neurological evaluation | Decreased neurological deficit scores | |
| Histopathology (triphenyl tetrazolium chloride (TTC) assessment of infarct size) | Decreased cerebral infarct volume | |||||||
| Biochemical level (pro inflammatory cytokine/BDNF/GABA in rat hippocampus) | Upregulated the expression of GABA receptor in hippocampusDecreased rate of attenuation in BDNF expression | |||||||
| Liu, 2015 [20] | China | Clostridium butyricum | Mice | 12 | 12 | Neurological evaluation (behavioral tests, open field test, Morris water maze) | Improved spatial learning ability | |
| Histopathology (Hippocampal change) | Ameliorated the morphological changes in the HippocampusIncreased butyrate in the brain | |||||||
| Mei, 2017 [21] | China | Shuan tong ling | Rat | Not found | Not found | Neurological deficit | Increased neurological scores | |
| Histopathology (infarct volume) | Reduced infarct volume | |||||||
| Biochemical level (inflammatory cytokines including TNF alpha, IL 1 beta) | Decreased TNF alpha and IL 1 beta | |||||||
| Nagao, 2019 [22] | Japan | Fermented ginseng | Rat | Not found | Not found | Neurological evaluation (spatial memory evaluated using Morris water maze (MWM)) | Shortened the extended time to reach the platform in the MWM | |
| Histopathology (use of neuronal nuclei positive cells to assess hippocampus neuron loss, protein expression of caspase3/Iba1/glial fibrillary acidic protein) | Ameliorated loss of hippocampus cornu ammonis neurons and increased caspase-3/Iba1 | |||||||
| Pang, 2020 [23] | United Kingdom | Yam gruel | Rat | 9 | 18 | Neurological evaluation (MWM test (spatial learning and memory function)) | Improved cognitive function | |
| Biochemical markers (SOD and MDA, TNF alpha and IL 1 beta and LPS, characteristic of gut microbiota) | Increased relative content of probiotic bacteria and SCFAs in intestinal tract, cerebral cortexreduced oxidative stressand inflammatory responsePromoted the expression of neurotransmitters and brain derived neurotrophic factor | |||||||
| Park, 2016 [24] | Korea | Chungkookjang (fermented soybean) | Gerbil | Not found | Not found | Neurological evaluation | Prevented symptoms such as drooped eyelid/bristling hair/reduced muscle tone and flexor reflex/abnormal posture | |
| Histopathology (Neuronal cell death in hippocampus) | Prevent the neuronal cell deat | |||||||
| Biochemical markers (cytokine expression in hippocampus, serum cytokine levels, glucose metabolism) | Suppress cytokine expression, prevent the impairment of glucose metabolism | |||||||
| Rahmati, 2019 [25] | Netherlands | Commercial probiotics (LactoCare capsule, 109 CFU, ZIST TAKHMIR, Tehran, Iran), which are a mixture of seven probiotic bacteria strains, including Lactobacillus casei ZT-Lca.106, Lactobacillus acidophilus ZT-Lac.51, Lactobacillus rhamnosus ZT-Lrh.54, Lactobacillus bulgaricus ZT-Lbu.90, Bifidobacterium breve ZT-Bbr.22, Bifidobacterium longum ZT-Lca.106, and Streptococcus thermophilus ZT-Sth.20 | Mouse | 30 | 20 | Neurological evaluation (Spatial and learning memory) | Reduced spatial memory impairment and neurological dysfunction | |
| Histopathology (histological damage and apoptosis) | Reduced neuronal death | |||||||
| Biochemical markers malondialdehyde (MDA) content and brain-derived neurotrophic factor (BDNF) level | MDA and BDNF change was not significant | |||||||
| Sun, 2016 [26] | Netherlands | Clostridium butyricum | Diabetic mice | Not found | Not found | Neurological evaluaiton (Cognitive impairment) | Ameliorate cognitive impairment | |
| Histopathology (neuronal injury, apoptosis, expression of Akt/p-Akt/caspase3 level) | Ameliorate histopathologic change in the hippocampusIncrease p-Atk expression and decreased caspase-3 expression equal inhibit neuronal apoptosis | |||||||
| Biochemical markers (blood glucose level) | Decrease blood glucose level | |||||||
| Wanchao, 2018 [27] | China | Inactivated lactobacillus | Rat | 24 (divided into 4 groups with varied concentrations) | 6 | Neurological evaluationneurolobehavioral scores, | Improve neurobehavioral scores | |
| HistopathologyCerebral infarction volume, tunnel and TLR4/ IkB/A20 (cell apoptosis) | Decrease cerebral infarction volumeDecrease neural cells apoptosisInhibit expression of TLR4Promote the expression of IkB and A20 which | |||||||
| Biochemical markers (SOD + MDA levels), | MDA level decrease, SOD activity increase, reduce oxidative stress | |||||||
| Zhang, 2019 [28] | Netherlands | Chamomile | Rat | Not found | Not found | Neurological evaluation (neurological score, neurological deficits) | Improve neurological scores | |
| Histopathology (infarction size) | Decrease in both infarct volume | |||||||
| Biochemical markers(Protein levels of Nrf2/Keap1/HO1/ERK) | Increase the activity of HO1 and Nrf2 | |||||||
3.2.1. Neurological Test
Most of the studies [14,18,19,21,23,24,25,26,27,28] showed that probiotic use could decrease neurological deficits and some claimed that it can improve spatial learning ability and cognitive function. Tests used to measure cognitive functioning in the studies include neurological function score [14,18,19,20,21,22,23,24,25,26,27,28,29], Morris water maze [20,22,23], and open field test [20]. Morris water mazes were used to evaluate the capability of rodent spatial learning by navigating and locating the escape platform of the maze [30]. Open field tests were used in the observation of exploratory behavior by focusing on the movement activity of rodents, which could be related to motor power, emotional, and other instinctive behaviors such as fight and fear [31].
3.2.2. Biochemical Level
The inflammatory response was also found to be impacted by probiotic use, including Tumor necrosis factor (TNF) alpha [32,33] and Interleukin 1 (IL-1) [32,33], which show an anti-inflammatory effect with probiotics [21,23]. TNF alpha is secreted by macrophages and is responsible for acute inflammation processes that lead to cell necrosis or apoptosis [32]. IL-1 is known to be the regulator of inflammation and is involved in a variety of pathways, including the innate immune process which causes a range of functions from leukocytic pyrogen and fever to an activated immune system [34]. Other studies found that probiotics are involved in decreasing multiple factors in metabolic pathways such as SCFAs, lipid profile and blood sugar. SCFAs have been studied extensively in gut-associated effects including stabilized intestinal membrane integrity, anti-inflammation and production of mucous. Moreover, SCFAs were found to cross the blood–brain barrier and control the blood–brain barrier’s integrity which impacts the gut–brain axis [35,36]. Similarly, hyperglycemia has been found to cause a larger infarction area in a mouse model which may be due to its toxicity, but the mechanism is still not clear [37].
Another dimension was associated with that involved in the coagulation pathway, which could impact the resolution of stroke in animal models. A high fibrinogen level was associated with an increased risk of mortality and predicted poor outcome in an ischemic stroke model [38,39]. Decreased oxidative stress has also been reported after using probiotics [15,21] by using serum malondialdehyde (MDA), which is a product in lipid peroxidation that can be inferred as a biomarker for oxidative stress. Superoxide dismutase (SOD) is an antioxidant that has a major role in preventing both endothelial and mitochondrial dysfunction. SOD impacts ischemic penumbra by reducing superoxide anion and abnormal glutamate release [40,41,42].
Nuclear factor erythroid 2-related factor 2 (Nrf2) [28] was one of the novel targets in the treatment of stroke due to its ability to induce the antioxidative potential of the cell especially in neurons by cooperating with its actin-binding protein Keap1 [43,44]. Brain-derived neurotrophic factor (BDNF) level—one of the important factors involved in neuron differentiation and survival [45]—was used for measuring the outcome in two studies [19,25].
3.2.3. Histopathology
Some researchers have found that probiotics could be used to decrease cerebral infarction volume [27,28,30,32,46,47]. Using cerebral infarction volume is known as one of the stronger predictors in acute stoke outcomes from previous multivariate analysis, which suggests the reliability of using this outcome as a measurement in almost all of the study that had been included [48]. Moreover, probiotics have been found to inhibit neuronal apoptosis by detecting the expression of caspase-3, Akt, Iba1, TLR4, IkB and A20 [46,49]. Caspase-3 is known as a key factor in neuron cell death within the acute phase of stroke and could be used as a interesting marker of cell apoptosis [48]. Probiotics can also play a role in the immunologic pathway of the brain and decrease changes within the hippocampus [34,50].
4. Discussion
This systematic review showed that a variety of probiotic types have been used in interventions for potential stroke treatment, which makes it difficult to compare results between the studies. Despite these differences, we found similar results in multiple parameters including neurological score and brain volume, which suggests that probiotic supplementation could be a beneficial component of rehabilitation programs in post-stroke patients. For example, a systematic review of probiotic use in human stroke patients in other modalities was also published and found that probiotics in enteral nutrition can improve clinical parameters including infection events, intestinal dysbiosis, gastrointestinal complications, and nutritional status [30]. However, more research is needed due to the lack of literature available on probiotic supplementation in human specimens and its effect on neurological modalities.
4.1. Probiotic and Inflammatory Effects
There is growing evidence that supports beneficial effects of inflammatory processes in various aspects of ischemic cerebral disease. To illustrate a bidirectional process that involved peripheral immune response and the cerebral ischemic brain model, many pathways were found in the inflammatory process including: interleukin 1 as protection of hippocampal cortical area 1 (CA1) in gerbil, T-reg in postischemic anti-inflammatory effect through use of IL-10 secretion in ischemic brain tissue, and the complex role of neutrophil that could destabilize the blood–brain barrier by enzyme secretion and create inflammation by blocking vascular supply [31]. These pathways are linked to the essential role of inflammation in ischemic brain disease.
Although not fully understood, the gut–brain axis has been known to be the novel bidirectional pathway involving the gut and brain. Moreover, it is thought to involve the vagus nerve in multiple complex pathways including the enteric nervous system, neuronal-glial endothelial interaction, and immune and inflammatory cell response involving damage-associated molecular pattern (DAMPS) and cytokine. Previous associations have also been found to suggest gut dysbiosis or an altered strain of gut microbiota to be preset in stroke patients [32]. Thus, some studies have included probiotic supplementation as a primary form of intervention and found probiotic use to improve neurological outcomes by using bidirectional pathways [33].
4.2. The Use of the Hippocampus in Measured Outcomes
The hippocampus is a known part of the limbic system and plays a critical role in many neurological diseases, including Alzheimer’s disease. This part of the brain has various functions, including memory, mood, emotional drive, and spatial navigation. Thus, much of the research presented in our study used many components from the hippocampus, such as neuron loss as the neurological outcome, to evaluate the effect of probiotics in stroke animal models [34].
4.3. Probiotic Strain and Its Benefit
In this study we found that using different types of probiotic could impact the outcome of the disease, for example Panax Notoginsenoside Extract (PNE) was found to affect the BDNF expression [19]. On the other hand, commercial probiotics (LactoCare capsule) did not have the same impact [25] which shows the importance of selection of strain and type of probiotic that could be further studied in human or animal models. This is similar to a previous review article which showed the potential benefit of probiotics in various diseases according to the strain, from gastrointestinal-related diseases including colitis, inflammatory bowel disease, irritable bowel syndrome and diarrhea, to non-gastrointestinal diseases such as obesity and Alzheimer’s disease.
Several probiotics with anti-inflammation effects that could be further investigated in stroke-associated models include Lactiplantibacillus plantarum, Lacticaseibacillus rhamnosusc and Bifidobacterium infantis Bifidobacterium breve, which have been previously shown to have benefits in spontaneous colitis, Rotavirus infection and Alzheimer’s disease, respectively [51,52,53,54,55].
It is possible that these probiotics could apply the same anti-inflammation properties to the ischemic stroke model which correlates with our included paper [14] using a mixture of probiotics that included Bifidobacterium breve and which showed anti-inflammation outcomes in an ischemic stroke model.
4.4. Probiotic and Oxidative Stress
Oxidative stress is a result of a disrupted redox pathway creating more reactive oxygen species (ROS) than antioxidants can reduce [56]. This phenomenon happens frequently in the neuron tissue due to its high energy production. Oxidative stress can cause cellular damage by nucleic acid damage, protein oxidation and lipids peroxidation [57].
The brain is composed of high lipid composition which causes it to be vulnerable to oxidative stress, especially in the lipid peroxidation pathway [58]. This can lead to multiple neurodegenerative disease and neuronal damage which could be prevented by the gut microbiota due to its ability to regulate endogenous and exogenous ROS. In this article our focus was on probiotics that have this potential, i.e., Bifidobacterium breve, Lactobacillus casei, Lactobacillus bulgaricus (Lactobacillus delbrueckii subsp. bulgaricus), Lactobacillus acidophilus and inactivated lactobacillus [14,27]. This raises the importance of further study on probiotics that target this mechanism as a potential method to treat stroke patients in the future [59].
4.5. Low-Quality Paper with No Control or Vague Parameter
We found that many studies did not use case and control comparisons, which prevented us from being able to qualitatively interpret the results, and thus acts as a limitation of this review. Moreover, the variety of parameters used to evaluate the outcome of probiotic use made it difficult to form a conclusion from this review.
4.6. Further Research
This systematic review suggests that the lack of standardization among probiotic use in research poses a major obstacle in interpreting the role of probiotics in stroke treatment. Therefore, more in-depth studies of each probiotic are needed to consider changes to the mainstay of treatment in stroke patients. Moreover, the scarcity of in vivo studies further emphasizes the need to conduct more research on this topic. Investigating the effects of probiotic supplementation on stroke patients in greater detail could ultimately lead us to a cheaper and nonpharmacologic treatment option for post-stroke patients.
5. Conclusions
The current literature suggests that there are potential cognitive benefits of probiotic supplementation, especially among animal models, on decreasing cerebral volume, increasing neurological score, and decreasing the inflammatory response. However, more research is needed to confirm these suggestions in other populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14173661/s1, Table S1: Probiotic Search Term.
Author Contributions
Conceptualization, C.S. (Chatuthanai Savigamin), C.S. (Chatpol Samuthpongtorn) and K.P.; methodology, C.S. (Chatpol Samuthpongtorn), N.M. and T.N.; validation, C.S. (Chatuthanai Savigamin), N.M., T.N. and K.P.; formal analysis, C.S. (Chatuthanai Savigamin); resources, K.P.; data curation, C.S. (Chatuthanai Savigamin), C.S. (Chatpol Samuthpongtorn) and T.N.; writing—original draft preparation, C.S. (Chatuthanai Savigamin); writing—review and editing, K.P. and J.H.; visualization, K.P.; supervision, T.N. and K.P.; project administration, K.P.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviation
| A20 | ubiquitin E3 ligase A20 |
| Akt | Ak strain transforming |
| BDNF | Brain-Derived Neurotrophic Factor |
| CA1 | Cortical Area 1 |
| CENTRAL | Cochrane Central Register of Clinical Trials |
| DAMPS | Damage-Associated Molecular Pattern |
| DALYs | Disability-Adjusted Life Years |
| GABA | γ-Aminobutyric Acid |
| GFAP | Glial Fibrillary Acidic Protein |
| Iba1 | Ionized calcium binding adaptor molecule 1 |
| IkB | Inhibitor of nuclear factor kappa B |
| IL-1 | Interleukin 1 |
| IL-10 | Interleukin 10 |
| LPS | Lipopolysaccharide |
| MDA | Malondialdehyde |
| MCAO | Middle cerebral artery occlusion |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PNE | Panax Notoginsenoside Extract |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| SOD | Superoxide Dismutase |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| TLR4 | Toll-Like Receptor 4 |
| TNF alpha | Tumor necrosis factor alpha |
References
- 1.Lanas F., Seron P. Facing the stroke burden worldwide. Lancet Glob. Health. 2021;9:e235–e236. doi: 10.1016/S2214-109X(20)30520-9. [DOI] [PubMed] [Google Scholar]
- 2.Stinear C.M., Lang C.E., Zeiler S., Byblow W.D. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19:348–360. doi: 10.1016/S1474-4422(19)30415-6. [DOI] [PubMed] [Google Scholar]
- 3.Cramer S.C. Drugs to Enhance Motor Recovery After Stroke. Stroke. 2015;46:2998–3005. doi: 10.1161/STROKEAHA.115.007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson J.M. SSRI Antidepressant Medications: Adverse effects and tolerability. Prim. Care Companion J. Clin. Psychiatry. 2001;3:22–27. doi: 10.4088/PCC.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borovac J. Side effects of a dopamine agonist therapy for Parkinson’s disease: A mini-review of clinical pharmacology. Yale J. Biol. Med. 2016;89:37–47. [PMC free article] [PubMed] [Google Scholar]
- 6.Lye H.S., Balakrishnan K., Thiagarajah K., Ismail N.I.M., Ooi S.Y. Beneficial Properties of Probiotics. Trop. Life Sci. Res. 2016;27:73–90. doi: 10.21315/tlsr2016.27.2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mörkl S., Butler M.I., Holl A., Cryan J.F., Dinan T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020;9:171–182. doi: 10.1007/s13668-020-00313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer L.M., Aravind L., Coon S.L., Klein D., Koonin E.V. Evolution of cell-cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004;20:292–299. doi: 10.1016/j.tig.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 10.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2021;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luck B., Engevik M.A., Ganesh B., Lackey E.P., Lin T., Balderas M., Major A., Runge J., Luna R.A., Sillitoe R.V., et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 2020;10:7737. doi: 10.1038/s41598-020-64173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durgan D.J., Lee J., McCullough L.D., Bryan R.M., Jr. Examining the Role of the Microbiota-Gut-Brain Axis in Stroke. Stroke. 2019;50:2270–2277. doi: 10.1161/STROKEAHA.119.025140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W., Dowd S.E., Scurlock B., Acosta-Martinez V., Lyte M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol. Behav. 2009;96:557–567. doi: 10.1016/j.physbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Akhoundzadeh K., Vakili A., Shadnoush M., Sadeghzadeh J. Effects of the Oral Ingestion of Probiotics on Brain Damage in a Transient Model of Focal Cerebral Ischemia in Mice. Iran. J. Med. Sci. 2018;43:32–40. doi: 10.30476/IJMS.2018.40511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q., Liang X. Food therapy and medical diet therapy of Traditional Chinese Medicine. Clin. Nutr. Exp. 2018;18:1–5. doi: 10.1016/j.yclnex.2018.01.001. [DOI] [Google Scholar]
- 16.Cassir N., Benamar S., La Scola B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016;22:37–45. doi: 10.1016/j.cmi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Bae E.-A., Hyun Y.-J., Choo M.-K., Oh J.K., Ryu J.H., Kim D.-H. Protective effect of fermented Red ginseng on a transient focal ischemic rats. Arch. Pharmacal Res. 2004;27:1136–1140. doi: 10.1007/BF02975119. [DOI] [PubMed] [Google Scholar]
- 18.Chen R., Wu P., Cai Z., Fang Y., Zhou H., Lasanajak Y., Tang L., Ye L., Hou C., Zhao J. Puerariae Lobatae Radix with Chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain–gut barriers of dietary capsaicin against chronic low-grade inflammation. J. Nutr. Biochem. 2019;65:101–114. doi: 10.1016/j.jnutbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Li H., Xiao J., Li X., Chen H., Kang D., Shao Y., Shen B., Zhu Z., Yin X., Xie L., et al. Low Cerebral Exposure Cannot Hinder the Neuroprotective Effects of Panax Notoginsenosides. Drug Metab. Dispos. 2017;46:53–65. doi: 10.1124/dmd.117.078436. [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Sun J., Wang F., Yu X., Ling Z., Li H., Zhang H., Jin J., Chen W., Pang M., et al. Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. BioMed Res. Int. 2015;2015:412946. doi: 10.1155/2015/412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei Z.-G., Tan L.-J., Wang J.-F., Li X.-L., Huang W.-F., Zhou H.-J. Fermented Chinese formula Shuan-Tong-Ling attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen. Res. 2017;12:425–432. doi: 10.4103/1673-5374.202946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagao M., Yamano S., Imagawa N., Igami K., Miyazaki T., Ito H., Watanabe T., Kubota K., Katsurabayashi S., Iwasaki K. Effect of Lactobacillus paracasei A221-fermented ginseng on impaired spatial memory in a rat model with cerebral ischemia and β-amyloid injection. Tradit. Kamp-Med. 2019;6:96–104. doi: 10.1002/tkm2.1220. [DOI] [Google Scholar]
- 23.Pang S., Luo Z., Wang C.C., Hong X., Zhou J., Chen F., Ge L., Li X., Dai Y., Wu Y., et al. Effects of Dioscorea polystachya ‘yam gruel’ on the cognitive function of diabetic rats with focal cerebral ischemia-reperfusion injury via the gut-brain axis. J. Integr. Neurosci. 2020;19:273–283. doi: 10.31083/j.jin.2020.02.69. [DOI] [PubMed] [Google Scholar]
- 24.Park S., Kim D.S., Kang S., Moon B.R. Fermented soybeans, Chungkookjang, prevent hippocampal cell death and β-cell apoptosis by decreasing pro-inflammatory cytokines in gerbils with transient artery occlusion. Exp. Biol. Med. 2015;241:296–307. doi: 10.1177/1535370215606811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahmati H., Momenabadi S., Vafaei A.A., Bandegi A.R., Mazaheri Z., Vakili A. Probiotic supplementation attenuates hippocampus injury and spatial learning and memory impairments in a cerebral hypoperfusion mouse model. Mol. Biol. Rep. 2019;46:4985–4995. doi: 10.1007/s11033-019-04949-7. [DOI] [PubMed] [Google Scholar]
- 26.Sun J., Wang F., Ling Z., Yu X., Chen W., Li H., Jin J., Pang M., Zhang H., Yu J., et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 27.Wanchao S., Chen M., Zhiguo S., Futang X., Mengmeng S. Protective effect and mechanism of Lactobacillus on cerebral ischemia reperfusion injury in rats. Braz. J. Med. Biol. Res. 2018;51:e7172. doi: 10.1590/1414-431x20187172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S., Xu S., Duan H., Zhu Z., Yang Z., Cao J., Zhao Y., Huang Z., Wu Q., Duan J. A novel, highly-water-soluble apigenin derivative provides neuroprotection following ischemia in male rats by regulating the ERK/Nrf2/HO-1 pathway. Eur. J. Pharmacol. 2019;855:208–215. doi: 10.1016/j.ejphar.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Kong C., Gong L., Zhang X., Zhu Y., Wang H., Qu X., Gao R., Yin F., Liu X., et al. The Association of Post-Stroke Cognitive Impairment and Gut Microbiota and its Corresponding Metabolites. J. Alzheimer’s Dis. 2020;73:1455–1466. doi: 10.3233/JAD-191066. [DOI] [PubMed] [Google Scholar]
- 30.Vorhees C.V., Williams M. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould T.D., Dao D.T., Kovacsics C.E. The Open Field Test. In: Gould T.D., editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Humana Press; Totowa, NJ, USA: 2009. pp. 1–20. [Google Scholar]
- 32.Idriss H.T., Naismith J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Ling Z., Wang F., Chen W., Li H., Jin J., Zhang H., Pang M., Yu J., Liu J. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci. Lett. 2016;613:30–35. doi: 10.1016/j.neulet.2015.12.047. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharosay A., Bharosay V.V., Bandyopadhyay D., Sodani A., Varma M., Baruah H. Effect of Lipid Profile Upon Prognosis in Ischemic and Haemorrhagic Cerebrovascular Stroke. Indian J. Clin. Biochem. 2013;29:372–376. doi: 10.1007/s12291-013-0372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piironen K., Putaala J., Rosso C., Samson Y. Glucose and Acute Stroke. Stroke. 2012;43:898–902. doi: 10.1161/STROKEAHA.111.631218. [DOI] [PubMed] [Google Scholar]
- 38.You S., Yin X., Liu H., Zheng D., Zhong C., Du H., Zhang Y., Zhao H., Qiu C., Fan L., et al. Hyperfibrinogenemia is Significantly Associated with an Increased Risk of In-hospital Mortality in Acute Ischemic Stroke Patients. Curr. Neurovascular Res. 2017;14:242–249. doi: 10.2174/1567202614666170621103604. [DOI] [PubMed] [Google Scholar]
- 39.Mehta V., Prabhakar R., Kumar R., Jyoti D., Sharma C.B. Fibrinogen as a Predictor of Early Neurological Deterioration and Poor Outcome in Acute Ischemic Stroke. J. Assoc. Physicians India. 2022;70:11–12. [PubMed] [Google Scholar]
- 40.Mao C., Yuan J.-Q., Lv Y.-B., Gao X., Yin Z.-X., Kraus V.B., Luo J.-S., Chei C.-L., Matchar D.B., Zeng Y., et al. Associations between superoxide dismutase, malondialdehyde and all-cause mortality in older adults: A community-based cohort study. BMC Geriatr. 2019;19:104. doi: 10.1186/s12877-019-1109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukai T., Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dohare P., Hyzinski-García M.C., Vipani A., Bowens N.H., Nalwalk J.W., Feustel P.J., Keller R.W.K., Jr., Jourd’Heuil D., Mongin A.A. The neuroprotective properties of the superoxide dismutase mimetic tempol correlate with its ability to reduce pathological glutamate release in a rodent model of stroke. Free Radic. Biol. Med. 2014;77:168–182. doi: 10.1016/j.freeradbiomed.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gugliandolo A., Bramanti P., Mazzon E. Activation of Nrf2 by Natural Bioactive Compounds: A Promising Approach for Stroke? Int. J. Mol. Sci. 2020;21:4875. doi: 10.3390/ijms21144875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alfieri A., Srivastava S., Siow R.C.M., Modo M., Fraser P.A., Mann G.E. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J. Physiol. 2011;589:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Björkholm C., Monteggia L.M. BDNF—A Key Transducer of Antidepressant Effects. Neuropharmacology. 2015;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu B., Arya A. Brain-gut axis after stroke. Brain Circ. 2018;4:165–173. doi: 10.4103/bc.bc_32_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhikav V., Anand K.S. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012;15:239–246. doi: 10.4103/0972-2327.104323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaidi S.F., Aghaebrahim A., Urra X., Jumaa M.A., Jankowitz B., Hammer M., Nogueira R., Horowitz M., Reddy V., Jovin T.G. Final Infarct Volume Is a Stronger Predictor of Outcome Than Recanalization in Patients with Proximal Middle Cerebral Artery Occlusion Treated with Endovascular Therapy. Stroke. 2012;43:3238–3244. doi: 10.1161/STROKEAHA.112.671594. [DOI] [PubMed] [Google Scholar]
- 49.Anrather J., Iadecola C. Inflammation and Stroke: An Overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X., Hu Y., Yuan X., Yang J., Li K. Effect of early enteral nutrition combined with probiotics in patients with stroke: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2021;76:592–603. doi: 10.1038/s41430-021-00986-3. [DOI] [PubMed] [Google Scholar]
- 51.Fan W., Dai Y., Xu H., Zhu X., Cai P., Wang L., Sun C., Hu C., Zheng P., Zhao B. Caspase-3 Modulates Regenerative Response After Stroke. Stem Cells. 2013;32:473–486. doi: 10.1002/stem.1503. [DOI] [PubMed] [Google Scholar]
- 52.Keubler L.M., Buettner M., Häger C., Bleich A. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm. Bowel Dis. 2015;21:1967–1975. doi: 10.1097/MIB.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu F., Li G., Wen K., Bui T., Cao D., Zhang Y., Yuan L. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010;23:135–149. doi: 10.1089/vim.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elian S.D., Souza E.L., Vieira A.T., Teixeira M.M., Arantes R.M., Nicoli J.R., Martins F.S. Bifidobacterium longum subsp. infantis BB-02 attenuates acute murine experimental model of inflammatory bowel disease. Benef. Microbes. 2015;6:277–286. doi: 10.3920/BM2014.0070. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi Y., Kuhara T., Oki M., Xiao J.Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes. 2019;10:511–520. doi: 10.3920/BM2018.0170. [DOI] [PubMed] [Google Scholar]
- 56.Halliwell B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 57.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24:1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams T.I., Lynn B.C., Markesbery W.R., Lovell M.A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol. Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Shandilya S., Kumar S., Jha N.K., Kesari K.K., Ruokolainen J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2021;38:223–244. doi: 10.1016/j.jare.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.