Abstract
Purpose:
The primary endpoint results from the comparing alternative ranibizumab dosages for safety and effcacy in retinopathy of prematurity (CARE-ROP) core study identified ranibizumab as an effective treatment to control acute retinopathy of prematurity (ROP). This study reports the 1- and 2-year follow-up data focusing on long-term functional outcomes and safety.
Methods:
The CARE-ROP trial compared 0.12 mg versus 0.20 mg ranibizumab in 20 infants with ROP in a multicentric, prospective, randomized, double-blind, controlled study design. Sixteen patients entered the follow-up period. An ophthalmologic assessment at one year postbaseline was acquired from all 16 patients and a neurodevelopmental assessment at two years postbaseline was acquired from 15 patients.
Results:
Fifteen of 16 infants were able to fixate and follow moving objects at one year postbaseline treatment. One child progressed to stage 5 ROP bilaterally between the end of the core study and the 1-year follow-up (first seen at PMA 75 weeks). Mean spherical equivalents were −1.9 diopters (D) and −0.75 D in the 0.12 mg and the 0.20 mg treatment arms. Strabismus was present in seven and nystagmus in five out of 16 infants. Mental development scores were within normal limits in six out of ten patients with available data. No statistically significant difference was observed between the two treatment arms.
Conclusion:
Neurodevelopmental and functional ocular outcomes 1 and 2 years after treatment with ranibizumab are reassuring regarding long-term safety. Late reactivation of ROP, however, represents a challenge during the follow-up phase and it is of utmost importance that regular follow-ups are maintained.
Keywords: anti-vascular, endothelial, growth, factor – long-term, outcomes – ranibizumab – retinopathy of prematurity
Introduction
Retinopathy of prematurity (ROP) is still, almost 80 years after its first description (Terry 1942), a potentially blinding eye disease affecting millions of infants worldwide. In regions with advanced neonatal care, ROP occurs mainly in extremely small and early-born preterm children. In low- and middle-income countries, infants with higher birth weight are also at risk for developing treatment-warranting ROP. Annually, 23 800–45 600 infants worldwide are diagnosed with irreversible visual impairment from ROP (Blencowe et al. 2013). Retinopathy of prematurity accounts for up to 40% of preventable childhood blindness in low- and middle-income countries (Quinn 2016). Since 2019, ranibizumab, an anti-VEGF (vascular endothelial growth factor) agent, is approved by the European Medicines Agency in the dose of 0.20 mg for the treatment of ROP zone I stage 1+, 2+, 3+/−, as well as zone II stage 3+ and aggressive posterior ROP (AP-ROP) (Stahl et al. 2019; EMA. Europa.eu 2020). Intravitreal anti-VEGF treatment has thus become a widely accepted alternative to laser coagulation which has been the standard of care since the 1990s. There are, however, still unsolved questions and ongoing controversies among ophthalmologists as well as neonatologists regarding the choice between laser coagulation and anti-VEGF medication (Pertl et al. 2015; Mueller et al. 2017; Kennedy et al. 2018; Li et al. 2018; Ling et al. 2019). In particular, data for long-term safety of anti-VEGF treatments beyond the initial months after treatment are scarce and more data are needed to build a better basis for informed treatment decisions.
Only very few randomized controlled prospective studies have been conducted in this field, one of them being the CARE-ROP trial (comparing alternative ranibizumab dosages for safety and effcacy in retinopathy of prematurity). The results of the core study demonstrated that two different ranibizumab doses (0.12 and 0.20 mg) were effective in controlling acute ROP without altering systemic VEGF levels (Stahl et al. 2018). The significantly larger randomized controlled trial RAINBOW (RAnibizumab Compared With Laser Therapy for the Treatment of INfants BOrn Prematurely With Retinopathy of Prematurity), which had a study protocol very similar to CARE-ROP but with an additional laser arm, confirmed the CARE-ROP primary endpoint data both regarding the short-term effectiveness of ranibizumab as well as the fact that systemic VEGF levels remain unchanged after ranibizumab (Stahl et al. 2019).
Regarding long-term effcacy of anti-VEGF drugs, mainly case reports have been published to date, several of them describing the problem of late reactivations (Wong, Hubschman & Tsui 2015; Chan et al. 2016; Lyu et al. 2017). Dataon long-term safety, particularly neurodevelopment outcomes are even more scarce and mainly limited to retrospective analyses which are inherently prone to bias (Morin et al. 2016). In this study, we report long-term outcomes on both effectiveness and safety of ranibizumab for ROP by analysing the ophthalmologic outcomes at 1 year and the neurodevelopmental outcomes at 2 years postbaseline treatment from the prospective randomized CARE-ROP trial.
Materials and Methods
Study design and endpoints
The CARE-ROP study is a randomized, double-blind, prospective, multicenter, phase II investigator-initiated trial, in which two different doses of ranibizumab (0.12 mg versus 0.20 mg) are compared in two parallel study arms. Details of the trial design and primary endpoint results have been published (Stahl et al. 2018). The study is conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Legal representatives gave their written informed consent before an infant was included into the study. The study consists of a core study and a long-term follow-up study. While the core study ended with the primary endpoint at 24 weeks post first injection, the long-term follow-up study is continued until a corrected age of 5 years with planned study visits at 1 year ( ± 2 months), 2 years ( ± 3 months) and 5 years ( ±6 months). The current analysis reports data from the follow-up visits at 1 and 2 years. The main focus of the 1- and 2-year follow-ups lies on the ophthalmologic and paediatric outcome as well as the long-term safety of ranibizumab.
Patient allocation
The core study was conducted in nine German hospitals, of which six centres recruited patients. Between September 2014 and July 2016, 20 infants were screened, and 19 were enrolled into the trial. Ten patients were allocated to the 0.12 mg ranibizumab arm and nine to the 0.20 mg ranibizumab arm. Three patients died during the core study (one patient in the 0.12 mg arm, two patients in the 0.20 mg arm). None of these deaths was considered connected to the treatment. Two patients in the 0.12 mg arm received one regular re-injection (a re-injection of the same dose of ranibizumab was allowed according to the study protocol ≥ 28 days post previous injection). In the 0.20 mg arm one patient received one re-injection, and one patient received two re-injections. In each study arm, one patient received rescue treatment for one eye. Rescue treatment was laser coagulation in one patient, in the other patient both laser coagulation and ranibizumab re-injection was applied. All surviving patients including the ones who received re-injections or rescue treatment were eligible to enter the follow-up period of the trial (nine patients in the 0.12 mg arm, seven patients in the 0.20 mg arm). The follow-up visit at year 1 was performed by all remaining 16 patients. At the follow-up visit at year 2, one patient from the 0.12 mg arm did not participate, as the child was a refugee child and had returned to the country of origin together with the parents before the follow-up visit at year 2 (Fig. 1).
Fig. 1.
Enrollment, allocation into the two study arms during the CARE-ROP core study and follow-up at one year ( ±2 months) and two years ( ±3 months).
Assessments during follow-up
At year 1, an ophthalmologic assessment was performed, including orthoptic status (fixation, objection to occlusion, strabismus, motility, nystagmus), cycloplegic retinoscopy, refraction, slit lamp exam, measurement of intraocular pressure and fundoscopy. During this assessment, late reactivations and treatments of ROP between the end of the core study and the 1-year ophthalmologic follow-up were documented. At year 2, the results of a developmental test, which is routinely done at this age by the treating neonatologists, were entered into the case report form together with additional information on the presence or absence of cerebral palsy, deafness or blindness. The Bayley-Test was suggested for this purpose but centres were free to choose alternative tests that were regularly performed at the respective site.
Statistical analysis
The data obtained during the follow-up period of the CARE-ROP study were mainly analysed by descriptive statistical methods, p-values were calculated only for spherical equivalent, astigmatism and intraocular pressure (t-tests). Statistical analyses are presented by treatment group and in total. Categorical data are given as frequency tables. Numerical data are shown with N (number of observations), median, standard deviation and minimum and maximum values.
Results
Baseline characteristics of infants entering the follow-up study
Median gestational age, birth weight, body length and head circumference of infants entering the follow-up study were comparable between the two study arms. In both study arms five infants were born with a gestational age below 25 weeks (56 versus 71%), and in each arm four infants were female (44% versus 57%). (Table S1).
ROP status of patients when entering the follow-up study
At the last study assessment during the core study (24 weeks postbaseline treatment), 18 eyes (56%; equally distributed between the two study arms) had no ROP. A zone III stage 1 ROP without plus disease was present in 12 eyes (38%) (6 eyes (33%) in the 0.12 mg study arm versus 6 eyes (43%) in the 0.20 mg study arm), an anterior zone II stage 1 ROP without plus disease was present in two eyes (6%), both in the 0.12 mg study arm. (Table 1).
Table 1.
ROP status of patients when entering the follow-up study.
| Last postbaseline assessment during core study (≤24 weeks posttreatment) | |||||
|---|---|---|---|---|---|
|
|
|||||
| ROP severity, n (%) | Baseline | No ROP | III, 1- | IIa, 1- | Total |
| Ranibizumab 0.12 mg | IIp, 3+ | 7 (54) | 6 (46) | 0 (0.0) | 13 (100) |
| (N = 18 eyes) | I, 3+ | 3 (60) | 0 (0.0) | 2 (40) | 5 (100) |
| Total | 10 (56) | 6 (33) | 2 (11) | 18 (100) | |
| Ranibizumab 0.20 mg | IIp, 3+ | 6 (55) | 5 (45) | 0 (0.0) | 11 (100) |
| (N = 14 eyes) | I, 3+ | 2 (67) | 1 (33) | 0 (0.0) | 3 (100) |
| Total | 8 (57) | 6 (43) | 0 (0.0) | 14 (100) | |
| Total (N = 32 eyes) | IIp, 3+ | 13 (54) | 11 (46) | 0 (0.0) | 24 (100) |
| I, 3+ | 5 (63) | 1 (13) | 2 (25) | 8 (100) | |
| Total | 18 (56) | 12 (38) | 2 (6) | 32 (100) | |
ROP = retinopathy of prematurity.
Retinal outcomes one year after baseline treatment
In the follow-up study, at one year after baseline treatment, no eye had a visible demarcation line between the vascularized and the peripheral avascular area (N = 28 eyes). In total, three eyes had a visible remaining ridge but no signs of vascular activity. One infant in the 0.20 mg arm had progressed to stage 5 ROP in both eyes after a late reactivation of ROP at around 4 months after the end of the core study (details see below). (Table 2).
Table 2.
Retinal outcomes at one year ( ±2 months) postbaseline injection.
| Ranibizumab 0.12 mg (9 infants/18 eyes) | Ranibizumab 0.20 mg (7 infants/14 eyes) | Total (16 infants/32 eyes) | ||
|---|---|---|---|---|
| Demarcation line between avascular and vascularized retina visible (ROP stage 1) | No [N, eyes (%)] | 16 (100) | 12 (100) | 28 (100) |
| Yes [N, eyes (%)] | – | – | – | |
| Missing values [N, eyes] | 2 | 2 | 4 | |
| Prominent ridge visible (ROP stage 2) | No [N, eyes (%)] | 15 (93.8) | 10 (83.3) | 25 (89.3) |
| Yes [N, eyes (%)] | 1 (6.3) (1 clock hour in IIa/III, with inactive proliferations) | 2 (16.7) (12 clock hours in IIa; without proliferations) | 3 (10.7) | |
| Missing values [N, eyes] | 2 | 2 | 4 | |
| Late recurrence of ROP* | No recurrence [N, infants (%)] | 9 (100.0) | 6 (85.7) | 15 (93.8) |
| In one eye [N, infants (%)] | – | – | – | |
| In both eyes [N, infants (%)] | – | 1 (14.3) | 1 (6.3) | |
| Progression to stage 4 or 5 ROP† | No progression [N, infants (%)] | 9 (100.0) | 6 (85.7) | 15 (93.8) |
| In one eye [N, infants (%)] | – | – | – | |
| In both eyes [N, infants (%)] | – | 1 (14.3) | 1 (6.3) |
ROP = retinopathy of prematurity.
ROP treatment performed after last visit of the core study.
Between end of core study and year 1 visit.
Peripheral vascularization
At the timepoint of entering the follow-up study, more eyes in the 0.12 mg ranibizumab arm (50%) than in the 0.20 mg study arm (21%) had complete vascularization of the retina up to one disc diameter of the ora serrata. The periphery was not assessable in four eyes.
At the follow-up assessment at one year, the percentage of eyes that were not assessable had increased to almost 50%, while all eyes that were assessable showed complete vascularization of the retina. (Table S2).
Late reactivations of ROP and re-treatments during follow-up period
During the follow-up period, one infant (14%) in the 0.20 mg ranibizumab group had a late reactivation of active ROP, while all other 15 infants did not receive any treatment after the end of the core study. The infant with late ROP reactivation had previously received two re-treatments within the core study at 7 and 17 weeks postbaseline. ROP stage at baseline had been 3+ in zone IIp in the right eye and 3+ in zone I in the left eye with severe plus disease in both eyes. After the initial treatment, plus disease and active proliferations had completely resolved by week 5. At 6 weeks, however, signs of active ROP reappeared bilaterally with stage 2+ in zone IIp. Only 5 days later, active proliferations re-developed bilaterally (zone IIp, stage 3+ in both eyes), and re-treatment with the study drug was reapplied as per core study protocol. After this re-treatment, signs of ROP dissolved within 1 week and remained absent until 8 weeks after re-treatment. At 10 weeks after re-treatment, ROP recurred bilaterally with stage 3+ in zone IIp and a third injection was applied as per protocol (at 17 weeks post initial injection). After this second re-treatment, signs of ROP disappeared more slowly than after the first re-injection: within one day plus disease resolved but the ridge took 4 weeks to disappear (stage 1 in zone IIa). At the end of the core study, which corresponds to 6 weeks after the second re-injection in this infant, no signs of ROP in any eye were visible and vascularization had proceeded beyond the site of the ridge in 7 clock hours in both eyes (Fig. S1).
After the end of the core study, follow-up exams took place at a local ophthalmologist. On the last available report from these visits, the infant was reported with new stage 1 ROP in zone III in both eyes at 35 weeks post initial injection (18 weeks after last re-treatment, 70 weeks PMA). Another follow-up exam was scheduled one week later but was postponed by the parents. When the exam eventually took place 5 weeks later, the infant had developed bilateral stage 5 ROP (40 weeks postbaseline treatment, 17 weeks after the end of the core study, 23 weeks after last re-treatment, 75 weeks PMA). The infant was referred back to the study centre and vitrectomy was performed in the right eye. The left eye was not treated as the treating ophthalmologists assessed that a treatment would not result in a favourable outcome.
Orthoptic status at one year after baseline injection
At the 1-year follow-up, 13 infants (81%) had equal fixation with both eyes. Two children in the 0.12 mg arm had unequal fixation, and the child who had progressed to stage 5 ROP was not able to fixate and follow moving objects with either eye. All infants assessed showed equal objection to occlusion (data missing for one infant). About half of the infants (56%) in both study arms had no strabismus. Slightly more children in the 0.12 mg arm had esotropia than in the 0.20 mg arm. In one child in the 0.12 mg study arm (11%), motility was restricted in both eyes. Nystagmus was present in both eyes of 31% of infants. (Tables 3 and S3).
Table 3.
Orthoptic status at 1 year.
| Ranibizumab 0.12 mg (N = 9) | Ranibizumab 0.20 mg (N = 7) | Total (N = 16) | ||
|---|---|---|---|---|
| Fixation | Equal fixation OD/OS [N, infants (%)] | 7 (78) | 6 (86) | 13 (81) |
| Unequal fixation [N, infants (%)] | 2 (22) | - | 2 (13) | |
| No fixation [N, infants (%)] | - | 1 (14) | 1 (6) | |
| Objection to occlusion | Equal objection [N, Infants (%)] | 8 (100) | 7 (100) | 15 (100) |
| Missing values [N, infants (%)] | 1 | - | 1 | |
| Strabismus | No strabismus [N, infants (%)] | 5 (56) | 4 (57) | 9 (56) |
| Exotropia [N, infants (%)] | - | - | - | |
| Esotropia [N, infants (%)] | 4 (44) | 2 (29) | 6 (38) | |
| Other [N, infants (%)] | - | 1 (14) | 1 (6) | |
| Motility | Both eyes fix and follow without problems [N, infants (%)] | 8 (89) | 6 (86) | 14 (88) |
| Motility restricted in one eye [N, infants (%)] | - | - | - | |
| Motility restricted in both eyes [N, infants (%)] | 1 (11) | - | 1 (6) | |
| Not applicable due to lack of fixation [N, infants (%)] | - | 1 (14) | 1 (6) | |
| Nystagmus* | No nystagmus [N, infants (%)] | 6 (67) | 5 (71) | 11 (69) |
| Nystagmus in both eyes [N, infants (%)] | 3 (33) | 2 (29) | 5 (31) |
See Table S3 for more information on nystagmus.
Refraction and intraocular pressure at one year after baseline treatment
In the large majority of infants (26 eyes), the spherical equivalent ranged between −3 diopters (D) and +3 D. One child (0.12 mg arm; rescue treatment with laser and ranibizumab due to progression to stage 4 in the right eye during the core study) had a spherical equivalent of −10.5 D and −10 D. The mean spherical equivalent did not differ significantly between the two study arms (−1.9 D in the 0.12 mg arm and −0.75 D in the 0.20 mg arm; p = 0.285, unpaired t-test). Astigmatism ranged from 0 to 3 D with a mean of 0.61 D in the 0.12 mg arm and 0.71 D in the 0.20 mg arm (p = 0.860, unpaired t-test). Intraocular pressure ranged from 10 to 20 mmHg for all eyes with available data, with a mean of 14.08 and 14.25 mmHg, respectively (p = 0.893, unpaired t-test) (Fig. 2).
Fig. 2.
Distribution of spherical equivalent, astigmatism and intraocular pressure by treatment arm. Mean values of the two study arms were compared with unpaired t-test; line and whiskers represent mean with standard deviation (SD).
Slit lamp and retinal exam at one year after baseline treatment
No abnormality on slit lamp exam was seen in 11 eyes (79%) in the 0.12 mg study arm, and in all eyes in the 0.20 mg study arm. On retinal exam, abnormalities were seen in about 40% of the eyes in both treatment arms. (Table S4).
Paediatric development
Paediatric development was assessed at 2 years (±3 months) after baseline treatment. No child was reported deaf in any ear nor needed to wear hearing aids. The child in the 0.20 mg study arm who progressed to stage 5 ROP in both eyes was reported blind in both eyes. The child in the 0.12 mg study arm, who had received rescue treatment during the core study due to a progression of ROP to stage 4 in the right eye but had recovered to no ROP thereafter and who had developed high myopia bilaterally (details see above), was able to see with both eyes wearing glasses. At the time of follow-up (postnatal age about two years), 88% of the children in the 0.12 mg study arm and 57% in the 0.20 mg study arm were able to walk, one child with walking aids. A cerebral palsy was not reported in any of the children. (Table 4).
Table 4.
Paediatric development: deafness, blindness, walking ability and cerebral palsy at year 2.
| Ranibizumab 0.12 mg (N = 8) | Ranibizumab 0.20 mg (N = 7) | Total (N = 15) | ||
|---|---|---|---|---|
| Deafness present | No deafness [N, infants (%)] | 8 (100) | 7 (100) | 15 (100) |
| Infant wearing hearing aids | Not present [N, infants (%)] | 8 (100) | 7 (100) | 15 (100) |
| Blindness present | No blindness [N, infants (%)] | 8 (100) | 6 (86) | 14 (93) |
| In both eyes [N, infants (%)] | - | 1 (14) | 1 (7) | |
| Infant wearing | Yes [N, infants (%)] | 1 (13) | - | 1 (7) |
| glasses | No [N, infants (%)] | 7 (88) | 7 (100) | 14 (93) |
| Infant able to walk | Yes [N, infants (%)] | 7 (88) | 4 (57)* | 11 (73) |
| No [N, infants (%)] | 1 (13) | 3 (43) | 4 (27) | |
| Cerebral palsy present | No cerebral palsy [N, infants (%)] | 8 (100) | 7 (100) | 15 (100) |
One child able to walk with walking aid.
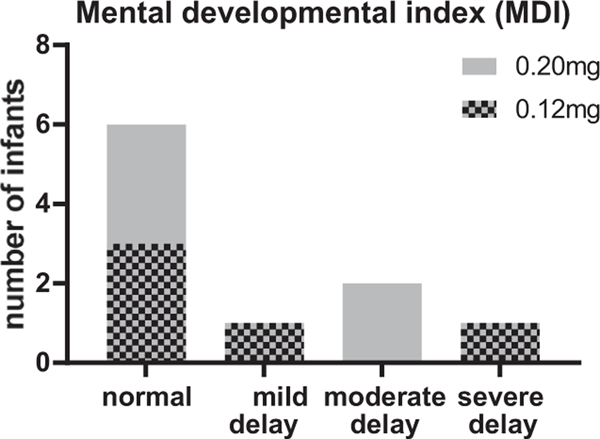
A mental developmental index (MDI) score was documented for five children in each study arm. In each arm, three children had an MDI ≥ 85 corresponding to a normal development. One child in the 0.12 mg study arm had a score of 80 corresponding to a mildly delayed development and one child had a score of less than 50, corresponding to a severe developmental delay. This child had an intraventricular haemorrhage (IVH) grade I and a cerebral haemorrhage before the child was included in the CARE-ROP core study. In the 0.20 mg arm, two children showed a moderately delayed development (MDI = 64 and 62; both with a diagnosis of IVH grade II). The psychomotor developmental index (PDI) was documented for three children only (two in the 0.12 mg arm versus one in the 0.20 mg arm). In the 0.12 mg arm, one child with normal MDI score showed a mild delay regarding the psychomotor development. The other child for whom the PDI was given, was the child with severe mental developmental delay, who was also severely delayed regarding the psychomotor development. Another infant in the 0.20 mg arm, who showed a normal MDI was severely delayed regarding psychomotor development. This infant was diagnosed with IVH grade I before inclusion in the CARE-ROP trial. (Table 5 and Fig. 3) For two children in the 0.12 mg ranibizumab arm, the Munich functional developmental test was performed instead of the Bayley-Test. The test was performed at around two years postnatal age. In one child, the developmental age estimated based on the test results ranged for manual dexterity from 9 to 17 months, the developmental age for perception was estimated with 21 months, the developmental age for speaking was 19 months, for understanding language 21 months and for social behaviour 15 months. In the second child, the developmental age for perception was estimated between 12 and 17 months and for manual skills 12.5 to 16 months (further developmental ages were not given for this child).
Table 5.
Developmental scores assessed at year 2.
| Patient | Bayley-test | Gestational age at birth | MDI | PDI | Medical history of IVH, cerebral haemorrhage or hydrocephalus | |
|---|---|---|---|---|---|---|
| 0.12 mg ranibizumab | 1 | Bayley III | 22 + 6 | 80 | - | No |
| 2 | Bayley II | 26 + 6 | 90 | - | No | |
| 3 | Bayley II | 26 + 6 | 94 | - | No | |
| 4 | Bayley II | 24 + 1 | <50 | <50 | IVH I and cerebral haemorrhage | |
| 5 | Bayley II | 24 + 1 | 102 | 73 | No | |
| 0.20 mg ranibizumab | 1 | Bayley II | 23 + 1 | 62 | - | IVH II |
| 2 | Bayley II | 24 + 4 | 64 | - | IVH II | |
| 3 | Bayley III | 23 + 5 | 100 | - | No | |
| 4 | Bayley III | 25 + 1 | 90 | - | No | |
| 5 | Bayley II | 24 + 5 | 90 | 50 | IVH I |
MDI = Mental Developmental Index; PDI = Psychomotor Developmental Index.
Fig. 3.
Visualization of mental developmental index by development categories.
Discussion
Anti-VEGF drugs have become a widely used treatment option for ROP in recent years. Prospective, randomized controlled trials, however, are still rare, especially concerning long-term data on safety and efficacy. The CARE-ROP trial is one of these few prospective trials with long-term follow-up. It is to our knowledge also the only trial with blinded treatment arms. Despite the small study population of only 19 infants, data from this trial therefore provides valuable information on long-term outcomes after ranibizumab in ROP.
The primary endpoint data of the CARE-ROP study provided evidence for the efficacy of ranibizumab in controlling acute ROP without altering systemic VEGF levels (Stahl et al. 2018). The current report adds data on long-term outcomes at one and two years postbaseline treatment.
The CARE-ROP core study reported a re-treatment rate of 21% until 24 weeks post initial treatment (Stahl et al. 2018). It is important to distinguish between regular re-treatments that become necessary due to a disease reactivation and rescue treatments that become necessary due to an insufficient initial treatment response. The latter was the case in only two out of 19 treated infants. By their nature, rescue treatments occur in timely association with an (insufficiently effective) primary treatment. Disease reactivation in contrast, can occur months after treatment (Hu et al. 2012; Wong, Hubschman & Tsui 2015; Walz et al. 2016; Walz et al. 2018). In CARE-ROP, infants were therefore followed after the end of the core study by either their local ophthalmologist or at the study centre, the location for follow-ups depending on joint decisions made by parents and ophthalmologists. Between the end of the core study and the 1-year visit, these follow-up exams were not defined as study visits but were scheduled and carried out based on the decisions made by the ophthalmologist performing the follow-ups following the German guidelines for ROP screening and treatment. The first mandatory study visit per protocol after the end of the core study was at one year postbaseline. This visit was completed by all infants who entered the follow-up study.
Between the end of the core study and the 1-year follow-up, one infant had an ROP reactivation that was not diagnosed early enough to allow for retreatment with either anti-VEGF or laser. This infant progressed to stage 5 ROP in both eyes. Since the follow-up exams between end of core study and 1-year follow-up were performed outside the study centre, limited detail is known about this case. We did receive, however, information on zone III, stage 1 disease 35.4 weeks postbaseline treatment. The follow-up appointment which was scheduled one week later, was cancelled by the patient’s family. Instead, the child was seen 5.1 weeks later when both eyes had developed stage 5 ROP. This unfortunate course of events emphasizes the importance of regular intensive follow-up exams over several months following anti-VEGF treatment and how important stringent and uninterrupted communication between physicians and parents is in this regard. In cases with re-treatments, the last treatment should be considered a new baseline for follow-ups with initial short-term exams and further follow-ups and based upon the current ROP stage and zone (Deutsche Ophthalmologische Gesellschaft e. V. (DOG), Retinologische Gesellschaft e. V. (RG) & Berufsverband der Augenärzte Deutschlands e. V. (BVA) 2020). Exams may need to continue for several months after each treatment, as late reactivations of ROP have been described up to an age of 35 weeks after anti-VEGF injection or 69 weeks postmenstrual age (Hu et al. 2012; Mintz-Hittner et al., 2016). In cases where the periphery of the retina remains avascular, an alternative approach can be to apply laser therapy to the remaining avascular retina (Hu et al. 2012; Chan et al. 2016; Akdogan et al., 2019). It should be noted that the follow-up period after anti-VEGF therapy remains a challenging time period to investigators, patients and parents with only limited data existing at present to guide examiners and with increasingly diffcult procedures when the infants get older.
The risk of late reactivations after anti-VEGF treatment challenges ophthalmologists because the retinal periphery becomes increasingly diffcult to assess in older infants. This is also illustrated by the fact that full vascularization to the ora serrata was confirmed in only 17 out of 32 eyes one year postbaseline with missing values for the remaining 15 eyes. As an additional word of caution, we would like to point out that in the infant progressing to stage 5 ROP, the retina was noted as fully vascularized in 3 clock hours at 3 visits between the first and second re-injection, but reported not reaching the ora serrata in any clock hour at subsequent visits. This illustrates how diffcult it can be to judge peripheral vascularization based on fundoscopy alone. It should be emphasized that repeated and detailed retinal exams are required after anti-VEGF injection, including scleral depression in order to ensure a good view of the retinal periphery and to not miss significant findings at the edge of the vascularized retina that can lead to a progression to stage 4 or 5 ROP. If in doubt, an examination under anaesthesia (EAU) can be performed. In addition, fluorescein angiography can be used to examine peripheral vascularization status, but is often not widely available (Klufas et al. 2015; Patel et al. 2015).
Regarding functional outcomes, our study reports relatively mild myopia of −1.9 D and −0.75 D after ranibizumab treatment in the 0.12 mg and the 0.20 mg arm, respectively, similar to what has been published for bevacizumab (Harder et al. 2013; Geloneck et al. 2014; Gunay et al. 2015; Hwang et al. 2015; Krohne et al. 2018). Only one infant in our study developed high myopia of −10.0 D and −10.5 D and this infant had needed rescue therapy with laser and ranibizumab re-injection for stage 4 ROP in the core study. Similarly, our results for posttreatment astigmatism are comparable to values published for bevacizumab (Harder et al. 2013). All infants except the one infant with stage 5 ROP were able to fixate and follow moving objects at one year and had no negative structural outcomes of the macular region like macular dragging or macular folds.
Regarding neurodevelopmental outcomes, our study reports data on only a subgroup of ten patients. Limitations when interpreting these results are the small sample size, the large number of comorbidities that can affect neurodevelopment outcomes in ROP infants (Jarjour 2015), the relatively wide time interval for the assessment at 2 years of ±3 months and the fact that different tests were used for the paediatric assessments at two years, which makes statistical comparisons diffcult. Due to the well-known and prolonged effect of intravitreally applied bevacizumab on systemic VEGF-levels (Sato et al. 2012; Hoerster et al. 2013), and the discussed effect of anti-VEGF on brain development (Morin et al. 2016) it is important to assess paediatric outcome data. Reassuringly, ranibizumab does not lead to prolonged systemic VEGF suppression (Stahl et al. 2018; Stahl et al. 2019). Regarding paediatric outcomes at two years, no infant in this trial was reported deaf or with cerebral palsy. It needs to be noted that cerebral palsy is in some infants only diagnosed after the age of 2 years, indicating the need for even longer follow-up visits which are planned for this cohort (Granild-Jensen et al. 2015). It is positive to note that MDIs were within normal limits for six out of ten infants for whom this data was reported. In infants with the lowest MDI scores, significant co-morbidities affecting the brain development were noted which might be the explanation for the low scores (IVH and cerebral haemorrhage). Only one infant in the 0.12 mg arm was reported to have severe developmental delay. This infant had been diagnosed with pre-existing intraventricular and cerebral haemorrhages at the CARE-ROP screening visit (i.e. prior to baseline treatment). In comparison to the bevacizumab and the laser group in the retrospective analysis of Morin et al. (2016) less severe neurodevelopmental impairment was documented in our cohort. The 2-year data from the BEAT-ROP trial reported no severe neurodevelopmental disability in any of the two treatment arms. (Kennedy et al. 2018). The EPICE cohort, a cohort that includes all infants born below 32 weeks from 15 regions in 10 European countries, reported neurodevelopmental impairment in 17.3% of infants (Draper et al. 2020). In general, it is extremely diffcult to compare neurodevelopmental outcomes across different trials as both the trial cohorts as well as the neurodevelopmental tests differ between trials. Standardization of test procedures and data presentation would help improving the comparability of trial results in the future.
In conclusion, the results of the 1- and 2-year follow-up data from CARE-ROP show that anti-VEGF treatment with ranibizumab appears to be safe and effective. Late reactivations, however, have to be taken very seriously and follow-up exams need to be conducted with utmost care. For example, an ROP follow-up ID card for treated infants can be used to plan and document all scheduled ophthalmological exams. The ROP ID card can be carried by the parents along with other essential medical information so that it is readily available in emergency situations, for example in case of unexpected inpatient admissions that may interfere with a scheduled ophthalmological follow-up visit. The German ROP screening guidelines have adopted such an ROP ID card that can be downloaded from the respective websites (Deutsche Ophthalmologische Gesellschaft e. V. (DOG), Retinologische Gesellschaft e. V. (RG) & Berufsverband der Augenärzte Deutschlands e. V. (BVA) 2020). Further results on the ophthalmological and paediatric outcomes for treated ROP infants from the CARE-ROP trial will be reported as soon as the 5-year follow-up data become available.
Supplementary Material
Figure S1. Graphic representation of the ROP time course during core study and follow-up for the patient progressing to stage 5 ROP.
Table S1. Baseline characteristics of patients who entered the follow-up study.
Table S2. Peripheral vascularization at the end of the core study and at 1 year.
Table S3. Detailed information on nystagmus.
Table S4. Abnormalities on slit lamp and retinal exam at 1 year.
Funding/support:
The University Hospital Freiburg is the study sponsor. Novartis Pharma GmbH, Germany, provided financial support and study medication. Novartis Pharma GmbH was not involved in the design and conduct of the study or collection, management, analysis, and interpretation of the data; Novartis Pharma GmbH was able to review the manuscript prior to submission but was not involved in preparation or approval of the manuscript or the decision to submit the manuscript for publication.
Financial disclosures:
AS: speaker fees/honoraria from Allergan, Bayer, Novartis; research funding from Novartis. MCB: speaker fees from Alcon, WAL: speaker fees/honoraria Santhera, Infectopharm, Boehringer Ingelheim. FEM: none. TB: advisory board and research funding from Novartis. NE: research funding/speaker fees from Novartis, Bayer, Allergan, Roche; consultant fees from Bayer; advisor for Apellis Pharmaceuticals, Alcon, Bayer, Novartis, Roche; lecture fees from Apellis Pharmaceuticals, Bayer, Novartis, Roche. RG: none. TUK: consultancy fees/speaker fees/travel reimbursement from Alimera Sciences, Allergan, Heidelberg Engineering, Roche; grants and personal fees from Bayer, Novartis. JMP: none
Appendix 1
Comparing Alternative Ranibizumab Dosages for Safety and Effcacy in Retinopathy of Prematurity (CARE-ROP) Study Group:
The CARE-ROP Study Group members are as follows: University of Freiburg, Ophthalmology: Anima Bühler, MD, Moritz Daniel, MD, Susanne Felzmann, Nikolai Gross, MD, Stefanie Horn, MD, Wolf Lagrèze, MD, Fanni Molnár, MD, Claudia Müller, Sabine Reichl, MD, Charlotte Reiff, MD, Olga Richter, MD, Andreas Stahl, MD, Milena Stech, MD; University of Freiburg, Neonatology: Roland Hentschel, MD, Dimitra Stavropoulou, MD, Juliane Tautz, MD; University of Bonn, Ophthalmology: Kerstin Bartsch, Jennifer Braunstein, MD, Ralf Brinken, Christian K. Brinkmann, MD, Joanna Czauderna, Wiebke Dralle, MD, Martin Gliem, Arno Goebel, MD, Philipp Heymer, MD, Martina Hofmann, Frank G. Holz, MD, Tim U. Krohne, MD, David Kupitz, MD, Philipp Müller, MD, Michael Petrak, MD, Eva J. Schmitz, MD, Steffen Schmitz-Valckenberg, MD, Moritz Schröder, MD, Julia Steinberg, MD, Julia Supé; University of Bonn, Neonatology: Evelyn Kant, MD, Diana Kunze, MD, Andreas Müller, MD; University of Münster, Ophthalmology: Adeline Adorf, Anne Alex, MD, Florian Alten, MD, Christoph R. Clemens, MD, Nicole Eter, MD, Silvia Falkenau, Caroline Friedhoff, Desiree Sandra Loos, MD, Natasa Mihailovic, MD, Julia Termühlen, Constantin Uhlig, MD; University of Münster, Neonatology: Isabell Hörnig-Franz, MD, Esther Rieger-Fackeldey, MD, Maria Tekaat, Claudius Werner; University of Regensburg, Ophthalmology: Mathias Altmann, MD, Teresa Barth, MD, Christiane Blecha, MD, Sabine Brandl, Horst Helbig, MD, Karsten Hufendiek, MD, Herbert Jägle, MD, Julia Konrad, MD, Eva Kopetzky, MD, Fabian Lehmann, MD, Isabel Oberacher-Velten, MD; Barmherzige Brüder Hospital Regensburg, Neonatology: Annette Keller-Wackerbauer, MD, Jochen Kittel, MD, Hugo Segerer, MD; University of Düsseldorf, Ophthalmology: Phillip Ackermann, Jemina Benga, Rainer Guthoff, MD, Tanja Guthoff, MD, Elena Kleinert, Ertan Mayatepek, MD, Stefan Schrader, MD, Magdalena Völker, MD; University of Düsseldorf, Neonatology: Thomas Höhn, MD, Klaus Lohmeier, MD, Hemmen Sabir, MD; Ertan Mayatepek, MD, University of Duisburg, Neonatology: Francisco Brevis, Tina Mönig, MD, Simone Schwarz, MD; University of Magdeburg, Ophthalmology: Angela Ehmer, Synke Meltendorf, MD, Claudia Schuart, MD; University of Magdeburg, Neonatology: Stefan Avenarius, MD, Ralf Böttger, MD; University of Magdeburg, Pharmacy: Christoph Apel, Anne Bergmann, Karsten Herrmann, Franziska Ockert-Schön, Sabine Wegener; Ludwigs-Maximilan University Munich, Ophthalmology: Oliver Ehrt, MD, Martin Nentwich, MD, Angelika Pressler, Günther Rudolph, MD; Ludwigs-Maximilan University Munich, Neonatology: Orsolya Genzel-Boroviczeny, MD, Susanne Schmidt, MD; Hauner’sches Kinderspital Munich, Neonatology: Hans-Georg Münch, MD, Claude Thilmany, MD; University of Tübingen, Ophthalmology: Sabine Aisenbrey, MD, Anna Bruckmann, MD, Spyridon Dimopoulos, MD, Ulrike Hagemann, Werner Inhoffen, PhD, Michael Partsch, MD, Merle Schrader, MD, Daniela Süsskind, MD, Michael Völker, MD; University of Töbingen, Neonatology: Anja Bialkowski, MD, Ingo Müller-Hansen, MD; University of Kiel, Ophthalmology: Andrea Gerberth, Heike Christine Hasselbach, MD, Solveig Lindemann, MD, Konstantine Purtskhvanidze, MD, Yvonne Raffel, Johann Roider; Data Safety Monitoring Board (DSMB): Heinrich Gerding MD, Claudia Jandeck MD, Lois Smith MD.
Footnotes
CARE-ROP study group members are listed in Appendix 1
References
- Akdogan M, Cevik SG & Sahin O (2019): The safety and effectiveness of 0.16 mg bevacizumab plus or minus additional laser photocoagulation in the treatment of retinopathy of prematurity. Indian J Ophthalmol 67: 879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H, Lawn JE, Vazquez T, Fielder A & Gilbert C (2013): Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 74: 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JJ, Lam CP, Kwok MK, Wong RL, Lee GK, Lau WW & Yam JC (2016): Risk of recurrence of retinopathy of prematurity after initial intravitreal ranibizumab therapy. Sci Rep 6: 27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsche Ophthalmologische Gesellschaft e. V. (DOG), Retinologische Gesellschaft e. V. (RG) & Berufsverband der Augenärzte Deutschlands e. V. (BVA) (2020, May): Stellungnahme von DOG, RG und BVA zur Anti-VEGF-Therapie der Frühgebore-nenretinopathie; ROP-Pass-Formular zum Download. Available at: https://www.dog.org/wp-content/uploads/2013/03/ROPPass_Version-3_4_FORMULAR.pdf. (Accessed on 15 Mar 2021).
- Draper ES, Zeitlin J, Manktelow BN et al. (2020): EPICE cohort: two-year neurodevelopmental outcomes after very preterm birth. Arch Dis Child - Fetal Neonatal Ed 105: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA.Europa.eu (2020, January 28): Lucentis 10 mg/ml solution for injection SmPC. Available at: https://www.ema.europa.eu/en/documents/product-information/lucentis-epar-product-information_en.pdf. (Accessed on 15 Mar 2021).
- Geloneck MM, Chuang AZ, Clark WL et al. (2014): Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol 132: 1327–1333. [DOI] [PubMed] [Google Scholar]
- Granild-Jensen JB, Rackauskaite G, Flachs EM & Uldall P (2015): Predictors for early diagnosis of cerebral palsy from national registry data. Dev Med Child Neurol 57: 931–935. [DOI] [PubMed] [Google Scholar]
- Gunay M, Celik G, Gunay BO, Aktas A, Karatekin G & Ovali F (2015): Evaluation of 2-year outcomes following intravitreal bevacizumab (IVB) for aggressive posterior retinopathy of prematurity. Arq Bras Oftalmol 78: 300–304. [DOI] [PubMed] [Google Scholar]
- Harder BC, Schlichtenbrede FC, von Baltz S, Jendritza W, Jendritza B & Jonas JB (2013): Intravitreal bevacizumab for retinopathy of prematurity: refractive error results. Am J Ophthalmol 155: 1119–1124 e1. [DOI] [PubMed] [Google Scholar]
- Hoerster R, Muether P, Dahlke C, Mehler K, Oberthur A, Kirchhof B & Fauser S (2013): Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol 91: e74–e75. [DOI] [PubMed] [Google Scholar]
- Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM & Kapur R (2012): Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol Chic Ill 1960 130: 1000–1006. [DOI] [PubMed] [Google Scholar]
- Hwang CK, Hubbard GB, Hutchinson AK & Lambert SR (2015): Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology 122: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour IT (2015): Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol 52: 143–152. [DOI] [PubMed] [Google Scholar]
- Kennedy KA & Mintz-Hittner HA & BEAT-ROP Cooperative Group (2018): Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus 22: 61–65.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klufas MA, Patel SN, Ryan MC et al. (2015): Influence of fluorescein angiography on the diagnosis and management of retinopathy of prematurity. Ophthalmology 122: 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne TU, Müller A, Larsen PP & Holz FG (2018): Long-term effects of anti-VEGF therapy for retinopathy of prematurity. Ophthalmol Z Dtsch Ophthalmol Ges 115: 464–468. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Liao Y, Zeng R, Zeng P & Lan Y (2018): Comparison of effcacy between anti-vascular endothelial growth factor (VEGF) and laser treatment in Type-1 and threshold retinopathy of prematurity (ROP). BMC Ophthalmol 18: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling K-P, Liao P-J, Wang N-K et al. (2019): Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti–vascular endothelial growth factor monotherapy. Retina 40: 1793–1803. [DOI] [PubMed] [Google Scholar]
- Lyu J, Zhang Q, Chen C-L, Xu Y, Ji X-D, Li J-K, Huang Q-J & Zhao P-Q (2017): Recurrence of retinopathy of prematurity after intravitreal ranibizumab monotherapy: timing and risk factors. Invest Ophthalmol Vis Sci 58: 1719–1725. [DOI] [PubMed] [Google Scholar]
- Mintz-Hittner HA, Geloneck MM & Chuang AZ (2016): Clinical management of recurrent retinopathy of prematurity after intravitreal bevacizumab monotherapy. Ophthalmology 123: 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J, Luu TM, Superstein R et al. (2016): Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics 137: e20153218–e20153218. [DOI] [PubMed] [Google Scholar]
- Mueller B, Salchow DJ, Waffenschmidt E et al. (2017): Treatment of type I ROP with intravitreal bevacizumab or laser photocoagulation according to retinal zone. Br J Ophthalmol 101: 365–370. [DOI] [PubMed] [Google Scholar]
- Patel SN, Klufas MA, Ryan MC et al. (2015): Color Fundus Photography Versus Fluorescein Angiography in Identification of the Macular Center and Zone in Retinopathy of Prematurity. Am J Ophthalmol 159: 950–957.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertl L, Steinwender G, Mayer C et al. (2015): A Systematic review and meta-analysis on the safety of vascular endothelial growth factor (VEGF) inhibitors for the treatment of retinopathy of prematurity. PLoS One 10: e0129383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GE (2016): Retinopathy of prematurity blindness worldwide: phenotypes in the third epidemic. Eye Brain 8: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C & Kusaka S (2012): Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol 153(327–333): e1. [DOI] [PubMed] [Google Scholar]
- Stahl A, Krohne TU, Eter N et al. (2018): Comparing alternative ranibizumab dosages for safety and effcacy in retinopathy of prematurity. JAMA Pediatrics 172: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl A, Lepore D, Fielder A et al. (2019): Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet 394: 1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry TL (1942): Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens. Am J Ophthalmol 25: 203–204. [DOI] [PubMed] [Google Scholar]
- Walz JM, Bemme S, Pielen A et al. (2016): The German ROP Registry: data from 90 infants treated for retinopathy of prematurity. Acta Ophthalmol 94: e744–e752. [DOI] [PubMed] [Google Scholar]
- Walz JM, Bemme S, Reichl S et al. (2018): Treated cases of retinopathy of prematurity in Germany: 5-year data from the Retina.net ROP registry. Ophthalmol Z Dtsch Ophthalmol Ges 115: 476–488. [DOI] [PubMed] [Google Scholar]
- Wong RK, Hubschman S & Tsui I (2015): Reactivation of retinopathy of prematurity after ranibizumab treatment. Retina 35: 675–680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Graphic representation of the ROP time course during core study and follow-up for the patient progressing to stage 5 ROP.
Table S1. Baseline characteristics of patients who entered the follow-up study.
Table S2. Peripheral vascularization at the end of the core study and at 1 year.
Table S3. Detailed information on nystagmus.
Table S4. Abnormalities on slit lamp and retinal exam at 1 year.