Abstract
BACKGROUND:
Intraoperative molecular imaging (IMI) has been shown to improve lesion detection during pulmonary sarcomatous metastasectomy. Our goal in this study was to evaluate whether data garnered from IMI-guided resection of pulmonary sarcoma metastasis translate to improved patient outcomes.
STUDY DESIGN:
Fifty-two of 65 consecutive patients with a previous history of sarcomas found to have pulmonary nodules during screening were enrolled in a nonrandomized clinical trial. Patients underwent TumorGlow the day before surgery. Data on patient demographics, tumor biologic characteristics, preoperative assessment, and survival were included in the study analysis and compared with institutional historical data of patients who underwent metastasectomy without IMI. p values < 0.05 were considered significant.
RESULTS:
IMI detected 42 additional lesions in 31 patients (59%) compared with the non-IMI cohort where 25% percent of patients had additional lesions detected using tactile and visual feedback only (p < 0.05). Median progression-free survival (PFS) for patients with IMI-guided pulmonary sarcoma metastasectomy was 36 months vs 28.6 months in the historical cohort (p < 0.05). IMI-guided pulmonary sarcoma metastasectomy had recurrence in the lung with a median time of 18 months compared with non-IMI group at 13 months (p < 0.05). Patients with synchronous lesions in the IMI group underwent systemic therapy at a statistically higher rate and tended to undergo routine screening at shorter interval.
CONCLUSIONS:
IMI identifies a subset of sarcoma patients during pulmonary metastasectomy who have aggressive disease and informs the medical oncologist to pursue more aggressive systemic therapy. In this setting, IMI can serve both as a diagnostic and prognostic tool without conferring additional risk to the patient.
Despite the advances in the management of primary peripheral extremity sarcomas over the last decade, more than 20% to 40% of these patients will develop pulmonary metastases.1 Since the results of landmark article by Marcove in 19702 showing that pulmonary metastasectomy is correlated with overall and progression-free survival (PFS), more than 500 studies have confirmed this hypothesis.2–9 Depending on disease type, pulmonary metastasectomy has become a well-accepted intervention that can improve survival for these patients with 30% to 50% 5-year disease-free survival.1,10,11 The best improvement from pulmonary metastasectomy occurs if all metastatic disease can be resected; thus, the thoracic surgeon is obligated to aggressively seek out all disease.12,13
In the operating room, thoracic surgeons have to rely on their visual and tactile assessment to accurately identify the metastatic lesions of interest as well as any occult lesions that may exist.14 Although development of fine-cut 1-mm CT scans has contributed to identification of additional smaller lesions, it remains imperfect as investigations by Cerfolio and colleagues5,6 have shown that 37% of patients still have occult lesions detected at the time of surgery. This is significant because nearly 60% of patients will experience a relapse in the lung requiring further reoperation thought to be related to incomplete disease clearance.1,15,16
Recently, our group proposed a new approach to improve identification of occult lesions: intraoperative molecular imaging (IMI).17 IMI entails injecting patients with a near infrared (NIR) tracer that targets pulmonary metastases. These NIR tracers visually enhance tumors to draw the attention of the thoracic surgeon to these occult lesions. In previous reports, we have used NIR tracers such as TumorGlow and OTL38 to visualize sarcoma metastases.18 TumorGlow is one approach using indocyanine green (ICG) 24 hours before surgery at high doses. It is an appealing optical agent given its excellent safety profile and its propensity to accumulate in a diverse array of sarcoma histologies via the enhanced permeability and retention (EPR).18 ICG has an excitation wavelength of 805 nm and an emission wavelength of 830 nm. Previous findings have shown that IMI is superior to our current techniques in detecting synchronous occult lesions, particularly in minimally invasive settings.19,20
Our previous reports have described the technology.21 Here, our goal is to determine whether detection of synchronous occult pulmonary metastases using IMI offers a clinical value to patients. The long-term survival impact of IMI on pulmonary metastasectomy has not been studied and can be informative for surgeons looking to incorporate the technology into their armamentarium.1 We describe our experience of a cohort of 52 patients who underwent IMI-guided resection of sarcomatous pulmonary metastasis, which is the largest study, and one of few studies, in the literature to date exploring IMI in this patient population. Our objective was to explore the effectiveness of the NIR tracer in detecting occult lesions, long-term survival, and change in postsurgical management when occult lesions are discovered only by IMI.
METHODS
Summary of study design and patient selection
This study is a longitudinal follow-up for patients in a nonrandomized open label study (NCT02280954; 2014–2019) approved by the University of Pennsylvania Institutional Review Board.21 Sixty-five consecutive patients with pulmonary nodules were identified during screening with previous history of nonpulmonary primary malignancy, of whom 52 consented for the study and had postsurgical follow up at UPHS facilities. All patients had their primary nonpulmonary disease under remission with no active disease (Fig. 1A).
Figure 1.
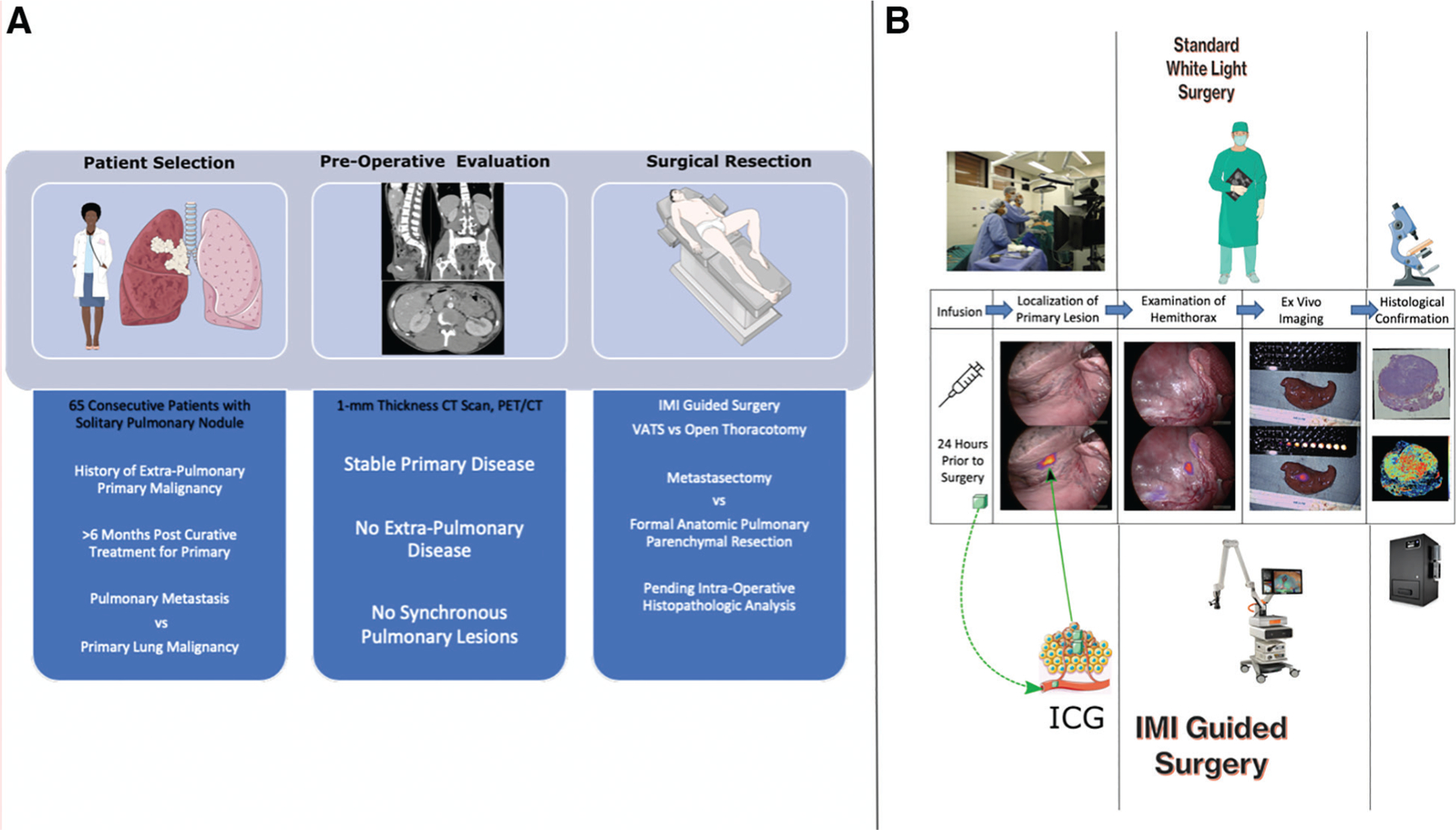
(A) Flowchart depicting the patient selection process, which involves evaluation of patients with pulmonary nodules with a history of primary nonpulmonary sarcoma who were in remission after treatment for primary disease. Differential for the patients included pulmonary metastasis vs primary lung malignancy. All the patients were evaluated with high-fidelity 1-mm thickness cut CT scan and positron emission tomography (PET)/CT for synchronous lesions or extra-pulmonary disease. Appropriate patients were then scheduled for intraoperative molecular imaging (IMI)–guided resection of the nodule (metastasectomy if nonpulmonary lesion or anatomic resection if primary lung disease). (B) Pictorial description of differences between standard white light surgery (top column) vs IMI-guided resection (bottom column) for patients enrolled in the study. ICG, indocyanine green; VATS, video-assisted thoracoscopic surgery.
Data from the IMI cohort were compared with a historical institutional retrospective database of patients who underwent pulmonary sarcomatous metastasectomy. From 2005 through 2019, 79 patients with adequate follow-up data who underwent non–IMI-guided pulmonary metastasectomy per institutional and societal guidelines and had postsurgical follow-up at UPHS facilities were included in the final analysis.
Study drug
Patients underwent infusion of high dose ICG (Akorn Pharmaceuticals, Decatur, IL; 5 mg/kg) up to 24 hours before surgery in a previously described TumorGlow protocol (Fig. 1B).18 ICG is a well-tolerated optical contrast agent, approved by the FDA.
Imaging device
In situ, real-time fluorescent imaging was performed using the Iridium (Medtronic Corp, Minneapolis, MN) imaging system optimized for detection of ICG. IMI-based optical devices are high definition, dual band (white light and NIR) camera systems capable of concurrent NIR emission and detection while generating real-time video.
Surgical approach
All subjects were scheduled for a resection via median sternotomy, video-assisted thoracic surgery (VATS), or open unilateral anterolateral thoracotomy. During surgery, surgeons used standard visualization and finger palpation (when applicable) to identify known tumors. After identification of the tumor, NIR imaging was used to confirm lesion fluorescence. If the preoperatively identified nodule was unidentifiable by white-light visualization or palpation, localization using fluorescence guidance was attempted. After identifying the primary lesions, fluorescence imaging was used to assess the hemithorax for occult lesions (Fig. 1B).
Statistical analysis
Data on patient demographics, tumor biologic characteristics, preoperative assessment, systemic and local treatment details for primary and distant lesions, and PFS and IMI lesion fluorescence quantification (tumor to background ratio [TBR]) were included in the study analysis and compared with historical UPHS data from 1997 through 2019. Patients lost to follow-up were not included in the final analysis. To avoid confounders on postoperative survival analysis, patients who were 18 years or older with Karnofsky performance status greater than 70%, median American Society of Anesthesiologists (ASA) class 3 or lower, no other preexisting terminal diagnoses, ejection fraction greater than 35%, and anticipated life expectancy of more than 12 weeks were selected for final statistical survival analysis.
Data are presented as mean (standard deviation) unless otherwise noted. Data were analyzed for parametric distribution. Parametric correlation of dichotomous variables was compared using Student’s t-test, and ANOVA for 3 or more continuous variables. Nonparametric categorical variables between groups were compared using Kruskal–Wallis test, whereas Mann–Whitney U test was conducted for continuous variables. Ordinal associations between variables were analyzed using Somers’ D, Kendall’s tau-b, and Kendall’s tau-c tests. Categorical associations between variables were analyzed using chi-squared–based measure of association including Phi, Creamer’s V, and McNemar’s Test. Survival analysis Kaplan–Meier survival curves were used to estimate patient survival and were compared using the Logrank, Gehan-Breslow-Wilcoxon, and Taron-Ware’s test based on time distribution of deaths between the groups. Univariate and multivariate analyses were performed by using the Cox proportional hazard model. The parameters that proved to be significant on the univariate analysis were subsequently tested with the multivariate model.
Statistical analyses were performed using R version 3.5.3 and packages tidyverse, ROCR survival, and survminer (R Foundation for Statistical Computing, Vienna, Austria). p-values < 0.05 were considered statistically significant.
RESULTS
Demographics
Between 2014 and 2021, 52 consecutive patients with pulmonary nodules who had known history of primary extra-pulmonary malignancies were evaluated. After thorough evaluation for synchronous lesions by high-fidelity 1-mm cross-sectional imaging, 65 total lesions were deemed amenable for IMI guided surgical resection (Table 1). Procedures in this group included open unilateral anterolateral thoracotomy (11), open unilateral posterolateral thoracotomy (1), and unliteral VATS guided resection (40).
Table 1.
Demographic Data for Both Sarcoma and Non-Sarcoma Metastases Analyzed During the Study
| Demographic | IMI-guided pulmonary sarcoma metastasectomy | Historical non-IMI group |
|---|---|---|
| Total patients, n | 52 | 79 |
| Lesions identified preoperatively, n | 65 | 116 |
| Additional lesions, n | 42 | 31 |
| Percent malignant for additional lesions, n (%) | 34 (82) | 22 (71) |
| Total lesions, n | 107 | 147 |
| Age at operation, y, median (IQR) | 53 (31.8–66.9) | 49 (36.4–63.6) |
| Sex, male, n (%) | 36 (70) | 51 (64) |
| Sex, female, n (%) | 16 (30) | 28 (36) |
| Age at death, y, median (IQR) | 55.5 (40.6–67.8) | 53.1 (45.8–62.4) |
| Follow-up, mos, median (IQR) | 41.2 (20.67–51.81) | 31.9 (17.48–44.11) |
IMI, intraoperative molecular imaging; IQR, interquartile range.
In the IMI group, distribution of gender was skewed towards males (n = 36,~70%, p < 0.05). Median age of the cohort was 53 years. Twenty-four patients in the cohort were deceased at the time of analysis, with a median age of death 55.5 years. Median follow-up time for sarcoma patients was 41.2 months (range, 6–83.1 months). There was no statistical preponderance of a particular lobe or location of metastases in the thoracic cavity (Fig. 2). The most common primary tumor histology for the sarcoma group was osteosarcoma (21.2%; Fig. 2), which is similar to previous reports in the literature and institutional experience. Mean size of sarcomatous metastases was 1.9 cm (range 0.46–3.9, SD 0.58 cm). Mean time from last surveillance imaging to surgery was 47.29 days. Details of the patients in the study are shown in Table 1.
Figure 2.
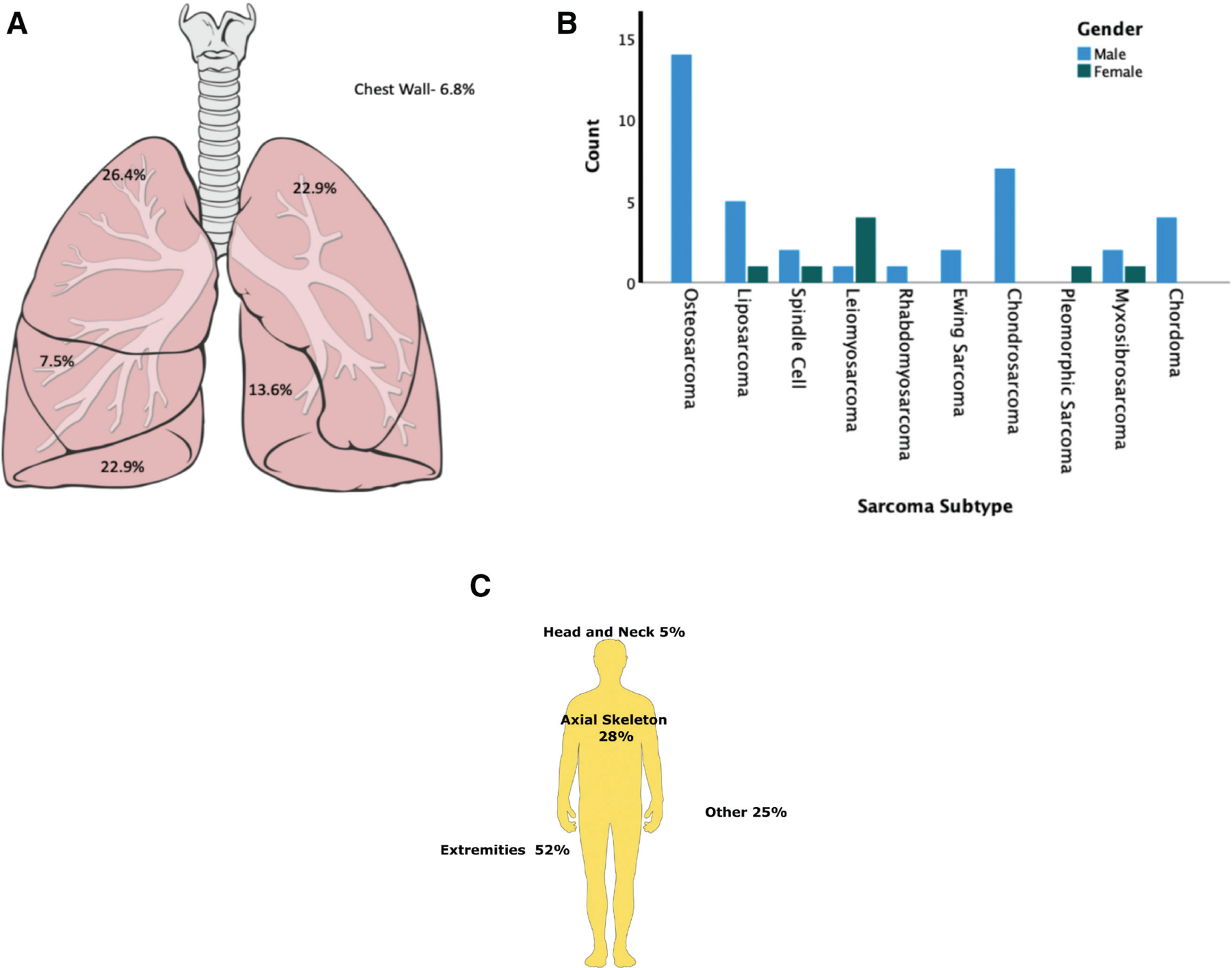
(A) Anatomic distribution of the sarcoma metastases in the thoracic cavity, which shows nonspecific and nonstatistically significant distribution of lesions in all 5 lobes with right middle lobe having the lowest frequency. (B) Distribution of sarcoma subtype by sex in the study group. (C) Anatomic distribution primary sarcomas in the cohort with more than half of the primary lesions arising from the extremities correlating with sarcoma subtypes.
From 2005 through 2019, 79 patients underwent non–IMI-guided pulmonary metastasectomy per institutional and societal guidelines. Procedures in this group included bilateral exploration through median sternotomy,8 initial staged bilateral thoracotomies several weeks apart,6 open unilateral anterolateral thoracotomy,14 and unilateral VATS guided resection (51). A total of 147 metastasectomies were performed, of which 116 were planned preoperatively (identified on preoperative work-up). There were no statistically significant differences between the age and gender distribution of the groups. Details of the demographics in the study are shown in Table 1.
IMI lesion detection
In the IMI group, 65 suspicious lesions were identified on preoperative high-fidelity cross-sectional imaging. Fifty-seven of those lesions (~87%) fluoresced intraoperatively with a mean TBR of 3.1(Figs. 3 and 4 and Video 1). On subgroup analysis, the 8 lesions that did not fluoresce with IMI were found to be deeper than 3.32 cm from the parietal pleural surface (p < 0.03). All 8 lesions fluoresced after tumor bisection during back table assessment.
Figure 3.
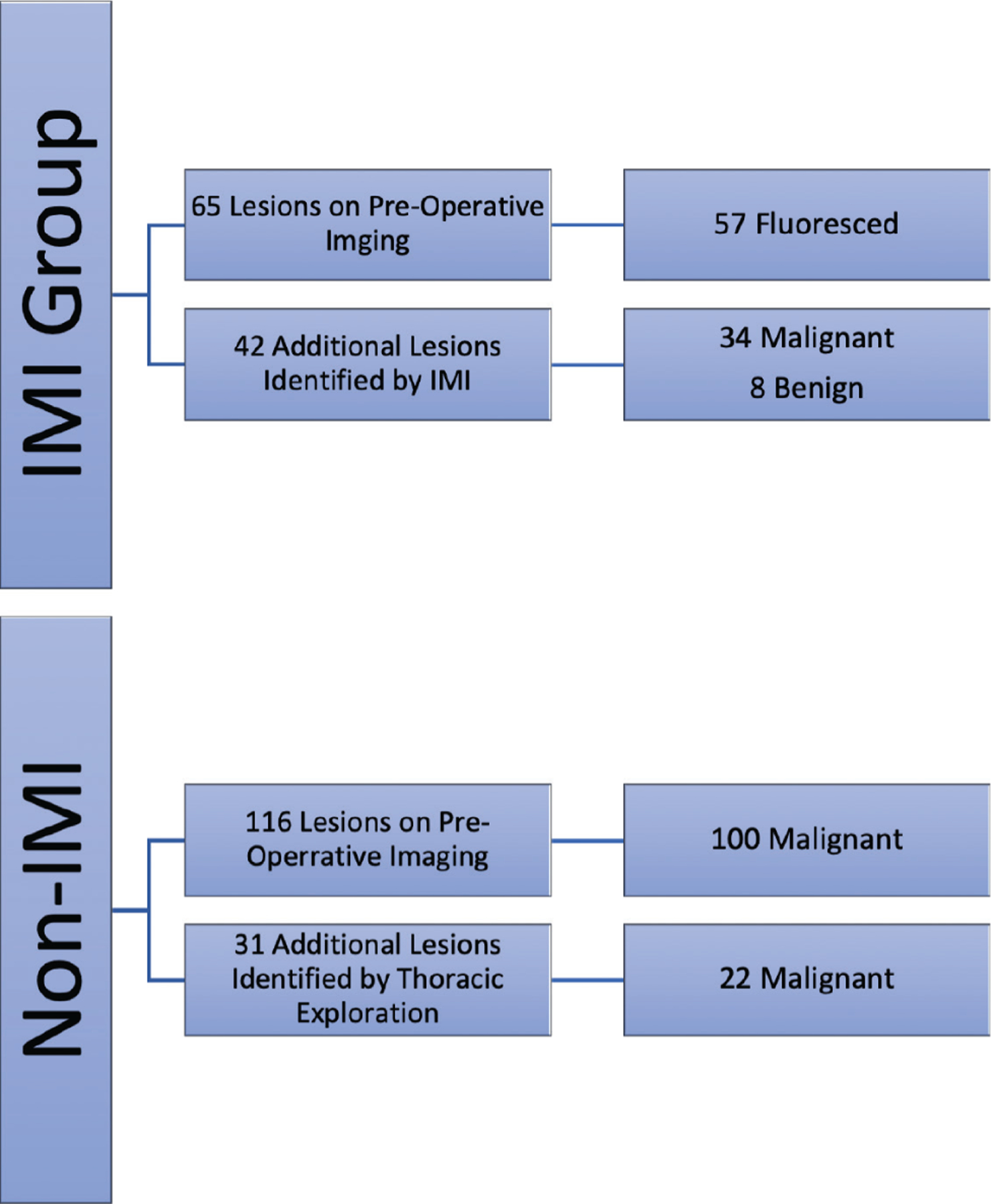
Summary of metastases identified in both sarcoma and non-sarcoma pulmonary metastases using gold-standard techniques and intraoperative molecular imaging (IMI). IMI identified 34 malignant sarcomatous pulmonary metastases that would otherwise be missed.
Figure 4.
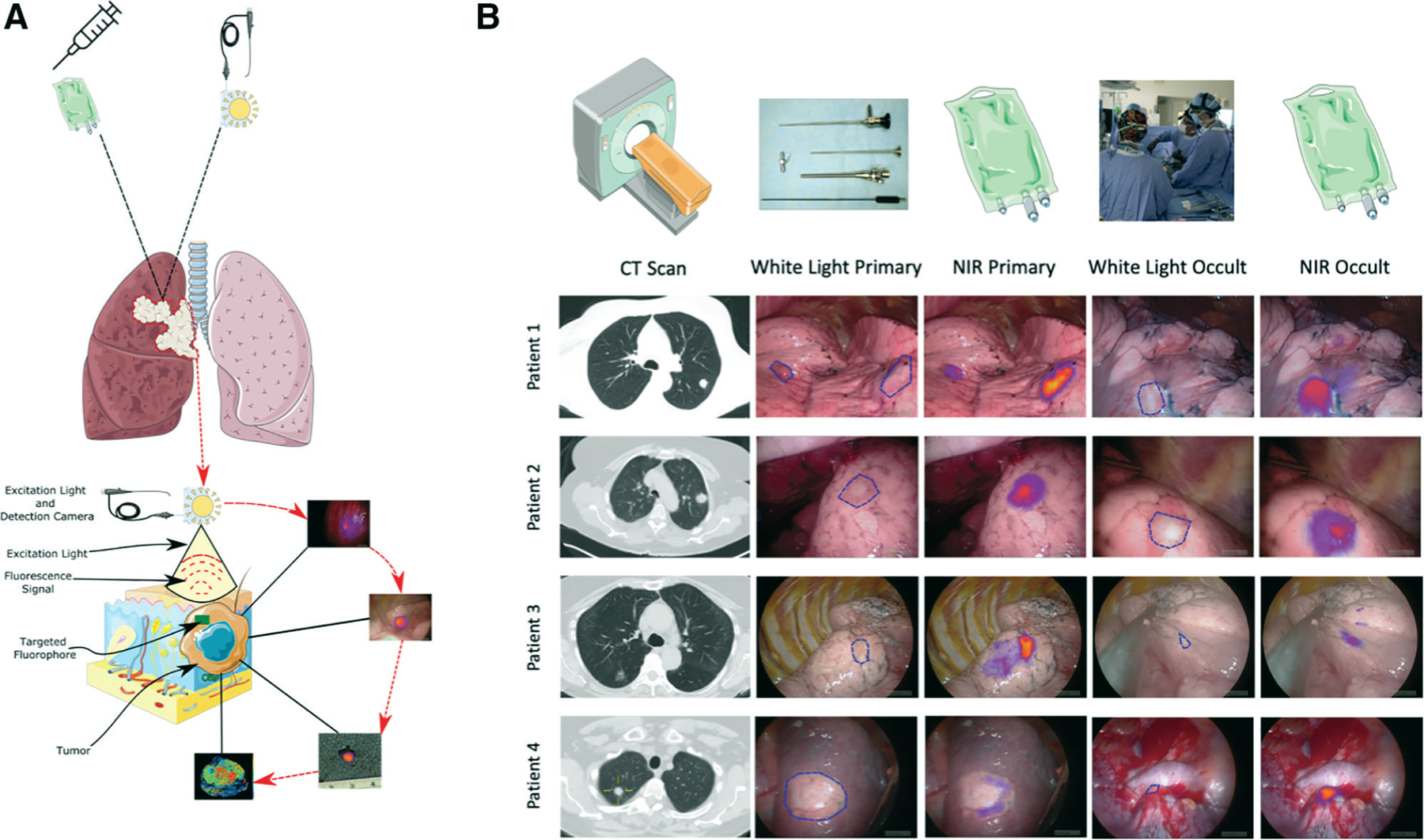
Representative intraoperative findings of intraoperative molecular imaging (IMI)–guided metastasectomy of sarcomatous pulmonary lesions. (A) Technological pictorial overview of IMI-guided pulmonary sarcoma metastasectomy. (B) Sample pictorial representation of various IMI guided metastasectomy including preoperative 1-mm cut thickness high-fidelity CT scan images, traditional white light view of the lesions showing identification of known primary metastatic lesions during video-assisted thoracoscopic surgery or open thoracic resection as well as near infrared (NIR) merged views depicting real-time intraoperative visualization primary and occult lesions. As seen in Patients #3 and #4, white light view did not provide clear visual identification of occult lesions, particularly near critical structures which was easily identified using IMI.
Video 1.
Known lesion detection by intraoperative molecular imaging (IMI) guidance. The video demonstrates 2 nodules in a patient with aggressive rhabdomyosarcoma which were detected on preoperative CT scan identified in real-time IMI-guided video-assisted thoracoscopic surgery resection. Both the tumors fluoresced after TumorGlow infusion. NIR, near infrared.
Furthermore, IMI allowed detection of 42 additional lesions in 31 (59%) patients who were not identified on preoperative imaging, of which 34 (82%) were confirmed metastases on histopathologic analysis (Fig. 3). The benign lesions were overwhelmingly lymphoid aggregates (7 of 8), and one was atypical adenomatous hyperplasia. The mean TBR for synchronous occult lesions was 2.84 (2.43–3.25), with no statistical difference compared with known lesions. As expected, additional occult lesions identified with IMI were significantly smaller compared with those that were seen on preoperative imaging (4.7 mm [3.25–6.1] vs 11.2 mm, p < 0.01; Fig, 4 and Video 2). Subset analysis showed that the rate of occult lesion detection with IMI was much more efficacious in those undergoing VATS resection (25 of 42) compared with open thoracotomies (p < 0.05).
Video 2.
Occult lesion detection by intraoperative molecular imaging. The patient in Video 1 further underwent exploration of the ipsilateral thorax and showed fluorescence of occult nodule that was not detected with standard visual assessment (right column video). Histologic assessment confirmed metastatic rhabdomyosarcoma. NIR, near infrared.
The rate of synchronous occult lesion detection was compared with an historical cohort of pulmonary sarcoma metastasectomy who did not undergo IMI-guided resection. Thirty-one additional patients had occult lesions detected using conventional techniques. Twenty-five percent of patients had additional lesions detected using tactile and visual feedback, which is statistically significantly lower compared with our IMI cohort (p < 0.05; Table 2). There was a statistically significant higher distribution of undifferentiated pleomorphic sarcoma (formerly Malignant Fibrous Histiocytoma)18 in the historical control group compared with the IMI group6 (p < 0.05). In summary, the addition of IMI to standard techniques appears to be more effective in detecting occult synchronous lesions compared with standard-of-care alone.
Table 2.
Number of Patients with Additional Occult Lesion Detection During Intraoperative Molecular Imaging and Non-Intraoperative Molecular Imaging-Based Pulmonary Metastasectomy
| Variable | Historical cohort of non-IMI pulmonary sarcoma metastasectomy | IMI-guided pulmonary sarcoma metastasectomy |
|---|---|---|
| Patients with additional malignant occult lesion detected, n/N (%) | 20/79 (~25)* | 31/52 (59)* |
Statistically significant (p < 0.05) association between the groups.
IMI, intraoperative molecular imaging.
Survival advantage of IMI for pulmonary metastases
Given that IMI identified a group of patients with occult lesions, a subset analysis was performed to understand whether these interventions improved PFS.
Median PFS for patients with IMI-guided pulmonary sarcoma metastasectomy was 36 months compared with 28.6 months for the non–IMI-guided historical cohort (p < 0.05; Fig. 5A). In a subset analysis of patients with pulmonary sarcomas who were found to have more than 2 occult lesions with IMI, median survival was statistically shorter (29.8 months vs 38.1 months) than those who did not have synchronous lesions detected (p < 0.05; Fig. 5B).
Figure 5.
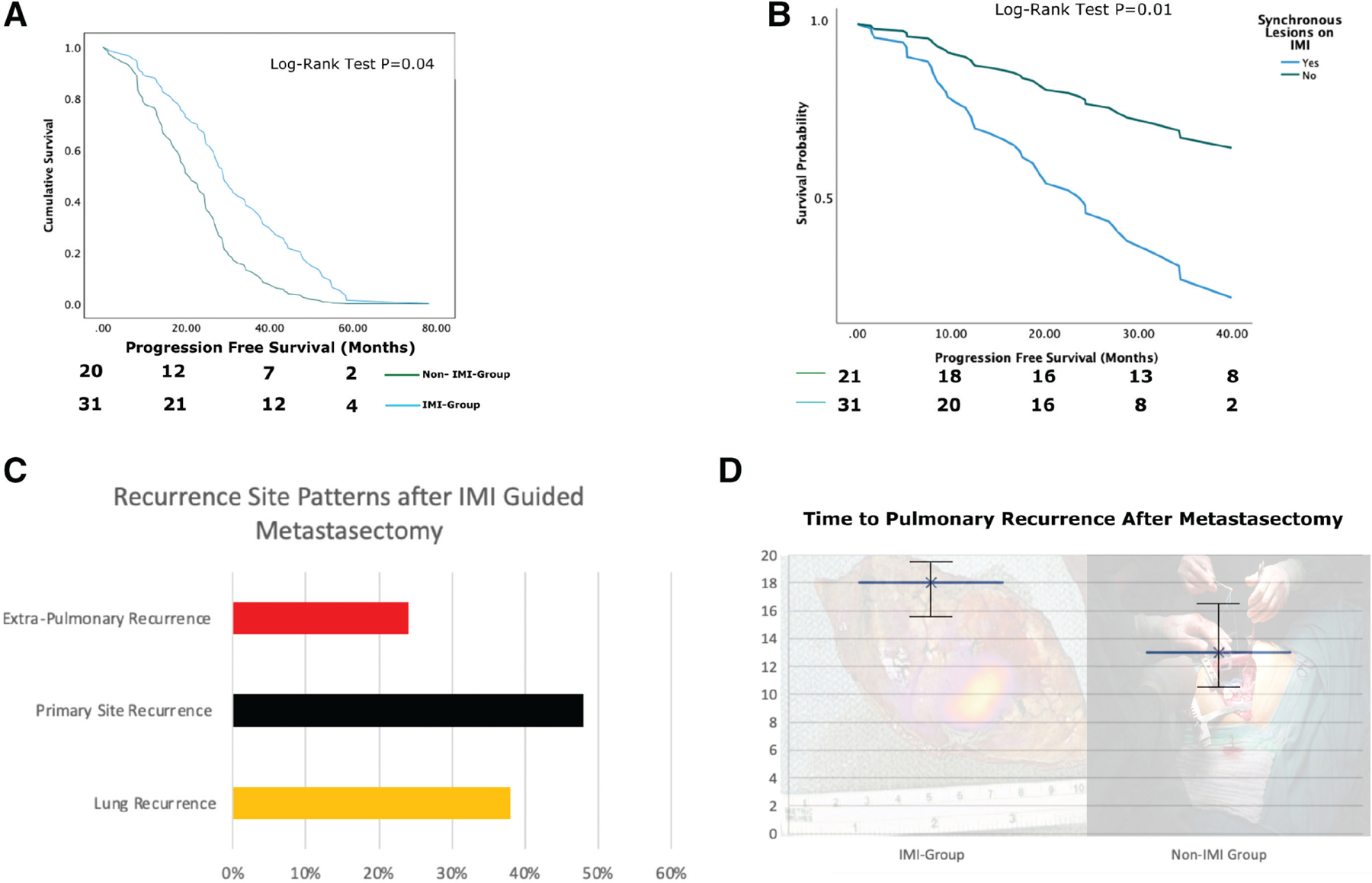
(A) Overall survival curves for the intraoperative molecular imaging (IMI) and non–IMI-based pulmonary metastasectomy groups. (B) Survival curves displaying worse overall survival for patients who had occult lesions detected with IMI vs those who had no occult lesions. (C) Initial recurrence patterns in patients with pulmonary sarcoma metastases after index resection of metastatic lesion. (D) Time to pulmonary recurrence after pulmonary metastasectomy for IMI and non-IMI groups.
Further analysis of patients who underwent IMI-guided pulmonary sarcoma metastasectomy had recurrence in the lung with a median time to recurrence of 18 months (Fig. 5D). The non-IMI group had a statistically significantly shorter time to recurrence at 13 months (p < 0.03). There was a higher risk of pulmonary recurrence on multivariate analysis in IMI patients who had more than 3 occult synchronous lesions detected at the index operation, but the difference was not statistically significant (p = 0.063). Additionally, on multivariate analysis, initial extrapulmonary recurrence was more common in patients who had occult lesions detected by IMI with primary site being the most common followed by lung and extra-pulmonary sites (liver most common), but again this did not reach the level of statistical significance (p = 0.071; Fig, 5C). The non-IMI group had a statistically higher initial recurrence in the lung compared with primary or extrapulmonary sites (p < 0.05).
A subset analysis of patients with complete follow-up information who underwent IMI-guided resection of pulmonary metastases found that of the 12 patients who developed lung recurrence, 10 (~83%) developed it on the contralateral lung first. Interestingly, all the 12 patients in the subset analysis had occult lesions detected during IMI.
Potential factors associated with improved survival
Although studies have looked at IMI’s effectiveness in occult lesion detection, its clinical impact on subsequent patient treatment patterns has not been analyzed.
For the 31 patients with sarcomatous pulmonary metastasis who had IMI-detected occult lesions, 21 (69%) underwent subsequent adjuvant systemic therapy (Fig. 6). These patients would not have undergone additional therapy if not for the identification of additional disease.
Figure 6.
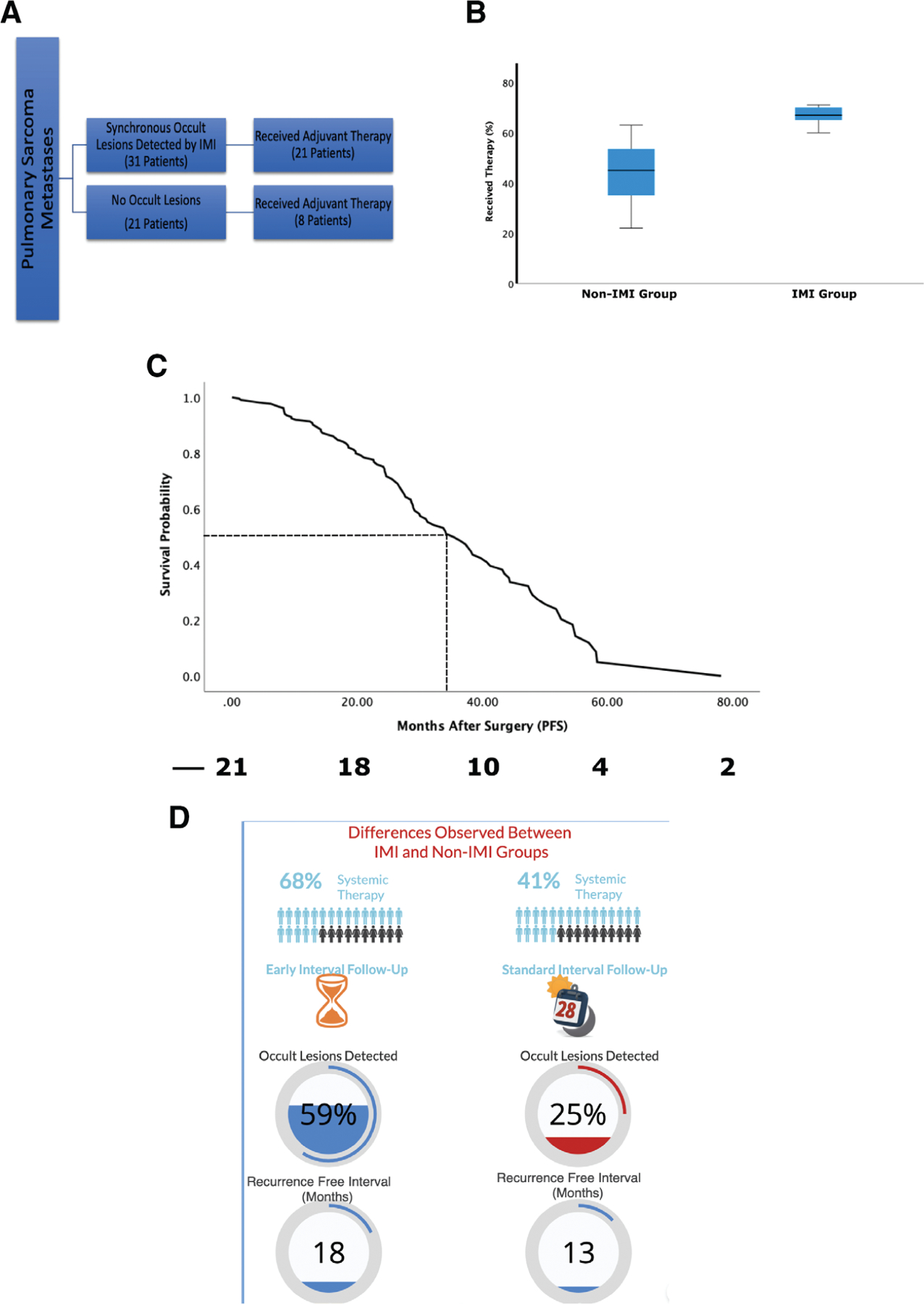
(A) Summary of postoperative systemic treatment trends for pulmonary sarcoma metastases based on occult lesion detection with intraoperative molecular imaging (IMI). (B) Box-whisker plot analyzing the rate of postoperative systemic treatment rates in those who had occult lesions detected by IMI showing statistically significant higher rate of postoperative systemic therapy compared with those in the non-IMI group. (C) Survival curve for patients with occult lesions with IMI who underwent postoperative systemic therapy. (D) Summary of differences observed between the IMI- and non–IMI-based pulmonary metastasectomy with IMI patients noted to undergo adjuvant systemic therapy at a higher rate, have a trend for shorter postoperative surveillance, having higher occult lesion detected (59% of patients in IMI group vs 25% in non-IMI group; p < 0.05), and having a higher recurrence-free interval from operation (18 months vs 13 months). PFS, progression-free survival.
On the other hand, the rate of administration of systemic therapy for sarcoma patients with no occult pulmonary metastasis was statistically lower compared with the occult sarcomatous metastasis group (6 of 21 [29%], p < 0.05). The most common chemotherapy regimens consisted of doxorubicin plus cisplatin for osteosarcomas and doxorubicin (with or without ifosfomide) for other soft tissue metastases. Immunotherapy has been used more often since 2017, but its increased use was not statistically significant between the two groups.
In the non-IMI group, of the 20 patients who had occult lesion detection, the rate of postoperative chemotherapy was statistically lower, with only 8 (~41%) patients undergoing postoperative systemic therapy (p < 0.05; Fig. 6B). Interestingly, the rate of postoperative therapy increased to 62% after recurrence (either pulmonary or extrapulmonary sites).
Further analysis demonstrated that the survival benefit in those with occult lesions detected on IMI was most evident in patients who underwent postoperative chemotherapy (37.2 months vs 24.0 months) compared with those who did not (p < 0.05; Fig. 6C). On univariate and multivariate analysis, the use of postoperative therapy has correlated with improved survival postresection (p < 0.05) in patients with occult lesions identified using IMI.
Even without controlling for inherent differences in practice patterns by different providers, there was a nonstatistically significant trend of more frequent follow-up and imaging in patients with synchronous lesions identified with IMI (p = 0.087). Of the 31 patients with occult synchronous lesions, 14 had their first imaging before the 6-month follow-up whereas 3 of 20 in non-IMI group had earlier interval follow-up.
In summary, patients with occult synchronous sarcomatous pulmonary metastases detected with IMI who undergo systemic postoperative therapy appear to have a favorable median survival compared with those who do not undergo such treatment. The summary of our findings is presented in Fig. 6D.
DISCUSSION
We performed this study to investigate whether complete resection of both known and occult lesions is critical to long-term survival after pulmonary metastasectomy for sarcomas.15 IMI has been a diagnostic tool for the thoracic surgeon in identifying occult synchronous lesions that would otherwise be missed by traditional means. This study comprises the largest cohort of IMI-guided pulmonary metastasectomy to date and indicates that high-dose ICG infusion followed by NIR imaging after 24 hours (TumorGlow) identified patients with occult disease who potentially after IMI-guided resection appeared to have a higher rate of systemic therapy. These patients were observed to have better clinical outcomes. To our knowledge, this is the first hypothesis-generating study to suggest that IMI has a potential in informing future therapeutic decisions value by identifying patients who could benefit from subsequent systemic treatment.
TumorGlow has been extensively studied as an NIR tracer of choice in various pulmonary and solid organ malignancies by our research group.14,17–20,22–24 Use of IMI for pulmonary metastasectomy offers unique advantages. These patients are less likely to be smokers and have otherwise normal appearing parenchyma. In our study, IMI was safe and feasible in wide array of tumor histologies, lesion size, demographics, and operation type (thoracotomy vs VATS; Fig. 2). Surgeons can easily incorporate IMI technology during pulmonary metastasectomy without compromising operative and oncologic safety (Fig. 4).
Even with the implementation of 1-mm fine-cut CT scanning and PET imaging, IMI identified additional 42 lesions in 20 patients (2.1 lesions per patient) that would otherwise be missed (Figs. 3 and 4). The occult lesions were significantly smaller than those captured on preoperative imaging (median 4.7 mm). Consistent with literature reports, 38% of patients in our cohort with pulmonary sarcomatous metastases had synchronous lesions when their hemithorax was examined, and this rate of detection was increased by IMI.1,6,12 Similar to a report published by Predina and colleagues,25 the rate of synchronous lesion detection was higher in patients who underwent VATS resection in the sarcoma group and was less successful in detecting lesions deeper than 3.32 cm. This finding is consistent with our previous IMI experience because the surgeons are not able to perform bimanual inspection during VATS. Some of the lesions are also located near critical cardiovascular structures that would be difficult to palpate during conventional techniques but were identified using IMI during VATS (Fig. 4, Patient 4). Furthermore, all the lesions removed during IMI were able to be palpated on back table after removal. Nevertheless, use of IMI did not compromise surgical resection as it identified more than 85% of known lesions, and lesions that did not fluoresce were all identified by conventional means. Knowing these limitations and optimal patient characteristics can allow the surgeon to make informed decisions regarding when and in whom to use IMI guided metastasectomy.
Findings produced by IMI informed and altered post-operative oncologic management of patients, as one possible confounding effect on the observed outcomes. Patients with completely resected pulmonary metastases are often referred to medical oncologists to discuss the risks, potential benefits, and uncertainties of postresection chemotherapy at the multi-disciplinary tumor board.10 Each institution uses different treatment algorithms based on the information available to the clinician, and IMI has the potential to provide updates about the potential aggressiveness of the disease that would otherwise be not available. This use of IMI as a prognostic indicator beyond diagnostic use has not previously been explored in the literature.
Although there was no randomization in this study, 21 of 31 (69%) patients who had additional lesions noted with IMI underwent subsequent systemic therapy. The rate of systemic therapy was significantly lower in those without synchronous lesions (p < 0.05) and in the historical cohort who had occult lesion detection using standard techniques (Fig. 5B). Postoperative systemic therapy after pulmonary sarcomatous metastasectomy is a controversial topic, with studies producing conflicting conclusions.12,26 Although not statistically significant, oncologists often screened these high-risk patients more frequently compared with those without additional lesions (3-month interval vs 6-month interval; Fig. 6E). However, this observation of uneven application of follow-up in this setting adds bias, which can confound PFS analysis.
PFS in patients with more than 2 occult synchronous lesion detected with IMI was significantly worse compared with those without synchronous lesions (Fig. 5B). Furthermore, patients with synchronous lesions who underwent postoperative systemic therapy had statistically significant longer survival compared with those who did not (37.2 months vs 24.0 months; Fig. 6C). Any potential survival benefit was not evident in those few patients with chondrosarcoma and pleomorphic sarcoma.27–30 The use and benefit of systemic chemotherapy is limited, particularly in patients with sarcoma. However, in a small number of patients who are selected to receive chemotherapy, response on surveillance imaging is found to be a good prognostic indicator.1 Additionally, in this patient population, where there is a considerable likelihood of recurrence and/or presence of occult lesions not detected on imaging or surgery, administration of systemic therapy may offer other advantages such as controlling the growth of lesions detected subsequently and abating disease progression, which could later be addressed with surgical resection.1 Stephens and associates,31 in their analysis, attempted to explore the benefit and prognostic value in patients who were started on chemotherapy after presentation with multiple metastases and found that those who progressed while on systemic therapy had a 4-fold increased risk of death and none was alive at 5 years. Nevertheless, there does not appear to be a clear consensus amongst the medical community on optimal postresection systemic therapy management. Even at our large cancer center, evaluation of practice patterns showed diverse treatment strategies over the last 2 decades which should be noted as a confounder and be recognized when interpreting the data. Nevertheless, data presented here are hypothesis-generating for a randomized trial of systemic therapy for patients in this situation.
There are several limitations for this study. Although patient accrual and perioperative data were collected prospectively, most of the postoperative data analysis was retrospective. Although this cohort consists of one of the largest IMI-guided pulmonary sarcoma metastasectomies, the size of the patients in the IMI and non-IMI occult groups are small and potentially underpowered to yield definitive recommendations without further follow-up studies. Accrual time, particularly in the non-IMI group, encompasses patients over the last decades, which has seen changes in preoperative selection and cross-sectional imaging quality (ie CT scan quality in 2005 vs 2019). The patient cohort, both in mesenchymal and nonmesenchymal groups, was heterogeneous. Different histological sarcomas are known to have widely variable natural histories that could have affected these outcomes. Furthermore, variable follow-up of patients over different time periods introduces a selection bias amongst this oncologically diverse group of patients. Additionally, IMI-guided pulmonary metastasectomy is best suitable for patients with lesions near the pleural surface who are deemed safe to undergo VATS resection rather than open thoracotomy, thus adding a potential selection bias to this population.
However, TumorGlow as it stands is easily integrated into current practice patterns used by the thoracic surgeons (Figs. 1 and 4). We propose that prospective, randomized, controlled studies should be conducted to further delineate the benefit of IMI in well-defined patient populations, studies that are best done at a multi-institutional or national level.
CONCLUSIONS
We conclude that IMI identifies a subset of sarcoma patients with pulmonary metastasectomy who may benefit from more aggressive management, such as greater use of systemic therapy. In this setting, IMI can serve both as a diagnostic and prognostic tool without conferring additional risk to the patient. Further randomized, controlled studies are needed to explore the ability of this technology in informing future therapeutic decisions in management of patients with pulmonary sarcoma metastases.
Acknowledgment:
We thank Lee Hartner, MD, for expertise and assistance throughout all aspects of our study and for help in writing the manuscript.
Support:
Dr Azari was supported by NIH training grant in surgical oncology T32CA251063–01, a Society of Thoracic Surgeons Thoracic Surgery Foundation Research Award, and a Stephen C Cheung Fellowship in Surgical Oncology. Dr Singhal was supported by NIH grant R01 CA193556 and the State of Pennsylvania Health Research Formula Fund. Dr Kennedy was supported by the American Philosophical Society and NIH grant F32 CA254210–01.
Abbreviations and Acronyms
- EPR
enhanced permeability and retention
- ICG
indocyanine green
- IMI
intraoperative molecular imaging
- NIR
near infrared
- PFS
progression free survival
- TBR
tumor to background ratio
- VATS
video-assisted thoracoscopic surgery
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Maki is a paid member of the data safety monitoring board to Aadi Bioscience, Karyopharm and at Daiichi-Sankyo.
Video content is available in the text.
Contributor Information
Feredun Azari, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Gregory T Kennedy, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Kevin Zhang, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Elizabeth Bernstein, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Robert G Maki, Department of Hematology and Medical Oncology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Colleen Gaughan, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Doraid Jarrar, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Taine Pechet, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
John Kucharczuk, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Sunil Singhal, Department of Thoracic Surgery, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
REFERENCES
- 1.Digesu CS, Wiesel O, Vaporciyan AA, et al. Management of sarcoma metastases to the lung. Surg Oncol Clin N Am 2016;25:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcove RC, Miké V, Hajek JV, et al. Osteogenic sarcoma under the age of twenty-one. A review of one hundred and forty-five operative cases. J Bone Joint Surg Am 1970;52:411–423. [PubMed] [Google Scholar]
- 3.Saltzman DA, Snyder CL, Ferrell KL, et al. Aggressive metastasectomy for pulmonic sarcomatous metastases: A follow-up study. Am J Surg 1993;166:543–547. [DOI] [PubMed] [Google Scholar]
- 4.Predina JD, Puc MM, Bergey MR, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol 2011;6:913–919. [DOI] [PubMed] [Google Scholar]
- 5.Cerfolio RJ, Bryant AS, McCarty TP, et al. A prospective study to determine the incidence of non-imaged malignant pulmonary nodules in patients who undergo metastasectomy by thoracotomy with lung palpation. Ann Thorac Surg 2011;91:1696–700; discussion 1700. [DOI] [PubMed] [Google Scholar]
- 6.Cerfolio RJ, McCarty T, Bryant AS. Non-imaged pulmonary nodules discovered during thoracotomy for metastasectomy by lung palpation. Eur J Cardiothorac Surg 2009;35:786–91; discussion 791. [DOI] [PubMed] [Google Scholar]
- 7.Pastorino U, Buyse M, Friedel G, et al. ; International Registry of Lung Metastases. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37–49. [DOI] [PubMed] [Google Scholar]
- 8.Marulli G, Mammana M, Comacchio G, et al. Survival and prognostic factors following pulmonary metastasectomy for sarcoma. J Thorac Dis 2017;9(Suppl 12):S1305–S1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martini N, Huvos AG, Miké V, et al. Multiple pulmonary resections in the treatment of osteogenic sarcoma. Ann Thorac Surg 1971;12:271–280. [DOI] [PubMed] [Google Scholar]
- 10.Okiror L, Peleki A, Moffat D, et al. Survival following pulmonary metastasectomy for sarcoma. Thorac Cardiovasc Surg 2016;64:146–149. [DOI] [PubMed] [Google Scholar]
- 11.Anon. Key Statistics for Soft Tissue Sarcomas Available at: https://www.cancer.org/cancer/soft-tissue-sarcoma/about/key-statistics.html. Accessed March 27, 2021.
- 12.Treasure T, Fiorentino F, Scarci M, et al. Pulmonary metastasectomy for sarcoma: A systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: What is the practice and where is the evidence for effectiveness? Thorax 2014;69:946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azari F, Kennedy G, Singhal S. Intraoperative detection and assessment of lung nodules. Surg Oncol Clin N Am 2020;29:525–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reza J, Sammann A, Jin C, et al. Aggressive and minimally invasive surgery for pulmonary metastasis of sarcoma. J Thorac Cardiovasc Surg 2014;147:1193–200; discussion 1200. [DOI] [PubMed] [Google Scholar]
- 16.Stahl M, Ranft A, Paulussen M, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 2011;57:549–553. [DOI] [PubMed] [Google Scholar]
- 17.Azari F, Kennedy G, Bernstein E, et al. Intraoperative molecular imaging clinical trials: A review of 2020 conference proceedings. JBO International Society for Optics and Photonics 2021;26:050901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton AD, Predina JD, Corbett CJ, et al. Optimization of second window indocyanine green for intraoperative near-infrared imaging of thoracic malignancy. J Am Coll Surg 2019;228:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Predina JD, Newton AD, Keating J, et al. Intraoperative molecular imaging combined with positron emission tomography improves surgical management of peripheral malignant pulmonary nodules. Ann Surg 2017;266:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton AD, Predina JD, Nie S, et al. Intraoperative fluorescence imaging in thoracic surgery. J Surg Oncol 2018;118:344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singhall S. A pilot and feasibility study of intraoperative imagery of solid tumors with indocyanine green 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT02280954.
- 22.Zeh R, Sheikh S, Xia L, et al. The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS One 2017;12:e0182034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaya T, Nakamura YA, Choyke PL, et al. Fluorescence-guided surgery. Front Oncol 2017;7:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton AD, Predina JD, Shin MH, et al. Intraoperative near-infrared imaging can identify neoplasms and aid in real-time margin assessment during pancreatic resection. Ann Surg 2019;270:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Predina JD, Newton AD, Corbett C, et al. Near-infrared intraoperative imaging for minimally invasive pulmonary metastasectomy for sarcomas. J Thorac Cardiovasc Surg 2019;157:2061–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter GA, Cantor SB, Walsh GL, et al. Cost-effectiveness of pulmonary resection and systemic chemotherapy in the management of metastatic soft tissue sarcoma: a combined analysis from the University of Texas M. D. Anderson and Memorial Sloan-Kettering Cancer Centers. J Thorac Cardiovasc Surg 2004;127:1366–1372. [DOI] [PubMed] [Google Scholar]
- 27.Jeong do S, Park DH, Kim CY. Cutaneous metastatic undifferentiated pleomorphic sarcoma from a mediastinal sarcoma. Ann Dermatol 2015;27:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Matsumine A, Yamada S, et al. Oncological outcome after lung metastasis in patients presenting with localized chondrosarcoma at extremities: Tokai Musculoskeletal Oncology Consortium study. Onco Targets Ther 2016;9:4747–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bindiganavile S, Han I, Yun JY, et al. Long-term outcome of chondrosarcoma: A single institutional experience. Cancer Res Treat 2015;47:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehme KA, Schleicher SB, Traub F, Rolauffs B. Chondrosarcoma: A rare misfortune in aging human cartilage? The role of stem and progenitor cells in proliferation, malignant degeneration and therapeutic resistance. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephens EH, Blackmon SH, Correa AM, et al. Progression after chemotherapy is a novel predictor of poor outcomes after pulmonary metastasectomy in sarcoma patients. J Am Coll Surg 2011;212:821–826. [DOI] [PubMed] [Google Scholar]


