Abstract
Objective
Steroidogenic factor 1 (SF1) expressing neurons in the ventromedial hypothalamus (VMH) have been directly implicated in whole-body metabolism and in the onset of obesity. The co-chaperone FKBP51 is abundantly expressed in the VMH and was recently linked to type 2 diabetes, insulin resistance, adipogenesis, browning of white adipose tissue (WAT) and bodyweight regulation.
Methods
We investigated the role of FKBP51 in the VMH by conditional deletion and virus-mediated overexpression of FKBP51 in SF1-positive neurons. Baseline and high fat diet (HFD)-induced metabolic- and stress-related phenotypes in male and female mice were obtained.
Results
In contrast to previously reported robust phenotypes of FKBP51 manipulation in the entire mediobasal hypothalamus (MBH), selective deletion or overexpression of FKBP51 in the VMH resulted in only a moderate alteration of HFD-induced bodyweight gain and body composition, independent of sex.
Conclusions
Overall, this study shows that animals lacking and overexpressing Fkbp5 in Sf1-expressing cells within the VMH display only a mild metabolic phenotype compared to an MBH-wide manipulation of this gene, suggesting that FKBP51 in SF1 neurons within this hypothalamic nucleus plays a subsidiary role in controlling whole-body metabolism.
Keywords: FKBP51, VMH, SF1, Mediobasal Hypothalamus, Obesity, High-fat diet
Highlights
-
•
Loss of FKBP51 in SF1 neurons of the VMH induces a mild metabolic phenotype.
-
•
Male and female mice develop similar metabolic responses to the loss of FKBP51.
-
•
VMH-specific overexpression of FKBP51 induces phenotypes comparable to knockout.
-
•
FKBP51 in the VMH mediates whole-body metabolism in a U-shaped manner.
1. Introduction
Since the ventromedial hypothalamus (VMH) was first described as a site for body weight regulation and food intake in 1942 by Hetherington and Ranson [26] it has been convincingly demonstrated that this mediobasal hypothalamic (MBH) nucleus directly steers food intake, whole-body metabolism, and energy homeostasis [10,34]. Hetherington's and other early VMH lesion studies concurringly observed hyperphagia, insulin resistance, and dramatic body weight gain following the loss of VMH function [1,8,32].
The heterogenous cytoarchitecture of the VMH is comprised of various cell types with different gene expression patterns [38]. Intriguingly, the ubiquitously expressed Nr5a1 gene, encoding steroidogenic factor 1 (SF1), is specifically and exclusively expressed within the VMH in the brain. Here, it acts as a transcription factor, which expression is essential for both VMH development and function [36,39,47]. Studies using postnatal VMH-specific SF1 knockout (KO) mice mirrored the metabolic phenotype of VMH lesion in rats, highlighting the cruciality of SF1 in the VMH for healthy energy homeostasis; together with its role as a primary satiety center [15,33]. Chemo- and optogenetic techniques, allowing for spatiotemporal neuronal manipulation, revealed that inactivation of Sf1-expressing neurons increased feeding behavior, reduced energy expenditure and thermogenesis, and blocked recovery from insulin-induced hypoglycemia via a plethora of different hormone receptors, including leptin- (LepR) and insulin receptor (IR), nutrient sensors and sympathetic nervous system (SNS) activation [16]. A recent study by Coupé et al. further demonstrated the importance of autophagy signaling within the VMH in response to fasting: mice lacking the autophagy-related gene 7 (Atg7) within the VMH displayed altered leptin sensitivity and disrupted energy expenditure in response to fasting. Additionally, from a cellular metabolism perspective, they had impaired mitochondrial morphology and activity [12].
In the periphery, SF1 is expressed in steroidogenic tissue of the adrenal cortex, in gonadotrope cells of the anterior pituitary, and gonads with differential roles during development. A global SF1 KO was shown to be lethal, with animals exhibiting a complex endocrine phenotype including gonadal and adrenal agenesis, impaired expression of pituitary gonadotropes and ablation of the VMH. Altogether, indicating that SF1 acts at multiple levels of this hypothalamic-pituitary-steroidogenic organ axis to regulate steroidogenesis, reproduction and energy homeostasis [27,37,43,59].
Another gene expressed at described sites along this axis, including the VMH, adrenal and pituitary is the FKBP506 binding protein 51 (FKBP51), encoded by the Fkbp5 gene [4,7,28,45]. This protein belongs to the superfamily of immunophilins, and as a co-chaperone of heat-shock protein 90 (Hsp90) it regulates the responsiveness of steroid hormone receptors [49]. Several studies identified FKBP51 as a strong inhibitor of glucocorticoid receptor (GR) function by reducing glucocorticoid (GC) binding, delaying nuclear translocation and decreasing GR-dependent transcriptional activity, thereby shaping (GR)-mediated negative feedback of the hormonal stress response system, the hypothalamic-pituitary-adrenal (HPA) axis [13,14,44,53,54]. The first cell type-specific murine KO studies recently published by our lab found that FKBP51 in the paraventricular nucleus (PVN) of the hypothalamus and in corticotrope proopiomelanocortin (POMC) cells of the anterior pituitary shape negative feedback and (re)activity of the HPA axis [7,25].
Besides its function along the HPA axis in humans and rodents, FKBP51 is abundantly expressed in metabolically relevant tissues in the periphery, such as adipocytes and skeletal muscle [3,5,40,48] and brain regions, like the arcuate nucleus (ARC) and VMH, both well-established as neuronal hubs controlling energy balance [4,19,45]. Today, there is an increasing body of research providing evidence on the role of FKBP51 in type 2 diabetes, insulin resistance, adipogenesis, browning of white adipose tissue (WAT), and bodyweight regulation [24,50]. We and other colleagues showed that FKBP51-null mice are resistant to diet-induced obesity and demonstrate improved glucose tolerance and increased insulin-signaling in skeletal muscle. Furthermore, chronic treatment with a highly selective FKBP51 antagonist, SAFit2, recapitulates the effects of FKBP51 deletion on both body weight regulation and glucose tolerance [3,22,51]. The investigation of central Fkbp5 mRNA expression in response to a metabolic challenge, either a high-fat diet (HFD) or fasting, revealed that hypothalamic Fkbp5 in the VMH, PVN, and arcuate nucleus (ARC) is increased following a metabolic stressor [4,45,55]. Based on the molecular interplay of FKBP51 with cellular autophagy [17,18,24], our lab recently uncovered that FKBP51 in the rodent MBH represents a novel regulatory link between central and peripheral autophagy signaling and the in vivo whole-body response to an obesogenic challenge, and that KO of Fkbp5 in the MBH results in opposite metabolic phenotypes to those observed in FKBP51-null mice [23].
Overall, there is strong evidence that central and peripheral FKBP51 is a molecular player in human and rodent metabolism, with a powerful role in MBH-mediated control of autophagy signaling. Thus, strategies aimed at manipulating FKBP51 could provide a new therapy approach to treat metabolic disorders such as obesity and type 2 diabetes. However, the MBH-specific FKBP51 manipulation study by Häusl and colleagues [23] once again exhibited the highly tissue-specific manner this protein acts in, together with its dynamic regulation by the environment. To address the lack of central cell type-specific studies on the role of FKBP51 on whole-body metabolism, we here investigated baseline and HFD-induced metabolic- and stress-related phenotypes in male and female mice with a conditional KO of Fkbp5 in all Sf1-expressing cells (Sf1Fkbp5-/-), mainly involving the VMH, adrenals, and pituitary [4,28,45]. To narrow down metabolic effects observed in Sf1Fkbp5-/- mice to the VMH, we virally overexpressed Fkbp5 exclusively in Sf1-expressing (Sf1Fkbp5 OE) cells within this nucleus. Overall, our results show that while a KO and OE of Fkbp5 in the VMH induced a slightly increased HFD-induced BW gain and decreased adrenal weights in Sf1Fkbp5-/- and Sf1Fkbp5 OE males and females, this nucleus does not account for the robust metabolic alterations observed following whole MBH FKBP51 manipulations.
2. Materials and methods
2.1. Animals and animal housing
All experiments and protocols were performed in accordance with the European Communities' Council Directive 2010/63/EU and were approved by the committee for the Care and Use of Laboratory animals of the Government of Upper Bavaria. All effort was made to minimize any suffering of the animals throughout the experiments. The mouse lines Fkbp5lox/lox, Sf1Fkbp5-/- and Sf1-Cre were obtained from the in-house breeding facility of the Max Planck Institute of Psychiatry and are all bred on C57/BL6N background. All animals were between 12 and 20 months old at the onset of the experiments. Male Sf1Fkbp5-/- animals were kept single housed and female mice were group housed throughout the experiments, unless indicated otherwise. All animals were held in individually ventilated cages (IVC; 30 cm × 16 cm x 16 cm; 501 cm2) serviced by a central airflow system (Tecniplast, IVC Green Line – GM500). Animals had ad libitum access to water (tap water) and food (see 2.3) and were maintained under constant environmental conditions (12:12 h light/dark cycle, 23 ± 2 °C and humidity of 55%). All IVCs had sufficient bedding and nesting material as well as a wooden tunnel for environmental enrichment. Animals were allocated to experimental groups in a semi-randomized fashion, data analysis and execution of experiments were performed blinded to group allocation.
2.2. Generation of Fkbp5lox/lox and Sf1Fkbp5-/- lines
Mice with a floxed Fkbp5 gene designated as Fkbp5lox/lox (Fkbp5tm1c(KOMP)Wtsi) were obtained by breeding Fkbp5Frt/Frt full KO mice to Deleter-Flpe mice [42]. The conditional Fkbp5Frt/Frt KO mice are derived from embryonic stem cell clone EPD0741_3_H03 which was targeted by the KO mouse project (KOMP). Frozen sperm obtained from the KOMP repository at UC Davis was used to generate KO mice (Fkbp5tm1a(KOMP)Wtsi) by in vitro fertilization. Finally, mice lacking Fkbp5 in SF1 cells (Sf1Fkbp5-/-) were obtained by breeding Fkbp5lox/lox mice to Sf1-Cre mice that express Cre recombinase under the control of the Sf1 promoter (approved mouse gene name, Nr5a1) [15]. Genotyping details are available upon request.
2.3. Diet for induced obesity
Baseline metabolic characterization of all experimental cohorts was performed under a standard chow diet (standard research diet by Altromin 1318, Altromin GmbH, Germany) with the following nutritional values: 14% fat and sucrose, 27% protein, and 59% carbohydrates. Animals under dietary challenge received a HFD diet (HFD, D12331, Research Diets, New Brunswick, NJ, USA) over a period of several weeks to induce overweight. Nutritional values HFD: 58% fat and sucrose, 17% protein, 25% carbohydrates. Bodyweight and food intake were measured weekly in all experimental cohorts.
2.4. Viral overexpression of Fkbp5 in the VMH
SF1-specific overexpression (OE) of human Fkbp5 (Sf1Fkbp5 OE) was achieved by bilateral injections of the Cre-dependent viral vector AAV1/2-Cre-dept-HA-FKBP51 (rAAV1/2-Cre-dependent-CAG-HA-human wildtype FKBP51 WPRE-BGH-polyA, titer: 1.3 × 1012 genomic particles/ml, Gene Detect GD1001-RV) into the VMH of male Sf1-Cre mice. As controls, Cre-negative animals of this mouse line were injected with an AAV2-eSyn-GFP control virus (CMV-hSYN1-eGFP, titer: 1.3 × 1012 genomic particles/ml, Vector Biolabs VB1107) for neuronal expression of a fluorescent reporter. Stereotactic surgeries were performed as described previously [25]. In brief, male mice between 3 and 5 months of age were anesthetized with isoflurane and fixated in a stereotactic apparatus. Then, 0.2 μl of the above-mentioned viruses were bilaterally injected into the VMH at a 0.05 μl/min flow rate with glass capillaries with a tip resistance of 2–4 MΩ. To target the VMH the following coordinates were used: 1.5 mm anterior to bregma, ± 0.4 mm lateral from midline, and 5.6 mm below the surface of the skull. After surgery, animals were treated with meloxicam for three days and were allowed to recover for four weeks before initiating the experimental phase.
2.5. RNAscope – Validation of Fkbp5 KO and OE
To validate successful KO of Fkbp5 mRNA in Sf1+ cells, we performed a RNAscope experiment on tissue sections of male Sf1Fkbp5-/- and Fkbp5lox/lox animals under basal conditions (3–4 months of age). Frozen tissue was sectioned at 20 μm at −20 °C in a cryostat microtome. Sections were thaw mounted on Super Frost Plus slides, dried, and stored at −80 °C. The RNA Scope Fluorescent Multiplex Reagent kit (cat. no. 320850, Advanced Cell Diagnostics, Newark, CA, USA) was used for mRNA staining. Probes used for staining were; Fkbp5 (Mm-Fkbp5-C1) for native Fkbp5 expression, human Fkbp5 (H-Fkbp5-C1) for detection of viral OE and Sf1 (Mm-Nr5a1-C2). The staining procedure was performed according to the manufacturer's specifications and as performed previously [25]. Images of the VMH (left and right side), pituitary, and adrenal were acquired by an experimenter blinded to the condition of the animals. Sixteen-bit images of each section were acquired on a Zeiss confocal microscope using a 20x and 40x objective (n = 2 animals per marker and condition). For every section, all images were acquired using identical settings for laser power, detector gain, and amplifier offset. Fkbp5 mRNA expression was analyzed using ImageJ with the experimenter blinded to the genotype of the animals and was counted manually.
The Fkpb5 probe targets Exon 9, which is deleted in our model, but it also spans neighboring exons. Consequently, the probe may still bind to truncated mRNA leading to a residual Fkbp5 mRNA signal in knock-out cells, even though no functional FKBP51 protein can be expressed. To assess resulting background signal, we performed an RNAScope in male global Fkbp5Frt/Frt KO mice, where exon 9 is replaced by a large selection cassette (see section 2.2) and therefore no functional protein is expressed. We then quantified the Fkbp5 signal in the VMH and hippocampus (HIP) of these global KO animals. This revealed a percentage of residual Fkbp5 signal of 80% in the VMH (t14 = 3.08, p = 0.008) and 60% in the HIP (t12 = 4.08, p = 0.002), ranging from 0 to 3 dots/cell (Suppl. Fig. 1 A – B). Therefore, Sf1-positive cells expressing 0–3 dots/cell were defined as background signal for all following quantifications. With this cut-off the efficiency of the KO lies at around 15% for both VMH (t14 = 10.45, p < 0.0001) and HIP (U = 0, p = 0.0003) in global Fkbp5Frt/Frt KO animals (Suppl. Fig. 1C). Accordingly, each Sf1+ cell containing more than 3 Fkbp5 mRNA dots/cell in our Sf1Fkbp5-/- model was counted as positive and calculated as a percentage of Fkbp5 positive cells from total number of Sf1-expressing cells.
2.6. In-situ hybridization in Sf1Fkbp5 OE
To quantify viral Fkbp5 mRNA OE, we performed in-situ hybridization (ISH). Frozen brains were processed as described for RNAscope and ISH using a 35S UTP labeled Fkbp5 ribonucleotide probe was performed as described previously [25,46]. All primer details are available upon request. For signal detection, the slides were exposed to Kodak Biomax MR films (Eastman Kodak Co., Rochester, NY) and developed. The autoradiographs were digitized, and expression was determined by optical densitometry utilizing the freely available NIH ImageJ software (NIH, Bethesda, MD, USA). The grey value of the left and the right side of the VMH was measured within a circular template (16 width × 16 height) in every slice analyzed (1 slice per animal, 2 animals per group). The data were analyzed blindly, always subtracting the background signal of a nearby structure not expressing the gene of interest from the measurements. Animals that did not overexpress viral Fkbp5 mRNA in the VMH were excluded from all analyses.
2.7. Tissue sampling procedure
On the day of sacrifice, animals were weighed, deeply anesthetized with isoflurane and, sacrificed by decapitation. Trunk blood (basal morning CORT and basal adrenocorticotropin hormone (ACTH)) was collected in labeled 1.5 ml EDTA-coated microcentrifuge tubes (Kabe Labortechnik, Germany) and kept on ice until centrifugation. After centrifugation (4 °C, 8,000 rpm for 15 min) plasma was removed and transferred to new, labeled 2 mL tubes and stored at −80 °C until hormone quantification. For mRNA analyses, brains were removed, and the pituitary was dissected from the skull and manually attached to the brain. Adrenals were dissected, weighed, and attached to a C57/BL6n holder brain. Tissues were snap-frozen in isopentane at −40 °C and stored at −80 °C until further processing.
2.8. Acute restraint stress paradigm
The acute restraint stress paradigm is perceived as a severe stressor robustly inducing the entire spectrum of known allostatic responses in rodents and was, therefore, the stress paradigm of choice. At 8:00 AM, 1 h after the lights were switched on, each animal was placed in a custom-made restrainer (50 ml falcon tube with holes at the bottom and the lid to provide enough oxygen and space for tail movement) for 15 min in their home cage. After 15 min, animals were removed from the tube and the first blood sample was collected by a tail cut. Subsequent blood samples were collected at 15, 30, and 60 min post stress in the home cage via tail cut. The animals were left undisturbed in between sampling procedures.
2.9. Hormone assessment
Baseline morning (measured between 08:00–12:00 a.m.) and post stress plasma CORT (ng/mL) and baseline ACTH (pg/mL) concentrations were determined by radioimmunoassay using CORT 125I RIA kit (sensitivity: 12.5 ng/ml, MP Biomedicals Inc) and ACTH 125I RIA kit (sensitivity: 10 pg/ml, MP Biomedicals Inc) following the manufacturers' instructions. The radioactivity of the pellet was measured with a gamma counter (Packard Cobra II Auto Gamma; Perkin–Elmer). Final CORT and ACTH levels were derived from the standard curve. Morning baseline testosterone concentrations (ng/mL) between 08:00–12:00 a.m. were measured with a testosterone ELISA (Demeditec, DEV991) according to the manufacturer's instructions. Final testosterone levels were derived from the standard curve.
2.10. Nuclear magnetic resonance
In addition to weekly measures of BW, the animal's body composition was assessed with a body composition analyzer (LF50 BCA NMR Minispec Analyzer, Bruker Optik) after several weeks on chow and HFD. This method applies time domain nuclear magnetic resonance (TD - NMR) to measure lean tissue mass, fat mass, and free fluids non-invasively and in vivo without the need for anesthetics in small rodents [21]. Body constituents were normalized to bodyweight for each group and the ratio of fat to lean mass was calculated.
2.11. Intraperitoneal glucose (GTT) and insulin (ITT) tolerance test
After several weeks under a chow diet, an intraperitoneal glucose tolerance test (GTT) was carried out after lights-on. A 20% D-(+)-Glucose solution (Sigma Aldrich, Merck, Darmstadt) was prepared, and animals were subjected to an overnight fast of 14 h (6 p.m. until 8 a.m.) prior to the experiment. Every animal was weighed and intraperitoneally injected with 2 g glucose per kg bodyweight. Blood glucose concentrations were measured from tail stitches at 0, 15, 30, 60, 90, and 120 min after the glucose injection using a handheld XT glucometer (Bayer Health Care, Basel, Switzerland).
An intraperitoneal insulin tolerance test (ITT) was performed 14 days after the GTT to ensure a complete recovery from the overnight fast. A similar procedure as for the GTT was applied as follows: An insulin stock solution of 0.5 IU/mL (Actrapid® Penfill®, Novo Nordisk Pharma GmbH, Bagsværd, Denmark) was prepared, and animals were fasted for 4 h (7 until 11 a.m.) before the onset of the ITT. Every animal was weighed and intraperitoneally injected with 1IU insulin per kg bodyweight. Blood glucose concentrations were measured at 0, 15, 30, 60, 90, and 120 min after the insulin injection.
2.12. Indirect calorimetry
Metabolic phenotyping and food intake after several weeks on HFD challenge was conducted by an automated PhenoMaster open-circuit indirect calorimetry system (TSE Systems) in single housed male Sf1Fkbp5-/- and Fkbp5lox/lox mice. All animals were allowed to acclimatize to the experimental setup for 2 days with a total experimental duration and data acquisition of 7 days. The statistical analysis was performed exclusively on data from day 3 to day 6 (total of 72 h) for all animals. The time plots were generated from hourly averages of all parameters over 72 h. Totals were calculated as the average of all measures over 72 h. HFD and water were available ad libitum. The data acquisition was carried out by TSE Phenomaster version 7.2.8.
2.13. Statistical analysis
The data presented are shown as means ± standard error of the mean (SEM) and samples sizes are indicated in the figure legends and the main text. All data were analyzed by the commercially available GraphPad Prism 9.0 software (GraphPad Software, San Diego, California, USA). When two groups were compared, the unpaired two-tailed student's t test was applied. If data were not normally distributed the non-parametric Mann–Whitney test (MW test) was used. Data based on repeated observations comparing two groups were tested by repeated measures ANOVA. Correlation analyses were performed using the Pearson's correlation coefficient. P values of less than 0.05 were considered statistically significant. Statistical significance was defined as: ∗p ≤ 0.05, ∗∗p ≤ 0.01. A statistical trend was accepted with a p value of 0.05 ≤ p ≤ 0.1 and indicated in the figures with the symbol “T”. Outliers were assessed with the online available Graph Pad outlier calculator performing the two-sided Grubb's outlier test. As this was an exploratory study, no statistical methods were used to predetermine sample sizes.
3. Results
3.1. Validation of successful Fkbp5 KO in VMH and metabolic phenotyping of male and female Sf1Fkbp5-/- mice
As hypothalamic Fkbp5 mRNA levels are highly responsive to dietary challenges such as prolonged HFD [4] and food restriction [20,45,55], this protein seems to sense the nutrient environment and to adjust its central expression to the given dietary conditions. Further, our group could show that hypothalamic FKBP51 can shape whole-body metabolism and steer central and peripheral autophagy [23]. Therefore, we hypothesized that a KO of Fkbp5 specifically in VMH SF1 neurons could alter homeostasis and resemble observed metabolic phenotypes following Fkbp5 manipulation in the whole MBH.
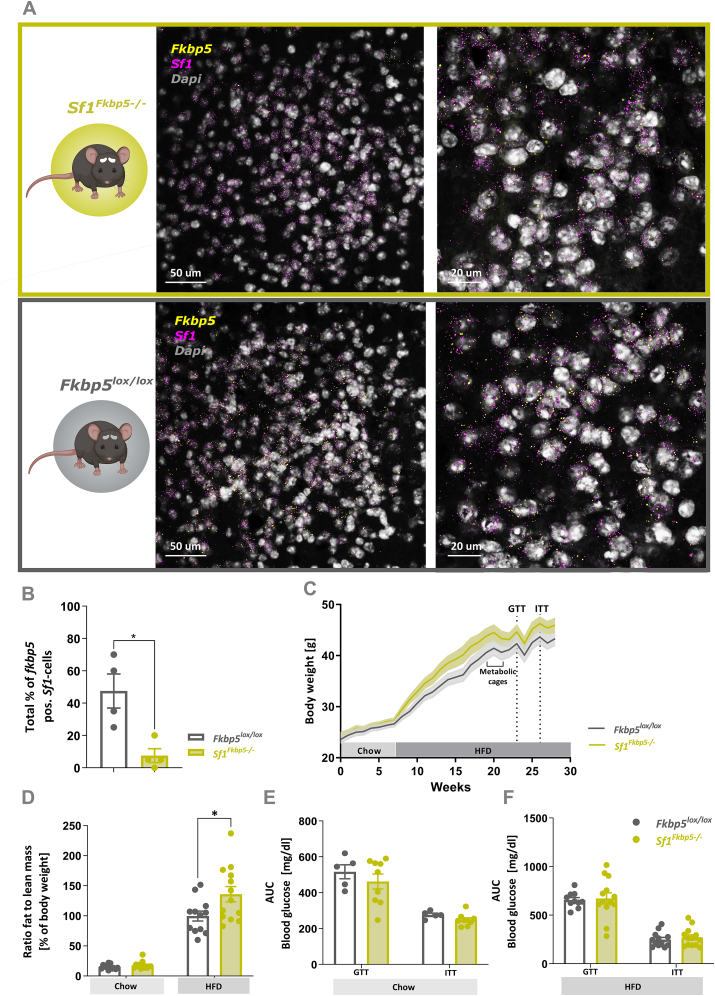
To study the effects of FKBP51 in Sf1-expressing neurons within the VMH on whole-body metabolism, we generated a SF1-specific conditional Fkbp5 KO mouse line (Sf1Fkbp5-/-). Qualitative co-expression analysis of Fkbp5 and Sf1+ neurons in the VMH revealed that Fkbp5 is almost exclusively expressed in Sf1+ neurons (Figure 1 A). Subsequent quantification of co-expression revealed that the number of Sf1+ cells expressing Fkbp5 within the VMH is significantly reduced in Sf1Fkbp5-/- mice compared to control Fkbp5lox/lox mice (t6 = 0.67, p = 0.01) confirming successful KO of Fkbp5 in this neuronal population (Figure 1 B). Note that the residual Fkbp5 signal in Sf1Fkbp5-/- mice is likely due to the detection of truncated Fkbp5 mRNA expression which is also present in global Fkbp5Frt/Frt KO animals (see Suppl. Fig. 1 A - C). Assessment of weekly BW in a first cohort revealed non-significant but slightly increased HFD-induced BW gain in mice with a KO of Fkbp5 (n = 13) compared to controls (n = 12), indicating a higher metabolic vulnerability to the dietary challenge (F1,23 = 2.63, p = 0.12) (Figure 1C). This slight increase of BW-gain in Sf1Fkbp5-/- animals could be confirmed by a significantly higher ratio of fat to lean mass in the KO animals after 8 weeks on a HFD (chow: U = 49, p = 0.12; HFD: t23 = 2.36, p = 0.03) (Figure 1 D). To assess the effects of our KO on glucose tolerance and insulin sensitivity under chow diet, we performed a GTT and ITT in a separate cohort (Figure 1 E), first confirming that these animals had the same phenotype as our 1st cohort (nSf1Fkbp5−/− = 5; nFkbp5lox/lox = 9) (Supl. Fig. 2 A – B). Further, we performed a GTT and ITT after several weeks on a HFD (GTT: 16 weeks, ITT: 18 weeks) in the 1st cohort (Figure 1 F). Under both dietary regimens, KO and control animals showed typical blood glucose curve progression after a single glucose or insulin bolus, but no difference between genotypes was detected (Chow: GTT t12 = 0.85, p = 0.42, ITT t12 = 1.67, p = 0.12; HFD: GTT t21 = 0.24, p = 0.81, ITT t23 = 0.66, p = 0.52). To allow deeper metabolic phenotyping, the animal's energy expenditure (EE), respiratory exchange ratio (RER), activity and food intake were measured in metabolic cages via indirect calorimetry. Repeated measures ANOVA did not detect significant differences between Sf1Fkbp5-/- KO animals and controls in any of the parameters assessed (RER: F1,14 = 0.91, p = 0.36, t13 = 0.4, p = 0.7; EE: F1,14 = 0.35, p = 0.56, t13 = 1.02, p = 0.33; activity: F1,14 = 0.02, p = 0.9, t13 = 0.04, p = 0.1; food intake: F1,12 = 0.91, p = 0.36, t12 = 1.26, p = 0.23) (Suppl. Fig. 3 A – H).
Figure 1.
Conditional KO of Fkbp5 in Sf1-expressing cells slightly increases BW gain under a HFD challenge in male mice. A Representative RNAscope confocal images of endogenous Fkbp5 mRNA expression in SF1 neurons within the VMH of Sf1Fkbp5-/- (green panel) and Fkbp5lox/lox controls (grey panel). B Quantification of the total percentage of Fkbp5 positive cells that co-express Sf1 mRNA revealed significant reduction of Fkbp5 expression in the Sf1Fkbp5-/- KO line (n = number of analyzed 40x confocal images of either left or right VMH, nKO = 4, nControl = 4; 2 animals/genotype). C In a first cohort under SD for 8 weeks and HFD for 21 weeks, Sf1Fkbp5-/- mice (n = 13) displayed slightly increased HFD-induced BW gain compared to controls (n = 12) which was stable over time but non-significant. D Higher BW was reflected in a significant increase in the ratio of fat to lean mass in Sf1Fkbp5 OE in this cohort compared to controls after 8 weeks on a HFD. Blood glucose levels remained unchanged in the GTT and ITT under chow, which were assessed in a separate cohort (nSf1Fkbp5−/− = 5; nFkbp5lox/lox = 9) (E) and HFD in the first cohort (F). Data are received from mice between 12 and 20 weeks of age and are presented as mean ± SEM. ∗p < 0.05. AUC area under the curve, GTT glucose tolerance test, ITT insulin tolerance test.
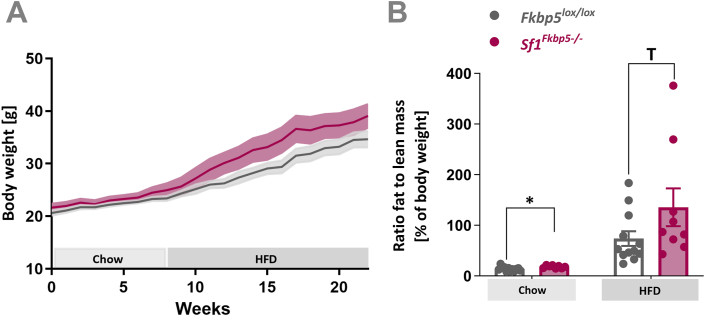
Conditional KO of Fkbp5 in female mice resembled metabolic phenotypes of male Sf1Fkbp5-/- with a non-significant mild increase in HFD-induced BW gain over time (F1,19 = 2.74, p = 0.11) (Figure 2 A), and an increase in the ratio of fat to lean mass already under chow (t18 = 2.48, p = 0.02) and after 8 weeks on HFD challenge (U = 29, p = 0.08) (nSf1Fkbp5−/− = 9; nFkbp5lox/lox = 12) (Figure 2 B). The group housing of mixed KO and control litters did not allow us to measure food intake in females.
Figure 2.
Conditional KO of Fkbp5 in Sf1-expressing cells of female mice resembles male metabolic phenotype. A Female Sf1Fkbp5-/- (n = 9) mice displayed higher HFD-induced weight gain than control Fkbp5lox/lox (n = 12), thus resemble BW phenotype of the male KO cohort. B Female Sf1Fkbp5-/- ratio fat to lean mass is higher under a chow diet and after a dietary HFD challenge (15 weeks total). Data are received from mice between 8 and 12 weeks of age and are presented as mean ± SEM. ∗p < 0.05, T < 0.1.
We further performed an RNAScope on the adrenal and pituitary as sites of peripheral Sf1-expression and quantified them according to KO validation in the Sf1Fkbp5-/- line. This revealed a significant reduction of Fkbp5 mRNA in the adrenal gland (t8 = 8.87, p < 0.0001) with a Sf1 co-expression of about 60%. In the pituitary, the co-expression of Fkbp5 and Sf1 mRNA was much lower at about 25%, which could lead to a floor effect that did not allow us to quantify the KO in this tissue (t12 = 1.72, p = 0.11) (Suppl. Fig. 4 A - D). In order to exclude that the mild metabolic phenotype of male and female Sf1Fkbp5-/- KO animals was affected by the loss of Fkbp5 in Sf1-expressing cells of the adrenal, we assessed common HPA axis parameters in all experimental cohorts (Suppl. Fig. 5 A – I). Adrenal weight was measured at the endpoint of each experiment and statistical analysis revealed significantly reduced relative adrenal weights in male KO mice under chow (t8 = 3.49, p = 0.008) and after 21 weeks on a HFD (t12 = 2.41, p = 0.03) in two individual cohorts (Suppl. Fig. 5 A). To determine if the function of the HPA axis was impaired by a KO of Fkbp5 in cortical adrenal- and gonadotrope pituitary SF1 cells, morning CORT on chow and HFD and morning ACTH on HFD were assessed between 08:00–12:00 a.m. Both endocrine measures remained unchanged in male Sf1Fkbp5-/- KO animals (CORT chow: U = 10, p = 0.37; CORT HFD: U = 67, p = 0.57; ACTH HFD: t23 = 1.25, p = 0.22) (Suppl. Fig. 5 B and C). In line with the effects observed in male KO mice, relative adrenal weight was significantly decreased in female Sf1Fkbp5-/- after 15 weeks on HFD (t11 = 4.09, p = 0.002) (Suppl. Fig. 5 D) without altering baseline CORT post HFD (t19 = 0.75, p = 0.46) (Suppl. Fig. 5 E), indicating that HPA axis function is unaffected by a KO of Fkbp5 in both sexes.
Together, data from male and female cohorts indicate that the KO of Fkbp5 in Sf1-expressing cells has a mild impact on BW progression under a dietary challenge without altering other metabolic parameters assessed by indirect calorimetry. Interestingly, relative adrenal weight was significantly reduced in Sf1Fkbp5-/- males and females under chow and after HFD, but this had no consequences on HPA axis (re)activity.
3.2. Viral Fkbp5 OE in SF1 neurons within the VMH induces similar phenotype to Sf1Fkbp5-/- males
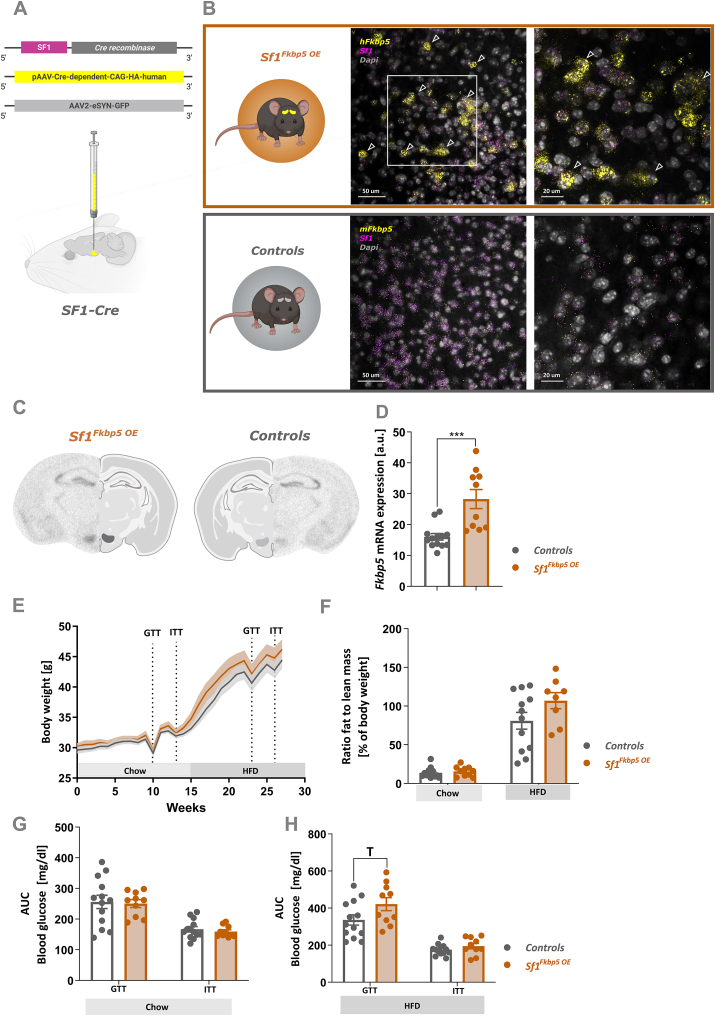
To gain deeper understanding of how changes in Fkbp5 expression levels within the VMH may control BW progression and changes in adrenal weight, and to narrow down observed effects on the VMH, we virally overexpressed Fkbp5 exclusively in VMH SF1 neurons. SF1-specific OE of Fkbp5 (Sf1Fkbp5 OE, n = 10) was achieved by bilateral injections of a Cre-dependent Fkbp5 OE virus into the VMH of male Sf1-Cre mice. Controls (n = 13), were injected with an AAV2-eSyn-GFP control virus (Figure 3 A). Qualitative analysis of viral Fkbp5 mRNA OE in RNAscope confocal images (Figure 3 B) and ISH autoradiographs (Figure 3C) revealed a robust and SF1-specific increase of Fkbp5 expression in the VMH of Sf1Fkbp5 OE mice. Quantification of ISH Fkbp5 mRNA expression within the VMH revealed a significant 1.7-fold increase of gene expression in Sf1Fkbp5 OE animals (U = 10, p = 0.0002) (Figure 3 D). Comparable to Sf1Fkbp5-/- KO mice, HFD-induced BW gain (F1,21 = 1.67, p = 0.21) and ratio of fat to lean mass in Sf1Fkbp5 OE after 6 weeks on HFD (chow: U = 54, p = 0.52; HFD: t18 = 1.64, p = 0.12) was mildly but non-significantly increased in group housed OE animals (Figure 3 E). Blood glucose levels assessed in the GTT and ITT were unaltered under chow diet (GTT t21 = 0.12, p = 0.84; ITT t21 = 0.69, p = 0.5) (Figure 3 G). However, on a HFD challenge, animals overexpressing Fkbp5 in their VMH displayed slightly but non-significantly impaired glucose tolerance (GTT t21 = 1.94, p = 0.07; ITT t21 = 1.23, p = 0.23) (Figure 3H).
Figure 3.
Viral OE of Fkbp5 exclusively in SF1 neurons within the VMH induces a similar metabolic phenotype to the KO under a HFD challenge. A VMH-specific Fkbp5 OE was achieved by bilateral injections of a Cre-dependent human Fkbp5 OE virus into the VMH of Sf1-Cre mice (Sf1Fkbp5 OE; n = 10). Control Sf1-Cre received an eGFP control virus (n = 13). B Representative RNAscope confocal images of Fkbp5 mRNA expression in SF1 neurons within the VMH of Sf1Fkbp5 OE (human Fkbp5; upper orange panel) and controls (mouse Fkbp5; lower grey panel). Arrowheads highlighting viral Fkbp5 OE in Sf1 positive neurons. C Qualitative and quantitative (D) ISH analysis confirmed successful viral Fkbp5 OE with a ∼1.6fold increase of Fkbp5 expression in Sf1Fkbp5 OE animals (n = 2) compared to controls (n = 2). ESf1Fkbp5 OE mice displayed a higher HFD-induced BW gain over time compared to controls. F Higher BW was reflected in a significant increase in the ratio of fat to lean mass in Sf1Fkbp5 OE after 6 weeks on a HFD. G Blood glucose levels in the GTT and ITT were unaltered under a chow diet but after 9 weeks on a HFD KO animals displayed significantly increased blood glucose levels in the GTT and a trend towards increased levels in the ITT (H). Data are received from mice between 16 and 20 weeks of age and are presented as mean ± SEM. ∗p < 0.05, T < 0.1. AUC area under the curve, GTT glucose tolerance test, ITT insulin tolerance test.
The group housing of mixed OE and control litters did not allow us to measure food intake in this cohort. Correlation analysis of Fkbp5 mRNA expression level with BW gain (r2 = 2.56, p = 0.002), ratio of fat to lean mass (r2 = 0.31, p = 0.06), GTT (r2 = 0.18, p = 0.15), and ITT (r2 = 0.27, p = 0.06) under HFD in control animals confirmed the negative relationship between metabolic parameters and Fkbp5 mRNA expression levels that we observed in Sf1Fkbp5-/- KO animals (Suppl. Fig. 6 A, C, E, G). In animals overexpressing Fkbp5 in SF1 neurons of the VMH, there is no significant correlation within this group of animals (BW gain: r2 = 0.03, p = 0.26; ratio fat to lean mass: r2 = 0.22, p = 0.24; GTT HFD: r2 = 0.05, p = 0.5; ITT HFD: r2 = 0.003, p = 0.87), which indicates a ceiling effect of the impact of Fkbp5 expression levels on observed changes in metabolic control (Suppl. Fig. 6 B, D, F, H).
As observed in male and female Sf1Fkbp5-/- animals, relative adrenal weight was significantly decreased in Sf1Fkbp5 OE after 13 weeks on HFD (t18 = 2.66, p = 0.02) (Suppl. Fig. 5 F), without affecting morning baseline CORT levels on chow (U = 49.5, p = 0.87) and HFD (t21 = 0.32, p = 0.75) nor ACTH on HFD (t21 = 1.65, p = 0.12) (assessed between 08:00–12:00 a.m.) (Suppl. Fig. 5 G - H). With an acute restraint stress and subsequent CORT measures at 15, 30, and 60 min post stress, we further confirmed that endocrine- and HPA axis function is unaffected by the OE of Fkbp5 in the VMH (T15: t20 = 0.56, p = 0.59; T30 t20 = 0.08, p = 0.94; T60 U = 51, p = 0.65) (Suppl. Fig. 5 I).
To assess a potential metabolic impact of our genetic manipulation of FKBP51 in Sf1-expressing cells in the peripheral sex organs [27,30,31,52,56,57], we assessed morning baseline testosterone levels in all experimental cohorts. As shown in supplementary figure 7 A - D testosterone levels were unaltered between controls and FKBP51 models (A Sf1Fkbp5-/- males on HFD: U = 49, p = 0.32; B Sf1Fkbp5-/- females on HFD: U = 15, p = 0.50; C Sf1Fkbp5 OE males on HFD: U = 58, p = 0.91; D Sf1Fkbp5-/- males on SD: U = 21.5, p = 0.94), excluding this sex hormone as a confounding factor for observed changes in metabolic control (Suppl. Fig. 7 A – D).
4. Discussion
Since a study led by Eriksson and colleagues in 2014 first revealed the metabolic impact of Fkbp5 expression in adipose tissue [40], our understanding of the peripheral role of this co-chaperone in adipogenesis, insulin signaling, autophagy, and type 2 diabetes (T2D) has rapidly improved. Preclinical full FKBP51 KO studies in rodents have further uncovered the role of this co-chaperone in regulating whole-body metabolism in vivo [24]. Despite this progress over the past 10 years and our knowledge that FKBP51 is highly expressed in metabolic brain centers such as the VMH, PVN, and ARC [4,7,25], the role of FKBP51 in these nuclei remains unexplored as cell type-specific studies are completely lacking. Here we manipulated FKBP51 in a cell type- and nucleus-specific manner to further narrow down its potential sites of action in whole-body metabolism; an indispensable step towards the development of targeted therapeutic interventions for metabolic disorders. Using a combination of systemic endogenous KO of FKBP51 in Sf1-expressing cells and viral OE of the gene exclusively in the VMH, we demonstrate that FKBP51 in VMH SF1 neurons induces only a mild metabolic phenotype triggered by a dietary challenge in male and female mice.
We have recently shown that deletion of FKBP51 in the MBH results in massive obesity within a few weeks already under chow conditions, while MBH-specific Fkbp5 OE resulted in protection from diet-induced obesity [23]. In this recent study, however, all cell types and regions of the MBH were affected. Data from the current study clearly indicate that while FKBP51 is strongly expressed in almost all SF1-positive neurons of the VMH, deletion of FKBP51 in these cells only had a moderate effect on weight gain and body composition under a HFD challenge, albeit in the same direction as a full MBH FKBP51 deletion. This effect was largely independent of sex and did not result in significant alterations of glucose or insulin tolerance in the animals. We can thus conclude that SF1 neurons in the VMH are not the main driver of an MBH FKBP51 deletion-induced obesity, and at most act in concert with other cell types or nuclei in the MBH to drive this phenotype. Interestingly, mice with a VMH-specific KO of SF1 showed increased weight gain and impaired thermogenesis, and in agreement with our results, a dietary challenge was necessary to induce this SF1-driven metabolic phenotype [15,33]. Since prolonged nutrient overload is considered a metabolic stressor activating inflammatory processes at metabolically active sites in the brain, it can trigger a central and behavioral stress response [2]. This response could in turn amplify the effects of stress-and diet-responsive Fkbp5 [4,45,55] in our KO model.
To gain a deeper understanding of the dose-dependent actions of FKBP51 in the VMH and its implications on homeostatic control, we overexpressed FKBP51 exclusively in Sf1-expressing neurons of the VMH by Cre-specific viral injections. Intriguingly, these male Sf1Fkbp5 OE developed a similar BW phenotype to our male and female Sf1Fkbp5-/- cohorts with a slightly accelerated weight gain under a HFD challenge, resulting in a mildly higher fat to lean mass ratio in animals overexpressing FKBP51 in the VMH. Further, viral Fkbp5 OE and mild differences in BW resulted in impaired glucose tolerance only under HFD. In line with MBH FKBP51 OE results, the BW phenotype was induced exclusively by a high-calorie challenge. However, resulting phenotypes diverged in opposite directions as the MBH FKBP51 OE mice were protected against an HFD-induced weight gain compared to controls. These results further underline that SF1 neurons in the VMH are not the main contributor to the overall metabolic role of FKBP51 in the MBH.
Interestingly, the recent paper by Häusl and colleagues [23] does offer possible explanations for the unexpected phenotypes observed in the current study. First, they detected an altered balance between hypothalamic and peripheral autophagy-mTOR signaling as a major contributor to the observed metabolic phenotype of MBH Fkbp5 OE and KO mice [23]. Recently, a knockout study of autophagy gene 7 (Atg7) in mouse VMH-SF1 neurons reported that loss of this gene alters the homeostatic response to fasting and that this metabolic challenge triggers autophagy in the VMH [12]. However, within the MBH, a role for proopiomelanocortin (POMC) and agouti-related protein (AGRP) neurons of the ARC in autophagy signaling has also been described. Atg7 deletion in POMC neurons of the ARC implied an obesogenic phenotype, especially when fed a HFD [11,41], whereas loss of this autophagy gene in AGRP neurons promoted leanness [29]. These studies suggest that autophagy signaling and homeostatic control within the MBH are regulated in a cell type-specific manner, and the resulting metabolic effects are highly dependent on the types of neurons involved. Nonetheless, it is tempting to speculate that alterations of autophagic signaling in the VMH are causally linked to the mild phenotype induced by FKBP51 manipulations in this region. Second, Häusl and colleagues described the effects of FKBP51 on autophagy in the MBH in an inversed u-shaped manner. Both deletion and robust OE of FKBP51 lead to significant reductions in autophagy in the MBH, while a moderate FKBP51 increase in the periphery of MBH OE mice stimulated autophagy and induced their lean phenotype [23]. It is therefore feasible that our current manipulations of FKBP51 in SF1 neurons both lead to a cell-type specific reduction of autophagic signaling. This could explain the similar metabolic phenotype of mice with either a SF1-specific Fkbp5 OE or deletion.
To rule out an impact of our Fkbp5 KO in Sf1-expressing cells within the adrenal and pituitary [7,27,43,59] on described phenotypes, we assessed adrenal weights and endocrine parameters of the HPA axis, including baseline and post-stress CORT and ACTH under HFD and SD in our cohorts. Intriguingly, adrenal weights were consistently lower in the experimental group, irrespective of sex, dietary condition and genetic manipulation. Since our viral OE exclusively affected the VMH and yet produced the same phenotype in the adrenal glands as the KO supports the hypothesis of a VMH-driven effect rather than a result of Fkbp5 KO in this organ itself. Further, there were no functional consequences of Fkbp5 manipulation on the HPA axis function in either cohort. Intriguingly, there are early studies demonstrating that compensatory adrenal growth after unilateral adrenalectomy, a common method to study adult adrenal growth mechanisms, is mediated by a neural loop including afferent and efferent limbs between the adrenals and the VMH [6]. Further, VMH-lesion studies indicated a gradual but strong increase in the efferent activity of the adrenal sympathetic nerves and increased catecholamine secretion from the adrenal medulla which could be involved in the development of metabolic disorders observed during the VMH syndrome [58]. A study by King et al. further established a key role for adrenal glucocorticoids in the development of obese phenotypes that resulted from VMH lesions [35]. However, the involvement of FKBP51 in the described VMH-mediated adrenal growth mechanisms and functions in the onset of obesogenic phenotypes is speculative at the moment. Nevertheless, our study opens a promising new avenue to study our mouse models in the context of VMH-induced metabolic phenotypes.
This study also comes with some limitations. It is important to note that the two approaches used for genetic manipulation of Fkbp5 expression might target a different set of neuronal subpopulations within the VMH. Developmental KO of Fkbp5 affects the entire VMH as SF1 acts as a transcription factor, which expression is essential for both VMH development and function [36,39,47]. Adult, viral OE of Fkbp5, on the other hand, only targets Sf1-expressing cells in the dorsomedial and central parts of the VMH [9]. Further, we exclusively assessed morning baseline CORT levels as a measure of HPA-axis function in our animal models, which means that we cannot exclude differences of HPA activity at other time points of the circadian rhythm.
5. Conclusion
In summary, this study shows that animals lacking and overexpressing Fkbp5 in Sf1-expressing cells within the VMH display a mild metabolic phenotype compared to an MBH-wide manipulation of this gene. Therefore, suggesting that FKBP51 in this hypothalamic nucleus plays a minor role in controlling whole-body metabolism. Therefore, future studies involving MBH cell type-specific KO and OE studies will shed light on MBH nuclei and cell populations that contribute significantly to the metabolic effects achieved with MBH-wide manipulation of FKBP51. Two promising candidates are POMC and AGRP populations within the ARC [11,29,41] and the ongoing metabolic phenotyping of FKBP51-specific KO mouse lines that we have generated [7] will help to further unravel the FKBP51 driven effects on MBH metabolism.
Author contributions
L.M.B and M.V.S.: Conceived the project and designed the experiments. J.M.D.: Provided scientific expertise for establishing the Sf1Fkbp5-/- mouse line. L.M.B. managed the mouse lines. L.M.B, I.T., L.A., and V.K. designed and performed RNAscope and ISH experiments and manual counting of cells. L.M.B. and I.T. performed experiments, surgeries and analysis of data. A.H., J.B., L.v.D., C.E., S.N., M.S., V.S., H.Y. and V.K. assisted with the experiments. L.M.B.: Wrote the initial version of the manuscript. M.V.S.: Supervised the research and all authors revised the manuscript.
Acknowledgments
The authors thank Prof. Dr. Alon Chen for financial and structural support and Dr. Rosa Hüttl, Rainer Stoffel, Daniela Harbich, Andrea Parl, Andrea Ressle and Bianca Schmid for their excellent technical assistance and support. We thank Shiladitya Mitra for his scientific and experimental support. We thank Stefanie Unkmeir, Sabrina Bauer and the scientific core unit Genetically Engineered Mouse Models for genotyping support. This work was supported by the “GUTMOM” grant of the ERA-Net Cofund HDHL-INTIMIC (INtesTInal MIcrobiomics) under the JPI HDHL (Joint Programming Initiative – A healthy diet for a healthy life) umbrella (01EA1805; MVS) and the SCHM2360-5-1 grant (MVS) from the German Research Foundation (DFG).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2022.101579.
Contributor Information
Lea M. Brix, Email: brix_lea-maria@psych.mpg.de.
Mathias V. Schmidt, Email: mschmidt@psych.mpg.de.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Anand B.K., Brobeck J.R. Hypothalamic control of food intake in rats and cats. Yale Journal of Biology & Medicine. 1951;24:123. [PMC free article] [PubMed] [Google Scholar]
- 2.Aslani S., Vieira N., Marques F., Costa P.S., Sousa N., Palha J.A. The effect of high-fat diet on rat's mood, feeding behavior and response to stress. Translational Psychiatry. 2015;511(5):e684. doi: 10.1038/tp.2015.178. e684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balsevich G., Häusl A.S., Meyer C.W., Karamihalev S., Feng X., Pöhlmann M.L., et al. Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nature Communications. 2017;81(8):1–12. doi: 10.1038/s41467-017-01783-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balsevich G., Uribe A., Wagner K.V., Hartmann J., Santarelli S., Labermaier C., et al. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. Journal of Endocrinology. 2014;222:15–26. doi: 10.1530/JOE-14-0129. [DOI] [PubMed] [Google Scholar]
- 5.Baughman G., Wiederrecht G.J., Chang F., Martin M.M., Bourgeois S. Tissue distribution and abundance of human FKBP51, an FK506-binding protein that can mediate calcineurin inhibition. Biochemical and Biophysical Research Communications. 1997;232:437–443. doi: 10.1006/BBRC.1997.6307. [DOI] [PubMed] [Google Scholar]
- 6.Beuschlein F., Mutch C., Bayers D.L., Ulrich-Lai Y.M., Engeland W.C., Keegan C., et al. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002;143:3122–3135. doi: 10.1210/ENDO.143.8.8944. [DOI] [PubMed] [Google Scholar]
- 7.Brix L.M., Häusl A.S., Toksöz I., Bordes J., van Doeselaar L., Engelhardt C., et al. The co-chaperone FKBP51 modulates HPA axis activity and age-related maladaptation of the stress system in pituitary proopiomelanocortin cells. Psychoneuroendocrinology. 2022;138:105670. doi: 10.1016/J.PSYNEUEN.2022.105670. [DOI] [PubMed] [Google Scholar]
- 8.Brobeck J.R. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiological Reviews. 1946;26:541–559. doi: 10.1152/PHYSREV.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- 9.Cheung C.C., Kurrasch D.M., Liang J.K., Ingraham H.A. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. The Journal of Comparative Neurology. 2013;521:1268–1288. doi: 10.1002/CNE.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y.H., Fujikawa T., Lee J., Reuter A., Kim K.W. Revisiting the ventral medial nucleus of the hypothalamus: the roles of SF-1 neurons in energy homeostasis. Frontiers in Neuroscience. 2013:71. doi: 10.3389/FNINS.2013.00071/BIBTEX. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coupé B., Ishii Y., Dietrich M.O., Komatsu M., Horvath T.L., Bouret S.G. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metabolism. 2012;15:247–255. doi: 10.1016/J.CMET.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coupé B., Leloup C., Asiedu K., Maillard J., Pénicaud L., Horvath T.L., et al. Defective autophagy in Sf1 neurons perturbs the metabolic response to fasting and causes mitochondrial dysfunction. Molecular Metabolism. 2021;47:101186. doi: 10.1016/J.MOLMET.2021.101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005 doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 14.Denny W.B., Valentine D.L., Reynolds P.D., Smith D.F., Scammell J.G. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- 15.Dhillon H., Zigman J.M., Ye C., Lee C.E., McGovern R.A., Tang V., et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/J.NEURON.2005.12.021/ATTACHMENT/61A9488A-C3F9-4A89-BBFF-7A603A5E7DEF/MMC1.PDF. [DOI] [PubMed] [Google Scholar]
- 16.Fosch A., Zagmutt S., Casals N., Rodríguez-Rodríguez R. New insights of SF1 neurons in hypothalamic regulation of obesity and diabetes. International Journal of Molecular Sciences. 2021;22:6186. doi: 10.3390/IJMS22126186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gassen N.C., Hartmann J., Schmidt M.V., Rein T. FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy. 2015;11:578. doi: 10.1080/15548627.2015.1017224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gassen N.C., Hartmann J., Zschocke J., Stepan J., Hafner K., Zellner A., et al. Association of FKBP51 with priming of autophagy pathways and mediation of antidepressant treatment response: evidence in cells, mice, and humans. PLoS Medicine. 2014;11 doi: 10.1371/JOURNAL.PMED.1001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gautron L., Elmquist J.K., Williams K.W. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161:133. doi: 10.1016/J.CELL.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guarnieri D.J., Brayton C.E., Richards S.M., Maldonado-Aviles J., Trinko J.R., Nelson J., et al. Gene profiling reveals a role for stress hormones in the molecular and behavioral response to food restriction. Biological Psychiatry. 2012;71:358. doi: 10.1016/J.BIOPSYCH.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halldorsdottir S., Carmody J., Boozer C.N., Leduc C.A., Leibel R.L. Reproducibility and accuracy of body composition assessments in mice by dual energy x-ray absorptiometry and time domain nuclear magnetic resonance. International Journal of Body Composition Research. 2009;7:147. [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann J., Wagner K., Liebl C., Scharf S., Wang X., Wolf M., et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–339. doi: 10.1016/J.NEUROPHARM.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 23.Häusl A.S., Bajaj T., Brix L.M., Pöhlmann M.L., Hafner K., De Angelis M., et al. Mediobasal hypothalamic FKBP51 acts as a molecular switch linking autophagy to whole-body metabolism. Science Advances. 2022;8:4797. doi: 10.1126/SCIADV.ABI4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Häusl A.S., Balsevich G., Gassen N.C., Schmidt M.V. Focus on FKBP51: a molecular link between stress and metabolic disorders. Molecular Metabolism. 2019;29:170–181. doi: 10.1016/J.MOLMET.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Häusl A.S., Brix L.M., Hartmann J., Pöhlmann M.L., Lopez J.-P., Menegaz D., et al. The co-chaperone Fkbp5 shapes the acute stress response in the paraventricular nucleus of the hypothalamus of male mice. Molecular Psychiatry. 2021:2021 1–202117. doi: 10.1038/s41380-021-01044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetherington A.W., Ranson S.W. The relation of various hypothalamic lesions to adiposity in the rat. The Journal of Comparative Neurology. 1942;76:475–499. doi: 10.1002/CNE.900760308. [DOI] [Google Scholar]
- 27.Hoivik E.A., Lewis A.E., Aumo L., Bakke M. Molecular aspects of steroidogenic factor 1 (SF-1) Molecular and Cellular Endocrinology. 2010;315:27–39. doi: 10.1016/J.MCE.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins S.A., Ellestad L.E., Mukherjee M., Narayana J., Cogburn L.A., Porter T.E. Glucocorticoid-induced changes in gene expression in embryonic anterior pituitary cells. Physiological Genomics. 2013;45:422–433. doi: 10.1152/physiolgenomics.00154.2012. [DOI] [PubMed] [Google Scholar]
- 29.Kaushik S., Rodriguez-Navarro J.A., Arias E., Kiffin R., Sahu S., Schwartz G.J., et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metabolism. 2011;14:173–183. doi: 10.1016/J.CMET.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawano H. The relationship between testosterone and metabolic syndrome. Hypertension Research. 2010;336(33):537–538. doi: 10.1038/hr.2010.52. [DOI] [PubMed] [Google Scholar]
- 31.Kelly D.M., Jones T.H. Testosterone: a metabolic hormone in health and disease. Journal of Endocrinology. 2013;217:R25–R45. doi: 10.1530/JOE-12-0455. [DOI] [PubMed] [Google Scholar]
- 32.Kennedy G.C. The hypothalamic control of food intake in rats. Proceedings of the Royal Society of London Series B Biological Sciences. 1950;137:535–549. doi: 10.1098/RSPB.1950.0065. [DOI] [PubMed] [Google Scholar]
- 33.Kim K.W., Zhao L., Donato J., Kohno D., Xu Y., Eliasa C.F., et al. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10673–10678. doi: 10.1073/PNAS.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King B.M. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiology & Behavior. 2006;87:221–244. doi: 10.1016/J.PHYSBEH.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 35.King B.M., Smith R.L. Hypothalamic obesity after hypophysectomy or adrenalectomy: dependence on corticosterone. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1985:18. doi: 10.1152/AJPREGU.1985.249.5.R522. [DOI] [PubMed] [Google Scholar]
- 36.Kurrasch D.M., Cheung C.C., Lee F.Y., Tran P.V., Hata K., Ingraham H.A. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. Journal of Neuroscience. 2007;27:13624. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majdic G., Young M., Gomez-Sanchez E., Anderson P., Szczepaniak L.S., Dobbins R.L., et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/ENDO.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 38.McClellan K.M., Parker K.L., Tobet S. Development of the ventromedial nucleus of the hypothalamus. Frontiers in Neuroendocrinology. 2006;27:193–209. doi: 10.1016/J.YFRNE.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Parker K.L., Rice D.A., Lala D.S., Ikeda Y., Luo X., Wong M., et al. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Progress in Hormone Research. 2002;57:19–36. doi: 10.1210/RP.57.1.19. [DOI] [PubMed] [Google Scholar]
- 40.Pereira M.J., Palming J., Svensson M.K., Rizell M., Dalenbäck J., Hammar M., et al. FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metabolism. 2014;63:1198–1208. doi: 10.1016/J.METABOL.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Quan W., Kim H.K., Moon E.Y., Kim S.S., Choi C.S., Komatsu M., et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012;153:1817–1826. doi: 10.1210/EN.2011-1882. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez C.I., Buchholz F., Galloway J., Sequerra R., Kasper J., Ayala R., et al. High-efficiency deleter mice show that FLPe is an alternative to Cre- loxP. Nature Genetics. 2000 doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 43.Sadovsky Y., Crawford P.A., Woodson K.G., Polish J.A., Clements M.A., Tourtellotte L.M., et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10939. doi: 10.1073/PNAS.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scammell J.G., Denny W.B., Valentine D.L., Smiths D.F. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. General and Comparative Endocrinology. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 45.Scharf S.H., Liebl C., Binder E.B., Schmidt M.V., Müller M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt M.V., Sterlemann V., Ganea K., Liebl C., Alam S., Harbich D., et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–429. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Segal J.P., Stallings N.R., Lee C.E., Zhao L., Socci N., Viale A., et al. Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. Journal of Neuroscience. 2005;25:4181. doi: 10.1523/JNEUROSCI.0158-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidibeh C.O., Pereira M.J., Abalo X.M., J. Boersma G., Skrtic S., Lundkvist P., et al. FKBP5 expression in human adipose tissue: potential role in glucose and lipid metabolism, adipogenesis and type 2 diabetes. Endocrine. 2018:1–13. doi: 10.1007/s12020-018-1674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinars C.R., Cheung-Flynn J., Rimerman R.A., Scammell J.G., Smith D.F., Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:868–873. doi: 10.1073/pnas.0231020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smedlund K.B., Sanchez E.R., Hinds T.D. FKBP51 and the molecular chaperoning of metabolism. Trends in Endocrinology and Metabolism. 2021;32:862–874. doi: 10.1016/J.TEM.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stechschulte L.A., Qiu B., Warrier M., Hinds T.D., Zhang M., Gu H., et al. FKBP51 null mice are resistant to diet-induced obesity and the PPARγ agonist rosiglitazone. Endocrinology. 2016;157:3888. doi: 10.1210/EN.2015-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Storer C.L., Dickey C.A., Galigniana M.D., Rein T., Cox M.B. FKBP51 and FKBP52 in signaling and disease. Trends in Endocrinology and Metabolism. 2011;22:481. doi: 10.1016/J.TEM.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westberry J.M., Sadosky P.W., Hubler T.R., Gross K.L., Scammell J.G. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. The Journal of Steroid Biochemistry and Molecular Biology. 2006;100:34–41. doi: 10.1016/J.JSBMB.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Wochnik G.M., Rüegg J., Abel G.A., Schmidt U., Holsboer F., Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. Journal of Biological Chemistry. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 55.Yang L., Isoda F., Yen K., Kleopoulos S.P., Janssen W., Fan X., et al. Hypothalamic Fkbp51 is induced by fasting, and elevated hypothalamic expression promotes obese phenotypes. American Journal of Physiology. Endocrinology and Metabolism. 2012;302:E987. doi: 10.1152/AJPENDO.00474.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeh W.C., Li T.K., Bierer B.E., McKnight S.L. Identification and characterization of an immunophilin expressed during the clonal expansion phase of adipocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11081–11085. doi: 10.1073/PNAS.92.24.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yong W., Yang Z., Periyasamy S., Chen H., Yucel S., Li W., et al. Essential role for Co-chaperone FKBP52 but not FKBP51 in androgen receptor-mediated signaling and physiology. Journal of Biological Chemistry. 2007;282:5026. doi: 10.1074/JBC.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshimatsu H., Oomura Y., Katafuchi T., Niijima A., Sato A. Lesions of the ventromedial hypothalamic nucleus enhance sympatho-adrenal function. Brain Research. 1985;339:390–392. doi: 10.1016/0006-8993(85)90112-X. [DOI] [PubMed] [Google Scholar]
- 59.Zhao L., Bakke M., Hanley N.A., Majdic G., Stallings N.R., Jeyasuria P., et al. Tissue-specific knockouts of steroidogenic factor 1. Molecular and Cellular Endocrinology. 2004;215:89–94. doi: 10.1016/J.MCE.2003.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.