Abstract
Peripheral arterial disease (PAD) has a strong relationship with inflammation. However, it is unclear whether the dietary inflammatory potential is associated with PAD. We aimed to address this knowledge gap. The dietary inflammatory index (DII) was obtained using a 24-h dietary recall interview for each individual. Logistic regression models and restricted cubic spline were performed to assess the relationship of DII with the prevalence of PAD. In addition, Spearman correlation analysis and subgroup analysis were also undertaken. In total, 5840 individuals from the 1999–2004 National Health and Nutrition Examination Survey (NHANES) were enrolled in our study. Participants in higher DII quartile tended to have higher rates of PAD. The increase in DII scores showed a positive association with PAD after fully multivariate adjustment (OR (odds ratios) = 1.094, 95% confidence interval (CI): 1.022–1.171). The multivariable-adjusted OR and 95% CI of the highest DII index quartile compared with the lowest quartile was 1.543 (95% CI: 1.116–2.133). Subgroup analysis demonstrated that the positive association between DII and PAD was persistent across population subgroups. In conclusion, we report that a proinflammatory dietary pattern is related to a higher risk of developing PAD among US adults.
Keywords: dietary inflammatory index, peripheral arterial disease, National Health and Nutrition Examination Survey, cross-sectional study, relationship
1. Introduction
Peripheral arterial disease (PAD) is a type of atherosclerosis characterized by fatty deposits along the walls of arteries, leading to narrowing and obstructive lesions in the lumen, primarily damaging the arteries of the lower extremities and feet [1]. In 2018, Jun Shu et al. estimated that more than 200 million people worldwide suffer from PAD [2]. PAD not only causes lower limb ischemic ulcers and amputations but also significantly increases the risk of cardiovascular and cerebrovascular events and death in patients [3,4]. The economic burden and health hazards caused by PAD cannot be ignored. Therefore, it is crucial to determine the etiology of PAD and take adequate measures. Previous studies have shown that inflammatory markers, endothelial dysfunction, and oxidative stress play an important role in the development of PAD [5,6,7,8,9,10,11]. Meanwhile, it was reported that dietary pattern, evaluated by the dietary inflammatory index (DII), was associated with various inflammatory markers, including CRP, IL-6, and homocysteine [12,13]. In addition, higher DII scores have been shown to be associated with poor health and higher risk of diseases, such as obesity [14], various cancer [15,16], cardiovascular disease (CVD) [14,17], chronic obstructive pulmonary disease [18], depression [19,20], metabolic syndrome [21], type 2 diabetes [14,22], and kidney stones [23].
Against this background, we hypothesized that the increased inflammatory potential of dietary intake might also be related to the prevalence of PAD. At present, the role of several common dietary indexes and some food in PAD has been investigated in some studies [24,25]. In 2014, Naqvi et al. reported that the prevalence of PAD was negatively associated with the intake of fiber, folate, and vitamins A, B6, C, and E after adjusting for age, sex, hypertension, diabetes, and smoking [26]. An epidemiological study demonstrated the inverse association between the greater frequency of fruit and vegetable consumption and the risk of PAD [27]. However, in contrast to the DII, which scores 45 nutrients and food components, these studies are limited to a few specific nutrients and fail to provide a comprehensive picture of the relationship between dietary patterns characterized as proinflammatory and anti-inflammatory and PAD. Therefore, our study aimed to assess the impact of DII on PAD based on data from the National Health and Nutrition Examination Survey (NHANES), a population-based cross-sectional survey designed to collect information on the health and nutritional status of adults and children in the United States.
2. Materials and Methods
2.1. Study Population
Consistent with previous studies investigating PAD using the NHANES database, we analyzed data from the 1999–2004 NHANES cycle (n = 31,126) [28]. The website of NHANES provides the details of the study design and protocols (http://www.cdc.gov/nchs/nhanes.htm (accessed on 1 May 2022). Briefly, the NHANES is a survey that uses a complex multistage probability sampling design to estimate the prevalence of major diseases and identify risk factors for diseases. This information will be used to assess the nutritional status of adults and children in the United States to achieve health promotion and disease prevention goals. A total of 7571 participants 40 years or older had a valid ankle–brachial index (ABI) measured. For this study, we excluded participants with a lack of dietary information (n = 171), participants with an ABI > 1.4 in at least one leg (n = 110, related to incompressible vessels in the leg) [29,30,31], and participants with missing data on covariates of interest (n = 1450). Finally, 5840 subjects were included in our study.
2.2. Exposure
DII, designed as the exposure variable, is a tool summarized from the literature to assess dietary inflammatory potential via 24-h dietary recall. DII calculates the inflammation effects of dietary consumption from 45 nutrients. The higher the DII score, the greater the proinflammatory effect while the lower the DII score, the greater the anti-inflammatory effect. The method for calculating DII has been reported in detail by N. Shivappa et al. [32]. We must first obtain the Z-score by the following equation: (daily mean intake reported—global daily mean intake)/standard deviation. To minimize the impact of “right skewing”, this value is transformed to a percentile score. Each percentile score is doubled, and then “1” is subtracted to achieve a symmetrical distribution with values centered on 0. The percentile value for each food parameter is then multiplied by its respective “overall inflammatory effect score” to obtain the “food parameter-specific DII score”. Finally, we can achieve an individual “overall DII score” by summing the “food parameter-specific DII score”. In this study, the NHANES 1999–2004 database provides 27 of the 45 food parameters to compute DII. These food parameters and other essential information for the calculation of DII are displayed in Table S1. Previous studies revealed that the DII scores were still available even if the nutrients used to calculate DII were <30 [33,34].
ABI was measured in subjects >40 years old. This measurement method has been reported in the previous studies [26]. In short, systolic blood pressures were measured on the right arm (brachial artery) and both ankles (posterior tibial artery) after a short rest. If the participant’s right arm readings were not available due to any condition that may interfere with accurate measurements (e.g., open wounds, dialysis shunts), the left arm was used for the brachial pressure measurement. Systolic blood pressure was measured twice in subjects aged 40–59 years but only once in subjects aged 60 years and older. The ABI was calculated by dividing the average systolic blood pressure (ASBP) of the ankle by the ASBP in the arm. Since participants aged 60 and older had only one reading, the first reading represented the mean values. PAD was defined as ABI < 0.9 [26,29,35,36].
2.3. Covariates
Sociodemographic and lifestyle information was obtained through standardized questionnaires. The Mobile Examination Center (MEC) provided the examination results of the body mass index, blood pressure, and other biochemical parameters. All details of these variables, including the measurement methods, questionnaire data, and variables list, can be found on the official NHANES website (www.cdc.gov/nchs/nhanes/ (accessed on 1 May 2022). Table S2 shows some questions about the sociodemographic and lifestyle information in the questionnaire. We used the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation to compute the estimated glomerular filtration rate (eGFR) [37]. CVD history was determined based on self-reported congestive heart failure, coronary heart disease, angina pectoris, heart attack, and stroke. Certified examiners measured the blood pressure using a mercury sphygmomanometer after resting in a seated position for 5 min. The average blood pressure was calculated by the following protocol in NHANES: (1) Diastolic reading with zero is not included in the calculation of the average diastolic blood pressure (ADBP). (2) The ADBP is zero when all diastolic readings are zero. (3) When there is only one blood pressure reading, this reading represents the mean. (4) When there are multiple blood pressure readings, the first reading is not used to calculate the mean value. Hypertension was defined as ASBP/ ADBP ≥ 140/90 mmHg or currently taking antihypertensive medications or previous diagnosis by a doctor or health professional. Diabetes was defined as fasting glucose >7 mmol/L or random glucose ≥11.1 mmol/L or glycated hemoglobin A1c ≥ 6.5% or the usage of hypoglycemic drugs or a history of diabetes. Physical activity was assessed by questions about vigorous activities that resulted in a large increase in breathing or heart rate (e.g., swimming or fast cycling) and moderate activities that resulted in a slight to moderate increase in breathing or heart rate (e.g., golf or recreational cycling). These activities lasted at least 10 min in the past 30 days. We classified physical activity into three levels: less than moderate activity (neither moderate activity nor vigorous activity), moderate activity (no vigorous activity with at least one moderate activity), and vigorous activity (at least one vigorous episode).
2.4. Statistical Analysis
Means ± standard deviations were used to represent continuous variables while frequencies or percentages were used to represent categorical variables. Based on the nature of data, we conducted Chi-square, ANOVA, or Kruskal–Wallis H-test to determine differences among participants in different DII quartiles. Three logistic regression models were constructed to assess the association between DII and PAD. Model 1 was an unadjusted model. Age, sex, and race were adjusted in Model 2. Model 3 was adjusted for age, sex, race, BMI, education level, PIR, the level of physical activity, marital status, hypertension, diabetes, CVD, medication use (hypotensive drugs and hypoglycemic drugs), ASBP, ADBP, smoking, TC, HDL-C, eGFR, HbA1c, and CRP. The restricted cubic spline (RCS) was performed to assess the potential non-linear relationship between DII and PAD. We also calculated the Spearman correlation coefficients to evaluate the correlation between DII and some cardiovascular risk factors. Stratification analysis was performed to assess whether the relationships between DII and PAD were affected by age, sex, hypertension, diabetes, CVD, PIR, physical activity, and smoking based on Model 3. A p value < 0.05 was considered statistically significant. We used R software (version 4.1, Vienna, Austria) and IBM SPSS statistics version 23.0 (Chicago, IL, USA) to perform all statistical analyses.
3. Results
3.1. Characteristics of the Study Population
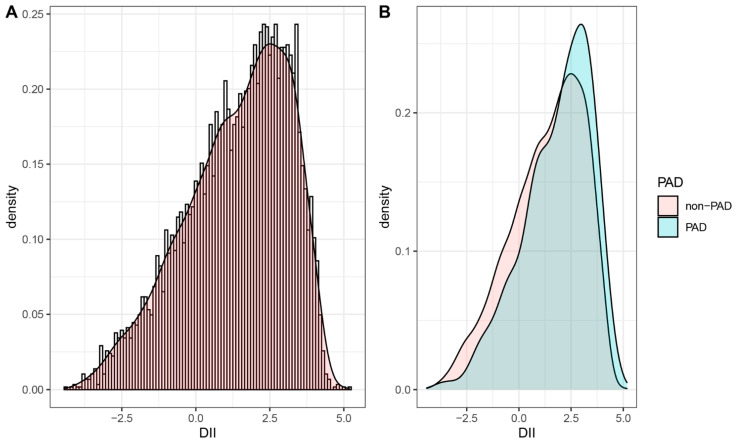
The baseline characteristics of the included participants are shown in Table 1. Among the different quartiles of DII, significant differences were observed in almost all characteristics except diabetes, hypotensive drugs, hypoglycemic drugs, ADBP, HDL, and eGFR. Compared with those with lower DII scores (Quartile 1 and 2), participants with higher DII scores (Quartile 3 and 4) were more likely to be older, female, smokers, and single. In addition, lower physical activity and poorer education levels were more common in participants with higher DII scores. These participants also tended to have higher levels of BMI, ASBP, TC, CRP, and HbA1c while lower levels of PIR (poverty income ratio). More importantly, we observed that participants with a higher DII quartile tended to have higher PAD rates (Quartile 1: 4.79%, Quartile 2: 6.96%, Quartile 3: 7.73%, Quartile 4: 10.33%, p < 0.001). Similar results were also observed in the prevalence of CVD and hypertension. Figure 1A illustrates the distribution of DII in the total population, and Figure 1B shows the distribution of DII stratified by PAD status. Participants with PAD preferred a proinflammatory diet.
Table 1.
Baseline characteristics stratified by the dietary inflammatory index (DII) quartiles (Q).
| Variables | Total (n = 5840) | Q1 (n = 1462) | Q2 (n = 1465) | Q3 (n = 1461) | Q4 (n = 1452) | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 59.78 ± 12.96 | 59.16 ± 12.98 | 59.44 ± 13.03 | 60.13 ± 12.92 | 60.4 ± 12.88 | 0.023 |
| Sex, male, n (%) | 3001 (51.39%) | 948 (64.84%) | 794 (54.20%) | 680 (46.54%) | 579 (39.88%) | <0.001 |
| Hypertension, n (%) | 3376 (57.81%) | 796 (54.45%) | 825 (56.31%) | 896 (61.33%) | 859 (59.16%) | 0.001 |
| Diabetes, n (%) | 1013 (17.35%) | 229 (15.66%) | 244 (16.66%) | 274 (18.75%) | 266 (18.32%) | 0.096 |
| MS, n (%) | <0.001 | |||||
| Married/Living with partner | 3895 (66.70%) | 1067 (72.98%) | 1006 (68.67%) | 929 (63.59%) | 893 (61.50%) | |
| Widowed/Divorced/Separated | 1615 (27.65%) | 319 (21.82%) | 373 (25.46%) | 443 (30.32%) | 480 (33.06%) | |
| Never married | 330 (5.65%) | 76 (5.20%) | 86 (5.87%) | 89 (6.09%) | 79 (5.44%) | |
| Education level, n (%) | <0.001 | |||||
| Less than high school | 1875 (32.11%) | 360 (24.62%) | 437 (29.83%) | 498 (34.09%) | 580 (39.94%) | |
| High school diploma or GED | 1376 (23.56%) | 316 (21.61%) | 343 (23.41%) | 344 (23.55%) | 373 (25.69%) | |
| More than high school | 2589 (44.33%) | 786 (53.76%) | 685 (46.76%) | 619 (42.37%) | 499 (34.37%) | |
| Physical activity, n (%) | <0.001 | |||||
| Less than moderate | 2623 (44.91%) | 519 (35.50%) | 612 (41.77%) | 705 (48.25%) | 787 (54.20%) | |
| Moderate | 1839 (31.49%) | 484 (33.11%) | 497 (33.92%) | 439 (30.05%) | 419 (28.86%) | |
| Vigorous | 1378 (23.60%) | 459 (31.40%) | 356 (24.30%) | 317 (21.70%) | 246 (16.94%) | |
| Race, n (%) | <0.001 | |||||
| Mexican American | 1205 (20.63%) | 307 (21.00%) | 299 (20.41%) | 283 (19.37%) | 316 (21.76%) | |
| Non-Hispanic white | 3261 (55.84%) | 873 (59.71%) | 851 (58.09%) | 806 (55.17%) | 731 (50.34%) | |
| Non-Hispanic black | 980 (16.78%) | 180 (12.31%) | 200 (13.65%) | 285 (19.51%) | 315 (21.69%) | |
| Other Hispanic | 228 (3.90%) | 56 (3.83%) | 64 (4.37%) | 52 (3.56%) | 56 (3.86%) | |
| Other races | 166 (2.84%) | 46 (3.15%) | 51 (3.48%) | 35 (2.40%) | 34 (2.34%) | |
| Hypotensive drugs, n (%) | 1531 (26.22%) | 389 (26.61%) | 381 (26.01%) | 406 (27.79%0 | 355 (24.45%) | 0.225 |
| Hypoglycemic drugs, n (%) | 612 (10.48%) | 146 (9.99%) | 144 (9.83%) | 160 (10.95%) | 162 (11.16%) | 0.553 |
| CVD, n (%) | 876 (15.00%) | 198 (13.54%) | 208 (14.20%) | 210 (14.37%) | 260 (17.91%) | 0.004 |
| Smoking, n (%) | <0.001 | |||||
| Never smoker | 2707 (46.35%) | 706 (48.29%) | 689 (47.03%) | 672 (46.00%) | 640 (44.08%) | |
| Past smoker | 2012 (34.45%) | 570 (38.99%) | 498 (33.99%) | 495 (33.88%) | 449 (30.92%) | |
| Current smoker | 1121 (19.20%) | 186 (12.72%) | 278 (18.98%) | 294 (20.12%) | 363 (25.00%) | |
| PIR | 2.80 ± 1.61 | 3.14 ± 1.61 | 2.94 ± 1.61 | 2.68 ± 1.59 | 2.46 ± 1.56 | <0.001 |
| BMI, kg/m2 | 28.43 ± 5.45 | 28.07 ± 5.23 | 28.37 ± 5.40 | 28.66 ± 5.53 | 28.61 ± 5.62 | 0.014 |
| ASBP, mmHg | 131.04 ± 20.66 | 129.13 ± 19.21 | 131.04 ± 20.61 | 132.04 ± 21.33 | 131.98 ± 21.33 | 0.002 |
| ADBP, mmHg | 72.91 ± 12.37 | 73.44 ± 11.68 | 72.75 ± 12.45 | 72.62 ± 13.08 | 72.83 ± 12.23 | 0.267 |
| Total cholesterol, mg/dL | 209.35 ± 41.68 | 207.68 ± 39.01 | 207.16 ± 39.98 | 211.95 ± 44.13 | 210.62 ± 43.24 | 0.009 |
| HDL, mmol/L | 52.69 ± 16.23 | 52.52 ± 16.44 | 52.38 ± 15.72 | 52.83 ± 16.52 | 53.03 ± 16.26 | 0.690 |
| CRP, mg/dL | 0.47 ± 0.87 | 0.38 ± 0.69 | 0.46 ± 0.74 | 0.48 ± 1.03 | 0.58 ± 0.95 | <0.001 |
| eGFR, mL/min/1.73 m2 | 83.61 ± 20.02 | 84.96 ± 18.57 | 83.59 ± 19.80 | 83.00 ± 20.91 | 82.88 ± 20.68 | 0.131 |
| HbA1c, % | 5.77 ± 1.12 | 5.69 ± 1.04 | 5.77 ± 1.17 | 5.80 ± 1.14 | 5.80 ± 1.11 | <0.001 |
| PAD, n (%) | 435 (7.45%) | 70 (4.79%) | 102 (6.96%) | 113 (7.73%) | 150 (10.33%) | <0.001 |
Values are given as mean ± standard deviation or numbers and percentages. Q1: DII ≤ 0.19; Q2: 0.19–1.66; Q3: 1.66–2.78; Q4: DII > 2.78. ASBP, average systolic blood pressure; ADBP, average diastolic blood pressure; BMI, body mass index; CVD, cardiovascular disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; GED, general educational development; HDL, high-density lipoprotein; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; MS, marital status; PIR, poverty income ratio; PAD, peripheral arterial disease.
Figure 1.
The population distribution of DII. (A) Density curve shows the distribution of DII in the total population. (B) The distribution of DII in the population with different PAD status. DII, dietary inflammatory index; PAD, peripheral artery disease.
3.2. Association between DII and PAD
Table 2 displays the results of the univariate and multivariable logistic regression analysis. When treating DII as a continuous variable, the increase in DII resulted in a higher prevalence of PAD in the unadjusted model (OR (odds ratios) = 1.190, 95% confidence interval (CI): 1.120–1.265). The association remained statistically significant in Model 2 and 3. When the DII was treated as a categorical variable based on quartiles and using the first quartile as a reference, participants in the third to the fourth quartile had a higher risk of PAD in all three models. The univariate analysis showed that the OR with 95% CI for PAD across increasing quartiles of DII was 1.488 (1.088–2.036), 1.667 (1.226–2.267), and 2.291 (1.708–3.073), respectively. After adjustment for underlying cofounding variables, the OR (95% CI) of PAD throughout the quartiles was 1.231 (0.880–1.722), 1.156 (0.828–1.613), and 1.543 (1.116–2.133), respectively.
Table 2.
Odds ratios (ORs) and 95% confidence interval (CI) of the DII quartiles for PAD.
| DII | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Continuous | 1.190 (1.120–1.265) | <0.001 | 1.168 (1.095–1.246) | <0.001 | 1.094 (1.022–1.171) | 0.010 |
| Categorical | ||||||
| Quartile 1 (≤−0.7680) | Reference | Reference | Reference | |||
| Quartile 2 (−0.7680–0.5475) | 1.488 (1.088–2.036) | 0.013 | 1.474 (1.066–2.038) | 0.019 | 1.231 (0.880–1.722) | 0.224 |
| Quartile 3 (0.5475–1.6080) | 1.667 (1.226–2.267) | 0.001 | 1.531 (1.112–2.109) | 0.009 | 1.156 (0.828–1.613) | 0.395 |
| Quartile 4 (>1.6080) | 2.291 (1.708–3.073) | <0.001 | 2.106 (1.546–2.869) | <0.001 | 1.543 (1.116–2.133) | 0.009 |
Data are presented as odds ratios, 95% CIs (confidence intervals), and p-value. Model 1 adjusted for none. Model 2 adjusted for age, sex, and race. Model 3 adjusted for all covariates. DII, dietary inflammatory index; PAD, peripheral arterial disease.
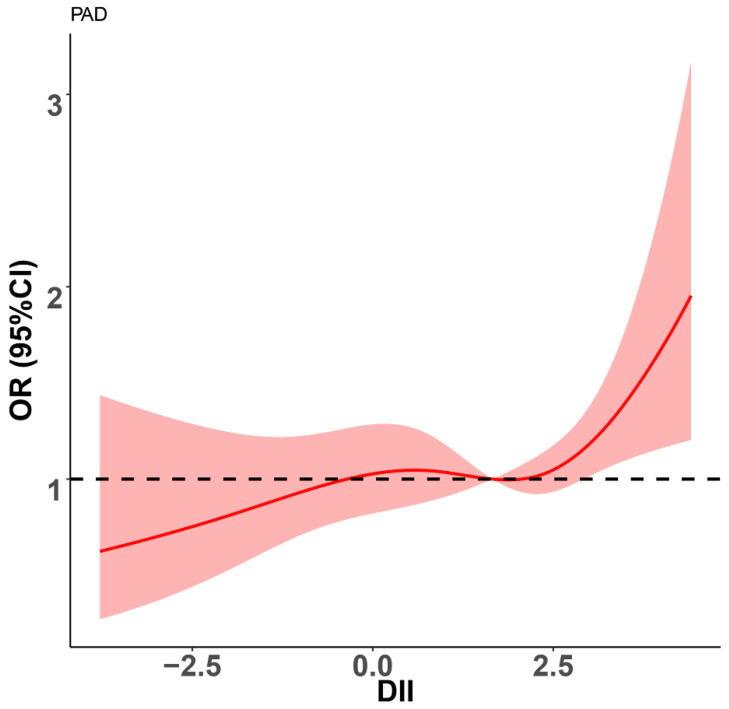
As shown in Figure 2, we also used a restricted cubic spline to visualize the association between DII and PAD. In the curve, we found that HR was less than 1 when diet was in anti-inflammatory mode (DII < 0). Thereafter, HR tended to the horizontal line with HR = 1 until DII reached approximately 2.5. Finally, HR sharply increased.
Figure 2.
Restricted spline curve shows the relationship between DII and PAD. Red line and red transparent area represent OR and 95% CI, respectively. ORs (95% CI) were adjusted based on Model 3. DII, dietary inflammatory index; PAD, peripheral arterial disease.
We also performed Spearman correlation analysis to evaluate the correlations between covariates related to CVD and DII. As shown in Table 3, DII was positively correlated with age, ASBP, TC, CRP, and HbA1c (r = 0.035, 0.042, 0.034, 0.140, and 0.083, respectively) while it was negatively correlated with PIR and eGFR (r = −0.167 and −0.027, respectively). However, ADBP and HDL were not significantly correlated with DII (r = −0.013, p = 0.333, and r = 0.01, p = 0.437).
Table 3.
Correlations of DII with some covariates.
| DII | ||
|---|---|---|
| r | p-Value | |
| Age | 0.035 | 0.007 |
| PIR | −0.167 | <0.001 |
| ASBP | 0.042 | 0.002 |
| ADBP | −0.013 | 0.333 |
| HDL | 0.01 | 0.437 |
| TC | 0.034 | 0.009 |
| CRP | 0.140 | <0.001 |
| HbA1c | 0.083 | <0.001 |
| eGFR | −0.027 | 0.040 |
ASBP, average systolic blood pressure; ADBP, average diastolic blood pressure; DII, dietary inflammatory index; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; PIR, poverty income ratio; TC, total cholesterol.
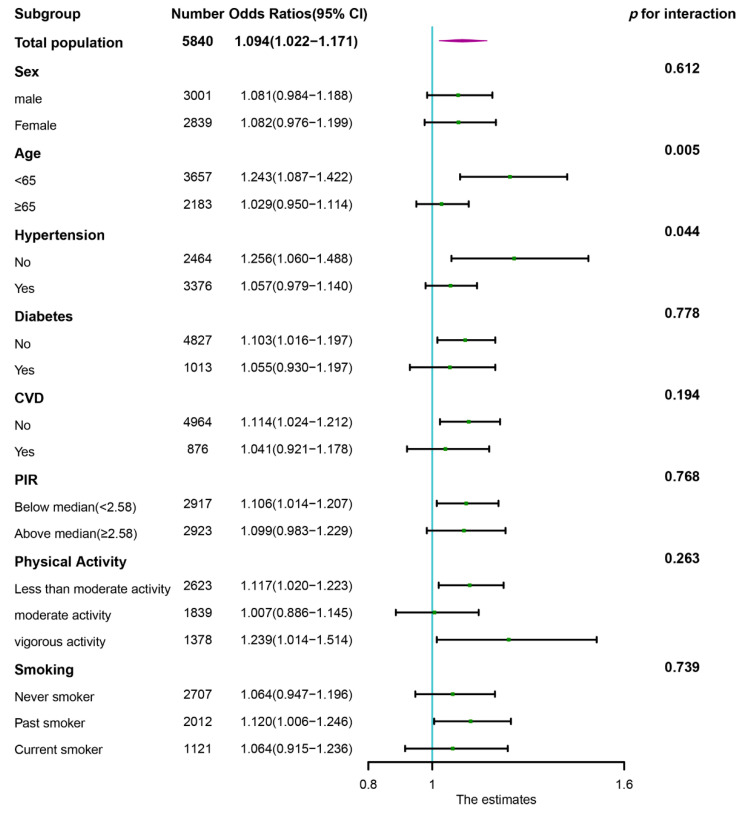
3.3. Stratification Analysis
Subgroup analysis was conducted according to the categories of age, sex, hypertension, diabetes, CVD, PIR, physical activity, and smoking. The DII was further treated as a continuous variable. As shown in the forest plot (Figure 3), the statistically significant positive associations between DII and PAD were found in participants under 65 years of age (OR = 1.243, 95% CI: 1.087–1.422), participants without hypertension (OR = 1.256, 95% CI: 1.060–1.488), participants without diabetes (OR = 1.103, 95% CI: 1.016–1.197), participants without CVD (OR = 1.114, 95% CI: 1.024–1.212), participants with PIR below the median (OR = 1.106, 95% CI: 1.014–1.207), participants with less than moderate activity (OR = 1.117, 95% CI: 1.020–1.223), participants with vigorous activity (OR = 1.239, 95% CI: 1.014–1.514), and past smokers (OR = 1.120, 95% CI: 1.006–1.246). Furthermore, we also found that the association between DII and PAD was more pronounced in the non-hypertensive population (p for interaction = 0.044) and middle-aged population (p for interaction = 0.005).
Figure 3.
Subgroup analysis of association of DII with PAD. The results were adjusted for all covariates except the corresponding stratification variable. CVD, cardiovascular disease; DII, dietary inflammatory index; PIR, poverty income ratio; PAD, peripheral arterial disease.
4. Discussion
In this large cross-sectional study, a significant positive association between DII and the prevalence of PAD was observed, indicating that a proinflammatory diet might lead to a higher risk of PAD. A restricted cubic spline visualized the relationship between DII and PAD. We observed that the risk of PAD dramatically increased when DII exceeded approximately 2.5. In addition, the stratified analysis revealed a positive association between DII and PAD in population subgroups, which was consistent with the main finding in the total population.
Available evidence suggests that one possible explanation for our results may be the impact of diet on inflammation factors. Ahmad Esmaillzadeh et al. reported that the “Western” diet was positively related to CRP and soluble intercellular adhesion molecule-1 while the healthy diet was inversely related to plasma concentrations of CRP and soluble intercellular adhesion molecule-1 [38]. Likewise, the Dietary Approaches to Stop Hypertension (DASH) diet has been reported to be associated with reduced concentrations of CRP [39]. Recently, more and more studies have demonstrated a positive association between a proinflammatory diet (higher DII scores) and levels of various inflammatory markers: CRP, IL-6, IL-1, IL-2, TNF-α, IFN-γ, and vascular cell adhesion molecule [12,13,40]. Our study also found that the DII was positively associated with CRP (r = 0.140, p < 0.001). Meanwhile, previous studies demonstrated that these inflammatory markers (CRP, IL-6, and soluble adhesion molecules) played an essential role in the development of PAD and predicting adverse outcomes in patients with PAD [7,11,41,42]. Therefore, we suspect that a possible mechanism for the prevalence of PAD caused by a proinflammatory diet is an increase in inflammatory markers.
Furthermore, it is well known that dietary nutrient intake plays an important role in the diversity, activity, features, and composition of human gut microbiota [43,44,45,46]. Some studies have demonstrated that the intestinal microbiota can contribute to the development of atherosclerosis through its metabolites [47,48,49]. For example, trimethylamine-N-oxide (TMAO), a pro-atherogenic metabolite formed by gut microbes, has been shown to correlate with PAD severity and prognosis [50,51]. Serum lipopolysaccharide (LPS), another gut derived metabolite, was also elevated in PAD patients and was strongly associated with atherosclerotic burden and oxidative stress [52]. Recently, Eelke Brandsma et al. reported that gut microbiota with proinflammatory characteristics could induce systemic inflammation and accelerate the process of atherosclerosis [53]. Therefore, we should pay attention to the dietary consumption of PAD patients in order to improve their gut microbiota and slow down the progression of PAD.
In this study, we observed that individuals with higher DII scores had a lower family income and poorer education levels, and higher rates of singleness. Meanwhile, growing studies have demonstrated that socioeconomic inequality played an important role in the burden of PAD. Among US adults, the lower the income and education level, the higher the risk of PAD [54,55]. A meta-analysis revealed that unmarried people had a higher risk of CVD and cardiovascular death compared to married people [56]. Moreover, lower physical activity was more common in participants with higher DII scores in our study, which also contributed to a higher risk of PAD. It has been observed that the prevalence of PAD and the incidence of adverse outcomes in patients with PAD are negatively associated with physical activity [28,57]. The intrinsic mechanism can be attributed to the fact that regular exercise reduces visceral fat and induces an anti-inflammatory environment, resulting in an anti-inflammatory effect [58,59]. In addition, Spearman correlation analysis demonstrated that participants with higher DII levels tended to have higher BMI, ASBP, TC, and HbA1c values, which might also increase the burden of PAD in the general population.
The restricted cubic spline visualized the association between DII and PAD. Interestingly, in the curve, we found that the risk of PAD was not increased until DII reached approximately 2.5, which suggests that there might be a threshold to the effect of a proinflammatory dietary pattern on PAD. The level of inflammation developed by the proinflammatory dietary pattern at a DII below 2.5 is not sufficient to cause PAD. On the other hand, the risk of PAD was significantly decreased when diet was in anti-inflammatory mode. Therefore, we need to focus more on people with DII above 2.5 to reduce the burden of PAD and encourage people to eat more foods that are rich in anti-inflammatory nutrients.
Subgroup analyses showed generally consistent associations with the primary results. The positive association between DII and PAD was similar in the population, with differences in age, sex, hypertension status, diabetes status, CVD status, PIR, physical activity, and smoking despite not being statistically significant in some subgroups. However, we found that the ORs in some subgroups, including middle-aged participants and non-hypertensive participants, were significantly higher than those in the corresponding subgroups. We think this may be due to aging and hypertension modifying and attenuating the effect of DII on PAD, which needs to be validated in a larger specific population in the future. Furthermore, we found a positive and statistically significant association between DII and the risk of PAD in participants with vigorous activity. Some studies revealed that excessive exercise and inadequate recovery induced the production and release of proinflammatory cytokines, which might have a detrimental effect on PAD [60,61]. In addition, Ronni E Sahl reported that prolonged excessive exercise could reduce the protective effects of exercise [62]. Therefore, a proinflammatory diet might aggravate systemic inflammation in individuals who participate in vigorous activity, leading to an increased risk of PAD. However, the reduction in sample size after stratification may have led to potential bias, so we need to validate this results in a larger sample size.
Despite the critical findings of our study, some limitations should be mentioned. First, causality cannot be obtained from the cross-sectional study design. Therefore, longitudinal studies with large samples are needed to confirm our results. Second, data on ABI for participants aged <40 years old was not available, which prevented us from analyzing this association for a broad age group. Third, as mentioned in some studies, the information used to calculate DII was collected using a detailed diet questionnaire; however, diet changes over time, so we could not explain the effect of dietary changes on PAD [34]. However, Asghar Z. Naqvi think that 24-h dietary recalls tend to provide highly reliable estimates of recent intakes, and if dietary recalls are collected on all days of the week and all seasons of the year, as is the case with NHANES, then the average of recent intake for a group can yield a reasonable estimate of the average of the usual nutrient intake for that group [26]. Fourth, 24-h dietary recall was used to calculate DII and some covariates were self-reported using validated questionnaires, which can lead to recall bias and social desirability bias. Fifth, physical activity was evaluated by a questionnaire, and actual physical activity could be unclear because of a lack of measurements by a pedometer and so on. Finally, we cannot arbitrarily generalize the results to other populations with different demographics because all participants were US residents.
5. Conclusions
In summary, our results demonstrated that a proinflammatory diet was associated with a higher risk of PAD among US adults. Our findings could provide valid information for large-scale prospective studies that could further draw attention to dietary health.
Acknowledgments
We acknowledge the staff at the National Center for Health Statistics at the CDC, who design, collect, administer the NHANES data and release the data available for public use. We are thankful to all study participants for their cooperation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14173490/s1, Table S1: Food parameters included in our study for the calculation of dietary inflammatory index (DII); Table S2: Some questions about sociodemographic and lifestyle information in the questionnaire.
Author Contributions
Conceptualization, H.F.; methodology, H.F.; software, H.F. and J.Z.; validation, Y.H. and X.F.; formal analysis, H.F.; investigation, Y.H. and X.F.; resources, G.L.; data curation, P.D.; writing—original draft preparation, H.F.; writing—review and editing, G.L. and J.Z.; visualization, J.Z.; supervision, G.L. and Z.Y.; project administration, Z.Y.; funding acquisition, Z.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB) (Protocol #98-12 and Protocol #2005-06).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study by the National Center for Health Statistics.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/ (accessed on 1 May 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Key R&D Program of China (2021YFA1301200, 2019YFA0802300), the National Natural Science Foundation of China (No. 81941005), the Key Project of Research and Development Plan (2017ZDCXL-SF-02-04-01), the Natural Science Foundation of Shaanxi Province (2020JM-373).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brevetti G., Giugliano G., Brevetti L., Hiatt W.R. Inflammation in peripheral artery disease. Circulation. 2010;122:1862–1875. doi: 10.1161/CIRCULATIONAHA.109.918417. [DOI] [PubMed] [Google Scholar]
- 2.Shu J., Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis. 2018;275:379–381. doi: 10.1016/j.atherosclerosis.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnelli G., Belch J.J.F., Baumgartner I., Giovas P., Hoffmann U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review. Atherosclerosis. 2020;293:94–100. doi: 10.1016/j.atherosclerosis.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Sigvant B., Lundin F., Wahlberg E. The Risk of Disease Progression in Peripheral Arterial Disease is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg. 2016;51:395–403. doi: 10.1016/j.ejvs.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Loffredo L., Marcoccia A., Pignatelli P., Andreozzi P., Borgia M.C., Cangemi R., Chiarotti F., Violi F. Oxidative-stress-mediated arterial dysfunction in patients with peripheral arterial disease. Eur. Heart J. 2007;28:608–612. doi: 10.1093/eurheartj/ehl533. [DOI] [PubMed] [Google Scholar]
- 6.Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.CIR.97.5.425. [DOI] [PubMed] [Google Scholar]
- 7.Tzoulaki I., Murray G.D., Lee A.J., Rumley A., Lowe G.D., Fowkes F.G. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112:976–983. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 8.Krzyzanowska K., Mittermayer F., Krugluger W., Schnack C., Hofer M., Wolzt M., Schernthaner G. Asymmetric dimethylarginine is associated with macrovascular disease and total homocysteine in patients with type 2 diabetes. Atherosclerosis. 2006;189:236–240. doi: 10.1016/j.atherosclerosis.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Ding N., Yang C., Ballew S.H., Kalbaugh C.A., McEvoy J.W., Salameh M., Aguilar D., Hoogeveen R.C., Nambi V., Selvin E., et al. Fibrosis and Inflammatory Markers and Long-Term Risk of Peripheral Artery Disease: The ARIC Study. Arterioscler. Thromb. Vasc. Biol. 2020;40:2322–2331. doi: 10.1161/ATVBAHA.120.314824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvaggio S., Abate A., Brugaletta G., Musso C., Di Guardo M., Di Guardo C., Vicari E.S.D., Romano M., Luca S., Signorelli S.S. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and monocyte-to-HDL cholesterol ratio as markers of peripheral artery disease in elderly patients. Int. J. Mol. Med. 2020;46:1210–1216. doi: 10.3892/ijmm.2020.4644. [DOI] [PubMed] [Google Scholar]
- 11.Hayfron-Benjamin C.F., Mosterd C., Maitland-van der Zee A.H., van Raalte D.H., Amoah A.G.B., Agyemang C., van den Born B.J. Inflammation and its associations with aortic stiffness, coronary artery disease and peripheral artery disease in different ethnic groups: The HELIUS Study. EClinicalMedicine. 2021;38:101012. doi: 10.1016/j.eclinm.2021.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin D., Lee K.W., Brann L., Shivappa N., Hébert J.R. Dietary inflammatory index is positively associated with serum high-sensitivity C-reactive protein in a Korean adult population. Nutrition. 2019;63–64:155–161. doi: 10.1016/j.nut.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Shivappa N., Hébert J.R., Rietzschel E.R., De Buyzere M.L., Langlois M., Debruyne E., Marcos A., Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br. J. Nutr. 2015;113:665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hariharan R., Odjidja E.N., Scott D., Shivappa N., Hébert J.R., Hodge A., de Courten B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022;23:e13349. doi: 10.1111/obr.13349. [DOI] [PubMed] [Google Scholar]
- 15.Syed Soffian S.S., Mohammed Nawi A., Hod R., Ja’afar M.H., Isa Z.M., Chan H.K., Hassan M.R.A. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients. 2022;14:1555. doi: 10.3390/nu14081555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayati Z., Jafarabadi M.A., Pirouzpanah S. Dietary inflammatory index and breast cancer risk: An updated meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022;76:1073–1087. doi: 10.1038/s41430-021-01039-5. [DOI] [PubMed] [Google Scholar]
- 17.Shivappa N., Godos J., Hébert J.R., Wirth M.D., Piuri G., Speciani A.F., Grosso G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients. 2018;10:200. doi: 10.3390/nu10020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C., Yang T., Wang C. The Dietary Inflammatory Index and Early COPD: Results from the National Health and Nutrition Examination Survey. Nutrients. 2022;14:2841. doi: 10.3390/nu14142841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao Y., Huang W. Association of Dietary Inflammatory Index with Depression and Suicidal Ideation in Older Adult: Results From the National Health and Nutrition Examination Surveys 2005–2018. Front Psychiatry. 2022;13:944154. doi: 10.3389/fpsyt.2022.944154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakya P.R., Melaku Y.A., Shivappa N., Hébert J.R., Adams R.J., Page A.J., Gill T.K. Dietary inflammatory index (DII®) and the risk of depression symptoms in adults. Clin. Nutr. 2021;40:3631–3642. doi: 10.1016/j.clnu.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Jia G., Wu C.C., Su C.H. Dietary inflammatory index and metabolic syndrome in US children and adolescents: Evidence from NHANES 2001–2018. Nutr. Metab. 2022;19:39. doi: 10.1186/s12986-022-00673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motamedi A., Askari M., Mozaffari H., Homayounfrar R., Nikparast A., Ghazi M.L., Nejad M.M., Alizadeh S. Dietary Inflammatory Index in relation to Type 2 Diabetes: A Meta-Analysis. Int. J. Clin. Pract. 2022;2022:9953115. doi: 10.1155/2022/9953115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C., Qiu S., Bian H., Tian B., Wang H., Tu X., Cai B., Jin K., Zheng X., Yang L., et al. Association between Dietary Inflammatory Index and kidney stones in US adults: Data from the National Health and Nutrition Examination Survey (NHANES) 2007–2016. Public Health Nutr. 2021;24:6113–6121. doi: 10.1017/S1368980021000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolbert L., Kreutzburg T., Zyriax B.C., Adegbola A., Westenhöfer J., Jagemann B., Alexander Neumann F., Preuß M., Debus E.S., Rieß H.C., et al. A cross-sectional survey study on the nutrition patterns of patients with peripheral artery disease. Vasa. 2022;51:239–246. doi: 10.1024/0301-1526/a001005. [DOI] [PubMed] [Google Scholar]
- 25.Wan D., Li V., Banfield L., Azab S., de Souza R.J., Anand S.S. Diet and Nutrition in Peripheral Artery Disease: A Systematic Review. Can. J. Cardiol. 2022;38:672–680. doi: 10.1016/j.cjca.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Naqvi A.Z., Davis R.B., Mukamal K.J. Nutrient intake and peripheral artery disease in adults: Key considerations in cross-sectional studies. Clin. Nutr. 2014;33:443–447. doi: 10.1016/j.clnu.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Heffron S.P., Rockman C.B., Adelman M.A., Gianos E., Guo Y., Xu J.F., Berger J.S. Greater Frequency of Fruit and Vegetable Consumption Is Associated with Lower Prevalence of Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2017;37:1234–1240. doi: 10.1161/ATVBAHA.116.308474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu C.J., Teng L.Q., Liu X.N., Zhang Y.B., Fang J., Shen C.Y. Dose-Response Relationship between Physical Activity and the Incidence of Peripheral Artery Disease in General Population: Insights from the National Health and Nutrition Examination Survey 1999–2004. Front. Cardiovasc. Med. 2021;8:730508. doi: 10.3389/fcvm.2021.730508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones M.R., Apelberg B.J., Samet J.M., Navas-Acien A. Smoking, menthol cigarettes, and peripheral artery disease in U.S. adults. Nicotine Tob. Res. 2013;15:1183–1189. doi: 10.1093/ntr/nts253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger J.S., Eraso L.H., Xie D., Sha D., Mohler E.R., 3rd Mean platelet volume and prevalence of peripheral artery disease, the National Health and Nutrition Examination Survey, 1999–2004. Atherosclerosis. 2010;213:586–591. doi: 10.1016/j.atherosclerosis.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zalawadiya S.K., Veeranna V., Panaich S.S., Afonso L. Red cell distribution width and risk of peripheral artery disease: Analysis of National Health and Nutrition Examination Survey 1999–2004. Vasc. Med. 2012;17:155–163. doi: 10.1177/1358863X12442443. [DOI] [PubMed] [Google Scholar]
- 32.Shivappa N., Steck S.E., Hurley T.G., Hussey J.R., Hébert J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z., Liu H., Deng Q., Sun C., He W., Zheng W., Tang R., Li W., Xie Q. Association between Dietary Inflammatory Index and Heart Failure: Results from NHANES (1999–2018) Front. Cardiovasc. Med. 2021;8:702489. doi: 10.3389/fcvm.2021.702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin Z., Chang K., Liao R., Jiang L., Yang Q., Su B. Greater Dietary Inflammatory Potential Is Associated with Higher Likelihood of Abdominal Aortic Calcification. Front. Cardiovasc. Med. 2021;8:720834. doi: 10.3389/fcvm.2021.720834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Min J.Y., Cho J.S., Lee K.J., Park J.B., Park S.G., Kim J.Y., Min K.B. Potential role for organochlorine pesticides in the prevalence of peripheral arterial diseases in obese persons: Results from the National Health and Nutrition Examination Survey 1999–2004. Atherosclerosis. 2011;218:200–206. doi: 10.1016/j.atherosclerosis.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 36.Selvin E., Köttgen A., Coresh J. Kidney function estimated from serum creatinine and cystatin C and peripheral arterial disease in NHANES 1999–2002. Eur. Heart J. 2009;30:1918–1925. doi: 10.1093/eurheartj/ehp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esmaillzadeh A., Kimiagar M., Mehrabi Y., Azadbakht L., Hu F.B., Willett W.C. Dietary patterns and markers of systemic inflammation among Iranian women. J. Nutr. 2007;137:992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 39.Soltani S., Chitsazi M.J., Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin. Nutr. 2018;37:542–550. doi: 10.1016/j.clnu.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Shivappa N., Hebert J.R., Marcos A., Diaz L.E., Gomez S., Nova E., Michels N., Arouca A., González-Gil E., Frederic G., et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017;61:1600707. doi: 10.1002/mnfr.201600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di X., Han W., Liu C.W., Ni L., Zhang R. A systematic review and meta-analysis on the association between C-reactive protein levels and adverse limb events after revascularization in patients with peripheral arterial disease. J. Vasc. Surg. 2021;74:317–326. doi: 10.1016/j.jvs.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 42.Kremers B., Wübbeke L., Mees B., Ten Cate H., Spronk H., Ten Cate-Hoek A. Plasma Biomarkers to Predict Cardiovascular Outcome in Patients with Peripheral Artery Disease: A Systematic Review and Meta-Analysis. Arterioscler. Thromb. Vasc. Biol. 2020;40:2018–2032. doi: 10.1161/ATVBAHA.120.314774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Mantrana I., Selma-Royo M., Alcantara C., Collado M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018;9:890. doi: 10.3389/fmicb.2018.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nova E., Gómez-Martinez S., González-Soltero R. The Influence of Dietary Factors on the Gut Microbiota. Microorganisms. 2022;10:1368. doi: 10.3390/microorganisms10071368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X., Gao Y., Chen W., Hu Q., He Z., Wang X., Li D., Lin R. Dietary variety relates to gut microbiota diversity and abundance in humans. Eur. J. Nutr. 2022:1–14. doi: 10.1007/s00394-022-02929-5. [DOI] [PubMed] [Google Scholar]
- 46.Bolte L.A., Vich Vila A., Imhann F., Collij V., Gacesa R., Peters V., Wijmenga C., Kurilshikov A., Campmans-Kuijpers M.J.E., Fu J., et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70:1287–1298. doi: 10.1136/gutjnl-2020-322670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonsson A.L., Bäckhed F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017;14:79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 48.Gui D.D., Luo W., Yan B.J., Ren Z., Tang Z.H., Liu L.S., Zhang J.F., Jiang Z.S. Effects of gut microbiota on atherosclerosis through hydrogen sulfide. Eur. J. Pharmacol. 2021;896:173916. doi: 10.1016/j.ejphar.2021.173916. [DOI] [PubMed] [Google Scholar]
- 49.Duttaroy A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients. 2021;13:144. doi: 10.3390/nu13010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roncal C., Martínez-Aguilar E., Orbe J., Ravassa S., Fernandez-Montero A., Saenz-Pipaon G., Ugarte A., Estella-Hermoso de Mendoza A., Rodriguez J.A., Fernández-Alonso S., et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019;9:15580. doi: 10.1038/s41598-019-52082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senthong V., Wang Z., Fan Y., Wu Y., Hazen S.L., Tang W.H. Trimethylamine N-Oxide and Mortality Risk in Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2016;5:e004237. doi: 10.1161/JAHA.116.004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loffredo L., Ivanov V., Ciobanu N., Deseatnicova E., Gutu E., Mudrea L., Ivanov M., Nocella C., Cammisotto V., Orlando F., et al. Is There an Association between Atherosclerotic Burden, Oxidative Stress, and Gut-Derived Lipopolysaccharides? Antioxid. Redox Signal. 2020;33:761–766. doi: 10.1089/ars.2020.8109. [DOI] [PubMed] [Google Scholar]
- 53.Brandsma E., Kloosterhuis N.J., Koster M., Dekker D.C., Gijbels M.J.J., van der Velden S., Ríos-Morales M., van Faassen M.J.R., Loreti M.G., de Bruin A., et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019;124:94–100. doi: 10.1161/CIRCRESAHA.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pande R.L., Creager M.A. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ. Cardiovasc. Qual. Outcomes. 2014;7:532–539. doi: 10.1161/CIRCOUTCOMES.113.000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fanaroff A.C., Yang L., Nathan A.S., Khatana S.A.M., Julien H., Wang T.Y., Armstrong E.J., Treat-Jacobson D., Glaser J.D., Wang G., et al. Geographic and Socioeconomic Disparities in Major Lower Extremity Amputation Rates in Metropolitan Areas. J. Am. Heart Assoc. 2021;10:e021456. doi: 10.1161/JAHA.121.021456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otto C.M. Marital status and cardiovascular disease risk. Heart. 2018;104:1893–1894. doi: 10.1136/heartjnl-2018-314336. [DOI] [PubMed] [Google Scholar]
- 57.Cha S., Grace S.L., Han K., Kim B., Paik N.J., Won-Seok K. Effect of physical activity and tobacco use on mortality and morbidity in patients with peripheral arterial disease after revascularisation: A Korean nationwide population-based cohort study. Eur. J. Vasc. Endovasc. Surg. 2022 doi: 10.1016/j.ejvs.2022.05.047. [DOI] [PubMed] [Google Scholar]
- 58.Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 59.Calcaterra V., Vandoni M., Rossi V., Berardo C., Grazi R., Cordaro E., Tranfaglia V., Carnevale Pellino V., Cereda C., Zuccotti G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int. J. Environ. Res. Public Health. 2022;19:6908. doi: 10.3390/ijerph19116908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.da Rocha A.L., Pinto A.P., Kohama E.B., Pauli J.R., de Moura L.P., Cintra D.E., Ropelle E.R., da Silva A.S.R. The proinflammatory effects of chronic excessive exercise. Cytokine. 2019;119:57–61. doi: 10.1016/j.cyto.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 61.Cheng A.J., Jude B., Lanner J.T. Intramuscular mechanisms of overtraining. Redox Biol. 2020;35:101480. doi: 10.1016/j.redox.2020.101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahl R.E., Andersen P.R., Gronbaek K., Morville T.H., Rosenkilde M., Rasmusen H.K., Poulsen S.S., Prats C., Dela F., Helge J.W. Repeated Excessive Exercise Attenuates the Anti-Inflammatory Effects of Exercise in Older Men. Front. Physiol. 2017;8:407. doi: 10.3389/fphys.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/ (accessed on 1 May 2022).