Abstract
Staphylococcus aureus was shown to transport iron complexed to a variety of hydroxamate type siderophores, including ferrichrome, aerobactin, and desferrioxamine. An S. aureus mutant defective in the ability to transport ferric hydroxamate complexes was isolated from a Tn917-LTV1 transposon insertion library after selection on iron-limited media containing aerobactin and streptonigrin. Chromosomal DNA flanking the Tn917-LTV1 insertion was identified by sequencing of chromosomal DNA isolated from the mutant. This information localized the transposon insertion to a gene whose predicted product shares significant similarity with FhuG of Bacillus subtilis. DNA sequence information was then used to clone a larger fragment of DNA surrounding the fhuG gene, and this resulted in the identification of an operon of three genes, fhuCBG, all of which show significant similarities to ferric hydroxamate uptake (fhu) genes in B. subtilis. FhuB and FhuG are highly hydrophobic, suggesting that they are embedded within the cytoplasmic membrane, while FhuC shares significant homology with ATP-binding proteins. Given this, the S. aureus FhuCBG proteins were predicted to be part of a binding protein-dependent transport system for ferric hydroxamates. Exogenous iron levels were shown to regulate ferric hydroxamate uptake in S. aureus. This regulation is attributable to Fur in S. aureus because a strain containing an insertionally inactivated fur gene showed maximal levels of ferric hydroxamate uptake even when the cells were grown under iron-replete conditions. By using the Fur titration assay, it was shown that the Fur box sequences upstream of fhuCBG are recognized by the Escherichia coli Fur protein.
With the possible exception of lactobacilli, all bacteria require iron for growth (5). Despite the fact that iron is plentiful in nature, under oxic conditions and at a neutral pH, iron readily oxidizes and precipitates, making it biologically unavailable. It has been estimated that the concentration of free iron under these conditions is 10−18 M (3), far below that which is required to support growth. Bacterial pathogens are faced with having to compete with host iron-binding proteins for available iron. Among successful pathogens, several different iron acquisition mechanisms are known by which bacteria overcome the iron-restricted environment of the host in order to multiply and cause infection. Many bacteria synthesize low-molecular-weight iron chelators known as siderophores, and, together with their cognate cell surface receptors and transporters, these systems provide an efficient means to acquire limiting concentrations of iron. There is considerable experimental evidence that siderophore-mediated iron transport systems are important contributors to the in vivo growth and pathogenicity of many disease-causing bacteria. Indeed, loss of the ability to produce siderophores is correlated with loss of virulence in Erwinia chrysanthemi in plants (16), Vibrio anguillarum in fish (11), and Pseudomonas aeruginosa (10, 38), Yersinia enterocolitica (23), and Escherichia coli (62) in mice. The direct binding of host iron-binding glycoproteins (e.g., transferrin and lactoferrin) at the bacterial cell surface provides an alternative to siderophores as a method to gain access to host iron supplies. Transferrin binding proteins have been identified in Neisseria spp., Haemophilus spp., and Staphylococcus aureus (for a recent review, see reference 17).
S. aureus is an important human pathogen that is capable of causing a wide spectrum of infections and diseases. Infections can range from minor (e.g., carbuncles) to severe (e.g., septicemia, endocarditis, and osteomyelitis). The diversity and severity of S. aureus infections can be attributed to this microorganism's ability to produce numerous cell surface-localized proteins that bind host tissues (e.g., fibronectin binding protein and collagen binding protein) as well as several different extracellular toxins (e.g., toxic shock syndrome toxin 1 and alpha-hemolysin) (for a review, see reference 12). S. aureus is currently a major threat to the quality of health care because of its resistance to many antibiotics and its frequent association with hospitalized patients, especially those who have undergone surgical procedures.
The production of three different siderophores, staphyloferrin A (30, 36), staphyloferrin B (14), and aureochelin (9), from strains of S. aureus has been demonstrated. S. aureus can also bind transferrin-iron complexes, and the protein involved in this binding, Tpn, has been identified as a multifunctional 42-kDa cell surface-localized protein (39, 40). Numerous other iron-regulated proteins with as yet unknown functions have been described in S. aureus (9, 26, 35).
We report here the identification, molecular cloning, and characterization of a locus of three genes (termed fhuC, fhuB, and fhuG; fhu stands for ferric hydroxamate uptake) from S. aureus that are involved in the acquisition of iron from hydroxamate siderophores. Predicted proteins from this locus share significant similarities with the FhuC, FhuB, and FhuG proteins of Bacillus subtilis. We provide evidence that fhuG is required for the transport of iron complexed to several different hydroxamate siderophores. Further, we show that expression of the S. aureus fhu operon is regulated by the external iron concentration via S. aureus Fur and that Fur box sequences upstream of the fhuC coding region are recognized by the E. coli Fur protein.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are described in Table 1. For experiments not directly involving the analysis of iron uptake systems, S. aureus strains were grown in tryptic soy broth (Difco) while E. coli was routinely grown in Luria-Bertani broth (Difco). Tris-minimal succinate medium (53) with glucose and iron omitted and supplemented with Casamino Acids (1%; Difco), pantothenic acid (0.5 μg/ml), biotin (0.01 μg/ml), thiamine (50 μM), MgCl2 (500 μM), and CaCl2 (100 μM) was the iron-deficient minimal medium used throughout. FeSO4 (50 μM) was added, as required, to generate iron-replete minimal media. Tetracycline (2 μg/ml), erythromycin (1 μg/ml), kanamycin (50 μg/ml), and lincomycin (20 μg/ml) were incorporated into the media, as required, for the growth of S. aureus, while tetracycline (10 μg/ml), erythromycin (300 μg/ml), and kanamycin (30 μg/ml) were incorporated into the media, as required, for the growth of E. coli. Solid media were obtained by the addition of 1.5% (wt/vol) Bacto agar (Difco). Unless otherwise stated, all bacterial growth was carried out at 37°C. Iron-free water was used for all experiments and was obtained by passage through a Milli-Q water filtration unit (Millipore Corp.).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| RN2564 | (80α) Ω25[Tn551] pig-131 | J. Iandolo |
| RN4220 | rK− mK+ | 34 |
| RN6390 | Prophage-cured wild-type strain | 45 |
| H256 | RN4220 fhuG::Tn917; Emr | This study |
| H287 | RN6390 fhuG::Tn917; Emr | This study |
| H295 | RN6390 fur::Km; Kmr | This study |
| E. coli | ||
| DH5α | φ80d lacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169 | Promega |
| H1717 | araD139 rpsL150 Δ(argF-lac) relA1 U169 flbB5301 deoC1 ptsF25 rbsR aroB fhuF::λplacMu53 | 55 |
| Plasmids | ||
| pAW8 | 5.1-kb E. coli-S. aureus shuttle vector; Tcr | 58 |
| pLTV1 | Temperature-sensitive vector containing Tn917-LTV1; Emr Apr Cmr | 6 |
| pMTS20 | S. aureus fhuCBG genes cloned into the BamHI site of pAW8; Tcr | This study |
| pMTS21 | S. aureus fhuG gene cloned into the BamHI site of pAW8; Tcr | This study |
| pBAD33 | Arabinose-regulatable expression vector; Cmr | 20 |
| pDG782 | Carries the kanamycin resistance gene; Apr Kmr | 19 |
| pAUL-A | 9.2-kb temperature-sensitive S. aureus suicide vector that is used for gene replacement experiments; Emr | 7 |
| pMDH6 | 3.2-kb fragment containing the insertionally inactivated S. aureus fur gene cloned into pAUL-A; Kmr Emr | This study |
| pJHCV75 | 0.7-kb HindIII-SalI fragment from pColV aerobactin promoter region cloned into pUC18; Apr | M. Valvano (56) |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Emr, erythromycin resistance; Kmr, kanamycin resistance; Tcr, tetracycline resistance.
Recombinant DNA methodology.
Restriction endonuclease digestion and DNA ligation were performed essentially as described by Sambrook et al. (49). Restriction enzymes were purchased from either Life Technologies, Inc. (Burlington, Ontario, Canada), New England Biolabs (Mississauga, Ontario, Canada), Roche Diagnostics (Laval, Quebec, Canada), or MBI Fermentas (Flamborough, Ontario, Canada). Plasmid DNA was prepared by using QIAprep plasmid spin columns (Qiagen Inc., Santa Clarita, Calif.) as directed by the manufacturer, except that lysostaphin (50 μg/ml) was incorporated into buffer P1 for lysis of S. aureus cells. Published methods were used to introduce plasmid DNA into E. coli, either by using CaCl2-competent cells (49) or by electroporation using conditions described elsewhere (24). Plasmids were introduced into S. aureus by electroporation using a method described previously (33). Chromosomal sequences flanking transposon insertions were identified by sequencing reactions initiated with an oligonucleotide primer (5′-CCA TAC GCA AGA CCA ATC ACT CTC GGA C-3′) that hybridized near the end of Tn917 sequences and primed outward. Chromosomal DNA, isolated from transposon insertion mutants, was purified by using the DNeasy tissue kit (Qiagen) (modified by the addition of lysostaphin to lyse cells) and was used as the sequencing template. The fhuCBG operon was amplified as a 3.7-kb PCR product by using primers that hybridized upstream of fhuC (FhuCBG2; 5′-TTT GGA TCC ACA AGT TTC AAA AGC AAA GC-3′) and downstream of fhuG (FhuCBG1; 5′-TTT GGA TCC GAT GTA AAT ACT TCG CCA CA-3′) and was cloned into the BamHI site of pAW8. The fhuG gene was amplified as a 1.7-kb PCR product by using a primer that hybridized near the 3′ end of fhuB (FhuG1; 5′-TTT GGA TCC TGC ATT TGT AGG TTT GAT GG-3′) and FhuCBG1 and was cloned into the BamHI site of pAW8. Automated sequencing was performed in the DNA sequencing facility at Mobix (Hamilton, Ontario, Canada. PCRs were performed by using a GeneAmp PCR system (Perkin-Elmer). PwoI DNA polymerase (Roche) was used in all PCRs.
Generation of a Tn917-LTV1 insertion library.
A Tn917-LTV1 insertion library was constructed in S. aureus RN4220 by using the temperature-sensitive vector pLTV1 (6) and the procedure of Watson, Antonio, and Foster (60).
Mutant selection.
A random Tn917-LTV1 insertion library of S. aureus RN4220 (constructed as described above) was plated onto erythromycin- and aerobactin-containing iron-deficient plates supplemented with ethylene diamine-di(o-hydroxyphenol acetic acid) (EDDHA) at 15 μM, and disks containing various concentrations of streptonigrin (a stock solution was prepared at 1 mg/ml in 10 mM Tris-HCl [pH 7.6]) were placed on the plates. After overnight incubation at 37°C, zones of growth inhibition were visible around the disks. Increasing zone diameters corresponded to increasing concentrations of streptonigrin. Scrapings were taken from within the zones of growth inhibition and streaked onto erythromycin-containing tryptic soy broth for isolated colonies. Colonies were then restreaked onto the same media before being patched to iron-deficient plates with or without aerobactin.
Phage preparation and transduction.
UV induction was used to obtain bacteriophage 80α lysates from RN2564, and bacteriophage were sterilized by filtration through 0.45-μm-pore-size filters and titered on S. aureus RN6390. Transductions were carried out as previously described (43).
Construction of an S. aureus fur mutant.
The S. aureus fur gene was identified on a contig present within the sequence available from S. aureus Genome Sequencing Project at the University of Oklahoma. The gene was PCR amplified from the RN6390 chromosome (by using primers Fur3, 5′-TCT CTT GCA CTG CTC TTA AAT C-3′, and Fur4, 5′-TTG CCA AAG AGT TAA CAC ATG T-3′) and was cloned into the SalI site (blunt ended with Klenow enzyme) in pBAD33 (Stratagene). The fur coding region was then disrupted by insertion of a kanamycin resistance cassette, obtained from plasmid pDG782 on a StuI-SmaI fragment, into the unique SalI site (blunt ended with Klenow enzyme) present within the fur coding region. A PstI-EcoRI fragment (blunted at both ends) containing the disrupted fur gene was then cloned into SmaI-digested pAUL-A, a temperature-sensitive S. aureus suicide plasmid. The resulting plasmid, designated pMDH6, was introduced into RN4220, and transformants were selected after growth at 30°C on appropriate antibiotics. To select for plasmid recombination into the chromosome, RN4220 containing pMDH6 was grown at 30°C to mid-log phase before the growth temperature was shifted to 42°C. The culture was allowed to grow for a further 5 h before being plated at 42°C on media containing kanamycin. Erythromycin- and kanamycin-resistant colonies were selected, and phage 80α was used to transduce the kanamycin resistance marker into RN6390. Kanamycin-resistant, erythromycin-sensitive colonies were selected for further study. The fur mutation was confirmed by PCR from chromosomal DNA (isolated by using the Instagene Matrix [Bio-Rad Laboratories, Mississauga, Ontario, Canada) obtained from individual Kmr Ems colonies.
Computer analyses.
DNA sequence analyses, PCR oligonucleotide primer identifications, hydropathy plots, and multiple sequence alignments were performed by using the Vector NTI Suite software package (Informax, Inc.). Microsoft Excel was used to generate graphs for 55Fe3+-ferrichrome transport assays.
Siderophores.
Ferrichrome, rhodotorulic acid, and 2,3-dihydroxybenzoic acid (2,3-DHBA) were purchased from Sigma Chemicals (Mississauga, Ontario, Canada), and desferrioxamine (used as Desferal [Novartis]) was obtained from the London Health Sciences Center. Aerobactin was a gift from T. Viswanatha (University of Waterloo), and enterobactin was a gift from Alain Stintzi (Oklahoma State University). Pyoverdine and pyochelin were prepared as previously described (25, 47). Siderophore was extracted from iron-starved S. aureus culture supernatants by the method of Rogers (48). Briefly, supernatants of 100-ml iron-starved cultures of S. aureus were acidified and extracted with 40 ml of ethyl acetate before the ethyl acetate was evaporated in a rotory evaporator. The crude preparation was dissolved in 400 μl of sterile water before being filter sterilized. Ten microliters of this extract was used in bioassay experiments.
Bioassays.
For plate bioassay experiments, stationary-phase cells of S. aureus (104/ml) were incorporated into plates of minimal media containing 25 μM EDDHA. Siderophores to be tested (10 μl of a 50 μM solution) were added to sterile paper disks and placed onto the surfaces of the plates. The uncharacterized S. aureus siderophore was used in bioassays as a crude extract (described above). Growth promotion was assessed after incubation for 24 h at 37°C.
Transport assays.
Transport assays were performed essentially as described previously (25). S. aureus cells used in 55Fe transport assays were grown overnight in minimal medium with or without FeSO4. For cultures grown without FeSO4, EDDHA was added to a final concentration of 0.75 μM. Cells were then diluted into 10-ml volumes of the same medium and grown to an optical density at 600 nm of 1.0. Cell cultures (5 ml) were harvested by filtration on 0.45-μm-pore-size membrane filters (GN-6; Gelman and were washed three times with an equal volume of Millipore-filtered water before being resuspended in an equal volume of Chelex 100 (Bio-Rad)-treated minimal medium. Cells were then shaken (at 150 rpm) at 37°C for 5 min prior to assay. Ferrichrome (200 μM) was mixed with 55FeCl3 (16 μM) in the presence of 2 μM nitrilotriacetic acid at least 30 min before the start of the assay. In some experiments, water replaced ferrichrome in the uptake mixture. A 10-μl portion of the 55Fe-ferrichrome (or water) mixture was then added to cells (1 ml) in a 10-ml disposable culture tube to initiate uptake. Cells were vortexed gently, and aliquots (200 μl) were removed at intervals, filtered onto 0.45-μm-pore-size membrane filters (GN-6; Gelman), and washed twice with 10 ml of 100 mM LiCl. The membranes were dried and counted in scintillation fluid by using the tritium channel of a scintillation system LS 6500 (Beckman). In some experiments, cells were treated with KCN (10 mM) 15 min prior to the uptake assay.
Fur titration assay (FURTA).
Plasmids were introduced into E. coli H1717 (55). LacZ expression was assessed by visualization of a change in colony color from white to red on MacConkey lactose plates (Difco) supplemented with 30 μM ferrous ammonium sulfate. Plates were examined after 24 h of growth at 37°C.
Nucleotide sequence accession number.
The 3,728-bp fhuCBG region sequenced in this study has been assigned GenBank accession no. AF251216.
RESULTS AND DISCUSSION
Utilization of heterologous siderophores by S. aureus.
In an earlier study, Brock and Ng (4) demonstrated that S. aureus was capable of utilizing desferrioxamine as an iron source. Interestingly, however, previous studies had failed to detect hydroxamate siderophore production from S. aureus (9). We confirmed this observation in S. aureus RN6390 by demonstrating that culture supernatants of iron-starved RN6390 give a negative result in the Csáky test (44) for hydroxamate compounds (data not shown).
We were interested in determining whether S. aureus could utilize several different hydroxamate-iron complexes, as has been shown for B. subtilis (50). By using the plate bioassay technique, RN6390 was demonstrated to readily utilize ferrichrome as an iron source (Fig. 1). Moreover, not only did RN6390 utilize ferrichrome, but it was also able to use other hydroxamate siderophores, such as aerobactin and desferrioxamine, to acquire iron (Table 2). Interestingly, rhodotorulic acid, a hydroxamate siderophore of fungal origin that is used by B. subtilis and E. coli (22, 50), failed to stimulate the growth of RN6390 in this assay. In addition to hydroxamate siderophores, RN6390 was shown to utilize catechol type siderophores, such as enterobactin and 2,3-DHBA (a precursor of enterobactin that is used as a siderophore by B. subtilis), and an uncharacterized siderophore(s) extracted from the culture supernatant of iron-starved RN6390. Although the identity of the S. aureus siderophore is not known, it was extracted by the same procedure as that used to identify aureochelin (9), suggesting that the siderophore isolated from RN6390 is aureochelin. The S. aureus siderophore(s) extracted in this manner reacted positively with chrome azurol S-shuttle solution (51), which is indicative of the presence of a siderophore(s). As a control to indicate that S. aureus was indeed producing a siderophore that could be utilized in plate bioassay experiments, minimal medium alone was extracted in the same manner as the supernatants from iron-starved S. aureus cultures and was tested in plate bioassays. No growth promotion of RN6390 was observed, indicating that the growth promotion of RN6390 observed in plate bioassays was not due to components of the medium (data not shown). S. aureus RN6390 was incapable of utilizing siderophores produced by members of the Pseudomonadaceae, such as pyoverdine and pyochelin (both produced by P. aeruginosa) (46), or cepabactin, produced by Burkholderia cepacia (37).
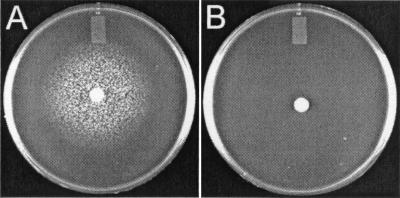
FIG. 1.
Ferrichrome plate bioassay for S. aureus strains RN6390 (A) and H287 (B).
TABLE 2.
Utilization of siderophores by RN6390 and H287
| Siderophore | Growth promotiona in:
|
|
|---|---|---|
| RN6390 | H287 | |
| Ferrichrome | ++++ | − |
| Aerobactin | ++++ | − |
| Desferal | ++++ | − |
| Rhodotorulic acid | − | − |
| Enterobactin | ++++ | ++++ |
| 2,3-DHBA | ++++ | ++++ |
| Pyoverdine | − | − |
| Pyochelin | − | − |
| Cepabactin | − | − |
| RN6390 siderophore(s)b | ++++ | ++++ |
++++, very good growth promotion; −, no growth promotion.
Uncharacterized siderophore(s) extracted from the culture supernatant of iron-starved RN6390.
Construction and characterization of a mutant deficient in the ability to transport iron-hydroxamate complexes.
The results indicating that S. aureus RN6390 could utilize heterologous hydroxamate siderophores to acquire iron suggested that a specific high-affinity uptake system for these compounds exists in this bacterium. In order to identify such a system, a streptonigrin enrichment procedure was used to select for mutants incapable of utilizing iron-hydroxamate complexes. Streptonigrin interacts with intracellular iron pools to cause the formation of reactive oxygen species that react with DNA and other macromolecules (61, 64). Accumulated damage eventually results in cell death. Not surprisingly, bacteria grown in high-iron media are significantly more sensitive to the damaging effects of streptonigrin than bacteria grown in low-iron media, since the bacteria grown in higher-iron environments accumulate higher intracellular iron pool concentrations. Conversely, mutants that are unable to utilize available iron sources are more resistant to the toxic effects of streptonigrin. Numerous researchers have taken advantage of the latter observation to select for mutants incapable of obtaining iron from various sources (2, 15, 27). To enrich for Fe3+-hydroxamate uptake-deficient S. aureus mutants, a Tn917-LTV1 insertion library in S. aureus RN4220 was plated onto media containing erythromycin, EDDHA, and aerobactin before disks containing various concentrations of streptonigrin were placed upon the plates. After overnight growth, zones of growth inhibition (or bacterial killing) were visible around the disks. It was surmised that mutants unable to obtain iron from aerobactin would fail to grow under the iron-limiting conditions of the media and would, presumably, resist the toxic effects of streptonigrin. Therefore, surviving bacteria were recovered from within the inhibition (killing) zones. To confirm that streptonigrin-resistant mutants could not utilize aerobactin as an iron source, the mutants were patched to iron-deficient media containing, or lacking, exogenously supplied aerobactin. Several mutants that failed to grow on plates containing aerobactin, but that grew on plates lacking aerobactin, were selected for further study. One mutant whose growth on iron-replete and iron-deficient media was identical to that of the parental strain, RN4220, and the S. aureus prototype strain RN6390 but whose growth on iron-deficient media supplemented with aerobactin was severely impaired, compared to that of RN4220 and RN6390, was eventually isolated and designated H256.
Phage transduction, using phage 80α, was used to transfer the Tn917-LTV1 insertion from H256 into the RN6390 parent background. Fifty transductants, in addition to RN6390 and H256, were tested for the ability to grow on iron-deficient plates containing aerobactin. All erythromycin-resistant transductants were unable to grow on this medium, indicating that the Tn917-LTV1 insertion correlates with the mutant phenotype (data not shown). One of the erythromycin-resistant RN6390 transductants, designated H287, was used throughout the remainder of these studies.
As observed in plate bioassays, H287 demonstrated an inability to utilize aerobactin, desferrioxamine (Desferal), and ferrichrome, while it retained the ability to utilize 2,3-DHBA, enterobactin, and the siderophore(s) isolated from iron-starved RN6390 culture supernatant (see Fig. 1 and Table 2). Not surprisingly, H287, like its parent, RN6390, failed to utilize pyoverdine, pyochelin, and cepabactin (Table 2).
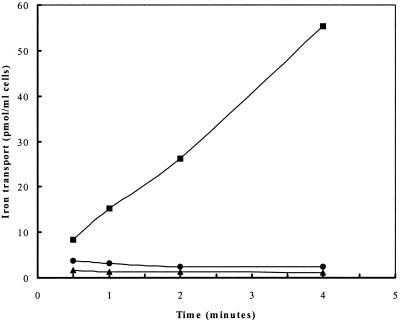
In an attempt to confirm the H287 mutant phenotype, and to rule out any possible artifactual results that may have interfered with our interpretation of the bioassay results (such as the production of endogenous S. aureus siderophores in RN6390 and not in H287), 55Fe3+-ferrichrome transport assays were performed. Strain H287, in contrast to its parent, RN6390, was completely devoid of ferrichrome-mediated iron uptake at 160 nM 55FeCl3 (Fig. 2).
FIG. 2.
Ferrichrome-mediated iron (55Fe3+) transport by S. aureus RN6390 (■) and H287 (●) cultured in iron-deficient medium. The uptake mixture contained ferrichrome (200 μM), nitrilotriacetic acid (2 μM), 55FeCl3 (160 nM), and 1 ml of cells at an optical density at 600 nm of 1.0. ▴, transport by RN6390 in the presence of 10 mM KCN or in the absence of any siderophore. Data are averages from experiments performed in triplicate.
Characterization of genes involved in ferric hydroxamate uptake in S. aureus.
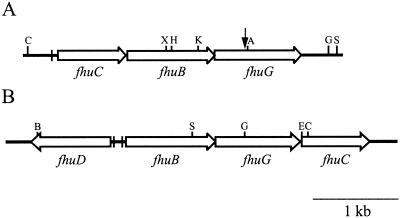
Genomic DNA was isolated from H287, and the nucleotide sequence of the junction between the Tn917-LTV1 insertion and the chromosome was determined. By using this sequence information in BLAST searches of the databases, it was determined that the disrupted gene encoded a protein with significant similarity to FhuG of B. subtilis, a protein involved in ferric hydroxamate uptake in this organism. This sequence information was also used to identify the region within the sequence available from the S. aureus Genome Sequencing Project at the University of Oklahoma (http://www.genome.ou.edu/staph.html). PCR was then used to amplify a larger region of DNA from RN6390 that would encompass the gene(s) of interest. PCR products were subsequently cloned and sequenced, and the data were analyzed. Computer analyses revealed that fhuG is the third of three open reading frames present within an operon designated fhu, for ferric hydroxamate uptake (Fig. 3).
FIG. 3.
Physical map of the fhu regions in S. aureus (A) and B. subtilis (B). The large open arrows represent coding regions, while the small vertical arrow indicates the position of the Tn917-LTV1 insertion present in H287. The filled vertical bars indicate the locations of Fur box sequences upstream of the S. aureus fhuCBG operon (63) and the two Fur box sequences present between the divergently transcribed fhuD and fhuBCG genes in B. subtilis (50). Selected restriction enzyme recognition sequences are indicated along the tops of the maps and are abbreviated as follows: A, AccI; B, BamHI; C, ClaI; E, EcoRI; G, BglII; H, HpaI; K, KpnI; S, SphI; X, XbaI.
The first open reading frame within the cloned operon (Fig. 3) encodes a predicted protein of 265 amino acids with a molecular mass of 29.5 kDa. BLAST searches of the databases revealed that the product of this open reading frame shares significant similarity with ATP-binding proteins that are involved in the transport of iron-siderophore complexes in numerous bacteria. These include FepC of E. coli (involved in ATP hydrolysis for ferric enterobactin transport) (52) and FecE of E. coli (involved in ATP hydrolysis for ferric citrate transport) (54), among numerous others. Most notably, the highest degree of similarity was shared with FhuC of B. subtilis (56.5% identity, 71.7% total similarity), followed by FhuC of E. coli (38.1% identity, 56.8% total similarity). The S. aureus FhuC contains conserved Walker A (36GPNGCGKS43) and Walker B (160IIFLDEPTTYLD171) motifs, as predicted for an ATP-binding protein functioning as part of a traffic ATPase (reviewed in reference 42). These amino acid residues are conserved between the FhuC proteins in S. aureus and in E. coli, and it has previously been shown that substitutions of amino acids in the predicted ATP binding site in E. coli FhuC abolish iron-hydroxamate uptake (1).
The second open reading frame within the S. aureus fhu operon (termed fhuB) (see Fig. 3) is predicted to encode a protein of 341 amino acids with a molecular mass of 36 kDa that shares significant similarity (39.6% identity, 63.2% total similarity) with the B. subtilis FhuB protein. Computer analyses indicate that the FhuB protein is highly hydrophobic and may possess as many as eight or nine transmembrane domains (data not shown). Of note, FhuB proteins from S. aureus and B. subtilis are approximately half the size of the E. coli FhuB protein (32). In E. coli and B. subtilis, it has been shown that FhuB acts as an integral membrane permease, allowing for the passage of iron-hydroxamate complexes across the cytoplasmic membrane (3, 18, 31). According to data currently available in the databases, the FhuB protein in S. aureus would appear, at first glance, to be significantly smaller (by 43 amino acids and 4.7 kDa) than its homolog in B. subtilis. An explanation for this lies in the fact that the size of the B. subtilis FhuB is currently predicted based upon the largest possible open reading frame (using a start codon of AUG) in that region of the chromosome. However, no experimental data have confirmed the size of the encoded FhuB protein in B. subtilis. Thirty-six codons downstream of the AUG in the same reading frame is a UUG codon, preceded by what would appear to be a strong ribosome binding region (AAGGAAGU) (41), and the two regions are separated by 7 intervening nucleotides. Given that approximately 30% of B. subtilis genes begin with UUG or GUG start codons (21, 41, 57), it is plausible that UUG is the initiation codon for FhuB in B. subtilis. If this were indeed the case, the predicted FhuB protein would be 348 amino acids long and would have a molecular mass of 36.4 kDa, bringing it more in line with the size of the predicted FhuB of S. aureus. Moreover, this would place the second of two Fur-binding consensus regions, originally identified upstream of fhuD (50), immediately upstream of the UUG codon, and this would serve to repress transcription of the fhuBGC operon in B. subtilis under iron-replete conditions. Although we acknowledge the lack of direct experimental evidence to support this suggestion, we nonetheless propose that UUG is the start codon for B. subtilis FhuB, and the comparisons of the two FhuB proteins (this study) reflect this assumption.
The third gene of the S. aureus fhu operon (termed fhuG) (Fig. 3) is predicted to encode a protein of 338 amino acids that has a molecular mass of 36.1 kDa. S. aureus FhuG shares 38.9% identity and 62.9% similarity with the B. subtilis FhuG protein. Like FhuB, FhuG is highly hydrophobic and may possess as many as eight or nine transmembrane domains (data not shown). Alignments between the S. aureus FhuB and FhuG proteins indicate that they share 28.9% identity and 51.3% similarity. There is a somewhat higher degree of conservation across the C-terminal halves of the proteins.
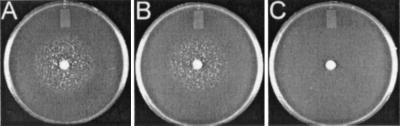
The Tn917-LTV1 insertion in H287 is positioned after the 379th bp of the fhuG gene, resulting in a truncated protein of only 126 amino acids. This suggests that there would be no active FhuG expressed in this strain. In order to confirm that it is the Tn917-LTV1 insertion that results in elimination of iron-hydroxamate transport ability in H287, and to rule out the possibility that the observed phenotype of this strain was due to mutation in another gene, complementation studies were performed. Using the plate bioassay technique, plasmid pMTS20, which contains the fhuC, fhuB, and fhuG genes, as well as 400 bp of DNA upstream of fhuC, was shown to complement the iron-hydroxamate transport defect of H287 (Fig. 4A). Moreover, the fhuG open reading frame alone, expressed from plasmid pMTS21, complements the H287 defect (Fig. 4B). Sequencing of the cloned DNA in pMTS21 showed that the fhuG coding region lay downstream of, and in the same orientation as, plac, and therefore expression of fhuG was presumably under the control of this vector-derived promoter. These data indicate that the iron-hydroxamate transport defect in H287 is due solely to the loss of fhuG as a result of its interruption by Tn917-LTV1. Plasmid pAW8 alone did not complement the defect in H287 (Fig. 4C).
FIG. 4.
Complementation of the ferrichrome uptake deficiency in S. aureus H287. The ferrichrome plate bioassay described in Materials and Methods was used for these experiments. Shown are results of ferrichrome bioassays of H287 carrying pMTS20 (A), pMTS21 (B), or pAW8 (C). Plasmids are described in Table 1.
Experimental evidence has shown that FhuG is required for ferric hydroxamate uptake (this study), while the B. subtilis FhuB protein is required for the transport of ferric hydroxamates (3). In both S. aureus and B. subtilis, the FhuB and FhuG proteins are predicted to form a heterodimeric membrane-localized permease complex that is involved in the transport of iron-hydroxamates across the cytoplasmic membrane of S. aureus. This is in contrast to iron-hydroxamate transport in E. coli, where the two halves of the functional permease are derived from a single polypeptide, FhuB, possessing two domains, FhuB(N) and FhuB(C). Like the permease component of previously characterized ferric hydroxamate transport systems (i.e., E. coli and B. subtilis), the S. aureus ferric hydroxamate permease appears to be of broad specificity. Indeed, complexes of iron with ferrichrome, aerobactin, and desferrioxamine are all transported into the S. aureus cell via this uptake system.
The B. subtilis fhu region consists of two divergently transcribed operons (see Fig. 3). One transcript contains the fhuBGC genes, while fhuD is transcribed in the opposite direction. Transcription in both directions is iron regulated by the presence of two Fur binding sites (3). In E. coli the FhuD protein is the periplasmic constituent of the binding protein-dependent iron-hydroxamate transport system, while in B. subtilis FhuD exists as a lipoprotein (50). Interestingly, the S. aureus fhu operon that was cloned and characterized in this study lacks a homolog of fhuD. Indeed, the DNA sequences on either side of the fhuCBG operon do not possess any potential coding sequences whose predicted products share similarities with FhuD homologs. It is conceivable that S. aureus (in contrast to B. subtilis) does not possess a FhuD homolog and that the putative transmembrane permease (consisting of FhuB and FhuG) acts as the ferrisiderophore receptor as well. This scenario is unlikely, however, and we are currently pursuing studies on two open reading frames, identified in ongoing genome sequencing projects, whose predicted products share similarities with FhuD homologs, with the goal of assessing their role, if any, in ferric hydroxamate transport.
Iron regulation of the fhu operon.
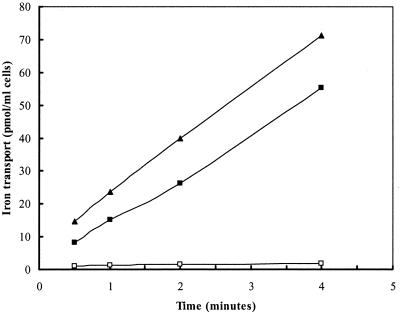
55Fe-ferrichrome transport assays were used to examine the ability of exogenous iron levels to regulate expression of the ferric hydroxamate uptake system in S. aureus. RN6390 was grown in either iron-deficient or iron-replete medium before 55Fe-ferrichrome transport was assessed. 55Fe-ferrichrome transport was completely absent in RN6390 cells that had previously been grown in iron-replete medium, whereas RN6390 grown under iron-deficient conditions showed rapid 55Fe-ferrichrome transport (Fig. 5). Conversely, transport of 55Fe-ferrichrome in H295 was maximal even in cells that were grown under iron-replete conditions, implicating fur in the control of the expression of genes necessary for Fe-ferrichrome transport. These data are in agreement with the findings of a recent study that identified a binding site for the S. aureus Fur protein upstream of the fhuC coding region (63).
FIG. 5.
Ferrichrome-mediated iron (55Fe3+) transport by S. aureus RN6390 cultured in iron-deficient (■) or iron-replete (□) medium and H295 cultured in iron-replete medium (▴). Data are averages from experiments performed in triplicate.
We were interested in determining whether Fur box sequences upstream of fhuCBG are recognized by the E. coli Fur protein, given that, of 19 nucleotides, there are 17 exact matches between the fhuCBG Fur box sequences and the E. coli consensus Fur box sequences. For these studies, we used E. coli H1717, a strain containing an iron-regulated fhuF::lacZ chromosomal fusion that is very sensitive to subtle changes in the iron concentration of the medium due to the presence of a weak Fur-Fe2+ binding region upstream of the fhuF coding region (55). Fur box sequences, present on multicopy plasmids, will deplete the cellular pool of Fur protein and allow transcription of the fhuF::lacZ fusion. When plasmid pMTS20 (which contains the fhuCBG genes as well as the fhu promoter region) was introduced into E. coli H1717 and the cells were plated onto MacConkey lactose plates containing 30 μM ferrous ammonium sulfate, the colony color was deep purple, indicative of LacZ expression (data not shown). Conversely, when plasmid pMTS21 (containing only the fhuG coding region) was introduced into H1717 and the cells were plated onto the same medium, the colonies remained white. As a positive control, colonies of H1717 carrying plasmid pJHCV75 (which contains the aerobactin promoter region from pColV) were deep purple, indicating strong Fur binding to the cloned aerobactin promoter. H1717 transformants containing pAW8 remained white in these experiments.
At least two other staphylococcal iron-regulated operons, whose products bear similarity to proteins involved in iron-siderophore uptake, have been reported (8, 26), but the substrates for these systems have not been elucidated. As a testament to their versatility, staphylococci are likely able to utilize a number of different iron complexes as a means to satisfy their requirement for this essential nutrient. Indeed, in this study we have shown that S. aureus can utilize, in addition to hydroxamate type siderophores, catechols such as enterobactin and 2,3-DHBA, and an uncharacterized S. aureus siderophore that may be aureochelin. Moreover, members of the staphylococci, including S. aureus, have been shown to produce staphyloferrin A and staphyloferrin B, siderophores with a polycarboxylate structure. It is noteworthy that transport components for these siderophores have not been identified.
We have shown here that the FhuG protein and, by extension, the FhuCBG proteins are required for the transport of desferrioxamine or, as used in this study, its mesylate salt Desferal. Desferal is widely used as an iron chelator in clinical situations of iron overload (5). Given that we, and others (4), have shown that S. aureus can readily use this compound for growth under iron-limiting conditions, it is reasonable to suggest that the use of Desferal would predispose patients to a higher risk of S. aureus infection. In one recent study of β-thalassemia patients undergoing desferrioxamine-mediated chelation therapy, S. aureus and coagulase-negative staphylococci caused 17 of 19 complications due to infection (13). In addition to our in vitro data showing that S. aureus can utilize desferrioxamine, exogenously supplied desferrioxamine B enhances infections by Klebsiella (29) and Salmonella (28) spp. and greatly increases the susceptibility of desferrioxamine-treated patients to infection with Y. enterocolitica (reviewed in reference 59). Taken together, these results illustrate one of the potential drawbacks to this strategy of iron chelation therapy.
ACKNOWLEDGMENTS
We thank John Iandolo, Ambrose Cheung, Akihito Wada, and Simon Foster for providing strains and plasmids and Thammaiah Viswanatha and Alain Stintzi for the gifts of aerobactin and enterobactin, respectively. We thank Miguel Valvano for helpful discussions and critical comments on the manuscript. Technical assistance provided by Deb Hayden is gratefully acknowledged.
This work was supported by operating grants from the Medical Research Council of Canada (MRC), Foundation Western, and the J. P. Bickell Foundation. D. E. H. is the recipient of an MRC scholarship.
REFERENCES
- 1.Becker K, Köster W, Braun V. Iron(III) hydroxamate transport of Escherichia coli K-12: single amino acid replacements at potential ATP-binding sites inactivate the FhuC protein. Mol Gen Genet. 1990;223:159–162. doi: 10.1007/BF00315810. [DOI] [PubMed] [Google Scholar]
- 2.Braun V, Gross R, Koster W, Zimmermann L. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol Gen Genet. 1983;192:131–139. doi: 10.1007/BF00327658. [DOI] [PubMed] [Google Scholar]
- 3.Braun V, Hantke K, Köster W. Bacterial iron transport: mechanisms, genetics, and regulation. Metal Ions in Biol Syst. 1998;35:67–145. [PubMed] [Google Scholar]
- 4.Brock J H, Ng J. The effect of desferrioxamine on the growth of Staphylococcus aureus, Yersinia enterocolitica, and Streptococcus faecalis in human serum. Uptake of desferrioxamine-bound iron. FEMS Microbiol Lett. 1983;20:439–442. [Google Scholar]
- 5.Bullen J J, Griffiths E. Iron and infection: molecular, physiological and clinical aspects. 2nd ed. New York, N.Y: John Wiley and Sons; 1999. [Google Scholar]
- 6.Camilli A, Portnoy D A, Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990;172:3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakraborty T, Leimeister-Wächter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockayne A, Hill P J, Powell N B L, Bishop K, Sims C, Williams P. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun. 1998;66:3767–3774. doi: 10.1128/iai.66.8.3767-3774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courcol R J, Trivier D, Bissinger M-C, Martin G R, Brown M R W. Siderophore production by Staphylococcus aureus and identification of iron-regulated proteins. Infect Immun. 1997;65:1944–1948. doi: 10.1128/iai.65.5.1944-1948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox C D. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982;36:17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosa J H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980;284:566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- 12.Crossley K B, Archer G L. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. [Google Scholar]
- 13.Davis B A, Porter J B. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood. 2000;95:1229–1236. [PubMed] [Google Scholar]
- 14.Dreschel H, Freund S, Nicholson G, Haag H, Jung O, Zähner H, Jung G. Purification and chemical characterization of staphyloferrin B, a hydrophilic siderophore from staphylococci. BioMetals. 1993;6:185–192. doi: 10.1007/BF00205858. [DOI] [PubMed] [Google Scholar]
- 15.Dyer D W, McKenna W, Woods J P, Sparling P F. Isolation by streptonigrin enrichment and characterization of a transferrin-specific iron uptake mutant of Neisseria meningitidis. Microb Pathog. 1987;3:351–363. doi: 10.1016/0882-4010(87)90005-2. [DOI] [PubMed] [Google Scholar]
- 16.Enard C, Diolez A, Expert D. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J Bacteriol. 1988;170:2419–2426. doi: 10.1128/jb.170.6.2419-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths E, Williams P. The iron-uptake systems of pathogenic bacteria, fungi and protozoa. In: Bullen J J, Griffiths E, editors. Iron and infection. 2nd ed. New York, N.Y: John Wiley and Sons, Ltd.; 1999. pp. 87–212. [Google Scholar]
- 18.Groeger W, Köster W. Transmembrane topology of the two FhuB domains representing the hydrophobic components of bacterial ABC transporters involved in the uptake of siderophores, haem and vitamin B12. Microbiology. 1998;144:2759–2769. doi: 10.1099/00221287-144-10-2759. [DOI] [PubMed] [Google Scholar]
- 19.Guérout-Fleury A M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–336. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 20.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hager P W, Rabinowitz J C. Translational specificity in Bacillus subtilis. In: Dubnau D A, editor. The molecular biology of the bacilli. II. New York, N.Y: Academic Press, Inc.; 1985. pp. 1–32. [Google Scholar]
- 22.Hantke K. Identification of an iron uptake system specific for coprogen and rhodotorulic acid in Escherichia coli K-12. Mol Gen Genet. 1983;191:301–306. doi: 10.1007/BF00334830. [DOI] [PubMed] [Google Scholar]
- 23.Heesemann J, Hantke K, Vocke T, Saken E, Rakin A, Stojiljkovic S, Berner R. Virulence of Yersinia enterocolitica is closely associated with siderophore production, expression of an iron-repressible outer membrane polypeptide of 65,000 Da and pesticin sensitivity. Mol Microbiol. 1993;8:397–408. doi: 10.1111/j.1365-2958.1993.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 24.Heinrichs D E, Monteiro M A, Perry M B, Whitfield C. The assembly system for the lipopolysaccharide R2 core-type of Escherichia coli is a hybrid of those found in Escherichia coli K-12 and Salmonella enterica. Structure and function of the R2 WaaK and WaaL homologs. J Biol Chem. 1998;273:8849–8859. doi: 10.1074/jbc.273.15.8849. [DOI] [PubMed] [Google Scholar]
- 25.Heinrichs D E, Young L, Poole K. Pyochelin-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. Infect Immun. 1991;59:3680–3684. doi: 10.1128/iai.59.10.3680-3684.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinrichs J H, Gatlin L E, Kunsch C, Choi G H, Hanson M S. Identification and characterization of SirA, an iron-regulated protein from Staphylococcus aureus. J Bacteriol. 1999;181:1436–1443. doi: 10.1128/jb.181.5.1436-1443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland J, Towner K J, Williams P. Isolation and characterisation of Haemophilus influenzae type b mutants defective in transferrin-binding and iron assimilation. FEMS Microbiol Lett. 1991;61:283–287. doi: 10.1016/0378-1097(91)90566-s. [DOI] [PubMed] [Google Scholar]
- 28.Jones R L, Peterson C M, Grady R W, Kumbaraci T, Cerami A, Graziano J H. Effects of iron chelators and iron overload on Salmonella infection. Nature. 1977;267:63–65. doi: 10.1038/267063a0. [DOI] [PubMed] [Google Scholar]
- 29.Khimji P L, Miles A A. Microbial iron-chelators and their action on Klebsiella infections in the skin of guinea-pigs. Br J Exp Pathol. 1978;59:137–147. [PMC free article] [PubMed] [Google Scholar]
- 30.Konetschny-Rapp S, Jung G, Meiwes J, Zähner H. Staphyloferrin A: a structurally new siderophore from staphylococci. Eur J Biochem. 1990;191:65–74. doi: 10.1111/j.1432-1033.1990.tb19094.x. [DOI] [PubMed] [Google Scholar]
- 31.Köster W, Böhm B. Point mutations in two conserved glycine residues within the integral membrane protein FhuB affect iron(III) hydroxamate transport. Mol Gen Genet. 1992;232:399–407. doi: 10.1007/BF00266243. [DOI] [PubMed] [Google Scholar]
- 32.Köster W, Braun V. Iron hydroxamate transport of Escherichia coli: nucleotide sequence of the fhuB gene and identification of the protein. Mol Gen Genet. 1986;204:435–442. doi: 10.1007/BF00331021. [DOI] [PubMed] [Google Scholar]
- 33.Kraemer G R, Iandolo J J. High-frequency transformation of Staphylococcus aureus by electroporation. Curr Microbiol. 1990;21:373–376. [Google Scholar]
- 34.Kreiswirth B N, Lofdahl S, Bentley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:680–685. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay J A, Riley T V. Staphylococcal iron requirements, siderophore production, and iron-regulated protein expression. Infect Immun. 1994;62:2309–2314. doi: 10.1128/iai.62.6.2309-2314.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meiwes J, Fiedler H-P, Haag H, Zähner H, Konetschny-Rapp S, Jung G. Isolation and characterization of staphyloferrin A, a compound with siderophore activity from Staphylococcus hyicus DSM 20459. FEMS Microbiol Lett. 1990;67:201–206. doi: 10.1111/j.1574-6968.1990.tb13863.x. [DOI] [PubMed] [Google Scholar]
- 37.Meyer J M, Hohnadel D, Halle F. Cepabactin from Pseudomonas cepacia, a new type of siderophore. J Gen Microbiol. 1989;135:1479–1487. doi: 10.1099/00221287-135-6-1479. [DOI] [PubMed] [Google Scholar]
- 38.Meyer J M, Neely A, Stintzi A, Georges C, Holder I A. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modun B, Kendall D, Williams P. Staphylococci express a receptor for human transferrin: identification of a 42-kilodalton cell wall transferrin-binding protein. Infect Immun. 1994;62:3850–3858. doi: 10.1128/iai.62.9.3850-3858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modun B, Williams P. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. 1999;67:1086–1092. doi: 10.1128/iai.67.3.1086-1092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moszer I, Rocha E P, Danchin A. Codon usage and lateral gene transfer in Bacillus subtilis. Curr Opin Microbiol. 1999;2:524–528. doi: 10.1016/s1369-5274(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 42.Nikaido H, Hall J A. Overview of bacterial ABC transporters. Methods Enzymol. 1998;292:3–20. doi: 10.1016/s0076-6879(98)92003-1. [DOI] [PubMed] [Google Scholar]
- 43.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 44.Payne S M. Detection, isolation, and characterization of siderophores. Methods Enzymol. 1994;235:329–344. doi: 10.1016/0076-6879(94)35151-1. [DOI] [PubMed] [Google Scholar]
- 45.Peng H L, Novick R P, Kreiswirth B, Kornblum J, Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988;170:4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole K, Dean C, Heinrichs D E, Neshat S, Krebes K, Young L, Kilburn L. Siderophore-mediated iron transport in Pseudomonas aeruginosa. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C.: American Society for Microbiology; 1996. pp. 371–383. [Google Scholar]
- 47.Poole K, Neshat S, Heinrichs D. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol Lett. 1991;78:1–6. [PubMed] [Google Scholar]
- 48.Rogers H J. Iron-binding catechols and virulence in Escherichia coli. Infect Immun. 1973;7:445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Schneider R, Hantke K. Iron-hydroxamate uptake systems in Bacillus subtilis: identification of a lipoprotein as part of a binding protein-dependent transport system. Mol Microbiol. 1993;8:111–121. doi: 10.1111/j.1365-2958.1993.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 51.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 52.Shea C M, McIntosh M A. Nucleotide sequence and genetic organization of the ferric enterobactin transport system: homology to other periplasmic binding protein-dependent systems in Escherichia coli. Mol Microbiol. 1991;5:1415–1428. doi: 10.1111/j.1365-2958.1991.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 53.Simon E H, Tessman I. Thymidine-requiring mutants of phage T4. Proc Natl Acad Sci USA. 1963;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Staudenmaier H, van Hove B, Yaraghi Z, Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989;171:2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stojiljkovic I, Bäumler A J, Hantke K. Fur regulation in Gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 56.Valvano M A, Crosa J H. Aerobactin iron transport genes commonly encoded by certain ColV plasmids occur in the chromosome of a human invasive strain of Escherichia coli K1. Infect Immun. 1984;46:159–167. doi: 10.1128/iai.46.1.159-167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vellanoweth R L. Translation and its regulation. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 699–711. [Google Scholar]
- 58.Wada A, Watanabe H. Penicillin-binding protein 1 of Staphylococcus aureus is essential for growth. J Bacteriol. 1998;180:2759–2765. doi: 10.1128/jb.180.10.2759-2765.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward C G, Bullen J J. Clinical and physiological aspects. In: Bullen J J, Griffiths E, editors. Iron and infection. 2nd ed. New York, N.Y: John Wiley & Sons, Ltd.; 1999. pp. 369–450. [Google Scholar]
- 60.Watson S P, Antonio M, Foster S J. Isolation and characterization of Staphylococcus aureus starvation-induced, stationary-phase mutants defective in survival or recovery. Microbiology. 1998;144:3159–3169. doi: 10.1099/00221287-144-11-3159. [DOI] [PubMed] [Google Scholar]
- 61.White J R, Yeowell H N. Iron enhances the bactericidal action of streptonigrin. Biochem Biophys Res Commun. 1982;106:407–411. doi: 10.1016/0006-291x(82)91125-1. [DOI] [PubMed] [Google Scholar]
- 62.Williams P H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979;26:925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiong A, Singh V K, Cabrera G, Jayaswal R K. Molecular characterization of the ferric-uptake regulator, Fur, from Staphylococcus aureus. Microbiology. 2000;146:659–668. doi: 10.1099/00221287-146-3-659. [DOI] [PubMed] [Google Scholar]
- 64.Yeowell H N, White J R. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982;22:961–968. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]