Abstract
Extracellular vesicles (EV), which include exosomes and microvesicles, are secreted from virtually every cell. EV contain mRNA, miRNA, lipids and proteins and can deliver this expansive cargo into nearby cells as well as over long distances via the blood stream. Great interest has been given to them for their role in cell to cell communication, disease progression, or as biomarkers, and more recent studies have interrogated their potential as a therapeutic that may replace paracrine-acting cell therapies. The retina is a conveniently accessible component of the central nervous system and the proposed paradigm for the testing of many cell therapies. Recently, several studies have been published demonstrating that the delivery of EV/exosomes into the eye can elicit significant therapeutic effects in several models of retinal disease. We summarize results from currently available studies, demonstrating their efficacy in multiple eye disease models as well as highlighting where future research efforts should be directed.
Keywords: Exosomes, Extracellular vesicles, Retina, Mesenchymal stem cells, Glaucoma, Optic nerve crush
1. Introduction
The retina, due to its diencephalic origin, is part of the central nervous system (CNS) and converts photons into an electrochemical signal in a process known as phototransduction, allowing organisms to see. As typical with the CNS, damage, which can arise through a variety of traumatic and degenerative reasons, is permanent alongside the subsequent visual loss (Berry et al., 2008, 2019). Retinal diseases have multiple (non-mutually exclusive) theories explaining their cause and progression, owing to their complicated and multifactorial nature. It can be argued that a successful therapy must consider these multiple mechanisms rather than focusing on one pathway or molecule. In the example of glaucomatous damage, the majority of studies and preclinical therapies however target just one particular mechanism or signaling pathway e.g. glutamate-mediated excitotoxicity, tumor necrosis factor-α (TNF-α)-mediated inflammation, neurotrophic factor (NTF) deprivation etc. Several combinatorial therapies have been devised to address the multifactorial nature of retinal disease, from the literal combination and delivery of multiple NTF (Logan et al., 2006) to cellular therapy, whereby transplanted mesenchymal/neural stem cells (MSC/NSC) secrete a combination of said NTF (Flachsbarth et al., 2018; Johnson et al., 2014; Lu et al., 2013; Mead et al., 2014, 2015; Mesentier-Louro et al., 2014) and anti/pro-inflammatory cytokines (Millan-Rivero et al., 2018; Uccelli et al., 2008). Our earlier studies demonstrated the potential for MSC (bone marrow- and dental pulpderived) transplantation in a model of glaucoma (Johnson et al., 2010a; Mead et al., 2016), with significant neuroprotection of degenerating retinal ganglion cells (RGC) observed. MSC remained in the vitreous with their therapeutic efficacy resulting from paracrine-mediated mechanisms (Fig. 1).
Fig. 1.

Retinal ganglion cell (RGC) counts in a rat model of glaucoma after mesenchymal stem cell (MSC) treatment. Glaucoma was modeled through intracameral injections of transforming growth factor-β for 35d. Treatments consisted of intravitreal transplantation of dental pulp stem cells (DPSC), bone marrow MSC (BMSC), adipose-derived stem cells (ADSC) and dead DPSC (sham-treated; A). Retinae were stained with the phenotypic RGC marker BRN3A (red) and the nuclear marker DAPI (blue; scale bar: 50 μm). In (B), GFP+ MSC stained for the MSC marker STRO1 are identified in the vitreous, adhering to the inner limiting membrane. In (C), the mean number of BRN3A+ RGC in a 1 mm region of retina either side of the optic nerve head is shown from each of the above groups. Note the significant neuroprotective effect elicited by the transplanted MSC. Black lines indicate significant difference between groups (p < 0.01). Modified Fig. 4 from Mead et al. (2016), re-used under the Creative Commons Attribution 4.0 International (CCBY4.0) licence.
However, another secreted product of MSC that is suggested to mediate the paracrine benefit has recently gained a large amount of research interest. Known as extracellular vesicles (EV), their strong therapeutic potential derives from their expansive cargo and ability to deliver said cargo into cells and act on multiple signaling pathways (Kalluri and LeBleu, 2020). EV are becoming established signaling mediators between cells, including in the eye (Reviewed in Klingeborn et al., 2017a), but only recently are they gaining traction as a candidate treatment for ocular disease. This review discusses current progress in utilizing EV as a therapy for retinal diseases.
2. Extracellular vesicles
EV is the collective term for secreted vesicles and includes exosomes, microvesicles, and apoptotic bodies. They have distinct biogenesis pathways (Fig. 2) and are often distinguished by their size, internal cargo, and surface proteins.
Fig. 2.

Schematic diagram detailing exosomal treatment of the retina. Exosomes and microvesicles are isolated through ultracentrifugation of culture medium, conditioned by the proposed cell source. Lower speeds of centrifugation can be used in protocols that utilize polyethylene glycol while other techniques such as passing through a sucrose gradient are employed to further specify the vesicle size obtained. To partially-purify the 30–150 nm exosomes from the 100–1000 nm microvesicles, passage through a 0.22 μm filter is utilized. Following purification, exosome identity can be confirmed with Nanoparticle Tracking Analysis and Western blot before injection into the eye (vitreous or subretinal).
Exosomes form via the fusion of multivesicular bodies (an intracellular vesicular structure derived from endosomes) with the cell membrane and their subsequent release into the extracellular space (Mathieu et al., 2019; Thery et al., 2006). Microvesicles are instead formed due to outward budding of the plasma membrane. Exosomes are typically 30–150 nm whereas microvesicles are 100–1000 nm, although the exact values vary greatly between studies. Analysis of their size can be done using electron microscopy (Osteikoetxea et al., 2015) or a nanoparticle tracking analysis instrument (Fig. 3). The third class of EV, apoptotic bodies, are >1000 nm and are released through the membrane blebbing of cells undergoing apoptosis (Battistelli and Falcieri, 2020; Caruso and Poon, 2018; Jiang et al., 2017), although smaller EV from apoptotic cells (termed apoptotic microvesicles) have also been suggested. Apoptotic bodies have so far not seen any therapeutic use in the eye and appear to mainly function as signals to recruit macrophages to aid in cell debris clearing, as well as antigen presentation (Caruso and Poon, 2018). They do however contain miRNA and proteins as well as represent a heterogeneous population of subtypes and further study into their therapeutic and/or deleterious effects should be explored. In contrast, exosomes have shown remarkable therapeutic potential in many diseases throughout the body (Reviewed in Keshtkar et al., 2018) including Alzheimer’s disease (de Godoy et al., 2018), spinal cord injury (Sun et al., 2018; Wang et al., 2018a) and stroke (Xin et al., 2013, 2017), amongst others.
Fig. 3.

Electron microscopy images of exosomes before and after filtration through a 0.22 μm filter along with corresponding Nanosight/Nanoparticle Tracking Analysis of quantity and size. Modified Fig. 2 from Mead et al. (2018) and Mead et al., 2017, re-used under the Creative Commons Attribution 4.0 International (CCBY4.0) licence. The figure inset shows a higher quality electron microscopy image of an exosome (EXO), microvesicle (MV), and apoptotic body (APO). Reused from Osteikoetxea et al. (2015) with permission under the Creative Commons Attribution 4.0 International (CCBY4.0) licence.
Despite these differing subpopulations of EV, much overlap exists in the literature, with the term “exosome” used interchangeably with “extracellular vesicle”. Interestingly, “exosome” has been referenced more often than “extracellular vesicle” in published manuscripts over the last several decades, reflecting its popularity, yet this gap has narrowed significantly in 2019, perhaps reflecting research groups new to the field adopting the correct terminology (Fig. 4). The Minimum Information for Studies of Extracellular Vesicles (MISEV2018) (Théry and Witwer, 2018) are a recent published set of guidelines that, in summary, state that the term “EV” should be used exclusively unless they are confirmed to originate from the exosome biogenesis pathway (Fig. 2). While we agree with the above guidelines, given that we are reviewing past and present literature that has yet to take these into consideration, certain concessions were made. Thus, while many studies refer to their preparations as “exosomes” as opposed to EV, we will refer to them as EV unless they fulfill the definition of exosomes detailed recently (Klingeborn et al., 2017a) which are 30–150 nm vesicles loaded with at least some of the exosomal proteins CD63, CD9, CD81, syntenin-1, and TSG101. We also add a further requirement to the definition that is often included in most recent studies which is the use of a 0.22 μm filter, removing contaminating microvesicles (albeit at the cost of a reduced overall yield) that are often isolated with exosomes in most techniques such as ultracentrifugation or polyethylene glycol precipitation (Konoshenko et al., 2018; Ma et al., 2020; Mead and Tomarev, 2017; Pan et al., 2019). Isolation of exosomes with more intricate techniques such as sucrose gradients, relying on their buoyant density of about 1.10–1.19 g/ml, are also employed but this is largely restricted to specialized studies into vesicle mechanics and not therapeutic assessment (Shurtleff et al., 2016).
Fig. 4.
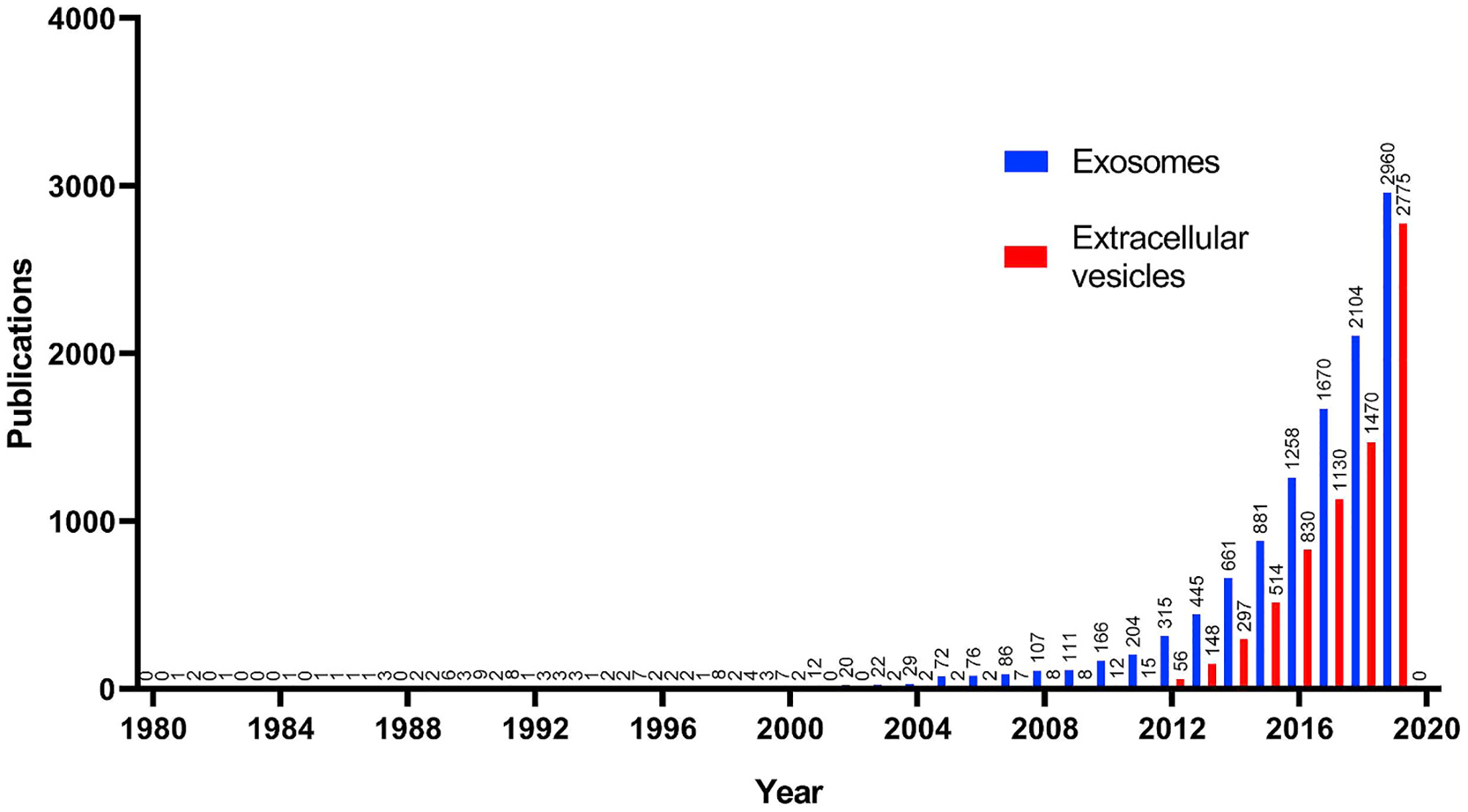
Publications with the keyword “exosome” or “extracellular vesicle” in the abstract/title from Jan 1st, 1980–Jan 1st, 2020. Note the exponential rise in publications referencing exosomes along with the historical popularity of “exosome” over “extracellular vesicle”, with the gap narrowing significantly in 2019.
A key detail regarding EV and the reason for their great research interest is that their cargo is expansive, containing proteins, mRNA, miRNA, and lipids. Furthermore, following secretion from a cell, these vesicles act as mediators of cell signaling, delivering their cargo into recipient cells and in the case of mRNA/miRNA, lead to the translation of new proteins/modulation of gene expression, respectively (Ratajczak et al., 2006; Skog et al., 2008; Valadi et al., 2007). By delivering multiple proteins, mRNA and miRNA (all of which target multiple different mRNA), EV are intrinsically a multifactorial treatment.
While the different types of EV are distinct, another variable that defines them is the source that the EV is secreted from. For example, we recently sequenced miRNA of exosomes from human BMSC and fibroblasts and identified over 40 candidates that were distinct between the two samples (Mead et al., 2018b). Several studies have been published detailing the miRNA within exosomes from multiple MSC types (Table 1). While many of the most abundant miRNA are consistent between studies, it is apparent that some variability even from the same cell type exists, demonstrating the heterogeneity of MSC cultures as well as the variability between miRNA library construction/analysis methodologies. Regarding the proteome and lipidome, distinct differences have been observed between exosomes isolated for U87 glioblastoma cells, Huh7 hepatocellular carcinoma cells, and BMSC (Haraszti et al., 2016). Exosomes from distinct retinal cells such as retinal astrocytes (Hajrasouliha et al., 2013), retinal pigment epithelium (RPE) cells (Klingeborn et al., 2017b), and retinal progenitor cells (Zhou et al., 2018) also contain distinct proteomes and this is reflected in their therapeutic efficacy (discussed below). It has equally been shown that cells secrete exosomes with variable cargo depending on the stage of differentiation they are in. For example, osteogenic differentiation of MSC leads to exosome secretion with extracellular matrix mineralization properties, but only in the late, not early phase of differentiation (Wang et al., 2018b). Even cell passage has been shown to have an effect on the neuroprotective efficacy of MSC exosomes, with it diminishing with increasing cell passage of the source (Venugopal et al., 2017). With the cargo of EV varying depending on their cellular origin, it could be thus assumed that EV cargo is just a sample of the cellular cytoplasm. However, proteomic analysis of adipose-derived MSC and EV demonstrated that over 100 proteins were more abundant in EV in comparison to the cell, suggesting that the loading of EV involves active and specific trafficking (Eirin et al., 2016). Interestingly, when comparing exosomes to microvesicles, it appears that this specific trafficking mechanism is more evident in exosomes as their protein population is more distinct from the host cell’s than the protein population of microvesicles (Haraszti et al., 2016). Comparison of mRNA, miRNA, and transfer RNA abundance between adipose-derived MSC/T cells and their EV leads to similar conclusions, select mRNA and miRNA enriched in EV in comparison to the cell (Chiou et al., 2018; Eirin et al., 2014). Likewise, exosomes isolated from HEK293 cells contain selectively packaged miRNAs compared with HEK293 cells and it was found that the RNA-binding Y-box protein (YBX1) binds to and is required for the sorting of certain miRNAs (mir-223) in exosomes (Shurtleff et al., 2016).
Table 1.
The ten most abundant miRNA in exosomes isolated from human mesenchymal stem cells (bone marrow-derived mesenchymal stem cells, BMSC; umbilical cord blood-derived mesenchymal stem cells, UCMSC; adipose-derived stem cells, ADSC).
| Study | Mead et al. (2018b) | Ferguson et al. (2018) | Baglio et al. (2015) | Wang et al. (2018) | Sun et al. (2017) | Qian et al. (2016) | Fang et al. (2016) | Baglio et al. (2015) |
|---|---|---|---|---|---|---|---|---|
| Source of exosomes (human) | BMSC | BMSC | BMSC | BMSC | UCMSC | UCMSC | UCMSC | ADSC |
| Ten most abundant miRNA | miR-221-3p | miR-1246 | miR-143-3p | miR-21-5p | miR-125b-5p | miR-21 | miR-21-5p | miR-486-5p |
| let-7a-5p | miR-23a-3p | miR-10b-5p | miR-125b-5p | miR-21-5p | miR-125b | miR-125b-5p | miR-10a-5p | |
| miR-21-5p | miR-451a | miR-486-5p | miR-221-3p | miR-24-5p | miR-23a | miR-23a-3p | miR-10b-5p | |
| miR-320a | miR-125b-5p | miR-22-3p | miR-16-5p | miR-16-5p | miR-100 | miR-100-5p | miR-191-5p | |
| miR-486-5p | miR-199a/b-3p | miR-21-5p | let-7a-5p | miR-92a-3p | let-7f-5p | miR-145-5p | miR-222-3p | |
| miR-423-5p | let-7a-5p | miR-222-3p | miR-23a-3p | miR-100-5p | let-7a-5p | let-7f-5p | miR-22-3p | |
| miR-21-5p | miR-4454/7975 | miR-191-5p | miR-100-5p | miR-106a-5p | miR-145 | let-7a-5p | let-7a-5p | |
| miR-1246 | miR-21-5p | miR-100-5p | miR-142-3p | miR-19b-3p | miR-1260b | miR-1260a | miR-21-5p | |
| miR-122-5p | let-7b-5p | let-7a-5p | miR-222-3p | miR-145-5p | miR-1260a | miR-1260b | miR-127-3p | |
| miR-92a-3p | miR-100-5p | miR-99b-5p | miR-24-3p | miR-25-3p | miR-199a | miR-199a-3p | miR-143-3p |
Along with their cargo, the number of exosomes released by cells can also vary greatly. In a comparison of several cell types including myoblasts and HEK cells, MSC secreted a significantly greater number (>10-fold) of exosomes (Yeo et al., 2013). Finally, along with the differences between the three types of EV, within each type they may be further divided into subtypes. Exosomal surface proteins can be analyzed using flow cytometry and antibody-bead conjugates which, although cannot quantify exosomes due to an inability in knowing how many exosomes have bound to each bead, does demonstrate considerable differences between sub populations that possess or lack exosomal proteins such as CD63 (Mead and Tomarev, 2017). To analyse these subtypes, more homogeneous population of exosomes are obtained through the inclusion of additional purification steps such as a flotation of exosomes to an interface between 20 and 40% sucrose and immunoprecipitation with CD63 antibody-immobilized beads (Shurtleff et al., 2016) or high-resolution iodixanol density gradient fractionation (Jeppesen et al., 2019). A distinct difference in the RNA cargo between the “high density” and “low density” exosomes (Jeppesen et al., 2019; Shurtleff et al., 2016) and distinct functional differences between them (Willms et al., 2016) has been reported, yet it remains to be seen if these distinctions are relevant when it comes to developing potential therapies for the eye. These additional purification and subtype separation techniques are not typically employed in research outside exosome-focused research groups and thus their therapeutic and biological relevance is largely unexplored.
One considerable benefit EV offer over cellular therapy as a treatment is their storage properties (Kusuma et al., 2018). EV RNA quality showed little to no deterioration after storage for 5 years at −20 °C in comparison to fresh EV while storage at 4 °C for 2 weeks led to significant degradation in some RNA (Ge et al., 2014). This can be partly explained by their bi-lipid membranes, which protects the cargo from enzymatic/chemical degradation. Another benefit is the safety in comparison to injections of dividing/differentiating cells into the eye. A recent report detailed three patients who received intravitreal injections of adipose-derived MSC as a treatment for age-related macular degeneration (AMD). Unfortunately, these patients subsequently went blind due to a variety of complications associated with the stem cell transplant including retinal detachment and hemorrhage (Kuriyan et al., 2017). The formation of a monolayer of cells on the inner limiting membrane of the retina is heavily involved in the pathology of retinal detachment and proliferative vitreoretinopathy (Yang et al., 2015) and these cells originate from the epithelial-mesenchymal transition of RPE cells. Given that transplanted MSC adhere and cluster to the inner limiting membrane (Mead et al., 2016), it is possible that these transplanted cells formed epiretinal membranes as observed in one patient receiving MSC (Kim et al., 2017). A more recent study transplanting MSC into the vitreous of rats also demonstrated significant vascular damage alongside glial activation and an inflammatory response (Huang et al., 2019). Cell therapy is also compounded by an unknown division rate and rate of death after transplantation, meaning a known number of cells quickly becomes unknown after administration. While the above does not mean cellular therapy is unfeasible, these complications are avoided by purifying the active secreted compound, believed to be EV (as well as neurotrophic proteins), and administering this in the place of the cells. It can be argued that EV therapy is more controlled regarding its dose since there is no risk of division occurring post-transplantation. However, both cells and EV share a dosing problem that is intrinsic to their role as deliverers of a multifactorial cargo. Given that this cargo varies between passages (Mead et al., 2014) and donors (Table 1), using the same number of cells/EV does not guarantee that the therapeutic cargo is being correctly dosed. Finally, a large disadvantage with retinal cell therapy is the lack of integration of transplanted cells into the retina (Emre et al., 2015; Johnson et al., 2010a; Mead et al., 2013) unless further measures are taken such as digestion of the inner limiting membrane and modulation of retinal glial activity (Johnson et al., 2010b), which may itself damage the retina. EV therapy avoids this complication and can pass through the inner limiting membrane with ease (Mead and Tomarev, 2017).
3. Retinal disease
EV are a strong candidate as a cell free therapy and below we discuss current evidence for their use in various diseases affecting the retina.
3.1. Optic nerve crush
Optic nerve crush is a model of traumatic optic neuropathy, a severe acute condition in which the delicate optic nerve, on its path from the retina to the lateral geniculate nucleus/superior colliculus, is physically injured. Crushing of the optic nerve in mice and rats leads to a 50% loss of RGC by 7 days and 90% loss by 14 days (Berkelaar et al., 1994; Leung et al., 2008; Rodriguez et al., 2014). Not only is it characterized by the selective loss of RGC but also the Wallerian degeneration of RGC axons that fail to regenerate (Berry et al., 2008). Finally, the optic nerve crush model appears to selectively kill certain RGC subtypes while largely preserving others, and in particular, α-RGC and melanopsin-expressing M1-RGC demonstrating robust survival in comparison to other RGC subtypes (Duan et al., 2015; Tran et al., 2019).
Recently we transplanted exosomes derived from BMSC into the vitreous of rats after optic nerve crush (Mead and Tomarev, 2017). Exosomes delivered their cargo into RGC, as shown by preloading the exosomes with a fluorescent marker, and provided significant neuroprotection and functional preservation, whereas long-distance axon regeneration was not observed. Fibroblast exosomes, which were used as control exosomes, provided no therapeutic effects. BMSC exosomes also preserved RGC function by over 50%, as measured by electroretinography. Since preventing RGC death does not inherently mean a prevention of RGC dysfunction (Fry et al., 2018), this result suggests exosomes work through multiple pathways to not only protect RGC but also distinctly preserve their function. Interestingly, exosomes appeared to be the therapeutically efficacious EV whereas microvesicles were not and were even toxic to RGC at higher concentrations (Mead and Tomarev, 2017) (Fig. 5b), an observation seen also in a retinal ischemic model (discussed below, van der Merwe et al., 2019) as well as in cortical neuron cultures (Lopez-Verrilli et al., 2016). The mechanism of action was determined to be, at least partially, due to the miRNA evident by the ablation of therapeutic efficacy if argonaute-2 (AGO2) is knocked down in BMSC. AGO2 is a protein that forms part of the miRNA complex and is necessary for their ability to inhibit mRNA translation. Knocking down AGO2 in cells prior to EV isolation leads to EV lacking in mature miRNA (Lv et al., 2014; Zhang et al., 2016). We can speculate that differences between the cargo packaged in exosomes and microvesicles (e.g. proteins and/or RNA) is the reason for their opposing effects on neurons but further investigations into the mechanism of action are needed before this can be corroborated.
Fig. 5.
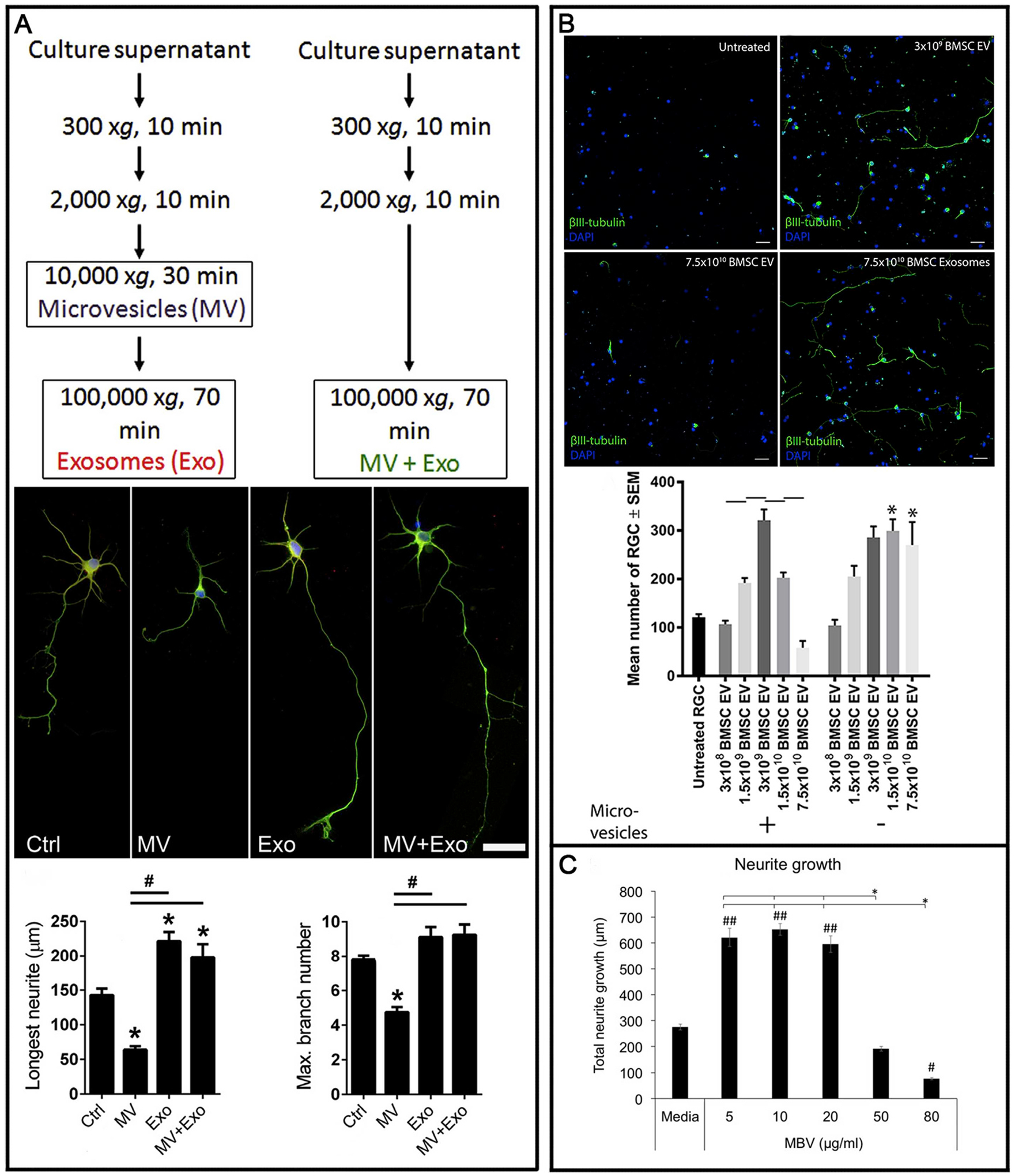
Differential effects of exosomes and microvesicles on retinal ganglion cells (RGC)/neurons. In three separate studies, one in cortical neurons (A) and 2 in RGC (B/C), exosomes demonstrated a neuritogenic/neuroprotective effect with microvesicles exerting the opposite. The first study (A) showed that exosomes were neuritogenic whereas the effect of microvesicles was worse than untreated controls. The second (B) demonstrated the efficacy of extracellular vesicles diminished at higher doses and this was due to the contamination of microvesicles. A third study (C) showed the same but did not confirm the effect was due to contaminating microvesicles. Modified Fig. 3 from Loppez-Verrilli et al. (2016) (A), Fig. 3 from Mead et al. (2017) (B), and Fig. 1 from van der Merwe et al. (2019), re-used under the Creative Commons Attribution 4.0 International (CCBY4.0) licence.
Further studies by Pan and coauthors transplanted exosomes derived from umbilical cord MSC into the vitreous of rats after optic nerve crush (Pan et al., 2019). As we had previously defined, by removing microvesicles through the use of a 0.22 μm filter, significant RGC neuroprotection was achieved and similarly, RGC axon regeneration was not. Authors also demonstrated significant glial activation. Interestingly the effect was not as significant as seen with BMSC exosomes and authors reasoned that this is due to the reported differences in exosomal miRNA between those isolated from BMSC (Baglio et al., 2015; Mead et al., 2018b) and UMSC (Fang et al., 2016).
A separate study utilized exosomes isolated from L-cells, a fibroblast cell line (Tassew et al., 2017). It is worth noting that in this study, authors did not filter their EV or fully define their preparation. Thus, their preparation is more accurately referred to as EV, a mixture of exosomes and microvesicles. Authors did not observe any significant neuroprotection of RGC but interestingly, observed significant regeneration of RGC axons. The mechanism of action appears to be due to the recruitment of Wnt10b to lipid rafts and subsequent activation of the axogenic mTOR pathway via GSK3β. This distinction between L cell exosomes/EV and MSC exosomes in the axogenic effect is likely due to a difference in their internal cargo. We recently sequenced L cell exosome miRNA and performed a comparison between them and BMSC/fibroblast exosomes. (Fig. 6). Their miRNA profile is shown with the majority distinct from that found in BMSC exosomes, although some similarities were observed (Fig. 6b). The difference in RGC neuroprotection observed could also be explained by the exosome isolation technique. Microvesicles were included in the authors preparation (i.e. not filtered out), and our observation of their toxicity on RGC (Mead and Tomarev, 2017) suggests L cell exosomes may indeed be neuroprotective but is obfuscated by microvesicle-induced RGC death.
Fig. 6.

miRNA in L cell exosomes. miRNAseq was performed on exosomes derived from L cells with those detected displayed (A) as mean estimated abundance (derived from the reads) ± standard error mean (SEM). Mouse L cell exosome miRNA that are homologues to their human miRNA counterpart were selected and compared to human bone marrow mesenchymal stem cell (BMSC) and dermal fibroblast exosome miRNA. Those miRNA also shown to be abundant in BMSC exosomes and L cell exosomes in comparison to fibroblast exosomes are displayed (B) as mean estimated abundance (derived from the reads) ± SEM. Comparative data for miRNA expression in BMSC exosomes/fibroblast exosomes is from a previous publication (Mead et al., 2018b).
In the above studies, BMSC (Mead and Tomarev, 2017) and umbilical cord MSC (Pan et al., 2019) exosomes both promoted neuroprotection without axon regeneration whereas L-cell exosomes (Tassew et al., 2017) did the opposite. This confirms the distinction between the pathways involved in neuroprotection and those for axon regeneration. It has been shown that Sox11 expression promotes axonal regeneration for some RGC subtypes yet for some subtypes promotes their death (Norsworthy et al., 2017). It is possible that despite the expansive cargo of MSC exosomes, they do not properly activate regeneration pathways which also include pten/socs3 modulation (Sun et al., 2011) and induction of neural activity (Lim et al., 2016). This may represent a benefit of cell therapy over EV as MSC have been demonstrated to reliably stimulate both regeneration and survival (Mesentier-Louro et al., 2014, 2019; Tan et al., 2015).
3.2. Glaucoma
Glaucoma bares some similarities to optic nerve crush in that it is also characterized by the selective death of RGC (Almasieh et al., 2012). In contrast, the death is a slow, progressive degeneration as opposed to acute loss and thus, is a more sinister condition. The principle risk factor is an elevation in intraocular pressure (IOP) which is believed to cause compression of the optic nerve at the lamina cribrosa. IOP is only a risk factor not a cause however, owing to the fact glaucoma can occur with normal IOP values (Coleman and Miglior, 2008). The mechanism by which RGC die in glaucoma is still not fully understood and studies demonstrate a myriad of processes responsible including NTF deprivation, excitotoxicity, inflammation, oxidative stress, and antero/retrograde axon transport dysfunction (Reviewed in Almasieh et al., 2012; Syc-Mazurek and Libby, 2019). For a treatment to be effective in preventing RGC death and dysfunction, it must be equally multifactorial to address these injury processes. Previous success has been found through transplantation of MSC (Fig. 1) which secrete a multitude of beneficial factors (Emre et al., 2015; Harrell et al., 2019; Johnson et al., 2010a; Mead et al., 2013; Mesentier-Louro et al., 2014, 2019).
We recently transplanted BMSC exosomes into the vitreous of three separate animal models of glaucoma: laser and microbead rat models (Mead et al., 2018b), and a genetic DBA/2J mouse model (Mead et al., 2018a). In all three models, BMSC exosomes promoted significant survival of RGC along with preventing their functional decline that is characteristic of glaucoma models. In the DBA/2J model, we also observed a protective effect on RGC axons. As with the optic nerve crush model, we used fibroblast exosomes as a negative control as they elicited no therapeutic effect in these three models of glaucoma. One interesting finding was that the efficacy of exosomes was maintained even when delivered on a monthly basis but failed to elicit neuroprotection if the treatment was delivered more infrequently. The DBA/2J mice are a 12-month model of glaucoma and exosomes were still efficacious over this time period.
The mechanism of action appeared to be, as before, due to the miRNA cargo they delivered into RGC. This was confirmed through AGO2 knockdown and the ablation of neuroprotection (Mead et al., 2018b). To determine which miRNA were responsible for these therapeutic effects, miRNAseq was performed, comparing miRNA in the efficacious MSC exosomes to the ineffective fibroblast exosomes. Previous studies have already profiled MSC EV/exosomes and mapped out the most abundant miRNA (Ferguson et al., 2018; Qian et al., 2016; Sun et al., 2017) (Table 1), and we identified 43 miRNA that were abundant in BMSC exosomes in comparison to fibroblast exosomes (Mead et al., 2018b). Given that miRNA target a great many different mRNA, it is difficult to determine which molecules and pathways are responsible for the therapeutic effects observed. Many of these targets are still only predictions with only a fraction tested and experimentally observed. Within these targets however, well known instigators of RGC death including the bcl2 family (Maes et al., 2017), tnf (Tezel, 2008), and pten/mtor (Morgan-Warren et al., 2016) exist and further study will determine to what extent exosome-derived miRNA is acting through these pathways.
This data suggests that exosomes may serve as a suitable neuroprotective strategy, both in glaucoma that is not amenable to IOP lowering therapies, or as an adjunctive treatment. Another important conclusion is that long-term exosome treatment could be developed that requires only a monthly injection, as is done with anti-vascular endothelial growth factor (VEGF) treatments for AMD. This is likely based on a combination of the stability of exosomes as well as miRNA whose stability is reported to be over several days (Bartel, 2018).
In an effort to determine if the therapeutic effects we and others have observed is also applicable to human retina, we tested exosomes in a human in vitro retinal culture (Sluch et al., 2015, 2017). Human embryonic stem cell lines were differentiated into retinal cells, which included RGC, and were injured using the microtubule poison colchicine (Mead et al., 2020). Delivery of BMSC-derived exosomes provided significant neuroprotection of human RGC (Fig. 7). While we would certainly not argue that this in vitro system models glaucoma, it does provide evidence that the efficacy we are seeing in animal models may indeed be translatable to the human condition. More studies are needed using human tissue to strengthen this argument.
Fig. 7.
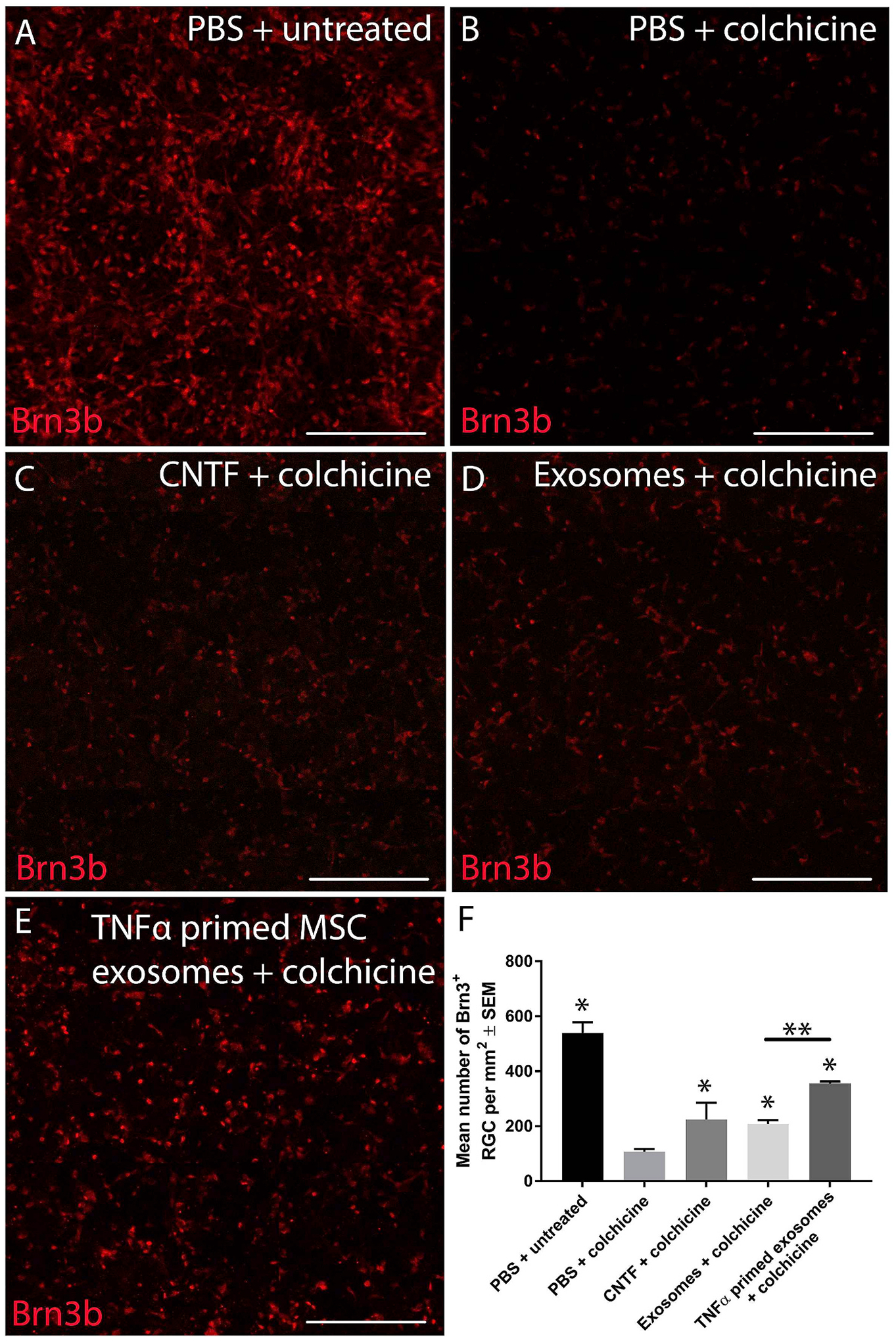
Exosome treatment of human retina. Heterogeneous retinal cultures were generated from a human embryonic stem cell line expressing a fluorescent marker under the brn3b (retinal ganglion cell (RGC) specific) promoter. To induce RGC degeneration, colchicine, a microtubule poison, was added to cultures (B) and led to significant RGC loss compared to uninjured controls (A). Ciliary neurotrophic factor (CNTF) led to significant neuroprotection of RGC (positive control, C), as did mesenchymal stem cell (MSC) exosomes (D), and tumor necrosis factor-α (TNF-α) primed MSC exosomes (E; scale bar: 250 μm). The quantified number of BRN3B+ RGC is shown in F. Fig. 2 from Mead et al. (2020) re-used under the Creative Commons Attribution 4.0 International (CCBY4.0) licence.
3.3. Retinal ischemia
Retinal ischemia, such as due to occlusion of the retinal artery or detachment of the retina, causes significant and irreversible damage. As with glaucoma, transplantation of MSC has shown efficacy at preventing retinal cell loss and dysfunction (Dreixler et al., 2014) and also, as with glaucoma, exosomes isolated from BMSC were able to recapitulate the effects of BMSC when transplanted into the vitreous of retinal ischemic mice, induced by hyperoxic conditioning (Moisseiev et al., 2017). These therapeutic effects included a significant reduction in retinal thinning and neovascularization and were present 14 days after the treatment. The ability of exosomes to prevent neovascularization is also seen in the choroid following delivery of retinal astrocyte-derived exosomes but is not seen when using RPE-derived exosomes (Hajrasouliha et al., 2013), again demonstrating the importance of the exosome source.
A more recent study utilized a brief elevation in IOP (15–150 mmHg for 60 min) to induce retinal ischemia in rats (van der Merwe et al., 2019). EV were isolated from bioscaffolds and in particular, decellularized porcine urinary bladder matrix. These EV, known as matrix bound nanovesicles, are similar to exosomes in that they are lipid membrane bound, containing protein and RNA, although their exact cargo profile may differ. The characterization of these matrix bound nanovesicles, including size or RNA/protein abundance was however not shown and thus it is unknown if these matrix bound nanovesicles are indeed just EV that have become associated with the scaffold following secretion. Evidence for this is shown when RGC in cultures are treated with membrane bound nanovesicles, which promoted neuritogenesis with increasing dosage, but a bi-phasic effect was observed with the neuritogenic effect dissipating at very high doses (Fig. 5c). This observation mirrored what we observed whereby MSC EV promoted neuritogenesis of RGC in a bi-phasic dose responsive manner (Mead and Tomarev, 2017). We had confirmed that this negative effect at increasing doses was due to microvesicles, and their removal from the EV sample, leaving just exosomes, ablated the bi-phasic dose response effect (Fig. 5b). Lopez-Verrilli and coauthors (Fig. 5a) also demonstrated a similar effect on cortical neurons with exosomes eliciting neuritogenesis while microvesicles did not (Lopez-Verrilli et al., 2016). Thus, it is possible microvesicles were present in the authors preparation. Despite this, authors demonstrated that EV treatment prevented microglia/astrocyte activation-induced release of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and TNF-α, significantly reducing subsequent RGC degeneration in vitro and in vivo (van der Merwe et al., 2019). Finally, authors also demonstrated that the intravitreal delivery of these EV reduced loss of cholera toxin b-subunit+ RGC axons as well as dysfunction in RGC, as measured by the photopic negative response.
Retinal ischemia can also occur when the retina becomes detached from the choroid, from which it depends on for its blood supply. In a rat model of retinal detachment, injection of BMSC-derived exosomes reduced the expression of pro inflammatory cytokines such as TNF-α while upregulating autophagy (Ma et al., 2020). Authors demonstrated a subsequent neuroprotective effect on photoreceptors, reducing cell loss despite the detached retina. While a mechanism of action was not deduced, authors did note the abundance of exosomal proteins with neuroprotective and anti-inflammatory properties.
3.4. Retinal laser injury
A separate model of retinal injury utilizes a laser, not to burn the outflow pathways like in glaucoma but to directly burn the retina. Several laser burn spots are delivered to the retina, which initiates indiscriminate rather than specific cellular degeneration alongside inflammation.
Delivery of MSC EV (unfiltered exosomes) into cultures of retinal cells after heat induced injury, or into the vitreous of mice after laser injury provided significant neuroprotection of retinal cells to the same efficacy as the MSC themselves (Yu et al., 2016). Along with a reduction in TUNEL+ retinal cells/thinning of retinal layers, MSC EV also prevented declines in a- and b-wave amplitudes, suggesting a preservation of photoreceptor and bipolar cell function, respectively. MSC EV diffused throughout the retina and RPE within 1 h. One mechanism identified by the authors was the exosome-induced down-regulation of MCP-1 retinal expression, whose upregulation is usually a consequence of retinal injury. MCP-1 is a chemotactic cytokine that attracts macrophages and microglial cells into the injury site, leading to further damage and degeneration. MSC EV reduced MCP-1 expression in vitro and in vivo, reducing macrophage infiltration and this effect was abolished if MCP-1 was delivered into animals. This study reveals another mechanism of action for EV, an anti-inflammatory one, yet did not determine if the down-regulation of MCP-1 was due to the EV protein or RNA cargo.
A separate study focused on the effects of laser damage to RPE, which causes choroidal neovascularization, a characteristic feature of wet AMD (He et al., 2018). In vitro, laser damage to RPE cells induced the production of VEGF, the principal growth factor responsible for the neo-vascularization and the basis for the anti-VEGF drugs used in the clinic. Treatment of laser-injured RPE cells with umbilical cord MSC-derived EV reduced the transcription and translation of VEGF whereas in vivo, delivery of EV did the same while reducing retinal damage as measured by fundus fluorescein angiography.
In the same in vivo model of choroidal neovascularization, Hajrasouliha and coauthors demonstrated that exosomes from retinal astrocytes can inhibit the formation of new blood vessels as well as suppress retinal vascular leakage (Hajrasouliha et al., 2013). Similar to other studies, a 0.22 μm filter was employed to filter out microvesicles. The authors state that the mechanism of action is likely through the inhibition of macrophage migration which is a major source of inflammatory cytokines as well as VEGF. Interestingly, authors attributed the therapeutic effects to the protein content of exosomes and identified several anti-angiogenic candidates abundantly found in retinal astrocyte-derived exosomes. By inhibiting the MMP-induced production of endostatin and utilizing the subsequently generated endostatin-free exosomes, suppression of vascular leakage was no longer observed, demonstrating a role for exosome-delivered endostatin.
3.5. Autoimmune uveitis
Uveitis is an inflammatory condition of the eye requiring immunosuppressive treatment. Since long-term use of immunosuppression comes with several side effects, there is still a need for new treatments. Interphotoreceptor retinol-binding protein immunization induces experimental autoimmune uveitis, and the inflammatory cell retinal infiltration (granulocytes, natural killer cells, macrophages, and T cells) is ameliorated after treatment with umbilical cord MSC exosomes (filtered and characterized) (Bai et al., 2017). MSC exosomes also prevented a loss in a- and b-wave amplitude, suggesting photoreceptor and bipolar cell function was preserved. Authors found that the MSC exosomes anti-inflammatory effects were specifically on T cell migration and not proliferation/apoptosis. While the study did not determine their mechanism of action, previous studies have shown that MSC exosomes inhibit macrophage activation through miRNA-mediated down-regulation of the toll-like receptor and nuclear factor kappa B (NF-kB) pathway (Phinney et al., 2015). Other mechanisms such as the polarization of CD4+ T cells to regulatory T cells has also been described (Bin et al., 2014).
A separate study testing the effects of MSC exosomes in experimental autoimmune uveitis delivered exosomes into the tail vein (Shigemoto-Kuroda et al., 2017). Exosomes were isolated by column fractionation and characterized using exosomal markers CD63 and CD81. MSC exosomes performed just as well as MSC in preventing photoreceptor layer disruption and inflammatory cell infiltration. Interestingly, only a single injection was administered at the beginning of the 21 day study, corroborating our own reports of MSC exosomes remaining efficacious for up to 1 month in the eye (Mead et al., 2018a, 2018b). MSC exosomes/MSC also reduced the transcription of many pro-inflammatory cytokines including interferon gamma (IFN-g), IL-17A, IL-2, IL-1b, IL-6, and IL-12A (Shigemoto-Kuroda et al., 2017). Unlike the previous study however, authors demonstrated that MSC exosomes suppressed T cell proliferation. One possible explanation for this discrepancy is that authors cultured their MSC in serum free medium designed to activate/prime the MSC prior to exosomes isolation, which would likely have changed their internal cargo and thus, therapeutic action.
The anti-inflammatory properties of EV have also been demonstrated by RPE, whose secreted EV provide immunomodulatory effects on monocytes and even induce their death (Knickelbein et al., 2016). Currently however, they have not been utilized as a potential therapeutic.
These studies suggest that MSC EV and in particular exosomes have potential as a treatment in inflammatory diseases of the eye. Further studies on their long-term efficacy, dose and ideal source of said exosomes are needed to improve the treatment. One exciting observation is MSC EV efficacy is still present when delivered into the blood stream rather than the eye, suggesting that they can home into an injured environment (Shigemoto-Kuroda et al., 2017). While this would be a more ideal route of administration from the patient’s perspective, the potential for off-target effects with pernicious consequences would need to be considered. The anti-inflammatory properties of EV are not just relevant to uveitis but also the retinal injury models discussed above. Retinal/optic nerve injury is followed by a polarization of microglia to a M1 pro-inflammatory phenotype, which secrete various inflammatory cytokines including TNF-α. These can not only directly induce the neurodegeneration of RGC (Tezel, 2008) but polarize astrocytes to a neurotoxic A1 phenotype which itself leads to RGC neurodegeneration (Liddelow et al., 2017). Further studies are required to determine if these anti-inflammatory effects are a relevant mechanism behind the EV-mediated neuroprotection previously discussed.
3.6. Diabetic retinopathy
Diabetic retinopathy, a consequence of diabetes mellitus that involves inflammation, microaneurysms, vasculature damage and subsequent neo-vascularization (Stitt et al., 2016) has also shown preliminary promise as an eye disease amenable to EV therapy. Delivery of MSC (adipose-derived) EV into the eye, either subconjunctival or intravitreous (but not intravenous) prevented significant retinal degeneration (Safwat et al., 2018) in a streptozotocin-induced model of diabetic retinopathy. Authors demonstrated that exosomes delivered miRNA-222 into the retina and restored falling levels typically associated with diabetic retinopathy. The discrepancy between this study’s inability to obtain a clinical effect after intravenous administration, and the positive effects seen in the above study (Shigemoto-Kuroda et al., 2017) emphasize the need for further investigation on this potential route of administration.
A separate study utilized the same model and delivered umbilical cord MSC exosomes intravitreally (Zhang et al., 2019). Hyperglycemia-induced inflammation is ameliorated by MSC exosomes in comparison to fibroblast exosomes, as measured by ELISA for the inflammatory markers IL-1β, IL-18, and caspase-1 in the vitreous. The mechanism of action appears to be miR-126-mediated inhibition of the high mobility group box 1 (HMGB1) signaling pathway. Diabetic retinopathy is associated with decreased miR-126 and over expression of miR-126 in MSC exosomes further augmented the therapeutic efficacy.
3.7. Clinical trials
As of this review 148 clinical trials have been listed looking at “exosomes” and 39 mentioning “extracellular vesicles”. However, very few are utilizing them as a therapy with the rest mostly focusing on the use of exosomes as biomarkers of disease.
Two of these clinical trials testing MSC EV therapies that have been published include steroid refractory graft-versus-host disease (Kordelas et al., 2014) and chronic kidney disease (Nassar et al., 2016). MSC EV reduced pro-inflammatory cytokine secretions including TNF-α, increased anti-inflammatory cytokines secretions including TGF-β, and improved patient recovery and kidney function.
Two clinical trials are listed using exosomes as a treatment for eye disease, one in diabetic retinopathy which is not yet recruiting (ClinicalTrials.gov Identifier: NCT03264976) and another for the treatment of macular holes which is still recruiting and has already published preliminary results (ClinicalTrials.gov Identifier: NCT03437759). Five patients with large and refractory macular holes were treated with an intravitreal delivery of MSC-derived exosomes (Zhang et al., 2018). Exosomal presence was confirmed using Western blot, staining for exosome markers such as CD63, CD9 and CD81. Since no size exclusion (e.g. 0.22 μm filter) was utilized, the preparation undoubtedly also included microvesicles and is thus more accurately described as MSC EV. Results of the study suggest that MSC EV stimulate the closure of macular holes although the mechanism of action was not elucidated, and control groups not included. The intravitreal MSC EV therapy was well tolerated with only one patient experiencing an inflammatory reaction which was not present when the dose was reduced.
As more studies demonstrate that EV have an active and potentially therapeutic role in the body, as opposed to only a passive one (Joo et al., 2020; Tieu et al., 2019), it is anticipated that there will be more clinical trials focusing on their clinical potential rather than their role solely as biomarkers.
4. Future considerations
While EV show great promise, many questions still remain unanswered.
4.1. Toxicology and dosing
While no evidence exists for any complications arising from delivery of EV into the eye, extensive toxicology studies are still needed. Some in vitro (Maji et al., 2017) and in vivo (Zhu et al., 2017) toxicology reports have been published detailing their safety after culture treatment or systemic delivery, but how true this is for ocular delivery is still not known. They also report toxicological differences between different cellular sources of EV which although is unsurprising given what we know, emphasizes the importance of treating EV from different cells as distinct agents.
Secondly, the large-scale production of clinical-grade EV represents a significant barrier to moving this experimental treatment into the clinic. Issues such as ensuring the batch-to-batch variability remains minimal as well as the detection of any viruses that will likely be enriched alongside EV remains paramount when moving forward (Rohde et al., 2019). Variations in the length of time in culture may also affect the cells and subsequently, the EV, increasing variability. For EV to be effectively dosed, it is not enough to simply consider their quantity but instead to dose for their cargo, ensuring that a controlled amount of the therapeutically efficacious elements are delivered irrespective of the number of EV particles. It is also important to consider that the therapeutically efficacious component of the isolate may indeed be an EV subtype that can be further purified, however techniques to achieve this are still lacking (Greening and Simpson, 2018) and the benefits would need to be balanced against the added cost. Along with the EV subtype, the subtypes of the target cells should also be taken into consideration. Using RGC as an example, just as different injuries affect different RGC subtype, it is also possible that EV treatment only protects specific RGC subtypes and given that over 40 subtypes have been identified (Reviewed in Sanes and Masland, 2015; Tran et al., 2019), these potential differential effects warrant investigation. Regarding large scale EV production, one research focus has been to target the MSC themselves, modifying them in such a way as to improve the isolated EV yield and efficacy (Phan et al., 2018).
4.2. Targeting EV to cells
For EV to exert their effects on the injured retina, they must be targeted to the correct cells and subsequently internalized. The above studies have demonstrated that EV deliver cargo into a whole range of retinal cells including RGC (Mead and Tomarev, 2017), microglia, astrocytes (van der Merwe et al., 2019), and RPE cells (He et al., 2018). However, many studies do not interrogate the exact cellular target, only referencing global changes in retinal expression, function, or morphology. Future studies should pay special attention to this aspect of EV, particularly as it is becoming apparent EV can preferentially bind to specific cells based on their protein cargo (Murphy et al., 2019). Thus, particular EV can be selected depending on the desired retinal cell target.
4.3. Mechanisms of action and the discovery of novel pathways
It is clear that EV contain an expansive cargo while unclear which of this cargo is responsible for the therapeutic effects observed in the above retinal diseases. It is tempting, and perhaps more feasible, to focus on clearly established pathways and delineate from this which of the EV cargo is likely responsible. However, EV also represent an opportunity to discover novel targets, particularly given most miRNA targets are untested and remain predicted rather than observed (Mead et al., 2018b).
Research has often used EV in a cross-species manner, in particular, human-derived EV in rodent models. It is unclear what interactions and effects are being excluded due to, for example, particular human-miRNA being incompatible with rodent mRNA. More studies using human EV on human cells may help refine the mechanisms or yield new candidates. If the mechanism of action can be limited to just a select few miRNA/mRNA/proteins, the treatment could be further simplified just using these particular candidates. Finally, it is currently unknown what the miRNA landscape of RGC (and their subtypes) is, as well as other specific retinal cells. This is important information considering the delivery of miRNA is one important mechanism of EV. It would be equally important to know the retinal mRNA/miRNA changes before and after EV treatment as well as under different injury conditions. Additionally, knowing the EV signaling that occurs to maintain eye homeostasis will help shape future EV therapies.
4.4. EV modification, priming, and loading
While it is clear EV are therapeutically efficacious in several disease models, how this effect can be improved further is of strong interest and may allow lower doses or less frequent administrations to be utilized. Modifying EV to better target cells of interest is one such approach and is demonstrated in a previous study involving the fusion of the exosomal protein lysosome-associated membrane protein 2 (Lamp2b) with the brain targeting peptide rabies viral glycoprotein peptide (Alvarez-Erviti et al., 2011). Subsequently generated EV were able to selectively target neurons, microglia, and oligodendrocytes in the brain after systemic administration. Priming or modifying the EV is another approach and we have recently demonstrated that by exposing MSC to the inflammatory cytokine TNF-α, the EV they release are more efficacious in the context of retinal neuroprotection (Mead et al., 2020) (Fig. 7). These “primed” EV warrant further investigation as it is expected that a cocktail of factors is required to maximally prime MSC and their EV. EV themselves can be also be modified directly, such as loaded with an abundance of a particular miRNA to increase their efficacy. This was achieved in a study described above, loading EV with miR-126 and increasing their efficacy further in a model of diabetic retinopathy (Zhang et al., 2019).
5. Conclusions
Exosomes/EV are strong candidates as a treatment for the injured retina. They circumnavigate the risk factors associated with delivering dividing cells into the eye while still possessing their multifactorial mechanism of action due to their expansive cargo. Further work is needed to characterize their mechanism of action including the mRNA, miRNA and proteins responsible alongside the myriad of therapeutic targets.
Acknowledgements
BM did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This work (ST) was supported by the Intramural Research Programs of the National Eye Institute. We would like to thank Dr. Zubair Ahmed for his critical reading of an early version of this manuscript.
Footnotes
Author statement
Ben Mead (70%): Conceptualization, Methodology, Investigation, Formal analysis.
Stanislav Tomarev (30%): Conceptualization, Formal analysis, Writing.
References
- Rohde E, Pachler K, Gimona M, 2019. Manufacturing and characterization of extracellular vesicles from umbilical cord–derived mesenchymal stromal cells for clinical testing. Cytotherapy 21, 581–592. [DOI] [PubMed] [Google Scholar]
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A, 2012. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res 31, 152–181. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA, 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol 29, 341–345. [DOI] [PubMed] [Google Scholar]
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Perez Lanzon M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM, 2015. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X, 2017. Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci. Rep 7, 4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP, 2018. Metazoan MicroRNAs. Cell 173, 20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistelli M, Falcieri E, 2020. Apoptotic bodies: particular extracellular vesicles involved in intercellular communication. Biology 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ, 1994. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci. : Off. J. Soc. Neurosci 14, 4368–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Ahmed Z, Lorber B, Douglas M, Logan A, 2008. Regeneration of axons in the visual system. Restor. Neurol. Neurosci 26, 147–174. [PubMed] [Google Scholar]
- Berry M, Ahmed Z, Logan A, 2019. Return of function after CNS axon regeneration: lessons from injury-responsive intrinsically photosensitive and alpha retinal ganglion cells. Prog. Retin. Eye Res 71, 57–67. [DOI] [PubMed] [Google Scholar]
- Bin Z, Yijun Y, Chai LR, Sim TS, Hwa CAB, Kiang LS, 2014. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cell. Dev 23, 1233–1244. [DOI] [PubMed] [Google Scholar]
- Caruso S, Poon IKH, 2018. Apoptotic cell-derived extracellular vesicles: more than just debris. Front. Immunol 9 1486–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou NT, Kageyama R, Ansel KM, 2018. Selective export into extracellular vesicles and function of tRNA fragments during T cell activation. Cell Rep 25, 3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman AL, Miglior S, 2008. Risk factors for glaucoma onset and progression. Surv. Ophthalmol 53, S3–S10. [DOI] [PubMed] [Google Scholar]
- de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB, Silva L.R.d.P., Leal RB, Monteiro VHS, Braga CV, de Araujo-Silva CA, Sinis LC, Bodart-Santos V, Kasai-Brunswick TH, Alcantara C.d.L., Lima APCA, da Cunha-E Silva NL, Galina A, Vieyra A, De Felice FG, Mendez-Otero R, Ferreira ST, 2018. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J. Biol. Chem 293, 1957–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreixler JC, Poston JN, Balyasnikova I, Shaikh AR, Tupper KY, Conway S, Boddapati V, Marcet MM, Lesniak MS, Roth S, 2014. Delayed administration of bone marrow mesenchymal stem cell conditioned medium significantly improves outcome after retinal ischemia in rats. Investig. Ophthalmol. Vis. Sci 55, 3785–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Qiao M, Bei F, Kim IJ, He Z, Sanes JR, 2015. Subtype-specific regeneration of retinal ganglion cells following axotomy: effects of osteopontin and mTOR signaling. Neuron 85, 1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirin A, Riester SM, Zhu X-Y, Tang H, Evans JM, O’Brien D, van Wijnen AJ, Lerman LO, 2014. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene 551, 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eirin A, Zhu X-Y, Puranik AS, Woollard JR, Tang H, Dasari S, Lerman A, van Wijnen AJ, Lerman LO, 2016. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep 6, 36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre E, Yuksel N, Duruksu G, Pirhan D, Subasi C, Erman G, Karaoz E, 2015. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow-derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 17, 543–559. [DOI] [PubMed] [Google Scholar]
- Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, Liu H, Xing X, 2016. Umbilical cord-derived mesenchymal stem cell-derived exosomal MicroRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/SMAD2 pathway during wound healing. Stem Cells Transl. Med 5, 1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J, 2018. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci. Rep 8, 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbarth K, Jankowiak W, Kruszewski K, Helbing S, Bartsch S, Bartsch U, 2018. Pronounced synergistic neuroprotective effect of GDNF and CNTF on axotomized retinal ganglion cells in the adult mouse. Exp. Eye Res 176, 258–265. [DOI] [PubMed] [Google Scholar]
- Fry LE, Fahy E, Chrysostomou V, Hui F, Tang J, van Wijngaarden P, Petrou S, Crowston JG, 2018. The coma in glaucoma: retinal ganglion cell dysfunction and recovery. Prog. Retin. Eye Res 65, 77–92. [DOI] [PubMed] [Google Scholar]
- Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z, 2014. miRNA in plasma exosome is stable under different storage conditions. Molecules (Basel, Switzerland) 19, 1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening DW, Simpson RJ, 2018. Understanding extracellular vesicle diversity – current status. Expet Rev. Proteonomics 15, 887–910. [DOI] [PubMed] [Google Scholar]
- Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang H-G, Shao H, 2013. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem 288, 28058–28067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszti RA, Didiot M-C, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A, 2016. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 5 32570–32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CR, Fellabaum C, Arsenijevic A, Markovic BS, Djonov V, Volarevic V, 2019. Therapeutic potential of mesenchymal stem cells and their secretome in the treatment of glaucoma. Stem Cell. Int 2019, 7869130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G-H, Zhang W, Ma Y-X, Yang J, Chen L, Song J, Chen S, 2018. Mesenchymal stem cells-derived exosomes ameliorate blue light stimulation in retinal pigment epithelium cells and retinal laser injury by VEGF-dependent mechanism. Int. J. Ophthalmol 11, 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Kolibabka M, Eshwaran R, Chatterjee A, Schlotterer A, Willer H, Bieback K, Hammes H-P, Feng Y, 2019. Intravitreal injection of mesenchymal stem cells evokes retinal vascular damage in rats. Faseb. J 33:12, 14668–14679 0, fj.201901500R. [DOI] [PubMed] [Google Scholar]
- Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ, 2019. Reassessment of exosome composition. Cell 177, 428–445 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Paone S, Caruso S, Atkin-Smith GK, Phan TK, Hulett MD, Poon IKH, 2017. Determining the contents and cell origins of apoptotic bodies by flow cytometry. Sci. Rep 7, 14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR, 2010a. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Investig. Ophthalmol. Vis. Sci 51, 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Martin KR, 2010b. Identification of barriers to retinal engraftment of transplanted stem cells. Invest. Ophthalmol. Vis. Sci 51, 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Dekorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI, 2014. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 137, 503–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo HS, Suh JH, Lee HJ, Bang ES, Lee JM, 2020. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int. J. Mol. Sci 21, 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, LeBleu VS, 2020. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshtkar S, Azarpira N, Ghahremani MH, 2018. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res. Ther 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, You YS, Kim SH, Kwon OW, 2017. Epiretinal membrane formation after intravitreal autologous stem cell implantation in a retinitis pigmentosa patient. Retin. Cases Brief Rep 11, 227–231. [DOI] [PubMed] [Google Scholar]
- Klingeborn M, Dismuke WM, Rickman CB, Stamer WD, 2017a. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res 59, 158–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingeborn M, Dismuke WM, Skiba NP, Kelly U, Stamer WD, Bowes Rickman C, 2017b. Directional exosome proteomes reflect polarity-specific functions in retinal pigmented epithelium monolayers. Sci. Rep 7, 4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein JE, Liu B, Arakelyan A, Zicari S, Hannes S, Chen P, Li Z, Grivel JC, Chaigne-Delalande B, Sen HN, Margolis L, Nussenblatt RB, 2016. Modulation of immune responses by extracellular vesicles from retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci 57, 4101–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP, 2018. Isolation of extracellular vesicles: general methodologies and latest trends. BioMed Res. Int 2018, 8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B, 2014. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 28, 970. [DOI] [PubMed] [Google Scholar]
- Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard REI, Parrott MB, Rosenfeld PJ, Flynn HWJ, Goldberg JL, 2017. Vision loss after intravitreal injection of autologous “stem cells” for AMD. N. Engl. J. Med 376, 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R, 2018. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front. Pharmacol 9 1199–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CK, Lindsey JD, Crowston JG, Lijia C, Chiang S, Weinreb RN, 2008. Longitudinal profile of retinal ganglion cell damage after optic nerve crush with blue-light confocal scanning laser ophthalmoscopy. Invest. Ophthalmol. Vis. Sci 49, 4898–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J-HA, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD, 2016. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci 19, 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A, Ahmed Z, Baird A, Gonzalez AM, Berry M, 2006. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain 129, 490–502. [DOI] [PubMed] [Google Scholar]
- Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M, 2016. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience 320, 129–139. [DOI] [PubMed] [Google Scholar]
- Lu B, Morgans CW, Girman S, Luo J, Zhao J, Du H, Lim S, Ding S, Svendsen C, Zhang K, Wang S, 2013. Neural stem cells derived by small molecules preserve vision. Transl. Vis. Sci. Technol 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Wei Y, Wang D, Zhang CY, Zen K, Li L, 2014. Argonaute 2 in cell-secreted microvesicles guides the function of secreted miRNAs in recipient cells. PloS One 9, e103599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Li B, Zhang M, Zhou L, Yang F, Ma F, Shao H, Li Q, Li X, Zhang X, 2020. Therapeutic effects of mesenchymal stem cell-derived exosomes on retinal detachment. Exp. Eye Res 191, 107899. [DOI] [PubMed] [Google Scholar]
- Maes ME, Schlamp CL, Nickells RW, 2017. BAX to basics: how the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin. Eye Res 57, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji S, Yan IK, Parasramka M, Mohankumar S, Matsuda A, Patel T, 2017. In vitro toxicology studies of extracellular vesicles. J. Appl. Toxicol 37, 310–318. [DOI] [PubMed] [Google Scholar]
- Mathieu M, Martin-Jaular L, Lavieu G, Théry C, 2019. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol 21, 9–17. [DOI] [PubMed] [Google Scholar]
- Mead B, Tomarev S, 2017. BMSC-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl. Med 6, 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA, 2013. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest. Ophthalmol. Vis. Sci 54, 7544–7556. [DOI] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA, 2014. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PloS One 9, e109305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Berry M, Logan A, Scott RAH, Leadbeater W, Scheven BA, 2015. Stem cell treatment of degenerative eye disease. Stem Cell Res 14, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Hill LJ, Blanch RJ, Ward K, Logan A, Berry M, Leadbeater W, Scheven BA, 2016. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 18, 487–496. [DOI] [PubMed] [Google Scholar]
- Mead B, Ahmed Z, Tomarev S, 2018a. Mesenchymal stem cell–derived small extracellular vesicles promote neuroprotection in a genetic DBA/2J mouse model of GlaucomaNeuroprotection by exosomes in DBA/2J mice. Investig. Ophthalmol. Vis. Sci 59, 5473–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Amaral J, Tomarev S, 2018b. Mesenchymal stem cell–derived small extracellular vesicles promote neuroprotection in rodent models of glaucoma. Investig. Ophthalmol. Vis. Sci 59, 702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Chamling X, Zack DJ, Ahmed Z, Tomarev S, 2020. Tnfα-mediated priming of mesenchymal stem cells enhances their neuroprotective effect on retinal ganglion cells. Investig. Ophthalmol. Vis. Sci 61 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueiredo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF, 2014. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PloS One 9, e110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesentier-Louro LA, Teixeira-Pinheiro LC, Gubert F, Vasques JF, Silva-Junior AJ, Chimeli-Ormonde L, Nascimento-Dos-Santos G, Mendez-Otero R, Santiago MF, 2019. Long-term neuronal survival, regeneration, and transient target reconnection after optic nerve crush and mesenchymal stem cell transplantation. Stem Cell Res. Ther 10 121–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan-Rivero JE, Nadal-Nicolas FM, Garcia-Bernal D, Sobrado-Calvo P, Blanquer M, Moraleda JM, Vidal-Sanz M, Agudo-Barriuso M, 2018. Human Wharton’s jelly mesenchymal stem cells protect axotomized rat retinal ganglion cells via secretion of anti-inflammatory and neurotrophic factors. Sci. Rep 8, 16299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisseiev E, Anderson JD, Oltjen S, Goswami M, Zawadzki RJ, Nolta JA, Park SS, 2017. Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr. Eye Res 42, 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Warren PJ, O’Neill J, de Cogan F, Spivak I, Ashush H, Kalinski H, Ahmed Z, Berry M, Feinstein E, Scott RAH, Logan A, 2016. siRNA-mediated knockdown of the mTOR inhibitor RTP801 promotes retinal ganglion cell survival and axon elongation by direct and indirect mechanisms. Investig. Ophthalmol. Vis. Sci 57, 429–443. [DOI] [PubMed] [Google Scholar]
- Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P, 2019. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp. Mol. Med 51, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad A-N, Essa W, Adel H, 2016. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res 20, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsworthy MW, Bei F, Kawaguchi R, Wang Q, Tran NM, Li Y, Brommer B, Zhang Y, Wang C, Sanes JR, Coppola G, He Z, 2017. Sox11 expression promotes regeneration of some retinal ganglion cell types but kills others. Neuron 94, 1112–1120 e1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteikoetxea X, Sódar B, Németh A, Szabó-Taylor K, Pálóczi K, Vukman KV, Tamási V, Balogh A, Kittel Á, Pállinger É, Buzás EI, 2015. Differential detergent sensitivity of extracellular vesicle subpopulations. Org. Biomol. Chem 13, 9775–9782. [DOI] [PubMed] [Google Scholar]
- Pan D, Chang X, Xu M, Zhang M, Zhang S, Wang Y, Luo X, Xu J, Yang X, Sun X, 2019. UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J. Chem. Neuroanat 96, 134–139. [DOI] [PubMed] [Google Scholar]
- Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A, 2018. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 7 1522236–1522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DWH, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA, 2015. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun 6 8472–8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Xu C, Fang S, Zhao P, Wang Y, Liu H, Yuan W, Qi Z, 2016. Exosomal MicroRNAs derived from umbilical mesenchymal stem cells inhibit hepatitis C virus infection. Stem Cells Transl. Med 5, 1190–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ, 2006. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856. [DOI] [PubMed] [Google Scholar]
- Rodriguez AR, de Sevilla Müller LP, Brecha NC, 2014. The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol 522, 1411–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat A, Sabry D, Ragiae A, Amer E, Mahmoud RH, Shamardan RM, 2018. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomarkers 7 1849454418807827–1849454418807827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Masland RH, 2015. The types of retinal ganglion cells: current status and implications for neuronal classification. Annu. Rev. Neurosci 38, 221–246. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Kuroda T, Oh JY, Kim D. k., Jeong HJ, Park SY, Lee HJ, Park JW, Kim TW, An SY, Prockop DJ, Lee RH, 2017. MSC-derived extracellular vesicles attenuate immune responses in two autoimmune murine models: type 1 diabetes and uveoretinitis. Stem Cell Rep 8, 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R, 2016. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry WT Jr., Carter BS, Krichevsky AM, Breakefield XO, 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol 10, 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluch VM, Davis C.-h.O., Ranganathan V, Kerr JM, Krick K, Martin R, Berlinicke CA, Marsh-Armstrong N, Diamond JS, Mao H-Q, Zack DJ, 2015. Differentiation of human ESCs to retinal ganglion cells using a CRISPR engineered reporter cell line. Sci. Rep 5, 16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluch VM, Chamling X, Liu MM, Berlinicke CA, Cheng J, Mitchell KL, Welsbie DS, Zack DJ, 2017. Enhanced stem cell differentiation and immunopurification of genome engineered human retinal ganglion cells. Stem Cells Transl. Med 6, 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes H-P, Simó R, Lois N, 2016. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res 51, 156–186. [DOI] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z, 2011. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480, 372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Li D, Song K, Wei J, Yao S, Li Z, Su X, Ju X, Chao L, Deng X, Kong B, Li L, 2017. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci. Rep 7, 2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B, 2018. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng C 89, 194–204. [DOI] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Libby RT, 2019. Axon injury signaling and compartmentalized injury response in glaucoma. Prog. Retin. Eye Res 73, 100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HB, Kang X, Lu SH, Liu L, 2015. The therapeutic effects of bone marrow mesenchymal stem cells after optic nerve damage in the adult rat. Clin. Interv. Aging 10, 487–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassew NG, Charish J, Shabanzadeh AP, Luga V, Harada H, Farhani N, D’Onofrio P, Choi B, Ellabban A, Nickerson PEB, Wallace VA, Koeberle PD, Wrana JL, Monnier PP, 2017. Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep 20, 99–111. [DOI] [PubMed] [Google Scholar]
- Tezel G, 2008. TNF-alpha signaling in glaucomatous neurodegeneration. Prog. Brain Res 173, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Witwer KW, 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A, 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol 30 (1), 1–29 Chapter 3, Unit 3 22. [DOI] [PubMed] [Google Scholar]
- Tieu A, Slobodian M, Fergusson DA, Montroy J, Burger D, Stewart DJ, Shorr R, Allan DS, Lalu MM, 2019. Methods and efficacy of extracellular vesicles derived from mesenchymal stromal cells in animal models of disease: a preclinical systematic review protocol. Syst. Rev 8, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NM, Shekhar K, Whitney IE, Jacobi A, Benhar I, Hong G, Yan W, Adiconis X, Arnold ME, Lee JM, Levin JZ, Lin D, Wang C, Lieber CM, Regev A, He Z, Sanes JR, 2019. Single-cell profiles of retinal ganglion cells differing in resilience to injury reveal neuroprotective genes. Neuron 104, 1039–1055 e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V, 2008. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol 8, 726–736. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol 9, 654–U672. [DOI] [PubMed] [Google Scholar]
- van der Merwe Y, Faust AE, Sakalli ET, Westrick CC, Hussey G, Conner IP, Fu VLN, Badylak SF, Steketee MB, 2019. Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function. Sci. Rep 9 3482–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal C, Shamir C, Senthilkumar S, Babu JV, Sonu PK, Nishtha KJ, Rai KS, K S, Dhanushkodi A, 2017. Dosage and passage dependent neuroprotective effects of exosomes derived from rat bone marrow mesenchymal stem cells: an in vitro analysis. Curr. Gene Ther 17, 379–390. [DOI] [PubMed] [Google Scholar]
- Wang L, Pei S, Han L, Guo B, Li Y, Duan R, Yao Y, Xue B, Chen X, Jia Y, 2018a. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via down-regulation of phosphorylated NFκB P65 subunit in spinal cord injury. Cell. Physiol. Biochem 50, 1535–1559. [DOI] [PubMed] [Google Scholar]
- Wang X, Omar O, Vazirisani F, Thomsen P, Ekström K, 2018b. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PloS One 13 e0193059–e0193059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M, Alaarg A, Smith CIE, Lehtiö J, El Andaloussi S, Wood MJA, Vader P, 2016. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep 6, 22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M, 2013. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cerebr. Blood Flow Metabol 33, 1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HQ, Katakowski M, Wang FJ, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M, 2017. MicroRNA-17–92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke J. Cereb. Circ 48, 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Li H, Li M, Wang F, 2015. Mechanisms of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Discov. Med 20, 207–217. [PubMed] [Google Scholar]
- Yeo RWY, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK, 2013. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev 65, 336–341. [DOI] [PubMed] [Google Scholar]
- Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, Zhang Y, Li Q, Zhang X, Li X, 2016. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep 6, 34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, Wu D, Zhang ZG, 2016. Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol. Neurobiol 54, 2659–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu J, Yu B, Ma F, Ren X, Li X, 2018. Effects of mesenchymal stem cells and their exosomes on the healing of large and refractory macular holes. Graefes Arch. Clin. Exp. Ophthalmol 256, 2041–2052. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang Y, Kong Y, 2019. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1MSC-exosomes ameliorate retinal inflammation. Investig. Ophthalmol. Vis. Sci 60, 294–303. [DOI] [PubMed] [Google Scholar]
- Zhou J, Benito-Martin A, Mighty J, Chang L, Ghoroghi S, Wu H, Wong M, Guariglia S, Baranov P, Young M, Gharbaran R, Emerson M, Mark MT, Molina H, Canto-Soler MV, Selgas HP, Redenti S, 2018. Retinal progenitor cells release extracellular vesicles containing developmental transcription factors, microRNA and membrane proteins. Sci. Rep 8, 2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle KL, Chalmers J, Schmittgen TD, Phelps MA, 2017. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 6, 1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]


