Abstract
Background
Grip strength is a widely used and well-validated measure of overall health that is increasingly understood to index risk for psychiatric illness and neurodegeneration in older adults. However, existing work has not examined how grip strength relates to a comprehensive set of mental health outcomes, which can detect early signs of cognitive decline. Furthermore, whether brain structure mediates associations between grip strength and cognition remains unknown.
Methods
Based on cross-sectional and longitudinal data from over 40,000 participants in the UK Biobank, this study investigated the behavioral and neural correlates of handgrip strength using a linear mixed effect model and mediation analysis.
Results
In cross-sectional analysis, we found that greater grip strength was associated with better cognitive functioning, higher life satisfaction, greater subjective well-being, and reduced depression and anxiety symptoms while controlling for numerous demographic, anthropometric, and socioeconomic confounders. Further, grip strength of females showed stronger associations with most behavioral outcomes than males. In longitudinal analysis, baseline grip strength was related to cognitive performance at ~9 years follow-up, while the reverse effect was much weaker. Further, baseline neuroticism, health, and financial satisfaction were longitudinally associated with subsequent grip strength. The results revealed widespread associations between stronger grip strength and increased grey matter volume, especially in subcortical regions and temporal cortices. Moreover, grey matter volume of these regions also correlated with better mental health and considerably mediated their relationship with grip strength.
Conclusions
Overall, using the largest population-scale neuroimaging dataset currently available, our findings provide the most well-powered characterization of interplay between grip strength, mental health, and brain structure, which may facilitate the discovery of possible interventions to mitigate cognitive decline during aging.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02490-2.
Keywords: Grip strength, Cognitive functioning, Mental health, Brain plasticity, Grey matter volume
Background
Identifying modifiable risk factors and the neurobiological underpinnings that preserve cognitive functioning has become a public health priority in an ever-increasing aging society [1]. Among potential candidates, handgrip strength, which is often assessed isometrically using a hydraulic hand dynamometer, serves as an easily administered and validated measure of muscle strength and overall health in clinical settings [2, 3]. Accumulating evidence shows that lower grip strength, as a proxy for muscle strength, imposes serious limitations on dependency, disability, and quality of life in older age [4, 5]. Individuals with weak grip strength are at higher risk of adverse health consequences like mobility, frailty, falls, hospitalization, and all-cause mortality [6, 7].
Epidemiological research has associated weaker grip strength with reduced cognitive functioning and increased risk for psychiatric conditions and dementia. For example, population-scale investigations reveal consistent associations between maximal grip strength and performance on cognitive tasks of verbal reasoning, reaction time, and working memory in both the general population and individuals with schizophrenia, bipolar disorder, or depression [8, 9]. This aligns with experimental studies, which have related greater grip strength to motivated behaviors, like effort and vigor, that are perturbed in psychotic and mood disorders [10, 11]. A recent community-based study also found that older adults in the lowest quantile of muscular strength have a higher likelihood of suffering from depressive symptoms and suicidal ideation [12]. Moreover, meta-analytic findings have consistently implicated the predictive value of grip strength for health outcomes, highlighting its crucial role as a clinically useful indicator for monitoring cognitive impairment and progression of neurodegenerative diseases [13–15].
Despite such potential, significant gaps remain in our knowledge of the links between grip strength and mental health. First, most studies on this topic have been either based on a small set of circumscribed cognitive domains or relied on relatively insensitive clinical measures, such as the mini-mental state examination [14], which may not detect subtle cognitive changes that occur in early stages of aging [16]. By comprehensively examining associations between grip strength and a wide repertoire of mental health-related outcomes (including cognitive functioning, anxiety/depression symptoms, subjective well-being, and life satisfaction), rather than only focusing on cognition, one could determine the sensitivity of grip strength to specific health domains. This, in turn, would aid early identification and intervention efforts for neurodegenerative disorders. Second, since existing studies are largely cross-sectional, the temporal associations between handgrip strength and mental health remain poorly characterized. Although many studies assume that baseline handgrip strength predicts future cognitive decline [17], others demonstrate the reverse association [18]. Moreover, a recent study of 5995 Korean participants confirmed a bi-directional relationship between grip strength and cognitive functions, suggesting the existence of common pathways underlying these two constructs [19].
While previous work has begun to unravel the relationship between grip strength and cognitive function during aging, relatively little attention has been paid to the underlying mechanism. Examining how the brain mediates the relationship between grip strength and cognitive function would advance mechanistic understanding of age-related health outcomes during senescence. More importantly, it may facilitate the discovery of novel interventions to mitigate cognitive decline during aging. In light of the well-documented evidence that MRI-derived measures of brain grey matter volume (GMV) serves as indicators of underlying neuropathological alterations in neurodegenerative disorders, emerging evidence has begun to establish the relevance of GMV to potential protective factors, including physical fitness, and muscular strength [20–22]. Nevertheless, associations between global or regional GMV and grip strength remain inconsistent [23], which may be partially attributed to low statistical power due to small sample sizes. Crucially, a large-scale investigation with both sensitive measures of behavioral outcomes as well as brain imaging indices is needed to comprehensively (1) establish the behavioral relevance of grip strength, (2) disentangle the directionality, (3) unravel the neurobiological correlates, and (4) examine the mediation role of these brain biomarkers.
To fill these gaps, we examine the behavioral and neural signatures of grip strength in one of the largest population-scale neuroimaging cohorts, the UK Biobank [24–26]. Using data from over 40,000 participants, we start by establishing how grip strength relates to a total of 30 mental health-related behavioral phenotypes. Based on longitudinal data, we further determine the directionality of these associations. We then investigate how grip strength is related to global and regional GMV and quantify the extent to which its neurobiological correlates are correlated with mental health outcomes. Finally, we examine whether GMV mediates any associations between grip strength and mental health outcomes.
Methods
Cohort and participants
The UK Biobank project is a population-scale, prospective cohort study of > 500,000 participants recruited from across the UK [26]. Between 2006 and 2010, all participants received a baseline assessment that collected a variety of phenotypic and health-related information (baseline visit) [24]. Since 2014, a subsample of participants has been invited back to four assessment centers for brain imaging and an extensive set of behavioral assessments (imaging visit). The baseline visit did not collect any brain imaging data, and some behavioral measures (e.g., matrix pattern completion, trail making, symbol digit substitution) were not available. Moreover, because a primary aim of the current study is to examine the neurobiological basis of grip strength and the mediation role of GMV, we restricted the main analysis to data from the imaging visit although there were more subjects at the baseline visit.
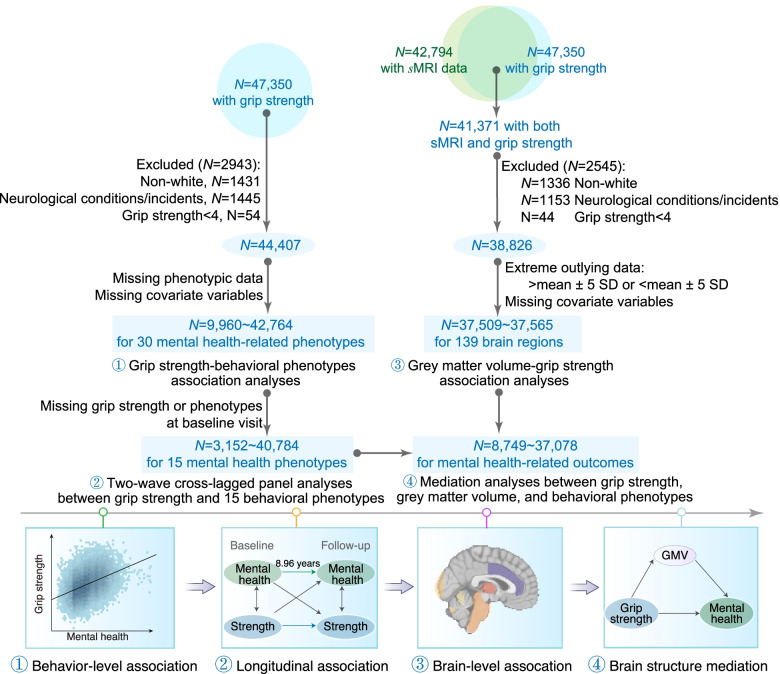
As per previous UK Biobank studies [8, 27, 28], we excluded participants who reported any of the listed severe neurological conditions/incidents (Additional file 1: Table S1). Additional exclusion criteria included missing MRI or relevant behavioral/demographic data. Since non-White participants constitute ~3% of this dataset, we only included White participants in the current study for further analysis. Overall, the behavioral analyses comprised a sample of 9960–42,764 participants per specific behavioral metric. In the longitudinal analyses, the number of participants was 3152–40,784 (age at baseline = 55.25±7.54 years, range = 40–70). In the brain imaging analyses, the number of participants was 37,509–37,565, 51% female, aged 64.21 ± 7.71 years (range: 45–82), and 48% had an education level of college (Fig. 1, and Additional file 1: Fig. S1).
Fig. 1.
Flowchart illustrating criteria for selection of samples as well as the four analyses performed in the current study. Using data from over 40,000 participants, we start by establishing how grip strength relates to a total of 30 mental health-related behavioral phenotypes. Based on longitudinal data, we further determine the directionality of these associations. We then investigate how grip strength is related to regional GMV and examine whether GMV mediates any associations between grip strength and mental health outcomes
Data acquisition and preprocessing
T1-weighted MPRAGE data were acquired on a 3T Siemens Skyra scanner using a standard 32-channel head coil. The detailed parameters were: Resolution: 1 × 1 × 1 mm, field-of-view (FOV): 208 × 256 × 256 matrix, duration: 5 min. The estimates of GMV were processed and quality controlled by the UK Biobank team, and they were made available to approved researchers as image-derived phenotypes. Specifically, cortical tissue-type segmentation was completed using FAST (FMRIB’s Automated Segmentation Tool), and subcortical structures were segmented using FIRST (FMRIB’s Integrated Registration and Segmentation Tool) [29]. An extensive overview of the data acquisition protocols and preprocessing steps can be found at https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/brain_mri.pdf and elsewhere [25, 30]. Overall, a total of 139 brain regions were extracted (Additional file 1: Fig. S2). Extreme outlying data points (further than mean ± 5 SD) were excluded from the imaging analysis.
Handgrip strength assessment
Following standard procedures [31], handgrip strength was assessed isometrically by a research assistant using a calibrated Jamar J00105 hydraulic hand dynamometer (Lafayette Instrument Company, IN, USA). With the participant seated upright with their elbow by their side and fixed at 90° (so that their forearm was facing forwards and resting on an armrest), a single trail indexing the maximal handgrip strength was acquired from each hand while allowing participants to select the most comfortable of 5 candidate grip positions [32]. In line with previous studies [8, 9], we used the reading from the self-reported dominant hand, or from the highest score from both hands if handiness is ambidextrous or unavailable. Participants with handgrip strength < 4 kg were identified as outliers and excluded from further analysis.
Behavioral data assessment
The behavioral assessment battery was administered using a brief and fully automated touchscreen computer without supervision. Some of the cognition or mental health assessments were specifically developed for the UK Biobank while others were adapted from commonly used tests. We briefly describe these phenotypes and provide their field ID in the UK Biobank in the Additional file 1: Table S2 [33–36], and detailed information can be found at the UK Biobank website and elsewhere [37]. Overall, a total of 30 behavioral phenotypes were included in the current study based on their relevance to cognition and mental health. They can be categorized into 4 groups:
Cognitive functioning (n = 17): fluid intelligence (reasoning), prospective memory, reaction time (processing speed), numeric memory (working memory), trail making (2 measures, executive function), symbol digit substitution (2 measures, processing speed), matrix pattern completion (non-verbal fluid reasoning), tower rearranging test (executive function), paired associate learning (verbal declarative memory), and pairs matching (6 measures, visual memory)
Life satisfaction (n = 6): health satisfaction, family relationship satisfaction, friendship satisfaction, financial situation satisfaction, work/job satisfaction, and happiness
Anxiety/depression (n = 4): neuroticism (12-item Eysenck Personality Questionnaire), depression symptoms (9-item Patient Health Questionnaire and the Composite International Diagnostic Interview [CIDI]), and anxiety symptoms (7-item Generalized Anxiety Disorder Questionnaire)
Subjective well-being (n=3): “general happiness,” “happiness with own health,” and “belief life is meaningful”
Association analysis between grip strength and 30 behavioral outcomes
Generalized linear mixed effect models (GLMMs) were employed to characterize how handgrip strength relates to each of the 30 behavioral outcomes adjusting for covariates, including age (in years), gender, education level, socioeconomic status (measured as Townsend deprivation index score), body mass index, height, and waist-to-hip ratio [32]. Following implementations in [8, 9], each behavioral phenotype was modeled as the response variable, and the grip strength and nuisance covariates were modeled as fixed effects. To account for the expected relatedness among data sites, the imaging site was modeled as a random effect, as recommended in studies using UK Biobank data [8, 9]. Depending on the distribution of behavioral phenotypes, LMM, GLMM with binomial error structure and logit link function, and GLMM with Poisson error structure were applied for continuous, binary (e.g., the prospective memory test) and count (e.g., the pairs-matching test: error made) phenotypes. Scores of behavioral phenotypes with significantly positive skew, like reaction time and trail making, were log-transformed. For the association analysis, we reported the relevant summary statistics, and associated two-tailed P values. Moreover, a Benjamini and Hochberg approach [38] was used to adjust for multiple comparisons to control false discovery rate (FDR), which was performed using the ‘p.adjust’ function in R. Specifically, when testing for m hypotheses, this approach firstly orders all P values from lowest to highest, and then identifies the minimum index k such that PFDR=Pk*m/k<significance level. Associations with FDR corrected P-values below 0.05 were considered as significant.
Longitudinal association between grip strength and behavioral phenotypes
Among all 30 behavioral measures, 15 have baseline data that were collected approximately 8.96 ± 1.82 years prior to MRI scanning (N = 3152–40,784). As in prior studies [39, 40], a classic two-wave cross-lagged panel model [6] was estimated using structural equation modeling in Mplus (version 8.3) [41] to determine the longitudinal relationship between grip strength and behavioral outcomes. Specifically, the model examines the relative strength of the cross-lagged correlations between baseline grip strength and each of the 15 phenotypes at 10-year follow-up, as well as between baseline behavioral measures and subsequent grip strength, while adjusting for confounding covariates and the baseline behavioral or grip strength measurements. The model was estimated via maximum likelihood estimation with robust standard errors [39]. We report the standardized regression coefficients and standard errors.
Association analysis of brain structure with grip strength and behavioral outcomes
We used the same analytical framework as described above to assess how regional GMV related to grip strength and behavioral outcomes while simultaneously accounting for confounders. To test whether associations between individual regional GMV and the grip strength or behavioral outcomes were confounded by the total intracranial volume (ICV), we repeated the GLMM analysis by additionally including the total ICV as a covariate. To investigate whether grip strength and 30 behavioral measures have common association maps, we calculated the Pearson’s correlation coefficient of the t-statistic maps between grip strength and each behavioral measure [42].
Mediation analysis
In light of the strong associations between GMV and both grip strength and the behavioral outcomes (see the “Results” section), mediation analysis was performed to examine whether the association can be explained by differences in brain structure while adjusting for confounders [43]. We first calculated the mean GMV of brain regions that were significantly associated with grip strength. Then, mediation analysis was performed using the “mediation” package in R (version 4.1.2) [44]. Specifically, grip strength was used as an independent variable, and each of the behavioral phenotypes was used as a dependent variable. The mean GMV constituted the mediator. The mediation analysis was only performed on grip strength-associated behavioral phenotypes. For behavioral phenotypes whose values showed a longitudinal association with subsequent grip strength, the role of predictor and response variable was exchanged. The same confounding variables as used in the association analysis were controlled here. The significance of the mediation effects was assessed based on 5000 bootstrap iterations.
Results
Behavioral relevance of grip strength to mental health
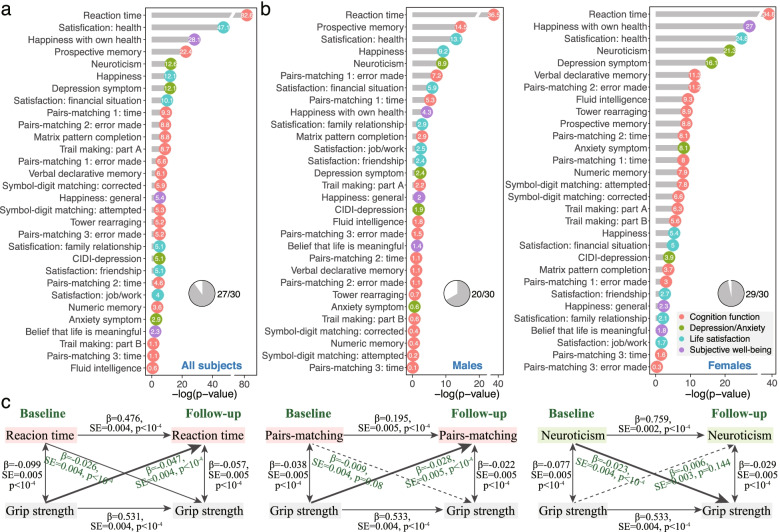
The behavioral analysis included a maximum of 42,843 participants who completed at least one mental health assessment. In general, increased grip strength were associated with young age (r = − 0.156, P < 10−10), male gender (t = 205.74, P < 10−10), above college education (t = 11.99, P < 10−10), low socioeconomical score (r=-0.019, P = 6.67 × 10−5), and with increasing height (r=0.658, P < 10−10), body mass index (r=0.097, P < 10−10), and waist-to-hip ratio (r = 0.418, P < 10−10). Among all covariates, gender showed the greatest association with grip strength. Figure 2a and Additional file 1: Table S3 display the results of the GLMMs in examining the association of grip strength with each of the 30 behavioral phenotypes. Of all these outcomes, 27 were significantly correlated with grip strength while controlling for confounding variables (FDR corrected P < 0.05). All correlations were in the expected direction, with stronger grip strength relating to improved cognitive performance, higher life satisfaction, greater subjective well-being, and lower depression and anxiety symptoms. The strongest effect for the behavioral outcomes was observed for reaction time (N = 40,278, t = − 19.56, FDR corrected P = 2.42 × 10−83) and followed by health satisfaction (N = 42,764, t = − 14.74, P = 7.16 × 10−48), happiness with own health (N = 29,501, t = − 11.35, P = 8.11 × 10−29), and prospective memory (N = 40,530, t = 10.12, odds ratio=1.27, P = 3.75 × 10−23, Additional file 1: Fig. S3). Negative effects were due to behavior task scoring where higher scores index worse performance.
Fig. 2.
Associations between handgrip strength and 30 mental health-related outcomes. a Of the 30 behavioral phenotypes, 27 showed significant association with grip strength and in the expected direction after controlling for confounders: stronger muscular strength was associated with better cognitive performance, higher life satisfaction, greater subjective well-being, and lower depression and anxiety symptoms. Significance is shown as -log10(FDR corrected P-value) and a value above 1.30 is considered statistically significant (-log10(0.05) = 1.30). b When the analyses were stratified by gender, a respective of 29 and 20 behavioral outcomes showed significant association with grip strength in females and males. c Three examples of the longitudinal association between grip strength and behavioral outcomes were revealed by a classic two-wave cross-lagged panel model. For the reaction time test, we observed a significant bi-directional association, i.e., stronger grip strength at baseline was related to better performance on reaction time at the 9-year follow-up, while the reverse was weaker but also significant (FDR corrected P < 10−4); for pairs matching, greater grip strength predicts higher task performance, while the reverse was nonsignificant; For neuroticism, a higher neuroticism score was associated with weaker grip strength measured 9 years later, but the reverse was nonsignificant
When the behavioral analyses were stratified by gender, similar results were found, with the association patterns highly correlated between males and females (r = 0.81, P = 6.64 × 10−8, Fig. 2b). However, the associations in females were generally stronger than those in males, and more behavioral outcomes showed significant associations with grip strength in females (N = 29) than in males (N = 20).
Longitudinal association between grip strength and mental health
Additional file 1: Fig. S4 details full results of the classic two-wave cross-lagged panel model in examining the longitudinal associations between grip strength and 15 mental health outcomes. Overall, results showed that poorer grip strength at baseline was a significant predictor of decreased cognitive performance in fluid intelligence (β = 0.019, FDR corrected P = 0.041), prospective memory (β = 0.025, P = 0.007), reaction time (β = − 0.047, P < 10−4), and pairs matching (β = − 0.028–β =− − 0.016, P < 0.003) at the 9-year follow-up after controlling for confounders and the corresponding baseline cognitive measure, but not for numeric memory (P = 0.952). The reverse was weaker and a significant bi-directional relationship was only found for reaction time (β = − 0.026, P < 10−4). Conversely, the analysis revealed a significant path from baseline mental status measures of neuroticism (β = − 0.023, P < 10−4), health satisfaction (β = − 0.025, P < 10−4), and financial situation satisfaction (β = − 0.024, P = 0.003) to subsequent grip strength, while the reverse was nonsignificant (Fig. 2c).
Neural signatures of grip strength and mental health outcomes
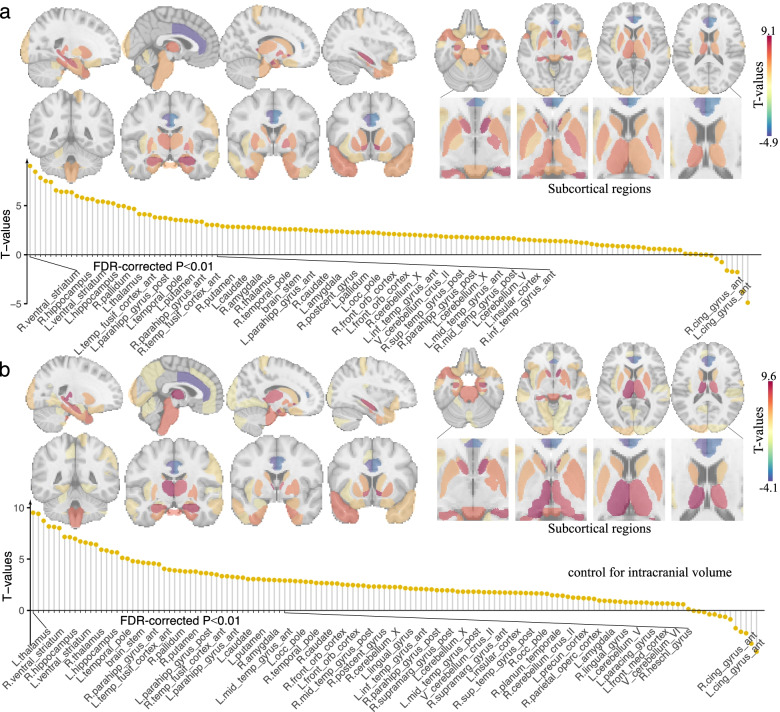
The regional analysis also revealed widespread significant associations between stronger grip strength and increased GMV (FDR corrected P < 0.01, Fig. 3a, Additional file 1: Table S4). When covarying for total ICV and the squared ICV, the results remained nearly unchanged (Fig. 3b, Additional file 1: Table S5), with the association maps highly correlated between the cases with and without adding the total ICV and ICV2 as covariates (r = 0.96, P < 10−30, Additional file 1: Fig. S5), suggesting that associations between grip strength and GMV reflect local variation in GMV across the cortex as opposed to overall brain size. Brain regions showing the highest correlations with grip strength primarily included: the ventral striatum, hippocampus, thalamus, temporal pole, parahippocampal gyrus, temporal fusiform cortex, brain stem, pallidum, and putamen. Regional distribution of associations between GMV and grip strength for males and females can be found in Additional file 1: Fig. S6 and S7.
Fig. 3.
Regional distribution of associations between grey matter volume and grip strength. a The regional analysis revealed widespread significant associations between grip strength and grey matter volume after controlling for potential confounders. T-statistics are visualized here. b The associations remained significant (FDR corrected P < 0.01) after additionally controlling for total intracranial volume, implying that grip strength relates to region GMV independently of overall brain volume. Brain regions showing the highest correlations with grip strength primarily included the ventral striatum, hippocampus, thalamus, temporal pole, parahippocampal gyrus, temporal fusiform cortex, brain stem, pallidum, and putamen
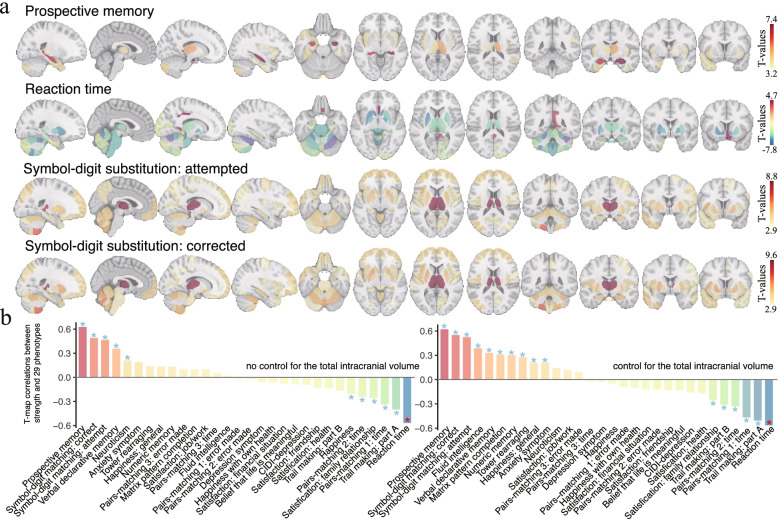
The associations between region-wise GMV and each of the 30 behavioral outcomes were also in the expected direction, regardless of whether the total intracranial volume was added as a covariate (Fig. 4a, Additional file 1: Fig. S8). Moreover, the association map of grip strength was significantly similar to that of the behavioral phenotypes, with absolute correlations ranging from 0.024 to 0.620 (16 of which reach statistical significance, FDR corrected P < 0.05, Fig. 4b). The top 4 behavioral phenotypes showing the highest similarities of association map with grip strength were prospective memory, reaction time, symbol-digit substitution: corrected, and symbol-digit substitution: attempted (Additional file 1: Fig. S9).
Fig. 4.
Regional distribution of associations between grey matter volume and cognitive function. a Significant associations were observed between behavioral phenotypes and regional grey matter volume after controlling for potential confounders (FDR corrected P < 0.01). The top 4 behavioral phenotypes showing the highest similarities of association map with grip strength were visualized here. b Comparison between GMV-grip strength and GMV-behavior association maps. The brain association map of grip strength was highly similar to that of the behavioral phenotypes regardless of whether the total intracranial volume was added as a covariate. The T-statistic map correlations reach statistical significance in 11 and 16 of all 30 behavioral outcomes in cases of including or not including the total intracranial volume as an additional covariate (*FDR corrected P < 0.05)
Mediation effect of GMV
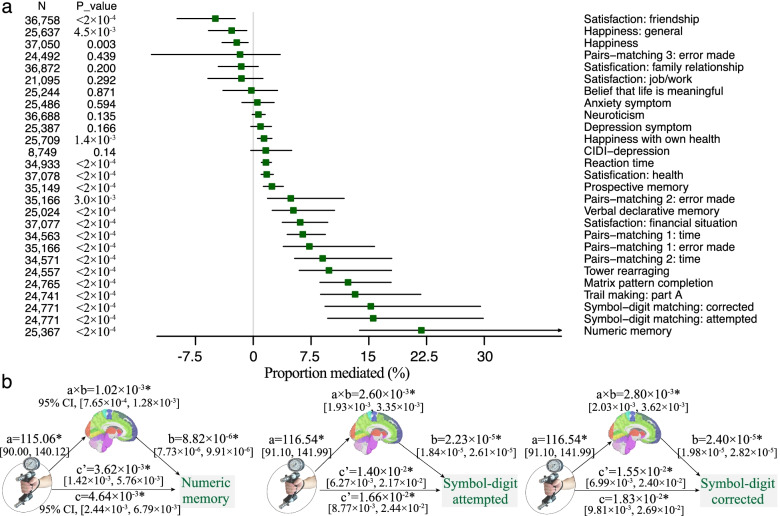
The mean GMV of the significant brain regions shown in Fig. 3 was highly correlated with grip strength (r = 0.485, P < 10−30). Mediation analyses revealed a significant indirect effect (a×b) of the mean GMV in 16 of all 27 behavioral outcomes (FDR corrected P < 0.05, the proportion of mediated effect size ranged from 1.40% to 21.83%, Fig. 5a, Additional file 1: Table S6), indicating that the brain GMV significantly and partially mediated the relationship between grip strength and some of the mental health outcomes. Specifically, the top three behavioral measures having the highest mediation effect size were numeric memory (proportion mediated = 21.83%), symbol-digit substitution: attempted (15.58%), and symbol-digit substitution: corrected (15.26%, Fig. 5b). Mediation effects based on the first principal component of 139 brain regions were provided in Additional file 1: Fig. S10.
Fig. 5.
Mediation effects of brain volume on the association between grip strength and behavioral outcomes. a Mediation effect of mean GMV on the association between grip strength and behavioral outcomes. The proportion of variance explained by the mediation as well as the lower and upper bound of 95% confidence interval was shown. b The top three behavioral phenotypes having the highest mediation effect size were numeric memory (proportion mediated = 21.83%; 95% CI = 13.80%~41.88%; bootstrapping test, FDR corrected P < 2 × 10−4), symbol-digit substitution: attempted (15.58%; 9.66%~29.87%; P < 2 × 10−4), and symbol-digit substitution: corrected (15.26%; 9.31%~29.51%; P < 2 × 10−4). a×b: the indirect effect; c: the total effect; c’: the direct effect
For females, of 29 behavioral outcomes that were significantly associated with grip strength, mediation analyses revealed a significant indirect effect of the mean GMV in 18 behaviors (FDR corrected P < 0.05, Additional file 1: Fig. S6). For males, the mean GMV significantly mediated the association between grip strength and 8 of 20 behavioral outcomes (FDR corrected P < 0.05, Additional file 1: Fig. S7).
Discussion
Using one of the largest available datasets (> 40,000 participants), this study dissected the complex interplay between grip strength, behavioral outcomes, and brain structure. We replicated and extended established relationships between stronger handgrip strength and better mental health, both cross-sectionally and longitudinally. Furthermore, we identified novel associations between grip strength and greater GMV across basal ganglia and limbic regions, and we characterized how patterns of regional GMV associated with grip strength were related to various mental health outcomes. Moreover, we demonstrated that mean regional GMV considerably mediates the association between grip strength and several measures of cognition and mental health.
The reported link between grip strength and cognition aligns with previous studies [13, 15, 45], which suggests that grip strength may serve as a complementary measure of cognitive ability in aging adults. Going beyond limited domains or insensitive cognitive measures, we investigated a broader constellation of cognitive metrics spanning domains of memory, executive function, reasoning, and processing speed that are sensitive to subtle, early changes related to aging. In addition, we investigated several behavioral measures that are closely related to health status but have never been directly related to grip strength [12]. Of the 30 behavioral outcomes examined here, reaction time, which reflects cognitive domains of processing speed [37], demonstrated the most robust association with grip strength. As suggested by Joesh et al. [8], the strong association may be partially explained by the high dependence of the reaction time task on motor speed and dexterity, which are closely related to muscular function of hands. Further, in contrast to other behavioral outcomes that were based on self-report, the cognitive task-measured reaction time scores may be more informative in capturing inter-individual variability. Moreover, according to the general slowing theory, the decline in reaction time is a leading and sensitive indicator of cognitive aging and can lead to decrements in other domains like executive functions and working memory [46]. Deficits in processing speed have been observed across many psychiatric disorders, including schizophrenia and other neurodevelopmental disorders [47, 48], and recent work in the UK Biobank suggests that the association between grip strength and reaction time is weaker in individuals with schizophrenia, depression, and bipolar disorder relative to the general population [8, 9]. In addition, we found that the associations between grip strength and mental health outcomes were stronger in females than in males, implying distinct mechanisms between them. Consistently, a recent study [49] based on Mendelian randomization analysis also identified shared pathways between grip strength and depression in females but not in males. In light of this, it is of interest for future investigations to ascertain any gender specificity of the beneficial effects stemming from physical exercise aiming at enhancing muscular fitness.
These findings increased current understanding on the use of grip strength not only as a proxy of physical fitness but also as a malleable indicator for health status in detecting early impairment of specific domains. Unsurprisingly, some of the associations were small and likely due in part to the fact that we controlled for numerous demographic, socioeconomic, and anthropometric measures that are expected to co-occur with health status [50, 51] and muscular strength [27]. Consequently, the association effects should be interpreted as the relevance of grip strength to these mental health outcomes beyond the contribution of covariates [28]. Moreover, this result also implies the power of large samples in identifying subtle effects that may not be detectable in smaller samples.
Our study also provides evidence for the directionality of the connection between grip strength and mental health. Specifically, the longitudinal analyses showed that baseline grip strength was related to cognitive performance at ~9 years follow-up, while the reverse effect was much weaker, and a significant bi-directional relationship was only found for reaction time. This is consistent with most longitudinal findings that implicate stronger grip strength as a protective factor against cognitive decline and dementia [13, 14, 52, 53] but also supports the notion that changes in these two constructs parallel each other over time [19, 54]. This finding supports the utility of grip strength as a potential treatment target for improved cognitive outcomes in older adults. Indeed, a recent intervention study found that 6 months of resistance training, but not computerized cognitive training, can significantly improve global cognition in older patients with dementia [55]. The bi-directional relationship was also encouraging as they implied that interventions that enhance either muscular fitness or cognitive capabilities may generate beneficial effects on the other [3, 19]. Furthermore, we demonstrated significant paths from baseline neuroticism, health and financial satisfaction to subsequent grip strength. As these measures were pertinent to mental health and life satisfaction, it may suggest that people with greater resilience and satisfaction were more likely to engage in physical activities [19]. Together, the behavioral analyses emphasized the need for increased awareness in clinical practice to incorporate muscular strength into routine assessment and provided insights into possible interventions to prevent cognitive decline during aging.
At the brain level, grip strength revealed mostly significant positive correlations with brain volume, which aligns with previous evidence at the global level [23]. As the global GMV is a general reflection of health status and GM atrophy is a signature of neurodegeneration [27, 28], the identified association pattern may suggest that having stronger muscular strength also relates to better overall brain health [22]. Furthermore, given that many regional GM associations reach a significant effect after controlling for the total ICV, it implies that grip strength may exert a region-specific beneficial effect on brain structure. Specifically, prominent among these select regions were the ventral striatum, hippocampus, thalamus, temporal pole, parahippocampal gyrus, pallidum, and putamen, evidencing the possibility that GMV underlies individual differences in muscular strength. These findings accord with previous evidence suggesting the crucial role these subcortical, limbic (especially the hippocampus), and temporal cortices play in muscular fitness [23, 56, 57].
In addition, we showed that the brain association map of grip strength was highly similar to that of the behavioral phenotypes and that the GMV significantly and partially mediated their associations. These findings raised the possibility that common neurobiological pathways underlie individual differences in grip strength and these behavioral outcomes [58]. Indeed, this hypothesis has gained support from neuroimaging studies linking higher grip strength and better cognitive performance to greater brain volume. For instance, a cross-sectional study in 835 older adults observed that weakness in strength was associated with reduced GMV in the hippocampus and fusiform cortex, which were implicated in high-order cognitive processing and social functioning [57]. Similarly, subcortical nuclei degeneration may underlie the pathogenesis of “cognitive frailty,” which was defined as the simultaneous presence of cognitive decline and physical frailty [56]. Also supportive is the finding showing the enhancement of brain plasticity in older adults following physical training [55, 59–61]. Specifically, Suo et al. showed that 6-month resistance training improved not only overall cognitive performance but also elicited GM expansion for participants at risk for dementia [55]. This study, together with ours, suggests that the relationship between muscular fitness and mental health may be mediated by increased GMV in regions having high plasticity like the hippocampus.
The present findings are consistent with existing evidence of how musculoskeletal strength relates to brain health. Skeletal muscle plays a crucial role in the production and secretion of many cytokines such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor-1 [14], which are involved in neuronal survival, synaptic development, angiogenesis, learning, and neural plasticity [62]. Higher levels of these peptides have been correlated with greater physical fitness and increased GMV [58], and more importantly, studies showed that resistance activities could stimulate the release of BDNF and evoke neuroplastic changes in frontal and hippocampal regions, which may further translate into cognitive improvements [63–65]. Therefore, cytokine-induced alterations in GMV may represent a mechanism through which muscular fitness influences cognition and mental status, yet further research is needed [66].
The results from both cross-sectional and longitudinal analyses indicated a significant association between grip strength and cognitive functioning and mental status. This implies that grip strength can be used as a complementary measure of mental health in aging adults and the routine assessment should be recommended in clinical practice. The large sample size (N > 40,000), sufficient control of confounders (including demographic, anthropometric, and socioeconomic covariates), use of multiple-comparisons correction, subgroup sensitivity analyses, and the longitudinal design ensure our current results are reliable and less likely to suffer from replication failure [67]. Moreover, our results regarding the association between grip strength, mental health, and brain structure are mostly consistent with existing small-sampled studies. There are some limitations to be acknowledged. First, we report statistical mediation effects that are strictly measures of association [39, 68], and causal inferences cannot be drawn from these models without further validation using randomized controlled trials. Nevertheless, these analyses represent a critical first step in characterizing associations between grip strength, brain structure, and mental health that can be further explored in longitudinal studies. To facilitate the use of grip strength in clinical settings, examining how interventions to enhance muscular strength would influence cognition capacities and brain health, especially in people with psychiatric disorders, is necessary. Second, as even small effects can reach statistical significance in a large sample, the magnitude of association may not be directly translated into clinical utility [69]. Third, as noted by Genon et al. [70], brain structure-behavior association studies are suffering from a replication crisis, where a poor replicability has been shown in both behavioral measurements and brain structure estimates. As such, reliability and replicability of the current findings merits further examination in external cohorts with great diversity in geographic, demographic, and sociocultural aspects. Further, going beyond statistical univariate approaches, further studies can take into account the multivariate nature of structural and behavioral measurements by leveraging machine learning techniques within cross-validated frameworks [71–73]. For in-depth discussion of this topic, we point the interested reader to [70]. Forth, some of the behavioral outcomes were assessed by ordinal measures, which represent different levels of fidelity [28]. It is possible that assessing these ordinal outcomes using continuous measures would prove more informative. Finally, there may be some other behavioral phenotypes that are related to health outcomes in the UK Biobank but not examined here. Future studies can examine the associations of grip strength with these less-commonly used outcomes.
Conclusions
In sum, the current study showed that stronger grip strength was associated with better mental health, cross-sectionally and longitudinally. At the brain level, we found widespread associations between grip strength and greater GMV in subcortical and temporal cortices. Moreover, these GMV also correlated with better mental health and considerably mediated the effect of grip strength on cognitive functioning. Overall, our finding provides insights into the complex interplay between grip strength, mental health, and brain structure.
Supplementary Information
Additional file 1: Table S1. Neurological conditions/incidents that are used to exclude participants in UK Biobank. Table S2. UK Biobank cognition and mental health measures used in the current study. Table S3. Association between grip strength and behavioral phenotypes. Table S4. Brain regions showing significant correlations with grip strength. Table S5. Brain regions showing significant correlations with grip strength after controlling for the total intracranial volume. Table S6. Results of the mediation analyses between grip strength, phenotypes, and mean grey matter volume. Fig. S1. A brief summary of the population characteristics of all participants used in the current study. Fig. S2. The 139 brain regions and their names. Fig. S3. The top four behavioral outcomes showing the strongest associations with grip strength. Fig. S4. Results of the classic two-wave cross-lagged panel model for 15 behavioral phenotypes that have complete data at two time points. Fig. S5. The correlation of association maps (T-maps) between the cases with and without including the total intracranial volume (ICV) and the squared ICV as covariates in examining the association of grip strength with regional grey matter volume across 139 regions. Fig. S6. Regional distribution of associations between grey matter volume and grip strength and the mediation effect of mean GMV in females. Fig. S7. Regional distribution of associations between grey matter volume and grip strength and the mediation effect of mean GMV in males. Fig. S8. The correlation of T-maps between the cases with and without including the total intracranial volume as a covariate in examining the association of grey matter volumes with behavioral outcomes across 139 regions. Fig. S9. Regional distribution of associations between grey matter volume and four representative behavioral phenotypes. Fig. S10. Mediation effects of the first principal component of 139 regional GMV on the association between grip strength and behavioral outcomes.
Acknowledgements
This research was conducted using the UK Biobank resources (application number 34175 and 49636). All data used in this study are publicly accessible from UK Biobank via their standard data access procedure (https://www.ukbiobank.ac.uk/).
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- FDR
False discovery rate
- GLMM
Generalized linear mixed effect model
- GMV
Grey matter volume
- ICV
Intracranial volume
- LMM
Linear mixed effect model
Authors’ contributions
R.J. and D.S. conceived and designed the experiment. R.J. performed the analyses with support from D.S., and W.D. R.J., M.W., and D.S. drafted the manuscript with contributions from S.N., M.W., M.R, and V.C. and comments from all other authors. All authors read and approved the final manuscript.
Funding
This work is supported in part by the National Institute of Mental Health (K00MH122372), NSF (2112455), NIH (R01MH118695), and the National Institute on Drug Abuse (T32DA022975).
Availability of data and materials
All data used in this study are publicly accessible from UK Biobank via their standard data access procedure at https://www.ukbiobank.ac.uk/. Researchers can apply for access to the UK Biobank data via the Access management System (AMS) (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). Code used in the current study is available from the authors upon reasonable request and can be found at can be found at https://github.com/Jiang-brain/Grip-strength-association.
Declarations
Ethics approval and consent to participate
The UK Biobank study was approved by the North West Multicenter Research Ethics Committee (No. 11/NW/0382), with written informed consent obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rongtao Jiang, Email: rongtao.jiang@yale.edu.
Dustin Scheinost, Email: dustin.scheinost@yale.edu.
References
- 1.Jiang R, Scheinost D, Zuo N, Wu J, Qi S, Liang Q, et al. A neuroimaging signature of cognitive aging from whole-brain functional connectivity. Adv Sci (Weinh). 2022:e2201621. (in press) [DOI] [PMC free article] [PubMed]
- 2.Willems SM, Wright DJ, Day FR, Trajanoska K, Joshi PK, Morris JA, Matteini AM, Garton FC, Grarup N, Oskolkov N, et al. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat Commun. 2017;8:16015. doi: 10.1038/ncomms16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGrath R, Johnson N, Klawitter L, Mahoney S, Trautman K, Carlson C, Rockstad E, Hackney KJ. What are the association patterns between handgrip strength and adverse health conditions? A topical review. Sage Open Med. 2020;8:2050312120910358. doi: 10.1177/2050312120910358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 5.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? - Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35(4):409–415. doi: 10.1093/ageing/afl024. [DOI] [PubMed] [Google Scholar]
- 6.Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ. 2012;345:e7279. doi: 10.1136/bmj.e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petermann-Rocha F, Lyall DM, Gray SR, Esteban-Cornejo I, Quinn TJ, Ho FK, Pell JP, Celis-Morales C. Associations between physical frailty and dementia incidence: a prospective study from UK Biobank. Lancet Health Longev. 2020;1(2):E58–E68. doi: 10.1016/S2666-7568(20)30007-6. [DOI] [PubMed] [Google Scholar]
- 8.Firth J, Firth JA, Stubbs B, Vancampfort D, Schuch FB, Hallgren M, Veronese N, Yung AR, Sarris J. Association between muscular strength and cognition in people with major depression or bipolar disorder and healthy controls. JAMA Psychiat. 2018;75(7):740–746. doi: 10.1001/jamapsychiatry.2018.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firth J, Stubbs B, Vancampfort D, Firth JA, Large M, Rosenbaum S, Hallgren M, Ward PB, Sarris J, Yung AR. Grip strength is associated with cognitive performance in schizophrenia and the general population: a UK Biobank study of 476 559 participants. Schizophr Bull. 2018;44(4):728–736. doi: 10.1093/schbul/sby034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clery-Melin ML, Schmidt L, Lafargue G, Baup N, Fossati P, Pessiglione M. Why don't you try harder? An investigation of effort production in major depression. PLoS One. 2011;6(8):e23178. doi: 10.1371/journal.pone.0023178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnelle V, Manohar S, Behrens T, Husain M. Individual differences in premotor brain systems underlie behavioral apathy. Cereb Cortex. 2016;26(2):807–819. doi: 10.1093/cercor/bhv247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyu-Man H, Jisoon C, Ho-Kyoung Y, Young-Hoon K, Byung-Joo H, Yong-Ku K, Changsu H. Relationships between hand-grip strength, socioeconomic status, and depressive symptoms in community-dwelling older adults. J Affect Disord. 2019;252:263–270. doi: 10.1016/j.jad.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Kunutsor SK, Isiozor NM, Voutilainen A, Laukkanen JA. Handgrip strength and risk of cognitive outcomes: new prospective study and meta-analysis of 16 observational cohort studies. Geroscience. 2022. (in press) [DOI] [PMC free article] [PubMed]
- 14.Cui M, Zhang S, Liu Y, Gang X, Wang G. Grip strength and the risk of cognitive decline and dementia: a systematic review and meta-analysis of longitudinal cohort studies. Front Aging Neurosci. 2021;13:625551. doi: 10.3389/fnagi.2021.625551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fritz NE, McCarthy CJ, Adamo DE. Handgrip strength as a means of monitoring progression of cognitive decline - a scoping review. Ageing Res Rev. 2017;35:112–123. doi: 10.1016/j.arr.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Veldsman M, Tai XY, Nichols T, Smith S, Peixoto J, Manohar S, Husain M. Cerebrovascular risk factors impact frontoparietal network integrity and executive function in healthy ageing. Nat Commun. 2020;11(1):4340. doi: 10.1038/s41467-020-18201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Oh JW, Son N-H, Chung W. Association between handgrip strength and cognitive function in older adults: Korean Longitudinal Study of Aging (2006–2018) Int J Environ Res Public Health. 2022;19(3):1048. doi: 10.3390/ijerph19031048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taekema DG, Ling CHY, Kurrle SE, Cameron ID, Meskers CGM, Blauw GJ, Westendorp RGJ, de Craen AJM, Maier AB. Temporal relationship between handgrip strength and cognitive performance in oldest old people. Age Ageing. 2012;41(4):506–512. doi: 10.1093/ageing/afs013. [DOI] [PubMed] [Google Scholar]
- 19.Kim GR, Sun J, Han M, Nam CM, Park S. Evaluation of the directional relationship between handgrip strength and cognitive function: the Korean Longitudinal Study of Ageing. Age Ageing. 2019;48(3):426–432. doi: 10.1093/ageing/afz013. [DOI] [PubMed] [Google Scholar]
- 20.Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Weitnauer L, Frisch S, Melie-Garcia L, Preisig M, Schroeter ML, Sajfutdinow I, Kherif F, Draganski B. Mapping grip force to motor networks. NeuroImage. 2021;229:117735. doi: 10.1016/j.neuroimage.2021.117735. [DOI] [PubMed] [Google Scholar]
- 22.Kant IMJ, de Bresser J, van Montfort SJT, Aarts E, Verlaan J-J, Zacharias N, Winterer G, Spies C, Slooter AJC, Hendrikse J. The association between brain volume, cortical brain infarcts, and physical frailty. Neurobiol Aging. 2018;70:247–253. doi: 10.1016/j.neurobiolaging.2018.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilgour AHM, Todd OM, Starr JM. A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatr. 2014;14(1):1–35. doi: 10.1186/1471-2318-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O'Connell J, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-41018-40579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, Bartsch AJ, Jbabdi S, Sotiropoulos SN, Andersson JL, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523–1536. doi: 10.1038/nn.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray JC, Thompson M, Bachman C, Owens MM, Murphy M, Palmer R. Associations of cigarette smoking with gray and white matter in the UK Biobank. Neuropsychopharmacology. 2020;45(7):1215–1222. doi: 10.1038/s41386-020-0630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchanan CR, Fawns-Ritchie C, Barbu MC, De Nooij L, Reus LM, et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019;40(28):2290–2300. doi: 10.1093/eurheartj/ehz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfaro-Almagro F, Jenkinson M, Bangerter NK, Andersson JLR, Griffanti L, Douaud G, Sotiropoulos SN, Jbabdi S, Hernandez-Fernandez M, Vallee E, et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 32.Firth JA, Smith L, Sarris J, Vancampfort D, Schuch F, Carvalho AF, Solmi M, Yung AR, Stubbs B, Firth J. Handgrip strength is associated with hippocampal volume and white matter hyperintensities in major depression and healthy controls: a UK biobank study. Psychosom Med. 2020;82(1):39–46. doi: 10.1097/PSY.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 33.Nagel M, Watanabe K, Stringer S, Posthuma D, van der Sluis S. Item-level analyses reveal genetic heterogeneity in neuroticism. Nat Commun. 2018;9(1):905. doi: 10.1038/s41467-018-03242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson LP, McCauley E, Grossman DC, McCarty CA, Richards J, Russo JE, Rockhill C, Katon W. Evaluation of the Patient Health Questionnaire-9 Item for detecting major depression among adolescents. Pediatrics. 2010;126(6):1117–1123. doi: 10.1542/peds.2010-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman MG, Zuellig AR, Kachin KE, Constantino MJ, Przeworski A, Erickson T, Cashman-McGrath L. Preliminary reliability and validity of the Generalized Anxiety Disorder Questionnaire-IV: a revised self-report diagnostic measure of generalized anxiety disorder. Behav Ther. 2002;33(2):215–233. doi: 10.1016/S0005-7894(02)80026-0. [DOI] [Google Scholar]
- 36.Davis KAS, Cullen B, Adams M, Brailean A, Breen G, Coleman JRI, Dregan A, Gaspar HA, Hubel C, Lee W, et al. Indicators of mental disorders in UK Biobank-a comparison of approaches. Int J Methods Psychiatr Res. 2019;28(3):e1796. doi: 10.1002/mpr.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. PLoS One. 2020;15(4):e0231627. doi: 10.1371/journal.pone.0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 39.Gong W, Rolls ET, Du J, Feng J, Cheng W. Brain structure is linked to the association between family environment and behavioral problems in children in the ABCD study. Nat Commun. 2021;12(1):1–10. doi: 10.1038/s41467-020-20314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng W, Rolls E, Gong W, Du J, Zhang J, Zhang XY, Li F, Feng J. Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol Psychiatry. 2021;26(8):3992–4003. doi: 10.1038/s41380-020-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kessler RC. Linear panel analysis: models of quantitative change. Cambridge: Elsevier; 2014.
- 42.Lee JJ, Kim HJ, Ceko M, Park BY, Lee SA, Park H, Roy M, Kim SG, Wager TD, Woo CW. A neuroimaging biomarker for sustained experimental and clinical pain. Nat Med. 2021;27(1):174–182. doi: 10.1038/s41591-020-1142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 44.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. 2014. [Google Scholar]
- 45.Taekema DG, Gussekloo J, Maier AB, Westendorp RGJ, de Craen AJM. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39(3):331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 46.Finkel D, McArdle JJ, Reynolds CA, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22(3):558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- 47.Kelleher I, Murtagh A, Clarke MC, Murphy J, Rawdon C, Cannon M. Neurocognitive performance of a community-based sample of young people at putative ultra high risk for psychosis: support for the processing speed hypothesis. Cogn Neuropsychiatry. 2013;18(1-2):9–25. doi: 10.1080/13546805.2012.682363. [DOI] [PubMed] [Google Scholar]
- 48.Knowles EEM, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167(7):828–835. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- 49.Jones G, Trajanoska K, Santanasto AJ, Stringa N, Kuo CL, Atkins JL, Lewis JR, Duong T, Hong S, Biggs ML, et al. Genome-wide meta-analysis of muscle weakness identifies 15 susceptibility loci in older men and women. Nat Commun. 2021;12(1):654. doi: 10.1038/s41467-021-20918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall AT, Betts S, Kan EC, McConnell R, Lanphear BP, Sowell ER. Association of lead-exposure risk and family income with childhood brain outcomes. Nat Med. 2020;26(1):91–97. doi: 10.1038/s41591-019-0713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daviet R, Aydogan G, Jagannathan K, Spilka N, Koellinger PD, Kranzler HR, Nave G, Wetherill RR. Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat Commun. 2022;13(1):1–11. doi: 10.1038/s41467-022-28735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chou MY, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, Otsuka R, Chen LK, Arai H. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019;19(1):186. doi: 10.1186/s12877-019-1199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sternang O, Reynolds CA, Finkel D, Ernsth-Bravell M, Pedersen NL, Dahl Aslan AK. Grip strength and cognitive abilities: associations in old age. J Gerontol B Psychol Sci Soc Sci. 2016;71(5):841–848. doi: 10.1093/geronb/gbv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGrath R, Vincent BM, Hackney KJ, Robinson-Lane SG, Downer B, Clark BC. The longitudinal associations of handgrip strength and cognitive function in aging Americans. J Am Med Dir Assoc. 2020;21(5):634–639. doi: 10.1016/j.jamda.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suo C, Singh MF, Gates N, Wen W, Sachdev P, Brodaty H, Saigal N, Wilson GC, Meiklejohn J, Singh N, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry. 2016;21(11):1633–1642. doi: 10.1038/mp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wan M, Xia R, Lin H, Qiu P, He J, Ye Y, Tao J, Chen L, Zheng G. Volumetric and diffusion abnormalities in subcortical nuclei of older adults with cognitive frailty. Front Aging Neurosci. 2020;12:202. doi: 10.3389/fnagi.2020.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishita Y, Nakamura A, Kato T, Otsuka R, Iwata K, Tange C, Ando F, Ito K, Shimokata H, Arai H. Links between physical frailty and regional gray matter volumes in older adults: a voxel-based morphometry study. J Am Med Dir Assoc. 2019;20(12):11587–11592. doi: 10.1016/j.jamda.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Lu SH, Herold F, Zhang YJ, Lei YR, Kramer AF, Jiao C, Yu Q, Doig S, Li JM, Yan Z, et al. Higher handgrip strength is linked to better cognitive performance in Chinese adults with hypertension. Brain Sci. 2021;11(8):985. doi: 10.3390/brainsci11080985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batouli SAH, Saba V. At least eighty percent of brain grey matter is modifiable by physical activity: a review study. Behav Brain Res. 2017;332:204–217. doi: 10.1016/j.bbr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35(Suppl 2):S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vancampfort D, Stubbs B, Firth J, Smith L, Swinnen N, Koyanagi A. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low- and middle-income countries. Int J Geriatr Psychiatry. 2019;34(4):609–616. doi: 10.1002/gps.5061. [DOI] [PubMed] [Google Scholar]
- 63.Jafarzadeh G, Shakerian S, Farbood Y, Ghanbarzadeh M. Effects of eight weeks of resistance exercises on neurotrophins and trk receptors in Alzheimer model male Wistar rats. Basic Clin Neurosci. 2021;12(3):349. doi: 10.32598/bcn.2021.2067.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I, Delecluse C, Bautmans I. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp Gerontol. 2015;70:144–149. doi: 10.1016/j.exger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements–a systematic review. Eur Rev Aging Phys Act. 2019;16(1):1–33. doi: 10.1186/s11556-019-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang X, Meng X, Xiao X, Xie Z, Yuan X. Grip training improves handgrip strength, cognition, and brain white matter in minor acute ischemic stroke patients. Clin Neurol Neurosurg. 2021;209:106886. doi: 10.1016/j.clineuro.2021.106886. [DOI] [PubMed] [Google Scholar]
- 67.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Hendrickson TJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lees B, Mewton L, Jacobus J, Valadez EA, Stapinski LA, Teesson M, Tapert SF, Squeglia LM. Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the adolescent brain cognitive development study. Am J Psychiatry. 2020;177(11):1060–1072. doi: 10.1176/appi.ajp.2020.20010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: a review. JAMA Psychiat. 2020;77(5):534–540. doi: 10.1001/jamapsychiatry.2019.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Genon S, Eickhoff SB, Kharabian S. Linking interindividual variability in brain structure to behaviour. Nat Rev Neurosci. 2022;23(5):307–318. doi: 10.1038/s41583-022-00584-7. [DOI] [PubMed] [Google Scholar]
- 71.Sui J, Jiang R, Bustillo J, Calhoun V. Neuroimaging-based individualized prediction of cognition and behavior for mental disorders and health: methods and promises. Biol Psychiatry. 2020;88(11):818–828. doi: 10.1016/j.biopsych.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woo CW, Chang LJ, Lindquist MA, Wager TD. Building better biomarkers: brain models in translational neuroimaging. Nat Neurosci. 2017;20(3):365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19(1):165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Neurological conditions/incidents that are used to exclude participants in UK Biobank. Table S2. UK Biobank cognition and mental health measures used in the current study. Table S3. Association between grip strength and behavioral phenotypes. Table S4. Brain regions showing significant correlations with grip strength. Table S5. Brain regions showing significant correlations with grip strength after controlling for the total intracranial volume. Table S6. Results of the mediation analyses between grip strength, phenotypes, and mean grey matter volume. Fig. S1. A brief summary of the population characteristics of all participants used in the current study. Fig. S2. The 139 brain regions and their names. Fig. S3. The top four behavioral outcomes showing the strongest associations with grip strength. Fig. S4. Results of the classic two-wave cross-lagged panel model for 15 behavioral phenotypes that have complete data at two time points. Fig. S5. The correlation of association maps (T-maps) between the cases with and without including the total intracranial volume (ICV) and the squared ICV as covariates in examining the association of grip strength with regional grey matter volume across 139 regions. Fig. S6. Regional distribution of associations between grey matter volume and grip strength and the mediation effect of mean GMV in females. Fig. S7. Regional distribution of associations between grey matter volume and grip strength and the mediation effect of mean GMV in males. Fig. S8. The correlation of T-maps between the cases with and without including the total intracranial volume as a covariate in examining the association of grey matter volumes with behavioral outcomes across 139 regions. Fig. S9. Regional distribution of associations between grey matter volume and four representative behavioral phenotypes. Fig. S10. Mediation effects of the first principal component of 139 regional GMV on the association between grip strength and behavioral outcomes.
Data Availability Statement
All data used in this study are publicly accessible from UK Biobank via their standard data access procedure at https://www.ukbiobank.ac.uk/. Researchers can apply for access to the UK Biobank data via the Access management System (AMS) (https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access). Code used in the current study is available from the authors upon reasonable request and can be found at can be found at https://github.com/Jiang-brain/Grip-strength-association.