Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the end of 2019, thousands of SARS-CoV-2 mutations have been increasingly observed. The virus epidemic has posed unprecedented challenges to global public health. As of September 19, 2022, over 609 million confirmed cases and about 6.50 million deaths worldwide have been reported to the World Health Organization. The development of novel antivirals capable of treating SARS-CoV-2 infections has been a central focus of current research. To cope with the current novel coronavirus pneumonia (coronavirus disease 2019 [COVID-19]) epidemic, the National Medical Products Administration granted conditional authorization for Azvudine developed by us to be used to treat COVID-19 on July 25, 2022, being the first domestic oral antiviral agent approved in China. On August 9, Azvudine was approved by the National Health Commission and National Administration of Traditional Chinese Medicine to be included in the “Diagnosis and Treatment Program for Novel Coronavirus Pneumonia (Ninth Edition)” for the treatment of adult patients with common COVID-19. Azvudine was later approved by the National Healthcare Security Administration on August 12, 2022, to be included in the medical reimbursement list.
Similar to natural nucleosides, nucleoside derivatives are first transformed into active nucleoside triphosphates by cellular kinases inside the host cells, which are then utilized as raw materials and incorporated into virus RNA, finally leading to virus replication inhibition. Unlike natural nucleosides, such modified nucleosides are generally believed to possess low toxicity. Mechanistically, nucleoside analogs also suppress viral replication through inhibiting DNA- and RNA-dependent polymerases. These characteristics make modified nucleosides therapeutically promising in antiviral therapy. Several modified nucleosides are currently being assessed for antiviral therapy.1 However, the emergence of resistant variants has posed new challenges to the nucleoside-based antiviral drug discovery. 4′-Modified nucleosides, capable of preventing the emergence of drug resistance, represent an important subclass of nucleoside analogs for antiviral therapies, and several 4′-modified nucleosides are being clinically assessed. The underlying mechanism is that the 3′-OH group of 4′-modified nucleosides is difficult to be discriminated by viruses and is chemically unreactive for chain elongation.
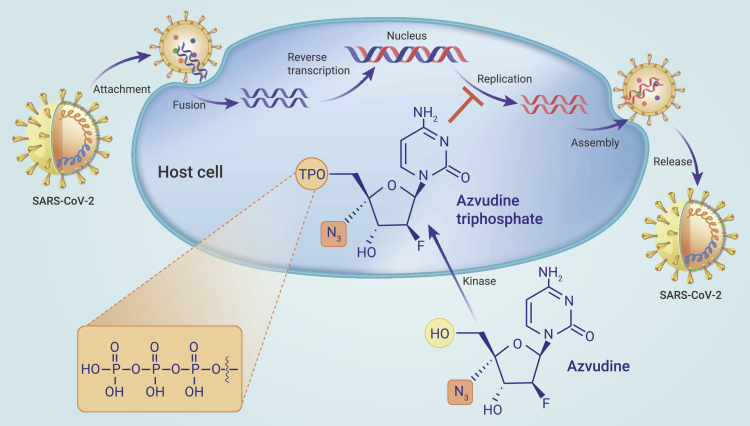
Based on above studies, our group initiated an antiviral drug discovery campaign and focused the discovery of novel nucleosides for antiviral therapies. Particularly, Azvudine (FNC, 2′-deoxy-2′-β-fluoro-4′-azidocytidine) targets reverse transcriptase and HIV-1 accessory protein (Vif) simultaneously, being the first-in-class dual inhibitor.2 In July 2021, Azvudine was approved by the National Medical Products Administration to treat adult patients infected by HIV-1. As an RNA-dependent RNA polymerase (RdRp) inhibitor, Azvudine was then investigated for its therapeutic potential against SARS-CoV-2. Studies demonstrated that Azvudine was initially phosphorylated by cellular kinases in the host cells, and the active triphosphate was then embedded into virus RNA during SARS-CoV-2 RNA synthesis, finally terminating RNA replication (Figure 1).3 Azvudine inhibited SARS-CoV-2 with an EC50 value ranging from 1.2 to 4.3 μM and a selective index of 15–83.4 In rats, the thymus was a key organ for Azvudine to fight against SARS-CoV-2 infection. The results showed that Azvudine and the active phosphorylated triphosphate were mainly concentrated in the thymus and peripheral blood mononuclear cells, showing an immune-targeting feature. This profile is unique among existing RdRp inhibitors. In SARS-CoV-2-infected rhesus macaque models, Azvudine reduced viral load, improved lymphocyte profiles, alleviated inflammation and organ damage, recuperated the thymus, and lessened ground-glass opacities. Single-cell sequencing studies demonstrated that Azvudine promoted thymus function. These studies indicate that Azvudine may cure COVID-19 patients through the thymus-homing feature and immunity promotion.
Figure 1.
Mechanism of Azvudine against SARS-CoV-2
In a preliminary randomized, open-label, controlled clinical trial, 20 mild and common COVID-19 patients were enrolled and randomly assigned to receive Azvudine (n = 10) and/or standard antiviral treatment (control group, n = 10). The group receiving Azvudine treatment had an average time of 2.60 days of the first nucleic acid negative conversion (NANC), while for patients receiving standard antiviral treatment (interferon alpha, kaletra and ribavirin, chloroquine phosphate, and hydroxychloroquine sulfate), the mean time of NANC was 5.60 days.5 A randomized, single-arm clinical trial on compassionate use (n = 31) showed that all COVID-19 patients receiving oral administration of Azvudine (5 mg, once daily, n = 15) were recovered, with 100% viral NANC in 3.29 ± 2.22 days and 100% hospital discharge rate in 9.00 ± 4.93 days.4 The clinical outcome was better than those who took Azvudine plus traditional Chinese medicine or/and other antivirals (interferon alpha, arbidol, Lopinavir/ritonavir, chloroquine, n = 16). In this study, minor and transient dizziness and nausea were observed in five patients receiving Azvudine treatment. In a phase 3 multicenter, randomized, double-blind, placebo-controlled study (n = 280), Azvudine significantly shortened the symptom improvement time of patients with moderate SARS-CoV-2 infection, increased the proportion of patients with improved clinical symptoms, and achieved clinically superior results. 40.43% of the COVID-19 patients (n = 142) who were first given Azvudine for 7 days had improved clinical symptoms, compared with 10.87% of those given a placebo (n = 138, p < 0.001). Relative to the placebo group, the median time of clinical symptom improvement in subjects given Azvudine was statistically significant (p < 0.001). Azvudine inhibited SARS-CoV-2 with the virus clearance time of about 5 days. Compared with the control group, the viral load of the Azvudine group decreased by an average of 1.56 log10 on the fifth day of medication. Azvudine was also effective against virus variants (Alpha, Beta, Delta, and Omicron). In terms of the safety, Azvudine (5 mg per person per day) was well tolerated and did not increase the risk of subjects, and the incidence of adverse events was not statistically different between the Azvudine group and the placebo group. The exposure or drug concentration of Azvudine at clinical therapeutic doses was far lower than the doses causing side effects in non-clinical trials, showing a good therapeutic safety window. The risk of carcinogenicity of Azvudine is currently being studied.
In addition to Azvudine, the FDA granted the emergency use of another two nucleoside-based RdRp inhibitors, Molnupiravir (developed by Merck) and Remdesivir (developed by Gilead Sciences), for treating hospitalized patients with mild-to-moderate and severe COVID-19, respectively. Molnupiravir is the first oral and direct-acting antiviral prodrug. The results from a phase 3, double-blind, randomized, placebo-controlled trial with a total of 1433 participants have shown that early Molnupiravir treatment reduces the risk of hospitalization or death in at-risk, unvaccinated COVID-19 adult patients. Remdesivir (Veklury), a broad-spectrum antiviral agent, is the first FDA-approved drug for hospitalized adults with COVID-19. Remdesivir shows superiority to placebo in shortening the recovery time and reduces respiratory tract infection. All these studies suggest that existing broad-spectrum nucleoside-based antiviral agents could be repurposed for COVID-19 treatment.
Apart from the polymerases, targeting the proteases in SARS-CoV-2 is another feasible strategy to inhibit virus replication for treating COVID-19. Paxlovid, developed by Pfizer, is a co-packaged oral medication consisting of Nirmatrelvir (PF-07321332) and Ritonavir. Paxlovid is used to treat mild-to-moderate COVID-19 pediatric and adult patients and those at high risk for progression to severe COVID-19. Nirmatrelvir acts as an orally active 3C-like protease inhibitor, while Ritonavir is a protease inhibitor, which helps maintain stability of Nirmatrelvir in the body at higher concentrations. Baricitinib (Olumiant), a Janus kinase (JAK1/2) inhibitor discovered by Incyte and later licensed to Eli Lilly, was first approved in 2018 for treating rheumatoid arthritis. As the first immunomodulatory treatment for COVID-19, Baricitinib, alone or in combination with Remdesivir, was authorized under the Emergency Use Authorization for hospitalized patients requiring breathing help.
Since the outbreak of the COVID-19 epidemic, numerous efforts have been devoted to coping with the challenges by developing highly efficacious and safe antiviral drugs and vaccines. To date, several drugs including small-molecule therapeutics and monoclonal antibodies have been approved globally for COVID-19 treatment. Most antivirals target the polymerases and proteases that are essential for SARS-CoV-2 replication. Particularly, modified nucleosides show great promise for antiviral therapies. As a broad-spectrum antiviral agent, Azvudine may provide hopes to treat patients with HIV/HBV and HIV/HCV co-infections. We believe that Azvudine could cope with the current COVID-19 epidemic and is potentially to be used to treat future coronavirus infections as well as other coronavirus-related diseases. Although great successes have been observed, issues such as the potential toxicity profiles of existing antivirals and SARS-CoV-2 mutations pose continuous challenges to our community. Mutations in SARS-CoV-2 variants already observed in infected people make the patients less susceptible to existing drugs. Therefore, a multipronged approach will be highly desirable to address these challenges.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 82130103 and U1804283), National Key R&D Program Emergency Response Project for Novel Coronavirus Pneumonia (no. 2021YFC0864700), and Henan Province Novel Coronavirus Control and Prevention Emergency Science and Technology Tackling Key Project (no. 201100311500).
Declaration of interests
The authors declare no competing interests.
Published Online: September 9, 2022
References
- 1.Chang J. 4′-Modified nucleosides for antiviral drug discovery: Achievements and perspectives. Acc. Chem. Res. 2022;55:565–578. doi: 10.1021/acs.accounts.1c00697. [DOI] [PubMed] [Google Scholar]
- 2.Sun L., Peng Y., Yu W., et al. Mechanistic insight into antiretroviral potency of 2′-deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention. J. Med. Chem. 2020;63:8554–8566. doi: 10.1021/acs.jmedchem.0c00940. [DOI] [PubMed] [Google Scholar]
- 3.Yu B., Chang J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Sig. Transduct. Target Ther. 2020;5:236. doi: 10.1038/s41392-020-00351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J.L., Li Y.H., Wang L.L., et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Sig. Transduct. Target Ther. 2021;6:414. doi: 10.1038/s41392-021-00835-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren Z., Luo H., Yu Z., et al. A Randomized, open-label, controlled clinical trial of Azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. 2020;7:2001435. doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]