Abstract
Following the report of a non-travel-associated cluster of monkeypox cases by the United Kingdom in May 2022, 41 countries across the WHO European Region have reported 21,098 cases and two deaths by 23 August 2022. Nowcasting suggests a plateauing in case notifications. Most cases (97%) are MSM, with atypical rash-illness presentation. Spread is mainly through close contact during sexual activities. Few cases are reported among women and children. Targeted interventions of at-risk groups are needed to stop further transmission.
Keywords: Monkeypox, MPX, European Region, outbreak, orthopoxvirus
Since detection of monkeypox virus (MPXV) transmission outside endemic areas in May 2022, a large multi-country monkeypox (MPX) outbreak has been ongoing worldwide, with 42,807 cases and 12 deaths reported in 97 Member States across six World Health Organization (WHO) Regions by 23 August 2022 [1]. On 23 July, the WHO Director General declared this outbreak a public health emergency of international concern (PHEIC) [2]. Here we describe the epidemiological features of MPX and analyse disease severity as well as the effect of prior smallpox vaccination on all cases in the WHO European Region reported in TESSy up to 23 August 2022 to inform optimal public health responses.
Epidemiological situation in the WHO European Region
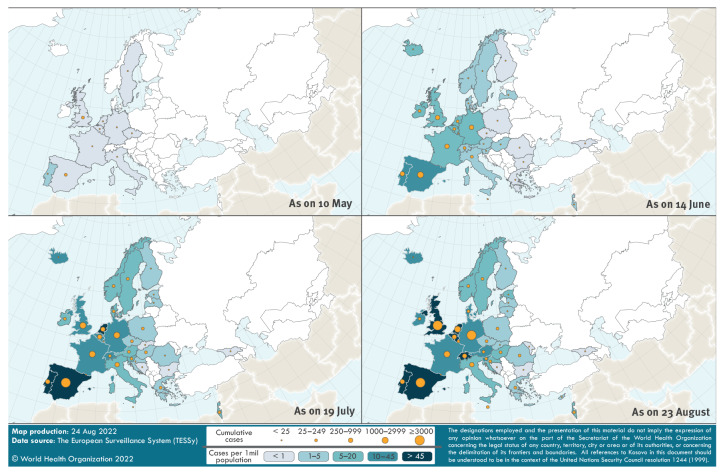
On 13 May 2022, the United Kingdom (UK) reported a non-travel-associated family cluster of MPX cases to the WHO through International Health Regulations (IHR) mechanisms [3]. Thereafter, the UK and other countries, including Portugal, Sweden, Belgium, Germany, Spain, France, Italy, the Netherland, Austria (chronological order) began detecting and reporting MPX cases of Clade II (formerly West African clade) [3,4], primarily among men who have sex with men (MSM). Subsequent retrospective testing of a residual sample in the UK dated the earliest known case back to 7 March 2022. Until end of July [1], Europe remained the epicentre of this large and geographically widespread outbreak, with a steady increase of cases and affected countries (Figure 1).
Figure 1.
Geographical distribution of monkeypox cases reported through The European Surveillance System (TESSy) by 36 WHO European Region countries, 7 March–23 August 2022 (n = 20,690 cases)
Distribution of cases by symptom onset or, if missing, the earliest date of diagnosis or notification.
Of 21,098 cases reported in the WHO European Region, case-based data for 20,690 cases (98.1%) from 36 of 41 countries were reported to the European Centre for Disease Prevention and Control (ECDC) and the WHO Regional Office for Europe, through The European Surveillance System (TESSy), using national (n = 9,831 cases) or WHO/ECDC case definitions (n = 1,314 cases) [5,6]. Information is missing or unknown for the other 9,545 cases. Of the total, 99.3% (20,545/20,690) were laboratory-confirmed.
Nowcasting of monkeypox cases reported in the WHO European Region
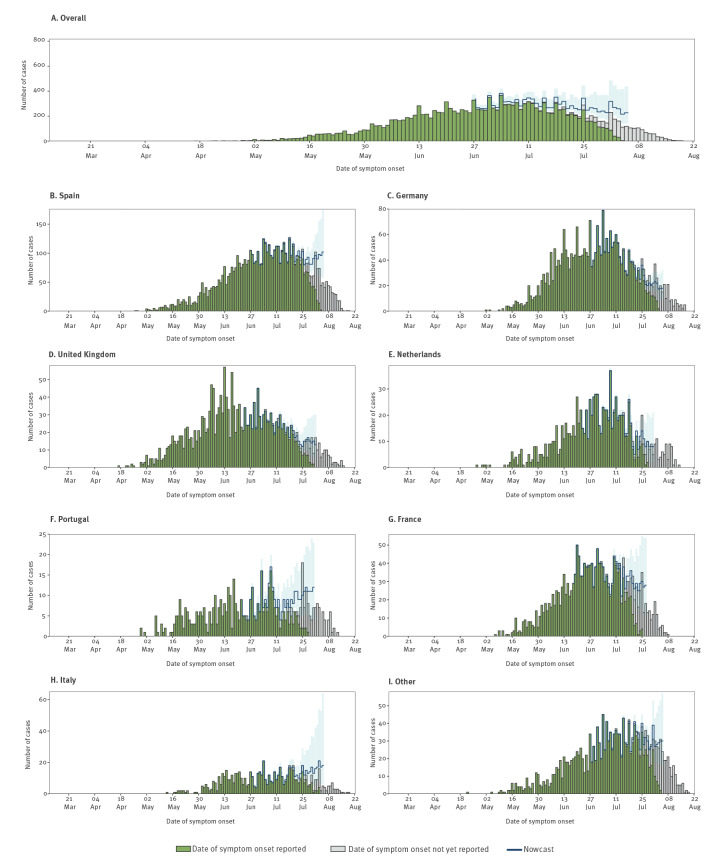
To assess the current epidemiological situation, we performed nowcasting on TESSy case-based data [7], with a prior negative binomial distribution (mean: 7 days and overdispersion 1.6 days) to adjust for reporting delay, and right truncation at 17 days, which corresponds to 95th percentile of reporting delay for cases in the last weeks. The median reporting delay, defined as the difference in days from date of symptom onset to date of notification at national level, was 7 days (range: 1–117 days) for 17,101 (82.6%) cases with complete date variables. Nowcast estimates suggest that the regional epidemic trend is plateauing overall, with some inter-country differences emerging (Figure 2).
Figure 2.
Distribution of reported and nowcasted cases of monkeypox by date of onset of symptoms, 36 WHO European Region countries in order of decreasing incidence, 7 March (week 10)–23 August (week 34) 2022
Nowcasting was performed up to 17 days before the last reported date of symptom onset. Reported cases are shown in green. Cases for which the date of symptom onset is not yet in the notification system at the time of nowcasting are shown in grey. Nowcasting point estimate (line) and 95% confidence interval (shaded area) are shown in blue.
Other reporting countries: Andorra, Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, Georgia, Greece, Hungary, Iceland, Ireland, Israel, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Republic of Moldova, Romania, Slovakia, Slovenia, Sweden, Switzerland.
Demographic characteristics, clinical presentation and outcome
Most cases (98.8%; 17,685/17,896) identified as male, and the median age of all cases was 37 years (interquartile range (IQR): 31–44; range: 0–88 years) and 37.2% (3,070/8,257) were HIV-positive (Table 1). Among male cases, 96.9% (8,771/9,053) self-identified as MSM. A small proportion of infections have consistently been reported in women and children. In total, 220 adult cases with a known gender were reported to be non-male (1.2%) and 41 cases aged under 18 years (0.2%) have been reported in TESSy. Of these, 15 cases were under 15 years of age.
Table 1. Demographic, clinical characteristics and disease-severity of confirmed and probable monkeypox cases, 36 WHO European Region countries, 7 March–23 August 2022, (n = 20,690 cases).
| Variables | Overall cases | Hospitalised | Not hospitalised | Unknown | Hospitalisation ratio (per 1,000 cases) |
p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||||
| Total cases | 20,690 | 100 | 197 | 100 | 10,601 | 100 | 9,892 | 100 | 10 | ||
| Age group (years) | 0–17 | 41 | 0.2 | 2 | 1.0 | 25 | 0.2 | 14 | 0.1 | 49 | 0.015 |
| 18–30 | 5,078 | 24.5 | 57 | 28.9 | 2,504 | 23.6 | 2,517 | 25.4 | 11 | ||
| 31–40 | 8,231 | 39.8 | 87 | 44.2 | 4,202 | 39.6 | 3,942 | 39.9 | 11 | ||
| 41–50 | 4,970 | 24.0 | 40 | 20.3 | 2,695 | 25.4 | 2,235 | 22.6 | 8 | ||
| 51–60 | 1,882 | 9.1 | 9 | 4.6 | 947 | 8.9 | 926 | 9.4 | 5 | ||
| > 60 | 442 | 2.1 | 2 | 1.0 | 209 | 2.0 | 231 | 2.3 | 5 | ||
| Unknown | 46 | 0.2 | 0 | 0.0 | 19 | 0.2 | 27 | 0.3 | 0 | ||
| Gendera | Female | 212 | 1 | 4 | 2 | 137 | 1.3 | 71 | 0.7 | 19 | 0.404 |
| Male | 17,685 | 85.5 | 193 | 98 | 10,457 | 98.6 | 7,035 | 71.1 | 11 | ||
| Other | 16 | 0.1 | 0 | 0 | 6 | 0.1 | 10 | 0.1 | 0 | ||
| Unknown | 2,777 | 13.4 | 0 | 0 | 1 | 0.0 | 2,776 | 28.1 | 0 | ||
| Prior smallpox vaccination | Vaccinated | 528 | 2.6 | 12 | 6.1 | 495 | 4.7 | 21 | 0.2 | 23 | 0.334 |
| Not vaccinated | 2,974 | 14.4 | 94 | 47.7 | 2,758 | 26.0 | 122 | 1.2 | 32 | ||
| Unknown | 17,188 | 83.1 | 91 | 46.2 | 7,348 | 69.3 | 9,749 | 98.6 | 5 | ||
| Smallpox vaccination for current event | PEPV | 42 | 0.2 | 0 | 0 | 40 | 0.4 | 2 | 0 | 0 | 0.461 |
| PPV | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| PEPV/PPV | 4 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | ||
| Not vaccinated | 3,017 | 14.6 | 101 | 51.3 | 2,798 | 26.4 | 118 | 1.2 | 33 | ||
| Unknown | 17,626 | 85.2 | 96 | 48.7 | 7,760 | 73.2 | 9,770 | 98.8 | 5 | ||
| HIV status | Positive | 3,070 | 14.8 | 37 | 18.8 | 2,697 | 25.4 | 336 | 3.4 | 12 | 0.441 |
| Negative | 5,187 | 25.1 | 52 | 26.4 | 4,536 | 42.8 | 599 | 6.1 | 10 | ||
| Unknown | 12,433 | 60.1 | 108 | 54.8 | 3,368 | 31.8 | 8,957 | 90.5 | 9 | ||
| STI | Yes | 93 | 0.4 | 8 | 4.1 | 81 | 0.8 | 4 | 0 | 86 | 0.67 |
| No | 625 | 3 | 44 | 22.3 | 537 | 5.1 | 44 | 0.4 | 70 | ||
| Unknown | 19,972 | 96.5 | 145 | 73.6 | 9,983 | 94.2 | 9,844 | 99.5 | 7 | ||
| Sexual orientation | MSM | 8,777 | 42.4 | 84 | 42.6 | 6,677 | 63 | 2,016 | 20.4 | 10 | Not calculated |
| Bisexual | 93 | 0.4 | 4 | 2 | 80 | 0.8 | 9 | 0.1 | 43 | ||
| Heterosexual | 276 | 1.3 | 9 | 4.6 | 242 | 2.3 | 25 | 0.3 | 33 | ||
| Unknown | 11,544 | 55.8 | 100 | 50.7 | 3,602 | 34.0 | 7,842 | 79.2 | 13 | ||
| Health worker | Yes | 64 | 0.3 | 0 | 0 | 56 | 0.5 | 8 | 0.1 | 0 | 0.64 |
| No | 3,645 | 17.6 | 80 | 40.6 | 3,334 | 31.4 | 231 | 2.3 | 22 | ||
| Unknown | 16,981 | 82.1 | 117 | 59.4 | 7,211 | 68 | 9,653 | 97.6 | 7 | ||
| Rash | Not reported | 657 | 3.2 | 4 | 2.0 | 424 | 4.6 | 229 | 2.0 | 6 | 0.085 |
| Reported | 12,415 | 60.0 | 187 | 94.9 | 8,367 | 90.1 | 3,861 | 34.4 | 15 | ||
| Unknown/no data on symptoms | 7,618 | 36.8 | 6 | 3.0 | 494 | 5.3 | 7,118 | 63.5 | 1 | ||
| Lymphadenopathy | Not reported | 7,837 | 37.9 | 91 | 46.2 | 5,118 | 55.1 | 2,628 | 23.4 | 12 | 0.005 |
| Reported | 5,235 | 25.3 | 100 | 50.8 | 3,673 | 39.6 | 1,462 | 13.0 | 19 | ||
| Unknown/no data on symptoms | 7,618 | 36.8 | 6 | 3.0 | 494 | 5.3 | 7,118 | 63.5 | 1 | ||
| Systemic symptomsb | Not reported | 4,596 | 22.2 | 91 | 46.2 | 2,917 | 31.4 | 1,588 | 14.2 | 20 | < 0.001 |
| Reported | 8,476 | 41.0 | 100 | 50.8 | 5,874 | 63.3 | 2,502 | 22.3 | 12 | ||
| Unknown/no data on symptoms | 7,618 | 36.8 | 6 | 3.0 | 494 | 5.3 | 7,118 | 63.5 | 1 | ||
MSM: men who have sex with men; PEPV: Post-exposure preventive vaccination; PPV: Primary preventive (pre-exposure) vaccination; STI: sexually transmitted infection.
a Gender collected in TESSy as female, male, other (e.g. transgender) or unknown.
b Fever, fatigue, muscle pain, chills and/or headache.
Based on case-based data reported in TESSy, hospitalisation ratios and p values were calculated for cases for whom hospitalisation status (i.e. not hospitalised, hospitalised for isolation purposes (n = 129 cases) or hospitalised for clinical management purposes (n = 197 cases)) was known. Cases whose hospitalisation status was reported as unknown or who were known to have been hospitalised, but purpose (isolation/clinical management) was unknown (n = 254) were not included in the analyses. ‘Hospitalisation’ is defined as hospitalisation for clinical care (n = 197 cases). Hospitalisation for known isolation (n = 129 cases) is included as ‘Not hospitalised’. P values were calculated by Fisher’s exact test. For each tabulation of hospitalisation (yes/no) by another variable, when one of the cells was equal to 0, 0.5 was added to all cells of the table in order to be able to conduct the statistical test.
All variables excluding vaccination are up to 23 August 2022. Smallpox vaccination variables combine data from 10 August 2022 and 23 August 2022 for completeness.
Of those reporting symptoms, most reported rash (95.0%; 12,415/13,072) and at least one systemic symptom (64.8%; 8,476/13,072) such as fever, fatigue, muscle pain, chills or headache. Some cases (48.1%; 5,973/12,415 reported rash in the anogenital region; of those, 554 reported no other symptom. Six percent of cases (576/9,732) were hospitalised (n = 129 for isolation purposes; n = 197 for clinical care and n = 250 for unknown reasons). Cases hospitalised for isolation purposes were considered as ‘not hospitalised’ in the analyses. Three cases were admitted to an intensive care unit (ICU) and two of these cases died with encephalitis.
To estimate predictors of severity, case hospitalisation ratios were calculated. The overall case hospitalisation ratio was 10 per 1,000 cases (Table 1) and did not vary over time (data not shown). Younger cases, those presenting with lymphadenopathy and those without systemic symptoms were at significantly higher risk of hospitalisation (p = 0.015, p = 0.005 and p<0.001, respectively). However, surveillance data does not allow capture of the full clinical course, therefore lack of systemic symptoms at the time of report cannot be interpreted as a predictor of severe disease without further in-depth clinical characterisation. No statistically significant difference was observed for other variables. Firth logistic regressions with hospitalisation as a binary outcome and age as a linear variable showed decreasing odds of hospitalisation with increasing age (odds ratio (OR): 0.97; 95% confidence interval (CI): 0.96–0.99). When considering those hospitalised for unknown reasons, HIV-positive cases were at higher risk of hospitalisation compared with HIV-negative cases (46 and 30/1,000 respectively, p < 0.001) (data not shown).
Exposure settings and transmission routes
Detailed data on possible exposure in the 21 days before symptom onset was only available for a minority of cases, limited to some countries. Sexual contact was reported as a possible route of transmission in 93.9% (6,385/6,797) of cases, followed by other person-to-person routes (PTP; non-sexual, non-mother-to-child and non-healthcare associated, 5.3%; 359/6,797) or fomites (0.2%; 11/6,797) (Table 2). Of the cases who reported ‘other’ as a route (0.3%; 41/6,797), 12 also reported likely exposure at a bar event, and one reported household fomite transmission. Many cases reported exposure at a private party/club (69.4%; 2,530/3,643) and/or a large event (28.3%; 1,030/3,643). Household exposure was reported by 233 (6.4%) cases, and these cases also reported sexual transmission (78.1%; 153/196) or PTP (21.4%; 42/196). Likely mode-of-transmission and exposure setting was reported for five cases under 15 years, which indicated transmission through contact with a parent or in the household.
Table 2. Exposure settings for monkeypox cases, 36 WHO European Region countries, 7 March–23 August 2022 (n = 20,690 cases).
| Variables | Exposure settinga
(n = 3,643 cases reporting at least one setting) |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Household | Work | School/nursery | Healthcare | Private party/club with sexual activity | Large event with sexual activity | Large event w/o sexual activity | Bar/restaurant w/o sexual activity | Other | Unknown | Missing | |||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||||
| Total cases | 20,690 | 100 | 233 | 100 | 48 | 100 | 0 | 0 | 0 | 0 | 2,530 | 100 | 378 | 100 | 652 | 100 | 199 | 100 | 1,007 | 100 | 1,008 | 100 | 16,129 | 100 | |
| Age group (years) | |||||||||||||||||||||||||
| 0–17 | 41 | 0.2 | 3 | 1.3 | 0 | 0.0 | 0 | 0 | 0 | 0 | 3 | 0.1 | 0 | 0.0 | 1 | 0.2 | 1 | 0.5 | 4 | 0.4 | 3 | 0.3 | 30 | 0.2 | |
| 18–30 | 5,078 | 24.5 | 59 | 25.4 | 18 | 37.5 | 0 | 0 | 0 | 0 | 633 | 25.1 | 82 | 21.8 | 152 | 23.4 | 43 | 21.8 | 243 | 24.2 | 262 | 26.2 | 3,947 | 24.5 | |
| 31–40 | 8,231 | 39.8 | 99 | 42.7 | 18 | 37.5 | 0 | 0 | 0 | 0 | 1,033 | 41.0 | 184 | 48.8 | 290 | 44.7 | 82 | 41.6 | 440 | 43.8 | 438 | 43.8 | 6,323 | 39.3 | |
| 41–50 | 4,970 | 24.0 | 53 | 22.8 | 6 | 12.5 | 0 | 0 | 0 | 0 | 585 | 23.2 | 82 | 21.8 | 154 | 23.7 | 50 | 25.4 | 228 | 22.7 | 229 | 22.9 | 3,907 | 24.3 | |
| 51–60 | 1,882 | 9.1 | 14 | 6.0 | 5 | 10.4 | 0 | 0 | 0 | 0 | 228 | 9.0 | 26 | 6.9 | 48 | 7.4 | 18 | 9.1 | 72 | 7.2 | 59 | 5.9 | 1,521 | 9.4 | |
| > 60 | 442 | 2.1 | 4 | 1.7 | 1 | 2.1 | 0 | 0 | 0 | 0 | 39 | 1.5 | 3 | 0.8 | 4 | 0.6 | 3 | 1.5 | 18 | 1.8 | 10 | 1.0 | 374 | 2.3 | |
| Genderb | |||||||||||||||||||||||||
| Male | 17,685 | 98.7 | 214 | 91.8 | 48 | 100.0 | 0 | 0 | 0 | 0 | 2,512 | 99.3 | 374 | 98.9 | 641 | 98.3 | 192 | 96.5 | 981 | 97.4 | 1,000 | 99.6 | 13,182 | 98.7 | |
| Female | 212 | 1.2 | 18 | 7.7 | 0 | 0.0 | 0 | 0 | 0 | 0 | 18 | 0.7 | 4 | 1.1 | 11 | 1.7 | 7 | 3.5 | 24 | 2.4 | 3 | 0.3 | 161 | 1.2 | |
| Other | 16 | 0.1 | 1 | 0.4 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.2 | 1 | 0.1 | 13 | 0.1 | |
| Sexual orientation | |||||||||||||||||||||||||
| MSM | 8,777 | 75.7 | 172 | 86.0 | 32 | 86.5 | 0 | 0 | 0 | 0 | 2,325 | 97.7 | 339 | 97.7 | 532 | 93.5 | 138 | 88.5 | 868 | 93.0 | 698 | 70.6 | 4,936 | 67.5 | |
| Bisexual | 93 | 0.8 | 12 | 6.0 | 1 | 2.7 | 0 | 0 | 0 | 0 | 21 | 0.9 | 4 | 1.2 | 13 | 2.3 | 5 | 3.2 | 20 | 2.1 | 16 | 1.6 | 35 | 0.5 | |
| Heterosexual | 276 | 2.4 | 14 | 7.0 | 3 | 8.1 | 0 | 0 | 0 | 0 | 30 | 1.3 | 4 | 1.2 | 23 | 4.0 | 13 | 8.3 | 35 | 3.8 | 44 | 4.4 | 147 | 2.0 | |
| Health worker | |||||||||||||||||||||||||
| Yes | 64 | 1.7 | 4 | 2.4 | 0 | 0.0 | 0 | 0 | 0 | 0 | 11 | 1.3 | 5 | 1.8 | 8 | 1.5 | 3 | 1.8 | 29 | 3.6 | 11 | 1.8 | 11 | 0.8 | |
| No | 3,645 | 98.3 | 162 | 97.6 | 46 | 100.0 | 0 | 0 | 0 | 0 | 865 | 98.7 | 270 | 98.2 | 528 | 98.5 | 168 | 98.2 | 787 | 96.4 | 601 | 98.2 | 1,285 | 99.2 | |
| Most likely mode of transmissionc | |||||||||||||||||||||||||
| PTP | 359 | 5.3 | 42 | 21.4 | 6 | 16.2 | 0 | 0 | 0 | 0 | 82 | 5.8 | 6 | 2.0 | 54 | 10.1 | 37 | 98.2 | 70 | 8.1 | 14 | 2.5 | 148 | 3.8 | |
| Sexual | 6,385 | 93.9 | 153 | 78.1 | 30 | 81.1 | 0 | 0 | 0 | 0 | 1,341 | 94.1 | 292 | 97.7 | 475 | 88.8 | 131 | 75.7 | 791 | 91.2 | 547 | 97.2 | 3,698 | 95.3 | |
| Fomite | 11 | 0.2 | 0 | 0.0 | 1 | 2.7 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 0.3 | 2 | 0.4 | 6 | 0.2 | |
| Otherd | 41 | 0.6 | 1 | 0.5 | 0 | 0.0 | 0 | 0 | 0 | 0 | 2 | 0.1 | 1 | 0.3 | 6 | 1.1 | 5 | 2.9 | 3 | 0.3 | 0 | 0.0 | 28 | 0.7 | |
| Sexual and PTP | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 | |
MSM: men who have sex with men; PTP: non-sexual person-to-person transmission; w/o: without.
a Possible exposure in the 21 days before symptom onset. Multiple exposures per case possible.
b Gender collected in TESSy as female, male, other (e.g., transgender) or unknown (not shown in table).
c No cases reported ‘most likely mode of transmission’ as zoonotic, occupational healthcare, occupational laboratory, vertical or transfusion.
d Many cases reporting ‘other’ route of transmission, also reported sexual, PTP or fomite transmission and exposure at a bar etc. (see text). Further details were not provided.
Sixty-four cases were health workers (1.7%; 64/3,708); of these 62 (96.9%) were male and 55 (85.9%) were MSM. While no occupational exposure in the healthcare setting or workplace has been reported through TESSy, three instances of occupational exposure have been reported to the WHO through other routes to date. Other modes of transmission, including zoonotic, vertical and laboratory transmission were not reported for any cases. Possible exposure settings and transmission routes are not mutually exclusive and local outbreak investigations will help identify clear transmission pathways.
Smallpox vaccination and disease severity
Only 16.8% (3,525/20,960) of cases reported on smallpox vaccination. Of these, most (81.8%; 2,577/3,152) self-reported as both unvaccinated prior to this outbreak and for this outbreak (median age: 36 years; IQR: 30–41), 423 reported receiving a vaccination before this outbreak (median age: 50 years; IQR: 39–56), one reported primary preventive (pre-exposure) vaccination (PPV) (aged 28 years) and 42 reported post-exposure preventative vaccination (PEPV) for this event (median age: 35.5 years; IQR: 30.3–43.8). We assessed the potential effect of prior smallpox vaccination on disease severity and hospitalisation (Table 3). Overall, 197 cases were hospitalised for clinical care, of which 12 cases (11.3%) reported prior vaccination. Firth logistic regressions to assess association between hospitalisation and vaccination were not statistically significant (adjusted OR: 1.07; 95% CI: 0.53–1.97) (Table 3).
Table 3. Outcome by prior smallpox vaccination status among monkeypox cases, 36 WHO European Region countries, 7 March–23 August 2022 (n = 3,502 cases).
| Variables | Vaccinated | Unvaccinated | Crude OR | 95% CI | Adjusted OR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||||
| Total cases | 528 | 15.1 | 2,974 | 84.9 | |||||
| Age group (years) | 18–30 | 49 | 5.7 | 817 | 94.3 | Ref | Ref | Ref | Ref |
| 0–17 | 0 | 0 | 10 | 100 | 0.79 | 0.01–6.26 | 7.95 | 1.46–30.44 | |
| 31–40 | 94 | 6.8 | 1,298 | 93.2 | 1.20 | 0.85–1.73 | 0.97 | 0.61–1.57 | |
| 41–50 | 130 | 16.0 | 680 | 84.0 | 3.17 | 2.26–4.50 | 0.87 | 0.50–1.51 | |
| 51–60 | 189 | 58.9 | 132 | 41.1 | 23.62 | 16.56–34.24 | 0.3 | 0.08–0.86 | |
| > 60 | 62 | 69.7 | 27 | 30.4 | 37.53 | 22.30–64.83 | 0.77 | 0.15–2.61 | |
| Unknown | 4 | 28.6 | 10 | 71.4 | Not calculated | Not calculated | |||
| Gendera | Male | 516 | 15.0 | 2,927 | 85.0 | Ref | Ref | Not calculated | |
| Female | 11 | 19.6 | 45 | 80.4 | 1.43 | 0.71–2.66 | |||
| Other | 1 | 33.3 | 2 | 66.7 | 3.40 | 0.31–25.62 | |||
| Hospitalisationb | Not hospitalised | 495 | 15.2 | 2,758 | 84.8 | Ref | Ref | Ref | Ref |
| Hospitalised | 12 | 11.3 | 94 | 88.7 | 0.74 | 0.39–1.29 | 1.07 | 0.53–1.97 | |
| Unknown | 21 | 14.7 | 122 | 85.3 | Not calculated | Not calculated | |||
| Health worker | No | 253 | 15.0 | 1,437 | 85.0 | Ref | Ref | Not calculated | |
| Yes | 2 | 4.9 | 39 | 95.1 | 0.36 | 0.07–1.07 | |||
| Unknown | 273 | 15.4 | 1,498 | 84.6 | Not calculated | ||||
CI: confidence interval; OR: odds ratio; Ref: reference.
a Gender collected in TESSy as female, male, other (e.g. transgender) or unknown (not shown in table).
b Hospitalisation is defined as hospitalisation for clinical care (n = 197). Hospitalisation for known isolation (n = 129) is included as not hospitalised for clinical care. Regressions were performed for cases for which there was complete data for the specific variables included in each model. Adjusted OR includes hospitalisation as a binary outcome and age (categorical) and vaccination (binary) as explanatory variables. Vaccinated include those vaccinated for smallpox prior to this outbreak.
Discussion
The MPXV is currently the most prevalent cause of orthopoxvirus infection in humans. MPX outbreaks have previously occurred largely in African countries, where the virus is enzoonotic. However, in recent years, sporadic cases and clusters of MPXV Clade II have occurred in other regions, largely linked to travel from endemic countries or imported animal to human transmission with limited onward human-to-human spread [8-16].
Transmission of MPXV is thought to occur primarily through close or direct physical contact with infected lesions, respiratory droplets or contaminated material [17]. Other transmission routes such as zoonotic or mother-to-child have been described [18]. Previously, typical clinical presentation was described as a prodromal phase, with fever, followed by a widespread, centrifugal, evolving maculopustular rash and lymphadenopathy [19]. People living with untreated HIV infection, pregnant women and young children have previously been identified to be at higher risk of severe MPX [20,21]. Epidemiological studies estimated that prior smallpox vaccination provides ca 85% cross-protection against MPXV and reduces the frequency and severity of symptoms [22,23]. However, routine vaccination was discontinued worldwide following the eradication of smallpox in 1980 and effectiveness of vaccination in the current outbreak remains to be assessed.
We describe an on-going multi-country outbreak of MPXV, mainly transmitted among MSM through close physical contact, often during sexual activities. A large proportion of cases (94%) reported sexual transmission, often at gatherings and events which provided the opportunity for amplification through sexual networks. A smaller number of cases were also steadily reported among women and children. Nowcasting estimates suggest that reported cases have plateaued overall in Europe, however, some countries continue to see an increase. Such variation in projections by country may reflect potential differential implementation and impact of local intervention measures.
Clinical presentation in the current epidemic is atypical compared with previous outbreaks [24,25]. Symptoms involve an atypical rash-illness presentation, with a relatively low, but still notable proportion of patients hospitalised. Severe manifestations such as encephalitis have been reported in a small number of cases [26]. This clinical picture may change in the event of spread into populations with increased risk of severe disease, including those with untreated HIV or otherwise immunosuppressed. Further investigations are required to assess disease severity in immunocompromised individuals and other potential vulnerable groups for the current outbreak. We found no evidence that prior smallpox vaccination significantly protects against severe disease and hospitalisation, which raises questions regarding potential waning protection following vaccination over 4 decades ago. As smallpox vaccines are currently rolled out to at-risk individuals, it is essential that studies are undertaken to understand vaccine effectiveness.
This study has some limitations. The analyses are based on surveillance data submitted to TESSy, which are dependent on availability of data at national level and vary in completeness. Indeed, for a number of variables, including vaccination, the level of missing data makes interpretation of analyses challenging. In addition, any clinical data reported in TESSy is of limited scope and will not reflect the full course of disease. Finally, while nowcasting is a valuable tool to account for delays in reporting, interpretation should consider that missing data and misclassification of symptom onset date and varying reporting delays over time can contribute to a considerable uncertainty around these estimates.
Conclusions
To interrupt transmission of MPXV, identification and testing, management of cases and contacts, targeted risk communication and strong community engagement with affected groups, implementation of targeted public health measures, combined with PPV/PEPV are fundamental [27-30]. However, the transmission patterns of the virus, coupled with the difficulty of tracing multiple often anonymous sexual contacts, likely under-ascertainment of cases, challenges to access and vaccinate priority groups and stigma complicate the public health response. An integrated response with strong collaboration among at-risk groups, communities, public health authorities, and international health organisations is required to overcome these challenges.
Ethical statement
Ethical approval was not needed for this study, which was based on surveillance data only.
Disclaimer
The authors affiliated with the World Health Organization (WHO) are alone responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of the WHO. The co-author is a fellow of the ECDC Fellowship Programme, supported financially by the European Centre for Disease Prevention and Control (ECDC). The views and opinions expressed herein do not state or reflect those of ECDC. ECDC is not responsible for the data and information collation and analysis and cannot be held liable for conclusions or opinions drawn.
Acknowledgements
This report would not have been possible without the contribution of many healthcare professionals, epidemiologists and public health workers across EU/EEA countries and areas of the WHO European Region. In particular, the authors would like to acknowledge (in no particular order): Heike Schulze and Doris Altmann (Robert Koch Institute, Berlin, Germany), Anna Marie Theut (Statens Serum Institut, Copenhagen, Denmark), Paula Vasconcelos (Directorate General of Health, Lisboa, Portugal), Malgorzata Stepien and Katarzyna Pancer (National Institute of Public Health NIH - National Research Institute, Warsaw, Poland), Martina Maresova (Regional Public Health Authority, Prague, Czech Republic), Katerina Fabianova, Jana Kostalova, Iva Vlckova, Helena Jirincova and Hana Zakoucka (National Institute of Public Health, Prague, Czech Republic), Giovanni Rezza, Alessia Mammone and Francesco Maraglino (Directorate General of Health Prevention, Ministry of Health, Italy), professionals of the Spanish National Epidemiological Surveillance Network (National Center for Microbiology (ISCIII), Spain), colleagues from the Infectious Diseases Division and Regional teams at Santé publique France and from Regional Health Agencies (France), Eve Robinson, Natasha Rafter, Paul McKeown and Kate O’Donnell, Health Protection Surveillance Centre (HPSC) and the National Monkeypox Incident Management Team (Republic of Ireland), Catherine Moore and Kathleen Pheasant (Wales Specialist Virology Centre, Public Health Wales); Slovenian Regional Epidemiological Units of National Institute of Public Health and Institute of Microbiology and Immunology, Medical Faculty, University of Ljubljana (Slovenia); colleagues at the Scottish and UK-level Incident Management Teams (United Kingdom) and colleagues from the National Institute for Public Health and the Environment (RIVM) (the Netherlands). We would like to acknowledge the contribution of colleagues at WHO: Amy Gimma, Laila Skrowny, Ara Tadevosyan, Lauren MacDonald, Michala Hegermann-Lindencrone, Charles Johnston, Catherine Smallwood, Jukka Pukkila, Karen Nahapetyan, Ana Hoxha, Nikola Sklenovska and Boris Pavlin and colleagues from the WHO Monkeypox Incident Management Support Team (IMST) at Regional Office for Europe and at WHO Headquarters Office. In addition, we would like to acknowledge ECDC monkeypox team and the contribution of colleagues at ECDC in setting up the TESSy reporting: Gianfranco Spiteri, Benjamin Bluemel, Zsolt Bartha.
Conflict of interest: None declared.
Authors’ contributions: AMV, OC, SC, LAdS, NF, JP, GS, CMG, RP and JMH drafted the manuscript. GA, MA, SB, PB, AC, EC, OC, AD, CD, ID, KK, MF, FF, RF, JF, CF, MGC, KG, MPG, BRGH, JH, EH, DI, MI, KJ, DGJ, TBJ, AK, AK, JK, JVM, AM, KM, ZM, ZM, JM, AN, HO, IPN, MKR, MST, CS, DS, AS, KS, AT, MT, MT, VU, CvE, JV, AV, RV and KZ conducted MPX surveillance and data collections in their respective countries. All authors read, revised and approved the final manuscript.
References
- 1.World Health Organization (WHO). 2022 Monkeypox Outbreak: Global Trends. Geneva: WHO. [Accessed: 5 Sep 2022]. Available from: https://worldhealthorg.shinyapps.io/mpx_global
- 2.World Health Organization (WHO). Second meeting of the International Health Regulations (2005) (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox. Geneva: WHO; 2022. Accessed: Available from: https://www.who.int/news/item/23-07-2022-second-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-regarding-the-multi-country-outbreak-of-monkeypox
- 3.United Kingdom Health Security Agency (UKHSA). Monkeypox cases confirmed in England – latest updates. London: UKHSA; 2022. Available from: https://www.gov.uk/government/news/monkeypox-cases-confirmed-in-england-latest-updates
- 4. Vivancos R, Anderson C, Blomquist P, Balasegaram S, Bell A, Bishop L, et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022;27(22):2200422. 10.2807/1560-7917.ES.2022.27.22.2200422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Monkeypox outbreak toolbox. Geneva: WHO; Jun 2022. Available from: https://www.who.int/emergencies/outbreak-toolkit/disease-outbreak-toolboxes/monkeypox-outbreak-toolbox
- 6.European Centre for Disease Control and Prevention (ECDC). Monkeypox multi-country outbreak. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Monkeypox-multi-country-outbreak.pdf
- 7. van de Kassteele J, Eilers PHC, Wallinga J. Nowcasting the number of new symptomatic cases during infectious disease outbreaks using constrained P-spline smoothing. Epidemiology. 2019;30(5):737-45. 10.1097/EDE.0000000000001050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141. 10.1371/journal.pntd.0010141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaughan A, Aarons E, Astbury J, Balasegaram S, Beadsworth M, Beck CR, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38):1800509. 10.2807/1560-7917.ES.2018.23.38.1800509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Durski KN, McCollum AM, Nakazawa Y, Petersen BW, Reynolds MG, Briand S, et al. Emergence of Monkeypox - West and Central Africa, 1970-2017. MMWR Morb Mortal Wkly Rep. 2018;67(10):306-10. 10.15585/mmwr.mm6710a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26(4):782-5. 10.3201/eid2604.191164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yong SEF, Ng OT, Ho ZJM, Mak TM, Marimuthu K, Vasoo S, et al. Imported Monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826-30. 10.3201/eid2608.191387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342-50. 10.1056/NEJMoa032299 [DOI] [PubMed] [Google Scholar]
- 14. Erez N, Achdout H, Milrot E, Schwartz Y, Wiener-Well Y, Paran N, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980-3. 10.3201/eid2505.190076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hobson G, Adamson J, Adler H, Firth R, Gould S, Houlihan C, et al. Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveill. 2021;26(32):2100745. 10.2807/1560-7917.ES.2021.26.32.2100745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yinka-Ogunleye A, Aruna O, Dalhat M, Ogoina D, McCollum A, Disu Y, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872-9. 10.1016/S1473-3099(19)30294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brown K, Leggat PA. Human Monkeypox: Current State of Knowledge and Implications for the Future. Trop Med Infect Dis. 2016;1(1):8. 10.3390/tropicalmed1010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mbala PK, Huggins JW, Riu-Rovira T, Ahuka SM, Mulembakani P, Rimoin AW, et al. Maternal and Fetal Outcomes Among Pregnant Women With Human Monkeypox Infection in the Democratic Republic of Congo. J Infect Dis. 2017;216(7):824-8. 10.1093/infdis/jix260 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization (WHO). Factsheet: Monkeypox. Geneva: WHO; 19 May 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/monkeypox
- 20. Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693-702. 10.1093/oxfordjournals.bmb.a011720 [DOI] [PubMed] [Google Scholar]
- 21. Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, Likos A, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742-51. 10.1086/498115 [DOI] [PubMed] [Google Scholar]
- 22. Ježek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293-8. 10.1093/infdis/156.2.293 [DOI] [PubMed] [Google Scholar]
- 23. Fine PEM, Jezek Z, Grab B, Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643-50. 10.1093/ije/17.3.643 [DOI] [PubMed] [Google Scholar]
- 24. Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox Virus Infection in Humans across 16 Countries - April-June 2022. N Engl J Med. 2022;387(8):679-91. 10.1056/NEJMoa2207323 [DOI] [PubMed] [Google Scholar]
- 25. Patel A, Bilinska J, Tam JCH, Da Silva Fontoura D, Mason CY, Daunt A, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. 10.1136/bmj-2022-072410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gobierno de Espana. Alerta sobre infección de viruela de los monos en España y otros países no endémicos. [Status report July 30, 2022. Alert on monkeypox infection in Spain and other non-endemic countries.] Centro de Coordinación de Alertas y Emergencias Sanitarias: Ministerio De Sanidad; 30 Jul 2022. Available from: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/alertaMonkeypox/docs/Informe_de_situacion_MPX_20220730.pdf
- 27.European Centre for Disease Prevention and Control (ECDC). Navigating monkeypox: considerations for gay and bisexual men and other men who have sex with men. Stockholm: ECDC. [Accessed: 10 Jun 2022]. Available from: https://www.ecdc.europa.eu/en/publications-data/navigating-monkeypox-considerations-gay-and-bisexual-men-and-msm
- 28.World Health Organization (WHO). Monkeypox: public health advice for gay, bisexual and other men who have sex with men. Geneva: WHO; 25 May 2022. Available from: https://www.who.int/news/item/25-05-2022-monkeypox--public-health-advice-for-gay--bisexual-and-other-men-who-have-sex-with-men
- 29.World Health Organization (WHO). Vaccines and immunization for monkeypox: Interim guidance. Geneva: WHO; 24 Aug 2022. Available from: https://apps.who.int/iris/bitstream/handle/10665/361894/WHO-MPX-Immunization-2022.2-eng.pdf
- 30.World Health Organization (WHO) Regional Office for Europe. Considerations for the control and elimination of monkeypox in the WHO European Region: policy brief No.1, 26 August 2022. Copenhagen: WHO Regional Office for Europe; 2022. Available from: https://apps.who.int/iris/handle/10665/361984