Abstract
The World Health Organization has highlighted the importance of an international standard (IS) for severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) neutralizing antibody titer detection to calibrate diagnostic techniques. We applied an IS to calibrate neutralizing antibody titers (NTs) (international units/mL) in response to coronavirus disease 2019 (COVID-19) vaccination. Moreover, the association between different factors and neutralizing antibodies was analyzed. A total of 1667 serum samples were collected from participants receiving different COVID-19 vaccines. Antibody titers were determined by a microneutralization assay using live viruses in a biosafety level 3 (BSL-3) laboratory and a commercial serological MeDiPro kit. The titer determined using the MeDiPro kit was highly correlated with the NT determined using live viruses and calibrated using IS. Fever and antipyretic analgesic treatment were related to neutralizing antibody responses in ChAdOx1-S and BNT162b2 vaccinations. Individuals with diabetes showed a low NT elicited by MVC-COV1901. Individuals with hypertension receiving the BNT162b2 vaccine had lower NTs than those without hypertension. Our study provided the international unit (IU) values of NTs in vaccinated individuals for the development of vaccines and implementation of non-inferiority trials. Correlation of the influencing factors with NTs can provide an indicator for selecting COVID-19 vaccines based on personal attributes.
Keywords: COVID-19 vaccines, Neutralizing antibody titers, International standard
Identification of the immune correlates of protection against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) remains a challenge. While neutralizing antibody (NAb) titer (NT) is not the only determinant of vaccine efficacy, the neutralization level is highly predictive of immune protection [[1], [2], [3]]. The NT can be determined using many approaches, such as plaque reduction assay, focus reduction assay, microneutralization assay using real viruses, and pseudovirus assay [4,5]. The assays differ substantially, including variations in protocols for similar assay types and among laboratories [6,7]. For calibration, establishment of international standards (ISs) for SARS-CoV-2 NT detection remains a major goal of the World Health Organization (WHO). Various standards are widely used to establish a baseline for comparing NTs across different datasets (laboratories, protocols, and assays) [6]. An IS was established based on the pooled human plasma data from convalescent patients [8]. For different pooled cohorts, different standard sera were obtained, each having predetermined international units (IUs) for converting NTs. This enabled the conversion of immunogenicity characteristics for accurate comparison across laboratories and vaccine developers. Although many laboratories have applied IUs to represent NTs in patients with coronavirus disease 2019 (COVID-19) or vaccinated individuals, the reference data are still insufficient. Furthermore, neutralization tests using live viruses in biosafety level 3 (BSL-3) laboratories are laborious and time-consuming. Binding assays based on the anti-spike (S) protein or anti-receptor-binding domain (RBD) are widely used to evaluate NAbs [[9], [10], [11]]. However, the correlation between antibody titers from enzyme-linked immunosorbent assay (ELISA) binding assays and NTs (IU/mL) remains unknown.
In this study, serum samples were obtained from participants vaccinated with different COVID-19 vaccines and were analyzed before vaccination, as well as after the first and second doses, to estimate the correlation between titers obtained via microneutralization assays using live viruses in a BSL-3 laboratory (IU/mL) and antibody titers measured using a commercial MeDiPro serological assay. Serum samples of vaccinated individuals from Taiwan, where the local COVID-19 infection rates were low during the period of sample collection, were also examined. We analyzed whether factors, such as sex, age, side effects of vaccines, and underlying diseases, could affect NAb response, and estimated the half-life of NTs after primary vaccination.
1. Materials and methods
1.1. Serum sample collection
A total of 1667 serum samples were collected from vaccinated individuals at the Chang Gung Memorial Hospital and Chang Gung University (ethics approval number: 202001041A3C) between January 20, 2021, and April 8, 2022. An additional 120 serum samples from vaccinated individuals were purchased from Access Biologicals (Vista, CA, USA). The samples were collected as per protocol SDP-003, Human Biological Specimens Collection, data September 22, 2017, and qualifications of the principal investigator (Robert Pyrtle, M.D.) were reviewed and approved by the Diagnostics Investigational Review Board (Cummaquid, MA, USA).
1.2. Cell culture and virus
African green monkey kidney (Vero E6) cells (CRL-1586) were purchased from the American Type Culture Collection (Bethesda, MD, USA) and maintained in Dulbecco's modified Eagle medium (DMEM; Gibco, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco) at 37 °C. The SARS-CoV-2/human/TWN/CGMH-CGU-01/2020 isolate was used in the live virus microneutralization assay.
1.3. Live virus microneutralization assay
Vero E6 cells (2 × 104 cells/well) were seeded in 96-well plates and incubated at 37 °C for 24 h. The medium was then replaced by 100 μL fresh DMEM containing 2% FBS. The assay was performed in a BSL-3 laboratory using the SARS-CoV-2/human/TWN/CGMH-CGU-01/2020 strain. All serum samples were heat-inactivated at 56 °C for 30 min and then serially diluted two-fold in DMEM without FBS. From a starting dilution of 1:8 for each sample, ten 2-fold dilutions were performed to obtain a final dilution of 1:8192. Each serum sample was incubated with 100 median tissue culture infectious doses of SARS-CoV-2 at 37 °C for 1 h prior to infection of Vero E6 cells. Subsequently, the virus-serum mixtures (100 μL) at each dilution were added to a 96-well plate containing a confluent Vero E6 monolayer. Infected cells were incubated at 37 °C for 5 d and then fixed with 10% formaldehyde and stained with crystal violet. The neutralization titer was calculated as logarithm of the 50% endpoint using the Reed–Muench method, based on the presence or absence of cytopathic effects. Each serum sample was tested in four replicates. Geometric mean titers (GMTs) were calculated with 95% confidence intervals (CIs) using GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA).
1.4. Serological assay
Each serum sample was analyzed by MeDiPro SARS-CoV-2 antibody ELISA according to the manufacturer's instructions. The assay detected antibodies against S1 and RBD, and values <34.47 IU/mL were considered negative.
1.5. WHO IU conversion
WHO IS sera (NIBSC code 20/136) and WHO Reference Panel for anti-SARS-CoV-2 antibody (NIBSC code 20/268, including NIBSC codes 20/150, 20/148, 20/144, and 20/140) were obtained from the National Institute for Biological Standards and Control (NIBSC). The WHO IS (NIBSC code 20/136) involves pooled plasma from 11 patients that recovered from SARS-CoV-2 infection and has an arbitrarily assigned unitage of 1000 IU/mL for neutralizing and binding activities. It is used to standardize and calibrate SARS-CoV-2 serological assays across different laboratories. The WHO Reference Panel (NIBSC code 20/268), including NIBSC codes 20/150, 20/148, 20/144, and 20/140, was prepared from four pools of plasma from convalescent patients that tested SARS-CoV-2-positive. Negative control plasma (NIBSC code 20/142) was obtained from healthy donors. Antibody titers of the WHO Reference Panel were determined via neutralization assays based on live virus or pseudovirus, and by ELISA that targeted S1, RBD, and N of SARS-CoV-2. Serum characteristics in the Reference Panel were as follows: NIBSC code 20/150 (high titer), 20/148 (mid titer), 20/144 (low anti-S, relatively high anti-N protein antibodies), and 20/140 (low titer). Convalescent plasma (NIBSC code 20/130) with a relatively high antibody titer was obtained from an individual donor. The 50% neutralization titer (NT50) values for the WHO IS and Reference Panel sera were determined using a live virus microneutralization assay (Table S1). Each standard serum sample was tested in duplicate, except for 20/130.
1.6. Statistical analysis
Statistical analyses were performed using GraphPad Prism version 8. Pearson's correlation coefficients (r) were used to determine the correlation between the titers obtained using different serological assays and those from live SARS-CoV-2 NT assay. Data were analyzed using Student's two-tailed unpaired t tests. Significance was set at P < 0.05.
2. Results
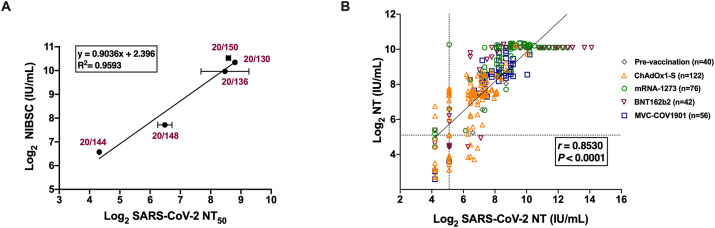
The NT is an important factor for evaluating the protection against viral infections after vaccination. Varying NTs were observed in fully vaccinated individuals. Serum samples were obtained from 336 individuals receiving homologous vaccinations with Vaxzevria (previously called COVID-19 vaccine, AstraZeneca, ChAdOx1-S), Spikevax (previously called COVID-19 vaccine Moderna mRNA-1273), Pfizer BioNTech162b2 (BNT162b2), and Medigen MVC-COV1901. To convert the NT to IU, NT50 values for the WHO IS and Reference Panel for anti-SARS-CoV-2 immunoglobulin (obtained from NIBSC) were determined using a live virus neutralization assay (Table S1) with linear regression, defining the conversion of NT50 values to IU/mL (Fig. 1 A). A total of 336 serum samples were tested to determine NTs using a live SARS-CoV-2 microneutralization assay. We obtained NT50 values that represented 50% protection against SARS-CoV-2-induced cell death. We further determined antibody titers using a commercial serological assay, the MeDiPro SARS-CoV-2 antibody ELISA [12]. MeDiPro is a Taiwan FDA-approved kit for quantifying S1- and RBD-binding antibodies. The assay analyzed data of S1 and RBD fusion proteins to accurately predict NTs. We used live virus NT50 (IU/mL) values as a standard to assess whether the MeDiPro assay reflected NTs based on the detection of antibodies against S1 and RBD (Fig. 1B). A good correlation was observed between titers obtained using MeDiPro and live SARS-CoV-2 NT assays (r = 0.853). The sensitivity and specificity of the MeDiPro assay were 92.5% and 91.1%, respectively (Table 1 ). Therefore, the MeDiPro assay represented an efficient tool for detecting SARS-CoV-2 NAbs without requiring a live virus neutralization assay in a BSL-3 laboratory.
Fig. 1.
Correlation analysis of commercial MeDiPro serological assay with SARS-CoV-2 NT. (A) A calibration curve (standard curve) was used for the conversion of NT50 values to IU/mL. Results are presented in technical duplicate and error bars show the standard deviation. (B) Correlation between the live virus neutralization titer (IU/mL) and titers obtained using MeDiPro SARS-CoV-2 antibody assay (IU/mL) in 336 serum samples. The vertical dashed line indicates the limit of detection (NT = 34.47 IU/mL). The horizontal dashed lines indicate the cut-off values for MeDiPro (34.47 IU/mL). Correlations were checked using Pearson's correlation coefficients (r). Geometric mean titers with 95% confidence interval are shown for pre-vaccination, after the first dose, and after the second dose. IU, international unit; NT, neutralizing antibody titer; SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2; NT50, 50% NT.
Table 1.
Comparison of a commercial serological assay with SARS-CoV-2 neutralizing antibody titer.
| Total samples (336) | MeDiPro SARS-CoV-2 antibody ELISA | ||
|---|---|---|---|
| Live virus NT | Positive | bNegative | |
| Positive | 280 | 259 | 21 |
| aNegative | 56 | 5 | 51 |
| Sensitivity = TP/(TP + FN) | 92.5% (88.8%–95.0%) | ||
| Specificity = TN/(TN + FP) | 91.1% (80.7%–96.1%) | ||
| PPV = TP/(TP + FP) | 98.1% (95.6%–99.2%) | ||
| NPV = TN/(TN + FN) | 70.8% (59.5%–80.1%) | ||
TP, true positive; FP, false positive; TN, true negative; FN, false negative; PPV, positive predictive value; NPV, negative predictive value.
a,bNegative < 34.47 IU/mL (limit of detection).
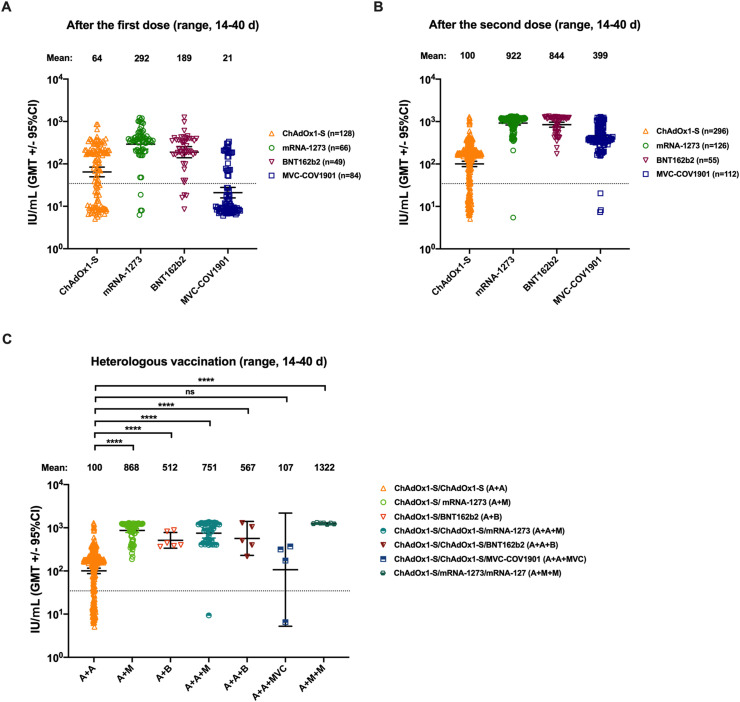
We collected serum samples from 916 individuals vaccinated with the first or second dose of COVID-19 vaccines in Taiwan to further compare homologous COVID-19 vaccination. Although the highest (292 IU/mL) and lowest (21 IU/mL) GMTs elicited by Moderna mRNA-1273 and MVC-COV1901 were observed after the first dose, respectively (Fig. 2 A), all GMTs elicited by different vaccines increased after the second dose (Fig. 2B). However, NTs elicited by AZ ChAdOx1-S varied to a greater extent than those elicited by the other three vaccines (Fig. 2), and approximately 22% of individuals had NTs below the cut-off value (34.47 IU/mL). We collected 144 serum samples from individuals vaccinated with heterologous primary or booster COVID-19 vaccines. Heterologous primary COVID-19 vaccination (ChAdOx1-S/mRNA-1273 or ChAdOx1-S/BNT162b2 combination) elicited higher GMTs than homologous primary COVID-19 vaccination (ChAdOx1-S/ChAdOx1-S) (Fig. 2C). Moreover, a significant increase in GMTs was observed after heterologous booster COVID-19 vaccination (booster mRNA-1273 or BNT162b2), except for MVC-COV1901, which was comparable to homologous primary COVID-19 vaccination (ChAdOx1-S/ChAdOx1-S) (Fig. 2C).
Fig. 2.
Antibody response in 1060 serum samples obtained from individuals receiving homologous or heterologous COVID-19 vaccination. The responses of neutralizing antibodies were determined using the commercial MeDiPro serological assay. (A) NT50 values for serum samples from recipients of ChAdOx1-S, mRNA-1273, BNT162b2, and MVC-CoV1901 after the first dose (range, 14–40 d). (B) NT50 values for serum samples from recipients of ChAdOx1-S, mRNA-1273, BNT162b2, and MVC-CoV1901 after the second dose (range, 14–40 d). (C) NT50 values for serum samples from recipients of heterologous primary or booster vaccines. The GMTs with 95% CI are shown, after the vaccination. Vertical dashed lines indicate the limit of detection (NT = 34.47 IU/mL). Data were analyzed using Student's two-tailed unpaired t tests. ∗∗∗∗, P < 0.0001. NT, neutralizing antibody titer; NT50, 50% NT; GMTs, geometric mean titers; CI, confidence interval; ns, not significant.
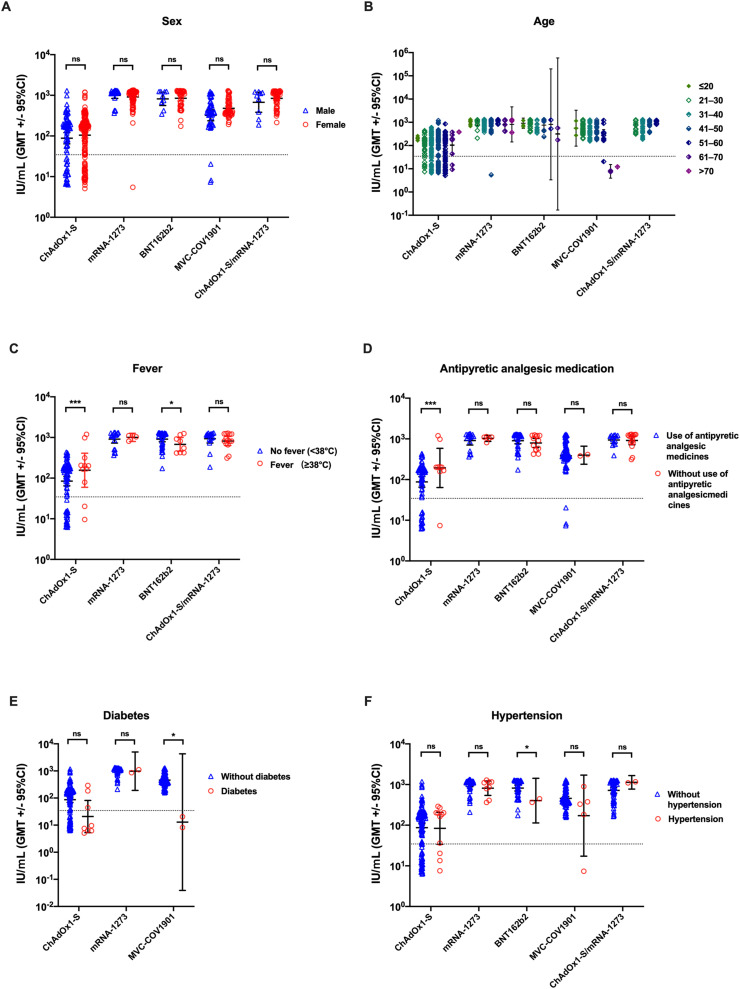
Next, we analyzed the factors that influence NTs elicited by COVID-19 vaccines, including sex, age, fever, antipyretic analgesic medication, and underlying diseases. Sex was independently related to NTs in different COVID-19 vaccination groups (Fig. 3 A). Participants vaccinated with MVC-COV1901 (aged ≥61 years) showed the lowest GMTs compared to those in other younger age groups (Fig. 3B). However, NTs elicited by two doses of COVID-19 vaccine were not correlated with age (Fig. 3B). Side effects of vaccines, such as fever, were positively associated with NTs in the ChAdOx1-S vaccination group; however, an opposite trend was observed for NTs in the BNT162b2 vaccination group (Fig. 3C). Fever was not observed in the MVC-COV1901 vaccination group. Antipyretic analgesic treatment increased the NTs elicited by ChAdOx1-S vaccines, but not by other vaccines (Fig. 3D). Underlying medical conditions are known to be associated with a high risk of severe COVID-19; however, the correlation between underlying medical conditions and NTs elicited by COVID-19 vaccines remains unknown. Our results indicated that underlying diabetes may not affect NAbs elicited by primary ChAdOx1-S and mRNA-1273 vaccination. However, participants with diabetes, who were vaccinated with MVC-COV1901, had lower GMTs than those without underlying diabetes (Fig. 3E). Moreover, BNT162b2 vaccination elicited fewer NAbs in participants with hypertension than in participants without hypertension (Fig. 3F).
Fig. 3.
Factors influencing the titers of neutralizing antibodies (range, 14–40 d). NTs elicited by homologous or heterologous primary COVID-19 vaccination were analyzed based on (A) sex, (B) age, (C) fever grade after vaccination, (D) use of antipyretic medicines, (E) diabetes, and (F) hypertension. The GMTs with 95% CI are shown, after the vaccination. Vertical dashed lines indicate the limit of detection (NT = 34.47 IU/mL). Data were analyzed using Student's two-tailed unpaired t tests. ∗, P < 0.05; ∗∗∗, P < 0.001. NT, neutralizing antibody titer; GMT, geometric mean titer; CI, confidence interval; ns, not significant.
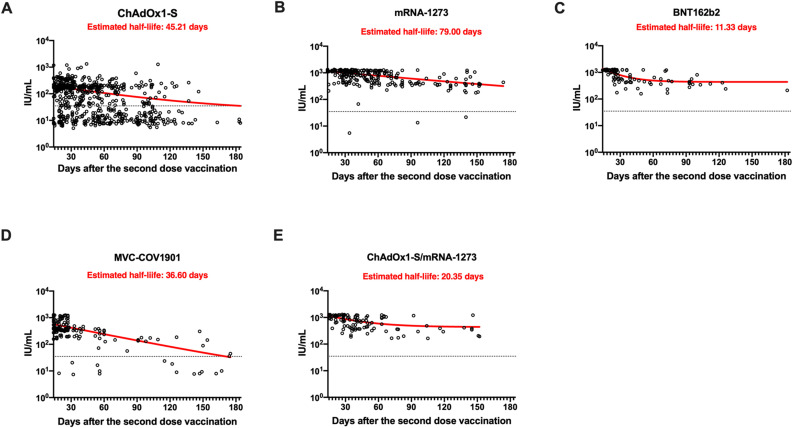
Since NTs are known to reduce over time, we analyzed the half-life of NTs in serum samples from participants vaccinated with different COVID-19 vaccines after 14 and up to 185 d. The NTs elicited by different COVID-19 vaccines reduced over time. However, the NTs elicited by the homologous mRNA-1273 vaccine reduced with an estimated half-life of 79 d during this period, representing the longest half-life of NTs compared to other vaccines. Although the NTs in participants vaccinated with different COVID-19 vaccines declined over time, very few NTs reached the cut-off value (34.47 IU/mL), except for those elicited by homologous ChAdOx1-S vaccination. Moreover, the one-phase decay curve of NTs elicited by ChAdOx1-S and MVC-COV1901 vaccination almost reached the cut-off value at 150 d after a second dose of vaccination (Fig. 4 A and D), in contrast to that by the mRNA-1273, BNT162b2, and ChAdOx1-S/mRNA-1273 combination group (Fig. 4B, C, and E).
Fig. 4.
Reduction in neutralizing antibody titers following homologous or heterologous primary COVID-19 vaccination. Estimated half-life of neutralizing antibody titers in the recipients of (A) ChAdOx1-S, (B) mRNA-1273, (C) BNT162b2, (D) MVC-CoV1901, and (E) ChAdOx1-S/mRNA-127 combination. The x-axis shows the 14–185-day period after the second-dose vaccination. The y-axis shows the NT50 values (IU/mL). Antibody half-life was estimated using a one-phase decay exponential regression on GraphPad Prism 8. NT50, 50% neutralizing antibody titer.
3. Discussion
Antibody titers gradually increase over a few weeks after vaccination and the time span may vary across individuals [13,14]. Therefore, testing for NAbs to determine whether protective antibody titers are elevated after vaccination is highly essential. Vaccinated individuals may still need to take measures to prevent infection. Hence, such assays are important for protecting vaccinated individuals and for the control and prevention of epidemics [15].
In this study, we used standard serum samples to develop an approach that utilizes a commercial kit to quantify antibody titers after vaccination. Titers determined using MeDiPro, which is designed to detect NTs, were strongly correlated with those determined using live SARS-CoV-2 NT assays via IS calibration. Previous studies had indicated that mRNA vaccines (mRNA-1273 and BNT162b2) elicit higher NTs than adenovirus-based vaccines (ChAdOx1-S) [16,17]. In the present study, we compared the protein subunit COVID-19 vaccine (MVC-COV1901), which has been approved for use in Taiwan [18], with other vaccines used worldwide. We obtained GMTs of 100, 922, 844, and 399 for NAbs in serum samples from recipients of ChAdOx1-S, mRNA-127, BNT162b2, and MVC-COV1901 vaccines, respectively, after two doses. Our results are consistent with other studies reporting low antibody titers after the first dose followed by their dramatic increase after the second dose [19]. Moreover, the NT for individuals vaccinated with heterologous COVID-19 vaccines was determined in our study. The estimates may be valuable to vaccine developers for implementing non-inferiority tests.
Studies have shown that antibody titers are correlated with the risk of COVID-19 infection and vaccine efficacy [1,3]. However, many factors may influence NAb responses. Individuals aged >80 years show a lower neutralization response than younger individuals after BNT162b2 vaccination [20,21]. The antibody response is higher in women than in men, and decreases with age in those receiving BNT162b2 and ChAdOx1-S [22,23]. However, we did not observe a significant difference in NTs between males and females, which was consistent with findings of a study in which participants received BNT162b2 vaccination [[24], [25], [26]]. Moreover, we did not observe an association between NTs and age of individuals vaccinated with ChAdOx1-S, mRNA-1273, BNT162b2, or ChAdOx1-S/mRNA-1273 combination, except for the MVC-COV1901 group. The results for MVC-COV1901 were consistent with those of a previous study [27]. In our study, fever intensity or antipyretic analgesic medication was significantly related to NTs after two doses of ChAdOx1-S or BNT162b2 vaccination. The results were inconsistent in the fact that fever was not significantly associated with anti-S IgG titers in individuals who received ChAdOx1-S or BNT162b2 [28]. However, Tani et al. observed fever grade to be positively associated with anti-RBD IgG titer and not with antipyretic medication after two doses of BNT162b2 vaccination [29]. Underlying medical conditions are associated with a high risk of COVID-19 [30]. NTs are higher in patients without diabetes than in those with type-2 diabetes mellitus, who received the BNT162b2 vaccine [24]. Hypertension is not associated with low NTs [22,24,31]. However, Watanabe et al. had reported that hypertension is associated with low Ab titers [26]. We found that NTs were reduced in individuals with diabetes that received MVC-COV1091 and in those with hypertension that received BNT162b2 vaccines. The difference in immunogenicity observed in our study may be attributed to ethnicity; the population analyzed in this study mainly represented Asians living in Taiwan. Although the NTs elicited by the homologous mRNA-1273 vaccine represented the longest estimated half-life of NTs compared to those of other vaccines, the higher initial NT levels elicited by mRNA-1273 vaccine were observed at 14 d after the second-dose vaccination compared to those of ChAdOx1-S and MVC-COV1091. The one-phase decay curve of NTs elicited by ChAdOx1-S and MVC-COV1091 reached the cut-off value after 150 days of vaccination and might depend on lower initial neutralization level. The lower estimated half-life of NTs elicited by BNT162b2 and ChAdOx1-S/mRNA-1273 combination might be caused by the smaller sample size than in the other vaccination group; however, the NTs were still maintained in high levels in BNT162b2 and ChAdOx1-S/mRNA-1273 combination groups compared to that in ChAdOx1-S and MVC-COV1091 groups.
In this study, we used commercially available kit for detecting COVID-19 antibodies based on its binding affinity to S1 and RBD. We found antibody titers, measured using the MeDiPro SARS-CoV-2 antibody ELISA, to be strongly correlated with NTs determined via IS calibration. The correlation between reactogenicity and NTs after COVID-19 vaccination would, however, require further investigation. Several factors, such as different types of COVID-19 vaccines, ethnicity, and collection period after vaccination, may affect the findings of different research groups. More evidence would be required to determine the factors associated with NTs after vaccination. Our current study described several factors that may lower the NAb response elicited by COVID-19 vaccines, especially MVC-COV1901, which is a protein subunit vaccine approved for use in Taiwan. Moreover, since the COVID-19 infection rates were low in Taiwan during the period of sample collection, it might have excluded some factors affected by SARS-CoV-2 infection. We also observed that vaccine efficacy and antibody level declined over time, following full immunization, which was consistent with previous studies [19,32]. NTs are highly correlated with protection. Therefore, monitoring the dynamics of antibody responses after vaccination would be important to determine whether an additional vaccine booster would be required. Moreover, our findings provided information that could be used to select vaccines based on the physical condition and personalized needs of individuals.
Author contributions
C.G.H., G.W.C., and S.R.S. designed the experiments. Y.A.K., S.Y.H., P.N.H., Y.T.L., and Y.C.L. performed the live virus neutralization assays at the BSL-3 facility. K.T.L., K.Y.Y., S.L.Y., C.P.C., and C.Y.C. performed the serological assays. S.Y.H., I.K.L., S.M.L., and H.P.C. collected the serum samples. Y.A.K., S.Y.H., K.T.L., and C.G.H. analyzed the data. Y.A.K., G.W.C., and S.R.S. wrote the manuscript. All authors have read and approved the manuscript to be submitted.
Declaration of competing interest
The MeDiPro SARS-CoV-2 antibody ELISA was transferred from the Research Center for Emerging Viral Infections, Chang Gung University, Taiwan to Formosa Biomedical Technology Corp., Taiwan. We hereby declare that Formosa Biomedical Technology Corp. did not financially support any research at the Research Center for Emerging Viral Infections, Chang Gung University, and Chang Gung Memorial Hospital, Taiwan.
Acknowledgements
This work was supported by the Research Center for Emerging Viral Infections and the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education, Taiwan; the Ministry of Science and Technology (MOST), Taiwan [MOST 111-2634-F-182-001 and 109-2221-E-182-043-MY2]; the Research Center for Epidemic Prevention Science by the MOST, Taiwan [MOST 111-2321-B-182-001]; the Chang Gung Memorial Hospital [grant numbers BMRP367, CMRPG3G1931 and CORPD1K0011-12], and the National Institutes of Health, USA (grant U01 AI151698) for the United World Antiviral Research Network.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micinf.2022.105044.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 2.Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.W., Le Bert N., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewley K.R., Coombes N.S., Gagnon L., McInroy L., Baker N., Shaik I., et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021;16:3114–3140. doi: 10.1038/s41596-021-00536-y. [DOI] [PubMed] [Google Scholar]
- 5.Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201–22011 e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO/BS.2020.2403 . 2020. Establishment of the WHO international standard and reference panel for anti-SARS-CoV-2 antibody. [Google Scholar]
- 7.WHO/BS.2020.2402 . 2020. Collaborative study for the establishment of a WHO international standard for SARS-CoV-2 RNA. [Google Scholar]
- 8.Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397:1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 10.Patel E.U., Bloch E.M., Clarke W., Hsieh Y.H., Boon D., Eby Y., et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021:59. doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huynh A., Arnold D.M., Smith J.W., Moore J.C., Zhang A., Chagla Z., et al. Characteristics of anti-SARS-CoV-2 antibodies in recovered COVID-19 subjects. Viruses. 2021:13. doi: 10.3390/v13040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K.T., Gong Y.N., Huang C.G., Huang P.N., Yu K.Y., Lee H.C., et al. Quantifying neutralizing antibodies in patients with COVID-19 by a two-variable generalized additive model. mSphere. 2022;7 doi: 10.1128/msphere.00883-21. e0088321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021:6. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartsch S.M., O'Shea K.J., Ferguson M.C., Bottazzi M.E., Wedlock P.T., Strych U., et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59:493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 17.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh S.M., Liu M.C., Chen Y.H., Lee W.S., Hwang S.J., Cheng S.H., et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir Med. 2021;9:1396–1406. doi: 10.1016/S2213-2600(21)00402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegu A., O’Connell S., Schmidt S.D., O’Dell S., Talana C.A., Lai L., et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373:1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier D.A., Ferreira I., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller L., Andree M., Moskorz W., Drexler I., Walotka L., Grothmann R., et al. Age-dependent immune response to the biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward H., Whitaker M., Flower B., Tang S.N., Atchison C., Darzi A., et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat Commun. 2022;13:907. doi: 10.1038/s41467-022-28527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J., Stoesser N., Matthews P.C., Ayoubkhani D., Studley R., Bell I., et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6:1140–1149. doi: 10.1038/s41564-021-00947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali H., Alterki A., Sindhu S., Alahmad B., Hammad M., Al-Sabah S., et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. 2021;12:752233. doi: 10.3389/fimmu.2021.752233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wall E.C., Wu M., Harvey R., Kelly G., Warchal S., Sawyer C., et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe M., Balena A., Tuccinardi D., Tozzi R., Risi R., Masi D., et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38:e3465. doi: 10.1002/dmrr.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lien C.E., Lin Y.-J., Lin Y.-L., Tai I.-C., Chen C. The age-dependent immunogenicity after two doses of MVC-COV1901 vaccine. medRxiv. 2021:2021. 12.12.21267573. [Google Scholar]
- 28.Hwang Y.H., Song K.H., Choi Y., Go S., Choi S.J., Jung J., et al. Can reactogenicity predict immunogenicity after COVID-19 vaccination? Korean J Intern Med. 2021;36:1486–1491. doi: 10.3904/kjim.2021.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tani N., Chong Y., Kurata Y., Gondo K., Oishi R., Goto T., et al. Relation of fever intensity and antipyretic use with specific antibody response after two doses of the BNT162b2 mRNA vaccine. Vaccine. 2022;40:2062–2067. doi: 10.1016/j.vaccine.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC . 2022. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. [Google Scholar]
- 31.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928. doi: 10.1016/j.eclinm.2021.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.