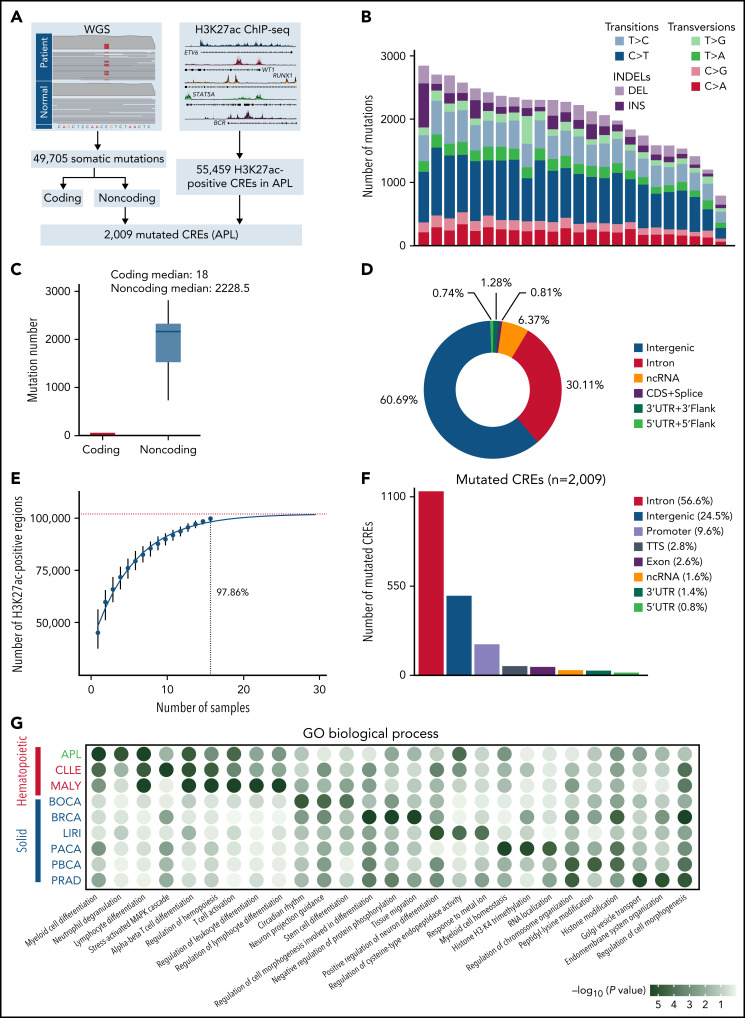
Figure 1.
Identification of mutated noncoding regulatory regions in APL by WGS and H3K27ac ChIP-seq data. (A) Schematic diagram for analyzing mutated CREs in APL. (B) The number of somatic single-nucleotide variants and short insertions/deletions in each patient. Different colors indicate different types of mutations. (C) Repartition of the 49 705 somatic mutations identified across the coding regions and noncoding regions. Each box plot represents the median, interquartile range, and minimum and maximum quartile of the mutation number. (D) Genomic localization of somatic mutations annotated using RefSeq hg38. (E) Saturation analysis for H3K27ac-positive regions identified from ChIP-seq across 16 APL samples. Individual points represent median peaks per sample added, and error bars represent standard deviations from the mean. (F) Genomic distribution of the mutated CREs over exons, promoter (-1 kb to +100 bp of the transcription start site), 3′UTR, 5′UTR, noncoding RNA (ncRNA), transcription termination site (TTS) (-100 bp to +1 kb of the TTS position), intron, and intergenic regions. (G) Enriched gene ontology (GO) terms within genes regulated by mutated CREs in APL compared with other types of hematopoietic malignancies and solid cancers. Other hematopoietic malignancies include chronic lymphocytic leukemia (CLLE) and malignant lymphoma (MALY). Solid cancers include bone cancer (BOCA), breast cancer (BRCA), liver cancer (LIRI), pancreatic cancer (PACA), pediatric brain cancer (PBCA), and prostate adenocarcinoma (PRAD). The variant call format files of WGS data for other cancer types were downloaded from the Pan-Cancer Analysis of Whole Genomes project, and the H3K27ac ChIP-seq data of these cancer types were downloaded from the Gene Expression Omnibus database. The bubble color indicates the P value. CDS, coding sequence.