Abstract
Objectives
Ageing populations have led to a growing prevalence of multimorbidity. Cardiometabolic multimorbidity (CM), the co-existence of two or more cardiometabolic disorders in the same person, is rapidly increasing. We examined the prevalence and risk factors associated with CM in a population-based sample of South African adults.
Study design
Data were analysed on individuals aged ≥15 years from the South African National Health and Nutrition Examination Survey (SANHANES), a cross sectional population-based survey conducted in 2011–2012.
Methods
CM was defined as having ≥2 of hypertension, diabetes, stroke and angina. Hypertension was defined as blood pressure ≥140/90 mmHg or self-reported antihypertensive medication use. Diabetes was defined by HbA1c ≥ 6.5% or self-reported medication use. Stroke and angina were assessed by self-report. Multivariable logistic regression was used to investigate the sociodemographic and modifiable risk factors associated with CM. The association of CM with the functional status of individuals was examined using logistic regression, where functional status was measured by the WHO DAS 2.0 12-item instrument.
Results
Of the 3832 individuals analysed, the mean age was 40.8 years (S.D. 18.3), 64.5% were female and 18% were ≥60 years. The prevalence of CM was 10.5%. The most prevalent CM cluster was hypertension and diabetes (7.3%), followed by hypertension and angina (2.6%) and hypertension and stroke (1.9%). Of the individuals with diabetes, nearly three quarters had multimorbidity from co-occurring hypertension, angina and/or stroke and of those with hypertension, 30% had co-occurring diabetes, angina and/or stroke. Age (30–44 years Adjusted Odds Ratio (AOR) = 2.68, 95% CI: 1.15–6.26), 45–59 years AOR = 16.32 (7.38–36.06), 60–74 years AOR = 40.14 (17.86–90.19), and ≥75 years AOR = 49.54 (19.25–127.50) compared with 15–29 years); Indian ethnicity (AOR = 2.58 (1.1–6.04) compared with black African ethnicity), overweight (AOR = 2.73 (1.84–4.07)) and obesity (AOR = 4.20 (2.75–6.40)) compared with normal or underweight) were associated with increased odds of CM. When controlling for age, sex and ethnicity, having ≥2 conditions was associated with significantly higher WHO DAS percentage scores (β = 5.4, S.E. = 1.1, p < 0.001).
Conclusions
A tenth of South Africans have two or more cardiometabolic conditions. The findings call for immediate prioritisation of prevention, screening and management of cardiometabolic conditions and their risk factors to avert large scale health care costs and adverse health outcomes associated with multimorbidity.
Keywords: Cardiometabolic multimorbidity, South Africa, Non-communicable diseases, Hypertension, Diabetes
1. Introduction
Advances in health care resulting in ageing populations, and lifestyle and behavioural changes resultant from globalisation have led to a growing prevalence of multimorbidity. Multimorbidity is associated with poor quality of life, higher healthcare costs, and a higher risk of mortality [[1], [2], [3]]. Cardiometabolic multimorbidity (CM), the co-existence of two or more cardiometabolic disorders in the same person, is rapidly increasing [4,5]. A recent study found that combinations of cardiometabolic conditions increased the risk of mortality multiplicatively, where each additional disease doubled the risk of mortality [6].
Many countries in Sub-Saharan Africa, including South Africa (SA), are currently undergoing nutrition transitions, marked by changes in dietary patterns and physical activity. The pace at which the nutrition transition is occurring in SA is particularly striking [7], [8], [7], [8] where rapid urbanisation and economic growth has led to shifts in diets toward more processed energy-dense foods. Over the past two decades, the prevalence of overweight or obesity in women aged 15 and older rose from 56% in 1998 to 68% in 2016; and in men, it increased from 29% to 31% [9]. The age-standardised death rates for non-communicable diseases (NCDs) now exceed those of HIV/AIDS and tuberculosis combined [10]. Cardiovascular disease is the leading cause of premature mortality and continual increases in diabetes mellitus-related mortality have been observed [10].
SA has the highest HIV/AIDS burden and most health-care spending is directed towards antiretroviral treatment, leaving limited spending for NCDs particularly at primary care level. Furthermore, the SA health care system is designed for handling single conditions where specialist departments deal with specific chronic conditions. These factors complicate the management of multimorbidity in the country. Quantifying the burden of multimorbidity of various conditions will inform policy and programmes to manage clusters of conditions. SA has begun implementing its strategic plan to decrease premature mortality from NCDs by one third by 2030 [11], which involves integrating chronic disease management into primary care.
Globally, little is known about CM and its risk factors, because observational studies typically focus on single disease outcomes. For instance, it is well documented that factors such as BMI and tobacco smoking increase the risk of cardiovascular disease, diabetes and stroke [12,13] but the magnitude of their risk on clusters of combinations are not often measured. The majority of studies on CM are conducted in high-income countries [6,[14], [15], [16], [17]] with a few in low-and-middle-income countries [18,19]. Most of these studies were conducted among older age groups. Multimorbidity research, in general, has received minor attention in South Africa. The national burden of disease studies focus on individual diseases. The current study is designed to bridge this gap by identifying patterns of CM in a population-based sample of South Africans and to examine its association with a range of socio-demographic and risk factors, which are likely to affect the ongoing health transition. CM was defined as having two or more of hypertension, diabetes, angina and stroke. We further examine the functional status of individuals with CM.
2. Methods
2.1. Sampling
This study is based on secondary analyses of data from individuals aged ≥15 years who participated in the South African National Health and Nutrition Examination Survey (SANHANES), a cross-sectional household survey conducted in 2011–2012 [20]. The survey included interviews, medical examination and blood sampling for biomarker analysis.
The SANHANES used a multi-stage disproportionate, stratified cluster sample design to select 10,000 households. Within the occupied households, 27,580 individuals of all ages were eligible to be interviewed and agreed to participate, 25,532 (92.6%) of whom completed the interview. Of the latter number, 12,025 (43.6%) and 8078 (29.3%) individuals volunteered to undergo a medical examination and provide a blood sample, respectively. A total of 15,353 participants aged ≥15 years completed the interview, of which 7043 underwent a physical examination that included blood pressure measurement and 4710 submitted a blood sample that included HbA1c measurement. Additional details of SANHANES methodology and laboratory procedures are reported elsewhere [20]. Data were analysed on the 3832 participants aged ≥15 years who underwent a physical examination and submitted a blood sample, and had non-missing data for all four of the conditions investigated; namely hypertension, diabetes, angina and stroke. Derivation of the analytic sample is presented in Supplementary file 1.
2.2. Measures
The primary outcome was CM, defined as having any two or more of the following conditions: hypertension, diabetes, stroke and angina. Hypertension was defined as having either a measured systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or a self-report of currently taking medication to lower blood pressure [21]. Three systolic and diastolic blood pressure measurements were taken after 5–10 min of rest using an Omron Automatic Digital BP monitor (model M2, Omron Healthcare, Bannockburn, Illinois, USA) [20]. The lower of the last two measurements was used as the final blood pressure reading. Diabetes was defined as having either an HbA1c ≥ 6.5% or a self-report of having been diagnosed and currently taking oral or glycaemic medication for high blood sugar or diabetes [22]. HbA1c was measured by high-performance liquid chromatography. Stroke and angina were assessed by self-report, using responses to the questions on whether a doctor, nurse or health worker at a clinic or hospital had told the respondent that they have or have had a stroke or a “heart attack/angina (chest pains)” respectively.
The selection of independent variables investigated in this study was guided by a review of the literature on risk factors associated with cardiometabolic conditions [7,[15], [16], [17], [18],23]. These risk factors are often categorised into modifiable and non-modifiable [23,24]. The non-modifiable risk factors investigated were the socio-demographic variables; sex, age, ethnicity, urban or rural residence, and household wealth quintile. Ethnicity was classified into black African, white, ‘coloured’ (mixed-race ancestry) and Indian/Asian (Indian or Asian ancestry). A household wealth index and corresponding wealth quintiles were constructed using a Multiple Correspondence Analysis [25]. Sixteen variables, including housing type, water and sanitation services, and asset ownership were used. The percentage inertia explained by the first dimension was approximately 90%. Asset ownership comprised thirteen assets, namely, ownership of a fridge, television, stove, mobile phone, radio, DVD, washing machine, computer, DSTV, motorcar, vacuum cleaner, landline telephone and internet access.
The modifiable risk factor variables measured in the study were tobacco smoking, risky alcohol use and body mass index (BMI). Tobacco smoking and alcohol use were assessed by self-report. Both the lifetime smoking and current smoking variables were used to categorize respondents as current, former or never smokers. High-risk alcohol use was assessed by the AUDIT-C, a 3-item alcohol screening tool [26]. Body mass index was calculated based on recommendations for adults by the Centers for Disease Control and Prevention [27]. The age-dependent BMI cut-off points were applied to classify respondents as underweight (BMI <18kg/m2), normal weight (BMI 18.0–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥30kg/m2).
The functional status of individuals with CM was measured by the WHO Disability Assessment Schedule (WHODAS 2.0), a 12-item interviewer-administered screening tool [28]. The WHODAS 2.0 measures health and disability at the population level by assessing the level of functioning over the past month in six domains of life, namely, cognition, mobility, self-care, getting along with other people, life activities and participation in society. Respondents rate the level of difficulty they experience in doing various activities, from none to extreme or cannot do. Composite scores ranging from 0 to 48 are transformed into percentages (0–100%) [28].
2.3. Statistical analysis
Data were analysed in Stata 15.0. (StataCorp, Texas, USA 2016) and all analyses applied sample weights to adjust for unequal probabilities of selection and nonresponse. The analytic sample comprised 3832 participants with non-missing data on the four cardiometabolic conditions. Significance levels were set at p < 0.05.
When investigating the association with risk factors, multiple imputations were used to impute values for missing data on the following variables: wealth index (17.9% missing), education (15.2% missing), BMI (4.2%), tobacco smoking (1.8%) and alcohol use (1.8%). Data on these risk factors were considered missing completely at random (MCAR).
Prevalence estimates of the combinations of the four cardiometabolic conditions were stratified by sex and chi-square tests were used to compare sex differences. Bivariable and multivariable logistic regression was used to investigate the risk factors associated with CM. Variables found to be significant in the bivariate models were added to the multivariable regression models, controlling for age, sex and ethnicity. Odd ratios including their 95% confidence intervals are reported.
The mean WHODAS percentage score was presented by the number of conditions. Multiple linear regression controlling for age, sex and ethnicity, was used to investigate the association of the number of conditions with the WHODAS percentage score and the beta regression coefficient with the associated standard error was reported.
3. Results
3.1. Description of the sample
The mean age of the sample was 40.8 years (S.D. 18.3), wherein 35% were 15–29 years old and 4% were 75 years or older (Table 1). Almost two-thirds lived in urban areas (64.6%). Over a third of the sample had less than secondary school education (35.8%) and 5.7% had tertiary education. In terms of BMI, 22.5% were overweight and 28.5% were obese. One in five (21.8%) were current tobacco smokers, 4.2% were former smokers and 18.7% were classified as high-risk users of alcohol.
Table 1.
Description of the sample (N = 3832).
| n (%) | |
|---|---|
| Age (years), mean (SD) | 40.8 (18.3) |
| 15-29 | 1342 (35.0) |
| 30-44 | 887 (23.2) |
| 45-59 | 910 (23.8) |
| 60-74 | 538 (14.0) |
| ≥75 | 155 (4.0) |
| Sex | |
| Male, n (%) | 1361 (35.5) |
| Female, n (%) | 2471 (64.5) |
| Ethnicity | |
| black African, n (%) | 2432 (63.5) |
| White, n (%) | 81 (2.1) |
| Mixed race, n (%) | 1134 (29.6) |
| Indian, n (%) | 185 (4.8) |
| Urban residence, n (%) | 2474 (64.6) |
| Wealth quintile | |
| 1 (Lowest), n (%) | 767 (20.0) |
| 2, n (%) | 773 (20.2) |
| 3, n (%) | 763 (19.9) |
| 4, n (%) | 763 (19.9) |
| 5 (Wealthiest), n (%) | 766 (20.0) |
| Education | |
| Primary/no formal education, n (%) | 1373 (35.8) |
| Secondary, n (%) | 2240 (58.5) |
| Tertiary, n (%) | 219 (5.7) |
| BMI status | |
| Underweight, n (%) | 300 (7.8) |
| Normal weight, n (%) | 1580 (41.2) |
| Overweight, n (%) | 861 (22.5) |
| Obese, n (%) | 1091 (28.5) |
| Tobacco smoking | |
| Never smoker, n (%) | 2835 (74) |
| Former smoker, n (%) | 160 (4.2) |
| Current smoker, n (%) | 837 (21.8) |
| High-risk alcohol use, n (%) | 715 (18.7) |
3.2. Combinations of cardiometabolic conditions
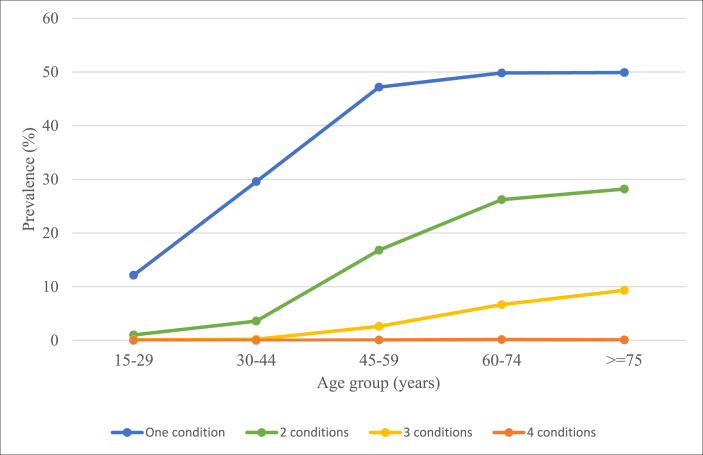
The prevalence of hypertension was 33.9% (95% CI: 31.8–36.1), diabetes 10.2% (8.9–11.7), angina 5.6% (4.5–6.9) and stroke 2.6% (2.0–3.5). The prevalence estimates of specific disease combinations (Table 2) show the 11 different combinations of CM in this study. The most prevalent dyad of disease was hypertension and diabetes, where 7.3% (6.2–8.5) had both these conditions. Hypertension and angina (2.6%,2.0–3.4), and hypertension and stroke (1.9%, 1.3–2.7) were the second and third most prevalent dyads. Of the individuals with diabetes, nearly three quarters (73.9%, 67.6–79.4) had multimorbidity from co-occurring hypertension, angina and/or stroke. Of the individuals with hypertension, 29.6% (26.2–33.3) had co-occurring diabetes, angina and/or stroke. A fifth of individuals with both hypertension and diabetes had co-occurring angina and/or stroke (19.6%, 13.7–27.1). With respect to sex differences, females had a significantly higher total prevalence of diabetes than males, as well as a significantly higher prevalence of co-occurring hypertension and diabetes. The prevalence of having one condition, as well as two or more conditions, increased with age (Fig. 1).
Table 2.
Prevalence of cardiometabolic combinations by sex.
| Cardiometabolic condition | Overall |
Male |
Female |
p-value for linearity | ||||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | obs. | % | 95% CI | % | 95% CI | ||
| One condition | 29.7 | [27.7–31.7] | 1192 | 30.3 | [27.1–33.8] | 29.2 | [26.8–31.7] | 0.581 |
| HTN | 23.9 | [22.0–25.9] | 986 | 24.8 | [21.8–28.2] | 23.3 | [21.0–25.6] | 0.415 |
| DM | 2.7 | [2.0–3.5] | 115 | 2 | [1.3–3.1] | 3.1 | [2.2–4.3] | 0.131 |
| Angina | 2.6 | [1.9–3.7] | 71 | 2.9 | [1.7–4.9] | 2.5 | [1.7–3.6] | 0.585 |
| Stroke | 0.4 | [0.2–0.8] | 20 | 0.5 | [0.2–1.4] | 0.4 | [0.2–0.8] | 0.612 |
| Two conditions | 8.7 | [7.5–10.1] | 426 | 7.7 | [6.2–9.6] | 9.4 | [8.0–11.1] | 0.102 |
| HTN, DM | 5.9 | [4.9–7.0] | 301 | 4.6 | [3.5–5.9] | 6.7 | [5.5–8.2] | 0.008a |
| HTN, Angina | 1.4 | [1.0–2.0] | 64 | 1.2 | [0.7–2.2] | 1.6 | [1.0–2.3] | 0.478 |
| HTN, Stroke | 1.1 | [0.7–1.7] | 46 | 1.7 | [0.9–3.2] | 0.6 | [0.4–1.1] | 0.058 |
| Angina, Stroke | 0.2 | [0.1–0.4] | 6 | 0.1 | [0.0–0.3] | 0.3 | [0.1–0.7] | 0.167 |
| DM, Stroke | 0.1 | [0.0–0.2] | 4 | 0.1 | [0.0–0.3] | 0.1 | [0.0–0.3] | 0.879 |
| DM, Angina | 0.1 | [0.0–0.7] | 5 | 0.1 | [0.0–0.3] | 0.2 | [0.0–1.2] | 0.464 |
| Three conditions | 1.7 | [1.2–2.4] | 70 | 1.7 | [0.9–3.0] | 1.7 | [1.2–2.4] | 0.924 |
| HTN, DM, Stroke | 0.5 | [0.3–1.0] | 19 | 0.8 | [0.3–2.0] | 0.3 | [0.1–0.7] | 0.275 |
| HTN, Stroke, Angina | 0.3 | [0.1–0.5] | 11 | 0.3 | [0.1–0.9] | 0.3 | [0.1–0.6] | 0.996 |
| HTN, DM, Angina | 0.9 | [0.5–1.4] | 39 | 0.6 | [0.3–1.5] | 1 | [0.7–1.7] | 0.158 |
| DM, Stroke, Angina | 0.1 | [0.0–0.4] | 1 | 0 | 0.1 | [0.0–0.6] | 0.318 | |
|
All four conditions |
<0.1 |
[0.0–0.1] |
4 |
<0.1 |
[0.0–0.1] |
<0.1 |
[0.0–0.2] |
0.413 |
| Totals | ||||||||
| HTN | 33.9 | [31.8–36.1] | 1470 | 34.1 | [30.8–37.5] | 33.9 | [31.3–36.5] | 0.918 |
| DM | 10.2 | [8.9–11.7] | 488 | 8.2 | [6.6–10.0] | 11.6 | [9.8–13.6] | 0.004a |
| Angina | 5.6 | [4.5–6.9] | 201 | 5.2 | [3.7–7.3] | 5.9 | [4.6–7.5] | 0.493 |
| Stroke | 2.6 | [2.0–3.5] | 111 | 3.5 | [2.2–5.4] | 2.1 | [1.5–2.8] | 0.059 |
HTN: Hypertension, DM: diabetes mellitus.
p < 0.05 indicating a significant difference between males and females.
Fig. 1.
Prevalence of number of cardiometabolic conditions by age group.
3.3. CM and related risk factors
The prevalence of CM, defined as having any two or more of the four conditions (hypertension, diabetes, stroke and angina) was 10.2% (Table 3) and ranged from 1.1% in 15–29-year olds to 37.6% in ≥75 year olds. In the bivariable logistic regressions, older age (30–44 years, 45–59 years, 60–74 years and ≥75 years compared with 15–29 years), Indian ethnicity (compared with black African ethnicity), increased wealth (highest wealth quintile compared with lowest/poorest quintile), overweight and obesity (compared with normal or underweight), and being a former tobacco smoker (compared to never smoker) were associated with increased odds of CM. Secondary level school education (compared with primary or no formal schooling) was associated with reduced odds of CM. In the adjusted model, ages 30–44 years (Adjusted Odds Ratio (AOR) = 2.68, 95% CI: 1.15–6.26, p = 0.023), 45–59 years (AOR = 16.32, 95% CI: 7.38–36.06, p < 0.001), 60–74 years (AOR = 40.14, 95% CI: 17.86–90.19, p < 0.001) and ≥75 years (AOR = 49.54, 95% CI: 19.25–127.5, p < 0.001) (compared with 15–29 years), Indian ethnicity (AOR = 2.58, 95% CI: 1.1–6.04, p = 0.029 compared with black African ethnicity), overweight and obesity (AOR = 2.73, 95% CI: 1.84–4.07, p < 0.001 and AOR = 4.20, 95% CI: 2.75–6.40, p < 0.001 respectively compared with normal or underweight) were associated with increased odds of CM.
Table 3.
Cardiometabolic multimorbidity by related factors.
| Prevalence |
Bivariable logistic regression |
Multivariable logistic regression |
||||||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | OR | 95% CI | p-value | AOR | 95% CI | p-value | |
| Total | 10.5 | [9.1–11.9] | ||||||
| Age (years) | ||||||||
| 15–29 | 1.1 | [0.3–1.9] | ref | – | – | ref | – | – |
| 30–44 | 3.8 | [2.2–5.4] | 3.52* | [1.55–7.99] | 0.003 | 2.68* | [1.15–6.26] | 0.023 |
| 45–59 | 19.5 | [15.9–23.1] | 21.51* | [10.36–44.65] | <0.001 | 16.32* | [7.38–36.06] | <0.001 |
| 60–74 | 33.0 | [27.4–38.6] | 43.83* | [20.66–92.97] | <0.001 | 40.14* | [17.86–90.19] | <0.001 |
| ≥75 | 37.6 | [24.5–50.7] | 53.55* | [21.84–131.29] | <0.001 | 49.54* | [19.25–127.5] | <0.001 |
| Sex | ||||||||
| Male | 9.4 | [7.4–11.4] | ref | – | – | ref | – | – |
| Female | 11.2 | [9.6–12.8] | 1.22 | [0.94–1.57] | 0.136 | 0.97 | [0.68–1.37] | 0.844 |
| Ethnicity | ||||||||
| black African | 9.9 | [8.4–11.4] | ref | – | – | ref | – | – |
| White | 17.7 | [6–29.3] | 1.94 | [0.85–4.44] | 0.114 | 0.61 | [0.29–1.27] | 0.185 |
| Mixed race | 9.6 | [6.9–12.2] | 0.96 | [0.68–1.35] | 0.805 | 0.99 | [0.68–1.44] | 0.951 |
| Indian | 26.6 | [16.4–36.8] | 3.28* | [1.88–5.72] | <0.001 | 2.58* | [1.1–6.04] | 0.029 |
| Place of residence | ||||||||
| Rural | 9.4 | [7.4–11.4] | ref | – | – | |||
| Urban | 11.2 | [9.3–13.1] | 1.21 | [0.89–1.65] | 0.215 | |||
| Wealth quintile | ||||||||
| 1 (Lowest) | 8.2 | [5.7–10.8] | ref | – | – | ref | – | – |
| 2 | 9.0 | [6.1–11.9] | 1.11 | [0.67–1.82] | 0.688 | 1.17 | [0.68–1.99] | 0.568 |
| 3 | 11.7 | [8.6–14.7] | 1.47 | [0.95–2.28] | 0.084 | 1.40 | [0.88–2.24] | 0.158 |
| 4 | 10.8 | [7.4–14.2] | 1.35 | [0.84–2.16] | 0.210 | 1.28 | [0.74–2.22] | 0.366 |
| 5 (Wealthiest) | 12.9 | [9–16.8] | 1.66* | [1.02–2.69] | 0.041 | 1.10 | [0.62–1.94] | 0.738 |
| Education | ||||||||
| Primary/no formal education | 15.3 | [12.7–17.9] | ref | – | – | ref | – | – |
| Secondary | 8.1 | [6.3–9.9] | 0.49* | [0.36–0.67] | <0.001 | 1.31 | [0.91–1.89] | 0.150 |
| Tertiary | 9.2 | [4.4–14] | 0.56 | [0.3–1.03] | 0.061 | 0.84 | [0.42–1.65] | 0.607 |
| BMI status | ||||||||
| Underweight/Normal weight | 4.1 | [3.1–5.2] | ref | – | – | ref | – | – |
| Overweight | 13.8 | [11–16.7] | 3.74* | [2.69–5.2] | <0.001 | 2.73* | [1.84–4.07] | <0.001 |
| Obese | 18.5 | [15.2–21.8] | 5.29* | [3.8–7.36] | <0.001 | 4.20* | [2.75–6.4] | <0.001 |
| Tobacco smoking | ||||||||
| Never smoker | 9.9 | [8.4–11.4] | ref | – | – | ref | – | – |
| Former smoker | 21.2 | [10.5–31.8] | 2.44* | [1.26–4.73] | 0.008 | 1.67 | [0.91–3.07] | 0.100 |
| Current smoker | 10.0 | [6.8–13.2] | 1.01 | [0.67–1.51] | 0.966 | 1.35 | [0.85–2.16] | 0.204 |
| Alcohol use | ||||||||
| Low risk | 10.9 | [9.3–12.4] | ref | – | – | |||
| High risk | 8.8 | [5.9–11.7] | 0.79 | [0.54–1.16] | 0.233 | |||
Odds Ratio (OR), Adjusted Odds Ratio (AOR).
3.4. Functional status and CM
The mean WHODAS percentage score, indicating the degree of difficulty in conducting routine activities, was higher for individuals with ≥2 conditions (13.9%, standard error (SE): 1.1) than for those with one condition (6.8%, SE: 0.5) and no condition (4.0%, SE: 0.3). (Table 4). After controlling for age, sex and ethnicity, having ≥2 conditions were associated with significantly higher WHODAS percentage scores (regression coefficient β = 5.39, S.E. = 1.1, p < 0.001, compared with having no condition).
Table 4.
WHODAS 2.0 mean percentage scores for individuals.
| Cardiometabolic condition | Mean | S.E. | B (S.E.) (p-value)a |
|---|---|---|---|
| Hypertension | 8.8 | 0.6 | |
| Diabetes | 11.0 | 1.1 | |
| Angina | 12.3 | 1.3 | |
| Stroke | 18.9 | 2.3 | |
| Number of conditions | |||
| None | 4.0 | 0.3 | ref |
| 1 condition | 6.8 | 0.5 | 0.1 (0.54) (0.883) |
| ≥ 2 conditions | 13.9 | 1.1 | 5.4 (1.1) (<0.001)b |
S.E. – standard error.
Adjusted for age, sex and ethnicity.
p < 0.05.
4. Discussion
This is the first study to provide data on multimorbidity of cardiometabolic conditions in SA using a large population-based sample. The prevalence of co-occurring clusters of conditions was estimated. The relationship between CM and its modifiable and non-modifiable risk factors was also examined. The prevalence of CM (defined as having two or more of the following conditions: hypertension, diabetes mellitus, angina and stroke) was 10.5%. Of the total sample, 60.1% had none of the four conditions; 29.7% had just one condition; 8.7% had two conditions; 1.7% had three conditions, and <0.1% had all four conditions. The most common form of multimorbidity was hypertension and diabetes, which was present in 7.3% of individuals. One in five individuals with hypertension and diabetes had co-occurring angina and/or stroke. These findings have implications for both the prevention and management of cardiometabolic conditions in SA, and can, therefore, inform health policy, in both health promotion and health systems design. Appropriate organisation of the health system at primary, secondary and tertiary levels is important in the optimal and cost-effective management of multimorbidity.
The prevalence rates of each of the four conditions reported in this study are similar to those reported in prior studies using data from SANHANES [20,29,30] and other South African national studies [9,31]. Hypertension was the single most prevalent condition at 33.9%. Hypertension alone was present in two-thirds of hypertensive individuals while the other third had one or more co-occurring condition(s). Diabetes was found to cluster more frequently with other conditions: 10.3% of individuals were diabetic and three-quarters of these people with diabetes had one or more co-occurring condition(s).
In the adjusted regressions, increasing age, Indian ethnicity, and overweight and obesity were significantly associated with increased odds of CM. The risk for cardiometabolic conditions was substantially higher in older age groups, as these conditions are ageing-related diseases [32]. This is particularly important in a country like South Africa that is experiencing significant increases in life expectancy [33], due to increased survival rates of patients receiving antiretroviral treatment for HIV/AIDS, declines in tuberculosis mortality, and reductions in extreme poverty. In addition, patients living longer on antiretroviral treatment suffer from the cardiometabolic side effects of these drugs. Consequently, growing numbers of older people may present with multiple cardiometabolic conditions.
The prevalence of multiple cardiometabolic conditions in people of Indian descent was more than twice that of the black African and mixed-race ethnic groups. Several studies in SA have confirmed a high risk for cardiometabolic diseases, particularly diabetes, in South Africans of Indian descent [9,30,34]. Indian Asians have been shown to have a higher risk than White Caucasians for the development of cardiometabolic diseases for the same unit of increase in BMI [35,36] and they may also have genetic markers that increase their susceptibility to diabetes [35].
The associations of CM with overweight and obesity were also found in other studies [16,17,37,38]. In this study, overweight and obesity were associated with double and quadruple the risk of CM respectively. Currently, more than two in five South African women are obese, half of whom are severely obese [9]; highlighting the need for urgent obesity prevention initiatives and screening of overweight and obese people for raised blood pressure and blood glucose. A pooled analysis of the USA and European cohorts found that overweight doubled the risk of CM, whereas mild and severe obesity resulted in five to ten times higher risk when compared to people of a healthy weight [17]. Dyslipidaemia and inflammation are common pathways through which obesity can lead to the development of multiple cardiometabolic conditions [39].
While CM generally increased with household wealth and was highest among former smokers and those with less than secondary school education, these associations were not statistically significant after adjusting for other factors. In HICs, the cardiometabolic risk is higher in lower socio-economic groups [40] whereas in LMICs, higher socioeconomic status is usually associated with increased risk of NCDs and cardiometabolic diseases [41]. The nutrition transition is still underway in SA, where economic growth and urbanisation have led to increased affordability for calorific foods and an inclination to more sedentary behaviours. These changes can in turn, lead to low physical activity and poor diets, particularly in individuals with low health education, that is, who are not aware of the health consequences of these behaviours. Consequently, some South African studies have found higher cardiometabolic and/or non-communicable disease risk in individuals with higher socioeconomic status [42,43] and lower education levels [43]. This sample included a wide age range while the former studies were based on older people.
Given that cardiometabolic conditions have shared risk factors, targeting these risk factors at primary and secondary prevention levels will lower the overall risk of developing any one or more condition(s). In primary prevention, health promotion programmes are needed to target the risk factors for obesity such as unhealthy eating and physical inactivity, as well as tobacco and alcohol use behaviours. Healthy lifestyles established early on in life will persist into old age thereby preventing or delaying hypertension, dyslipidaemia and cardiovascular diseases. In secondary prevention, for a patient with ischaemic heart disease and stroke, for example, addressing the risk factors such as smoking, blood pressure control, obesity, lack of exercise and poor diet will be of benefit for both the ischaemic heart disease (angina and heart attacks) as well as the risk of further stroke. Furthermore, many risk factors for cardiometabolic conditions are also risk factors for cancers and other chronic diseases. Hence, targeting risk factors for CM can, therefore, contribute to the prevention of other related chronic diseases.
CM was associated with the WHODAS measure of disability or reduced functionality, but having a single condition was not. Another SA study among elderly people with diabetes found that a larger number of comorbid chronic conditions increased disability and poor quality of life [44]. The optimal management of multimorbidity is important because comorbid conditions can interact with one another resulting in complications and thereby negatively impacting the quality of life [45]. Adequate care and management of people with CM can prevent the complications from these diseases, thereby preventing or reducing disability and poor quality of life.
The CM observed in this study has implications for clinical practice and health systems design in SA. The SA health system's inclination towards specialisation, with health professionals and clinics dedicated to specific types of non-communicable diseases, may not always be beneficial to the patient. This is particularly important for cardiometabolic diseases, as atherosclerotic narrowing of arteries and thrombotic or embolic occlusion of arteries that result in acute events arise from common disease processes. These common disease processes result in clinical syndromes in the various organs that the vasculature supplies. These clinical syndromes are currently managed by specialists in separate clinics or hospital departments, such as cardiology, renal and vascular surgery clinics. However, they are all caused by the same disease processes. A more optimal approach would be shifting work from specialist level down to the primary care level, where patients presenting with an index condition can be examined for other comorbidities and receive comprehensive care for all their conditions. This shift away from specialised units of care will also help to bridge the gap in access to quality health care in resource-limited settings.
It was difficult to compare the prevalence of CM with other studies, as the diseases included in the definition of CM differ between studies. Some studies argue that including hypertension in the definition may underscore the true effect of blood pressure on chronic disease [6]. However, we included hypertension to keep consistent with other South African studies on multimorbidity which often include hypertension as a disease rather than a risk factor [38,42,46].
The study has some limitations. Firstly, the cross-sectional nature of the study means that the findings on risk behaviours associated with CM cannot be interpreted as causational. Secondly, angina and stroke were based on self-reported data. Thirdly, the choice of explanatory variables in this study was restricted due to the data that was collected in the SANHANES. The strengths of the study are that it is based on a sample derived from a population-based survey and includes a wide age range. Diabetes and hypertension were based on clinical measures of blood glucose and blood pressure as well as self-reported diagnosis and medication use and therefore capture diagnosed and undiagnosed cases. Many studies on multimorbidity include only cases with known diagnoses. This study is therefore likely to reflect a more reliable estimate of CM, particularly for SA, where the majority of individuals are unscreened for cardiometabolic conditions and remain undiagnosed for many years [29,30].
5. Conclusion
The findings draw attention to the need to prioritise effective management of cardiometabolic conditions in SA and other developing countries to avert large scale health care expenses. Aggressive approaches are needed to upscale detection, treatment, and control of cardiovascular disease risk factors and diabetes in the primary health-care sector. This would entail empowering and training nurses, who are the frontline health workers in primary care.
Ethical approval
Ethical approval was obtained from the Research Ethics Committee (REC) of the South African Human Sciences Research Council (HSRC) (REC number: 6/16/11/11), which is in accordance with the Declaration of Helsinki. Informed written consent/assent was obtained from all the survey participants, and parental consent was obtained for participants below 18 years of age.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhip.2021.100193.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Marengoni A., Angleman S., Melis R., Mangialasche F., Karp A., Garmen A., et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res. Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Valderas J.M., Starfield B., Sibbald B., Salisbury C., Roland M. Defining comorbidity: implications for understanding health and health services. Ann. Fam. Med. 2009;7:357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff J.L., Starfield B., Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch. Intern. Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 4.Glynn L.G. Multimorbidity: another key issue for cardiovascular medicine. Lancet. 2009;374:1421–1422. doi: 10.1016/S0140-6736(09)61863-8. [DOI] [PubMed] [Google Scholar]
- 5.Weiss C.O., Boyd C.M., Yu Q., Wolff J.L., Leff B. Patterns of prevalent major chronic disease among older adults in the United States. J. Am. Med. Assoc. 2007;298:1158–1162. doi: 10.1001/jama.298.10.1160-b. [DOI] [PubMed] [Google Scholar]
- 6.Di Angelantonio E., Kaptoge S., Wormser D., Willeit P., Butterworth A.S., Bansal N., et al. Association of cardiometabolic multimorbidity with mortality. Jama. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steyn K., Sliwa K., Hawken S., Commerford P., Onen C., Damasceno A., et al. Risk factors associated with myocardial infarction in Africa: the INTERHEART Africa study. Circulation. 2005;112:3554–3561. doi: 10.1161/CIRCULATIONAHA.105.563452. [DOI] [PubMed] [Google Scholar]
- 8.Tibazarwa K., et al. A time bomb of cardiovascular risk factors in South Africa: results from the Heart of Soweto Study “Heart Awareness Days. Int. J. Cardiol. 2009;132:233–239. doi: 10.1016/j.ijcard.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 9.National Department of Health (NDoH) NDoH, Stats SA, SAMRC, and ICF; Pretoria, South Africa and Rockville, Maryland, USA: 2019. Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), ICF. South Africa Demographic and Health Survey 2016. [Google Scholar]
- 10.Nojilana B., Bradshaw D., Pillay-van Wyk V., Msemburi W., Somdyala N., Joubert J.D., et al. 2016. Persistent Burden from Non-communicable Diseases in South Africa Needs Strong Action. [DOI] [PubMed] [Google Scholar]
- 11.National Department of Health . 2019. National Strategic Plan for the Prevention and Control of Non-communicable Diseases 2020-25. Pretoria. [Google Scholar]
- 12.Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta: 2014. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. [Google Scholar]
- 14.Sakakibara B.M., Obembe A.O., Eng J.J. The prevalence of cardiometabolic multimorbidity and its association with physical activity, diet, and stress in Canada: evidence from a population-based cross-sectional study. BMC Publ. Health. 2019;19:1361. doi: 10.1186/s12889-019-7682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh-Manoux A., Fayosse A., Sabia S., Tabak A., Shipley M., Dugravot A., et al. Clinical, socioeconomic, and behavioural factors at age 50 years and risk of cardiometabolic multimorbidity and mortality: a cohort study. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staimez L.R., Wei M.Y., Kim M., Narayan K.M.V., Saydah S.H. Multimorbidity of four cardiometabolic and chronic pulmonary disease groups: prevalence and attributable fraction in US adults, 2007-2012. J. Comorbidity. 2017;7:22–32. doi: 10.15256/joc.2017.7.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kivimaki M., Kuosma E., Ferrie J.E., Luukkonen R., Nyberg S.T., Alfredsson L., et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Publ Health. 2017;2:e277–e285. doi: 10.1016/S2468-2667(17)30074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng L., Jehan I., de Silva H.A., Naheed A., Farazdaq H., Hirani S., et al. Prevalence and correlates of cardiometabolic multimorbidity among hypertensive individuals: a cross-sectional study in rural South Asia-Bangladesh, Pakistan and Sri Lanka. 2019;9 doi: 10.1136/bmjopen-2019-030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D., Tang X., Shen P., Si Y., Liu X., Xu Z., et al. Multimorbidity of cardiometabolic diseases: prevalence and risk for mortality from one million Chinese adults in a longitudinal cohort study. BMJ open. 2019;9 doi: 10.1136/bmjopen-2018-024476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shisana O., Labadarios D., Rehle T., Simbayi L., Zuma K., Dhansay A., et al. 2014 Edition. HSRC Press; Cape Town: 2014. South African National Health and Nutrition Examination Survey (SANHANES-1) [Google Scholar]
- 21.National Center for Health Statistics, Centers for Disease Control and Prevention Analytic and reporting guidelines. The national health and nutrition examination survey (NHANES). Prevention. 2006. http://www.cdc.gov/nchs/nhanes/nhanes2003-4/analytical_guidelines.htm Available at: 1-14.
- 22.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35 [Google Scholar]
- 23.Petersen S.E., Sanghvi M.M., Aung N., Cooper J.A., Paiva J.M., Zemrak F., et al. The impact of cardiovascular risk factors on cardiac structure and function: insights from the UK Biobank imaging enhancement study. PloS One. 2017;12 doi: 10.1371/journal.pone.0185114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Issaka A., Paradies Y., Stevenson C. Modifiable and emerging risk factors for type 2 diabetes in Africa: a systematic review and meta-analysis protocol. Syst. Rev. 2018;7:139. doi: 10.1186/s13643-018-0801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon T., Booysen F., Mbonigaba J. Socio-economic inequalities in the multiple dimensions of access to healthcare: the case of South Africa. BMC Publ. Health. 2020;20:289. doi: 10.1186/s12889-020-8368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bush K., Kivlahan D.R., Mcdonell M.B., Fihn S.D., Bradley K.A. The Audit alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention CDC 24/7: saving lives, protecting PeopleTM: division of nutrition, physical activity, and obesity. http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/ Available at:
- 28.World Health Organization . WHO; Geneva: 2010. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0) [Google Scholar]
- 29.Berry K.M., Parker W.A., Mchiza Z.J., Sewpaul R., Labadarios D., Rosen S., et al. Quantifying unmet need for hypertension care in South Africa through a care cascade: evidence from the SANHANES, 2011-2012. BMJ Glob. Health. 2017;2 doi: 10.1136/bmjgh-2017-000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stokes A., Berry K.M., McHiza Z., Parker W.A., Labadarios D., Chola L., et al. Prevalence and unmet need for diabetes care across the care continuum in a national sample of South African adults: evidence from the SANHANES-1, 2011-2012. PloS One. 2017;12 doi: 10.1371/journal.pone.0184264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruan Y., Guo Y., Zheng Y., Huang Z., Sun S., Kowal P., et al. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: results from SAGE Wave 1. BMC Publ. Health. 2018;18:778. doi: 10.1186/s12889-018-5653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Statistics South Africa . 2019. Mid - year population estimates. Pretoria. [Google Scholar]
- 34.Peer N., Balakrishna Y., de Villiers A., Naidoo P. Differential associations of cardio-metabolic diseases by population group, gender and adiposity in South Africa. PloS One. 2018;13 doi: 10.1371/journal.pone.0202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misra A., Khurana L. Obesity-related non-communicable diseases: South Asians vs white Caucasians. Int. J. Obes. 2011;35:167–187. doi: 10.1038/ijo.2010.135. [DOI] [PubMed] [Google Scholar]
- 36.Graham A., McCarthy M., Mohan V. The genetics of non-insulin dependent diabetes mellitus in South India. An overview. Annu. Mediaev. 1992;24:491–497. doi: 10.3109/07853899209167001. [DOI] [PubMed] [Google Scholar]
- 37.de Carvalho J.N., de Camargo Cancela M., de Souza D.L.B. Lifestyle factors and high body mass index are associated with different multimorbidity clusters in the Brazilian population. PloS One. 2018;13 doi: 10.1371/journal.pone.0207649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weimann A., Dai D., Oni T. A cross-sectional and spatial analysis of the prevalence of multimorbidity and its association with socioeconomic disadvantage in South Africa: a comparison between 2008 and 2012. Soc. Sci. Med. 2016;163:144–156. doi: 10.1016/j.socscimed.2016.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Gaal L.F., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 40.Sommer I., Griebler U., Mahlknecht P., Thaler K., Bouskill K., Gartlehner G., et al. Socioeconomic inequalities in non-communicable diseases and their risk factors: an overview of systematic reviews. BMC Publ. Health. 2015;15:914. doi: 10.1186/s12889-015-2227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogunsina K., Dibaba D.T., Akinyemiju T. Association between life-course socio-economic status and prevalence of cardio-metabolic risk ractors in five middle-income countries. J. Glob. Health. 2018;8 doi: 10.7189/jogh.08.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang A.Y., Gomez-Olive F.X., Payne C., Rohr J.K., Manne-Goehler J., Wade A.N., et al. Chronic multimorbidity among older adults in rural South Africa. BMJ Glob. Health. 2019;4 doi: 10.1136/bmjgh-2018-001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phaswana-Mafuya N., Peltzer K., Chirinda W., Musekiwa A., Kose Z., Hoosain E., et al. Self-reported prevalence of chronic non-communicable diseases and associated factors among older adults in South Africa. Glob. Health Action. 2013;6:20936. doi: 10.3402/gha.v6i0.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werfalli M., Kassanjee R., Kalula S., Kowal P., Phaswana-Mafuya N., Levitt N.S. Diabetes in South African older adults: prevalence and impact on quality of life and functional disability - as assessed using SAGE Wave 1 data. Glob. Health Action. 2018;11:1449924. doi: 10.1080/16549716.2018.1449924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin R.R., Peyrot M. Quality of life and diabetes. Diabetes Metab. Res. Rev. 1999;15:205–218. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 46.Chang A.Y., Gomez-Olive F.X., Manne-Goehler J., Wade A.N., Tollman S., Gaziano T.A., et al. Multimorbidity and care for hypertension, diabetes and HIV among older adults in rural South Africa. Bull. World Health Organ. 2019;97:10–23. doi: 10.2471/BLT.18.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.