The isolated cluster unit in the structure of [Nb6Cl12I2(H2O)4]·8THF consists of an {Nb6} atom octahedron coordinated by twelve chlorido, four aqua, and two iodido ligands. THF solvent molecules are bound via O—H⋯O hydrogen bonds.
Keywords: crystal structure, metal atom cluster, niobium, chloride, structure determination
Abstract
The title compound, [Nb6Cl12I2(H2O)4]·8THF (THF is tetrahydrofuran, C4H8O), comprises an uncharged niobium cluster unit surrounded by THF solvent molecules. The edges of the {Nb6} octahedron are μ
2-coordinated by twelve chlorido ligands. Four in-plane (equatorial plane) aqua ligands and two iodido ligands coordinating above and below the plane are bound at the corners of the {Nb6} atomic octahedron. O—H⋯O hydrogen bonds are formed between the aqua ligands and the THF solvent molecules; one THF molecule is disordered over two positions with the major component having a site occupancy of 0.64 (2).
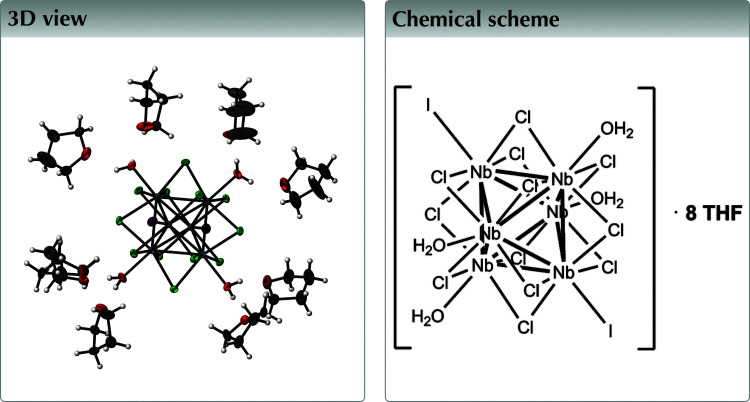
Structure description
Cluster complexes of the early transition metals have been the subject of intense research for decades. Hexanuclear {Nb6} cluster complexes represent an interesting field of research (Cotton, 1964 ▸; Simon, 1988 ▸). Such compounds are produced via solid-state reactions at high temperatures and then converted into more soluble species by solvent chemistry (Koknat et al., 1974 ▸; Lemoine et al., 2019 ▸). The title compound can be obtained by dissolving [Nb6Cl12I2(H2O)4]·4H2O in THF and recrystallization.
The {Nb6} atomic polyhedron is an octahedron (Fig. 1 ▸) in which two different Nb—Nb bond lengths have to be considered. The niobium atoms located in the equatorial plane (coordination by aqua ligands) have an average Nbeq—Nbeq bond length of 2.896 Å. The niobium atoms above and below this plane (Nbax), which are coordinated by iodido ligands, have Nbax—Nbeq bond lengths averaging at 2.938 Å. Thereby, the {Nb6} atomic octahedron is elongated, reflected also by the atomic distances between opposite niobium atoms. Within the equatorial plane they are 4.095 Å on average, and 4.2150 (8) Å between the axial sites. The twelve chlorido ligands of the inner ligand sphere are μ 2-bridging over the edges of the {Nb6} atom octahedron. The average Nbeq—Cl bond length is 2.469 Å and Nbax—Cl is 2.460 Å. Of the six outer coordination sites, four aqua ligands singly bond to the Nbeq atoms and two iodido ligands to the Nbax atoms with average Nb—O and Nb—I bond lengths of 2.223 and 2.944 Å, respectively. These atom distances indicate a cluster unit with 16 cluster-based electrons. Thus, there is no change of the oxidation state compared to the starting material. Rather strong hydrogen bonds (Steiner, 2002 ▸) with donor⋯acceptor distances in the range 2.530 (8)–2.68 (5) Å are found between the aqua ligands of the {Nb6} unit and the O atoms of the solvent THF molecules (Table 1 ▸). A view of the packing of cluster and THF solvent molecules is given in Fig. 2 ▸.
Figure 1.
The discrete cluster unit of [Nb6Cl12I2(H2O)4]·8THF with surrounding THF solvent molecules. Atoms are drawn as displacement ellipsoids at the 50% probability level. The {Nb6} metal atom octahedron is shown in a polyhedral representation, O—H⋯O hydrogen bonds are shown as red dashed lines. Of the disordered THF molecule, only the major component (A) is shown for better visibility.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯O5 | 0.85 | 1.79 | 2.642 (7) | 180 |
| O1—H1B⋯O6A_a | 0.85 | 1.75 | 2.60 (3) | 178 |
| O1—H1B⋯O6B_b | 0.85 | 1.83 | 2.68 (5) | 173 |
| O2—H2A⋯O7 | 0.85 | 1.83 | 2.639 (7) | 158 |
| O2—H2B⋯O8 | 0.85 | 1.78 | 2.634 (7) | 180 |
| O3—H3B⋯O9 | 0.85 | 1.75 | 2.601 (8) | 179 |
| O3—H3A⋯O10 | 0.85 | 1.88 | 2.637 (8) | 148 |
| O4—H4B⋯O11 | 0.85 | 1.92 | 2.613 (8) | 138 |
| O4—H4A⋯O12 | 0.85 | 2.09 | 2.530 (8) | 112 |
Figure 2.
Arrangement of neutral cluster units and THF solvent molecules in the unit cell in a view along the a axis. The {Nb6} metal atom octahedra are shown in a polyhedral representation, and O—H⋯O hydrogen bonds are shown as dashed red lines.
Synthesis and crystallization
Starting from the compound [Nb6Cl12I2(H2O)4]·4H2O (Schäfer et al., 1972 ▸; Brničević et al., 1981 ▸), the title compound [Nb6Cl12I2(H2O)4]·8THF can be synthesized in moderate yields. 50 mg (36.21 μmol) of [Nb6Cl12I2(H2O)4]·4H2O and 3 ml (36.86 mmol) of THF were placed in a 4 ml vial and heated in a sand bath at 333 K for two days. From the dark-green solution, small black crystals formed together with a larger amount of an amorphous sediment. The crystals were washed several times with THF. 32 mg (16.97 μmol, yield 64%) of [Nb6Cl12I2(H2O)4]·8THF were obtained.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. One of the solvent THF molecules, O6, C5–C8, is disordered over two sets of sites [ratio 0.64 (2):0.36 (2) for parts A:B], with constraints on some U ij parameters.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Nb6Cl12I2(H2O)4]·8C4H8O |
| M r | 1885.55 |
| Crystal system, space group | Orthorhombic, P b c a |
| Temperature (K) | 123 |
| a, b, c (Å) | 19.3389 (7), 18.1968 (7), 34.039 (1) |
| V (Å3) | 11978.6 (8) |
| Z | 8 |
| Radiation type | Mo Kα |
| μ (mm−1) | 2.72 |
| Crystal size (mm) | 0.23 × 0.16 × 0.14 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 94479, 15885, 11673 |
| R int | 0.050 |
| (sin θ/λ)max (Å−1) | 0.683 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.051, 0.132, 1.06 |
| No. of reflections | 15885 |
| No. of parameters | 602 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.47, −1.50 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314622006186/wm4165sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314622006186/wm4165Isup2.hkl
CCDC reference: 2178831
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We gratefully acknowledge the maintenance of the XRD equipment through Dr Alexander Villinger (University of Rostock).
full crystallographic data
Crystal data
| [Nb6Cl12I2(H2O)4]·8C4H8O | Dx = 2.091 Mg m−3 |
| Mr = 1885.55 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, Pbca | Cell parameters from 9812 reflections |
| a = 19.3389 (7) Å | θ = 2.5–29.0° |
| b = 18.1968 (7) Å | µ = 2.72 mm−1 |
| c = 34.039 (1) Å | T = 123 K |
| V = 11978.6 (8) Å3 | Block, black |
| Z = 8 | 0.23 × 0.16 × 0.14 mm |
| F(000) = 7328 |
Data collection
| Bruker APEXII CCD diffractometer | 11673 reflections with I > 2σ(I) |
| Radiation source: microfocus sealed tube | Rint = 0.050 |
| φ and ω scans | θmax = 29.0°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | h = −26→24 |
| k = −24→24 | |
| 94479 measured reflections | l = −36→45 |
| 15885 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.051 | Hydrogen site location: mixed |
| wR(F2) = 0.132 | H-atom parameters constrained |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0359P)2 + 173.2937P] where P = (Fo2 + 2Fc2)/3 |
| 15885 reflections | (Δ/σ)max = 0.001 |
| 602 parameters | Δρmax = 1.47 e Å−3 |
| 0 restraints | Δρmin = −1.50 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Hydrogen atoms were placed in idealized positions and refined using a riding model. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Nb1 | 0.59644 (3) | 0.17243 (3) | 0.36689 (2) | 0.0169 (1) | |
| Nb2 | 0.58974 (3) | 0.31709 (3) | 0.32845 (2) | 0.0171 (1) | |
| Nb3 | 0.58856 (3) | 0.30859 (3) | 0.41313 (2) | 0.0160 (1) | |
| Nb4 | 0.46988 (3) | 0.21109 (3) | 0.40986 (2) | 0.0171 (1) | |
| Nb5 | 0.47127 (3) | 0.21952 (3) | 0.32502 (2) | 0.0176 (1) | |
| Nb6 | 0.46288 (3) | 0.35529 (3) | 0.37133 (2) | 0.0163 (1) | |
| Cl1 | 0.67822 (8) | 0.22178 (9) | 0.31834 (5) | 0.0230 (3) | |
| Cl2 | 0.67647 (8) | 0.21119 (9) | 0.41928 (5) | 0.0218 (3) | |
| Cl3 | 0.53495 (8) | 0.09517 (8) | 0.41480 (5) | 0.0239 (3) | |
| Cl4 | 0.53829 (8) | 0.10741 (9) | 0.31292 (5) | 0.0236 (3) | |
| Cl5 | 0.67003 (8) | 0.38061 (9) | 0.37308 (4) | 0.0218 (3) | |
| Cl6 | 0.52879 (8) | 0.25339 (9) | 0.47005 (4) | 0.0224 (3) | |
| Cl7 | 0.38863 (8) | 0.14800 (9) | 0.36529 (5) | 0.0236 (3) | |
| Cl8 | 0.53035 (8) | 0.2760 (1) | 0.26799 (4) | 0.0244 (3) | |
| Cl9 | 0.52292 (8) | 0.43133 (9) | 0.32239 (4) | 0.0220 (3) | |
| Cl10 | 0.52290 (8) | 0.42292 (8) | 0.42386 (4) | 0.0204 (3) | |
| Cl11 | 0.38214 (8) | 0.30702 (9) | 0.42091 (4) | 0.0216 (3) | |
| Cl12 | 0.38156 (8) | 0.31507 (9) | 0.32027 (4) | 0.0229 (3) | |
| I1 | 0.68739 (2) | 0.04382 (3) | 0.36266 (2) | 0.0287 (1) | |
| I2 | 0.36838 (2) | 0.48292 (3) | 0.37360 (2) | 0.0274 (1) | |
| O1 | 0.6562 (2) | 0.3756 (3) | 0.2855 (1) | 0.028 (1) | |
| H1A | 0.6990 | 0.3682 | 0.2815 | 0.042* | |
| H1B | 0.6507 | 0.3929 | 0.2625 | 0.042* | |
| O2 | 0.6540 (2) | 0.3566 (3) | 0.4607 (1) | 0.024 (1) | |
| H2A | 0.6462 | 0.3344 | 0.4822 | 0.035* | |
| H2B | 0.6566 | 0.4015 | 0.4674 | 0.035* | |
| O3 | 0.4028 (3) | 0.1542 (3) | 0.4537 (2) | 0.030 (1) | |
| H3A | 0.4157 | 0.1653 | 0.4768 | 0.045* | |
| H3B | 0.3908 | 0.1092 | 0.4535 | 0.045* | |
| O4 | 0.4085 (2) | 0.1714 (3) | 0.2773 (1) | 0.029 (1) | |
| H4A | 0.3749 | 0.1999 | 0.2721 | 0.044* | |
| H4B | 0.4335 | 0.1661 | 0.2570 | 0.044* | |
| O5 | 0.7892 (3) | 0.3526 (3) | 0.2728 (2) | 0.033 (1) | |
| C1 | 0.8400 (4) | 0.3109 (5) | 0.2931 (3) | 0.045 (2) | |
| H1C | 0.8578 | 0.2710 | 0.2761 | 0.054* | |
| H1D | 0.8197 | 0.2887 | 0.3170 | 0.054* | |
| C2 | 0.8969 (4) | 0.3623 (5) | 0.3039 (2) | 0.040 (2) | |
| H2C | 0.9421 | 0.3367 | 0.3044 | 0.048* | |
| H2D | 0.8885 | 0.3851 | 0.3299 | 0.048* | |
| C3 | 0.8943 (4) | 0.4190 (4) | 0.2714 (2) | 0.034 (2) | |
| H3C | 0.9113 | 0.4673 | 0.2807 | 0.041* | |
| H3D | 0.9223 | 0.4034 | 0.2485 | 0.041* | |
| C4 | 0.8184 (4) | 0.4224 (4) | 0.2611 (2) | 0.033 (2) | |
| H4C | 0.7955 | 0.4632 | 0.2753 | 0.039* | |
| H4D | 0.8122 | 0.4302 | 0.2325 | 0.039* | |
| O6A_a | 0.643 (1) | 0.430 (1) | 0.2154 (8) | 0.030 (4) | 0.64 (2) |
| C5A_a | 0.670 (3) | 0.389 (3) | 0.179 (2) | 0.049 (6) | 0.64 (2) |
| H5A_a | 0.6924 | 0.3420 | 0.1866 | 0.059* | 0.64 (2) |
| H5B_a | 0.7026 | 0.4194 | 0.1639 | 0.059* | 0.64 (2) |
| C6A_a | 0.6005 (9) | 0.3771 (8) | 0.1577 (5) | 0.060 (5) | 0.64 (2) |
| H6A_a | 0.6086 | 0.3633 | 0.1300 | 0.072* | 0.64 (2) |
| H6B_a | 0.5732 | 0.3379 | 0.1706 | 0.072* | 0.64 (2) |
| C7A_a | 0.565 (4) | 0.447 (5) | 0.160 (3) | 0.044 (4) | 0.64 (2) |
| H7A_a | 0.5144 | 0.4417 | 0.1561 | 0.053* | 0.64 (2) |
| H7B_a | 0.5833 | 0.4831 | 0.1410 | 0.053* | 0.64 (2) |
| C8A_a | 0.5807 (4) | 0.4710 (5) | 0.2025 (3) | 0.041 (2) | 0.64 (2) |
| H8A_a | 0.5895 | 0.5246 | 0.2036 | 0.050* | 0.64 (2) |
| H8B_a | 0.5411 | 0.4596 | 0.2199 | 0.050* | 0.64 (2) |
| O6B_b | 0.631 (3) | 0.422 (3) | 0.212 (2) | 0.07 (2) | 0.36 (2) |
| C5B_b | 0.659 (5) | 0.389 (6) | 0.185 (3) | 0.049 (6) | 0.36 (2) |
| H5C_b | 0.6436 | 0.3372 | 0.1851 | 0.059* | 0.36 (2) |
| H5D_b | 0.7098 | 0.3901 | 0.1886 | 0.059* | 0.36 (2) |
| C6B_b | 0.639 (1) | 0.427 (1) | 0.1452 (6) | 0.038 (7) | 0.36 (2) |
| H6C_b | 0.6681 | 0.4699 | 0.1385 | 0.045* | 0.36 (2) |
| H6D_b | 0.6362 | 0.3925 | 0.1228 | 0.045* | 0.36 (2) |
| C7B_b | 0.566 (7) | 0.449 (8) | 0.162 (5) | 0.044 (4) | 0.36 (2) |
| H7C_b | 0.5457 | 0.4902 | 0.1470 | 0.053* | 0.36 (2) |
| H7D_b | 0.5338 | 0.4068 | 0.1614 | 0.053* | 0.36 (2) |
| C8B_b | 0.5807 (4) | 0.4710 (5) | 0.2025 (3) | 0.041 (2) | 0.36 (2) |
| H8C_b | 0.5976 | 0.5223 | 0.2039 | 0.050* | 0.36 (2) |
| H8D_b | 0.5396 | 0.4655 | 0.2196 | 0.050* | 0.36 (2) |
| O7 | 0.6621 (3) | 0.2999 (4) | 0.5319 (2) | 0.043 (1) | |
| C9 | 0.7253 (4) | 0.2924 (5) | 0.5529 (2) | 0.038 (2) | |
| H9A | 0.7568 | 0.3337 | 0.5468 | 0.046* | |
| H9B | 0.7485 | 0.2458 | 0.5459 | 0.046* | |
| C10 | 0.7064 (5) | 0.2929 (7) | 0.5958 (3) | 0.056 (3) | |
| H10A | 0.7235 | 0.2479 | 0.6090 | 0.068* | |
| H10B | 0.7262 | 0.3364 | 0.6091 | 0.068* | |
| C11 | 0.6288 (5) | 0.2956 (7) | 0.5964 (3) | 0.061 (3) | |
| H11A | 0.6089 | 0.2456 | 0.5986 | 0.073* | |
| H11B | 0.6118 | 0.3260 | 0.6185 | 0.073* | |
| C12 | 0.6110 (5) | 0.3297 (6) | 0.5579 (3) | 0.047 (2) | |
| H12A | 0.5637 | 0.3158 | 0.5495 | 0.056* | |
| H12B | 0.6142 | 0.3839 | 0.5593 | 0.056* | |
| O8 | 0.6622 (3) | 0.4959 (3) | 0.4812 (1) | 0.029 (1) | |
| C13 | 0.6032 (4) | 0.5277 (4) | 0.5017 (2) | 0.030 (2) | |
| H13A | 0.6162 | 0.5420 | 0.5288 | 0.036* | |
| H13B | 0.5646 | 0.4921 | 0.5030 | 0.036* | |
| C14 | 0.5828 (5) | 0.5943 (5) | 0.4780 (3) | 0.043 (2) | |
| H14A | 0.5618 | 0.6326 | 0.4949 | 0.052* | |
| H14B | 0.5499 | 0.5812 | 0.4569 | 0.052* | |
| C15 | 0.6514 (5) | 0.6195 (5) | 0.4614 (2) | 0.043 (2) | |
| H15A | 0.6779 | 0.6484 | 0.4809 | 0.052* | |
| H15B | 0.6449 | 0.6494 | 0.4374 | 0.052* | |
| C16 | 0.6869 (5) | 0.5471 (5) | 0.4522 (2) | 0.041 (2) | |
| H16A | 0.6747 | 0.5301 | 0.4255 | 0.050* | |
| H16B | 0.7377 | 0.5523 | 0.4540 | 0.050* | |
| O9 | 0.3659 (4) | 0.0167 (4) | 0.4525 (3) | 0.087 (3) | |
| C17 | 0.3983 (8) | −0.0391 (7) | 0.4734 (6) | 0.135 (9) | |
| H17A | 0.4090 | −0.0218 | 0.5003 | 0.161* | |
| H17B | 0.4423 | −0.0523 | 0.4603 | 0.161* | |
| C18 | 0.3548 (7) | −0.1018 (6) | 0.4753 (5) | 0.087 (4) | |
| H18A | 0.3423 | −0.1127 | 0.5029 | 0.104* | |
| H18B | 0.3784 | −0.1452 | 0.4640 | 0.104* | |
| C19 | 0.2917 (5) | −0.0831 (6) | 0.4520 (4) | 0.065 (3) | |
| H19A | 0.2914 | −0.1098 | 0.4266 | 0.078* | |
| H19B | 0.2492 | −0.0957 | 0.4667 | 0.078* | |
| C20 | 0.2968 (6) | −0.0021 (5) | 0.4456 (4) | 0.070 (4) | |
| H20A | 0.2834 | 0.0106 | 0.4184 | 0.084* | |
| H20B | 0.2660 | 0.0244 | 0.4640 | 0.084* | |
| O10 | 0.3910 (3) | 0.1938 (5) | 0.5279 (2) | 0.064 (2) | |
| C21 | 0.4469 (5) | 0.1819 (9) | 0.5540 (3) | 0.082 (4) | |
| H21A | 0.4679 | 0.2295 | 0.5615 | 0.098* | |
| H21B | 0.4828 | 0.1516 | 0.5410 | 0.098* | |
| C22 | 0.4213 (6) | 0.145 (1) | 0.5880 (4) | 0.122 (8) | |
| H22A | 0.4450 | 0.1624 | 0.6120 | 0.147* | |
| H22B | 0.4275 | 0.0911 | 0.5857 | 0.147* | |
| C23 | 0.3459 (5) | 0.1652 (7) | 0.5885 (3) | 0.065 (3) | |
| H23A | 0.3182 | 0.1277 | 0.6026 | 0.078* | |
| H23B | 0.3386 | 0.2136 | 0.6011 | 0.078* | |
| C24 | 0.3284 (5) | 0.1673 (7) | 0.5469 (3) | 0.058 (3) | |
| H24A | 0.3159 | 0.1178 | 0.5372 | 0.069* | |
| H24B | 0.2892 | 0.2011 | 0.5421 | 0.069* | |
| O11 | 0.4333 (3) | 0.1077 (5) | 0.2100 (2) | 0.063 (2) | |
| C25 | 0.4832 (4) | 0.1328 (6) | 0.1820 (3) | 0.050 (2) | |
| H25A | 0.5213 | 0.0968 | 0.1795 | 0.060* | |
| H25B | 0.5028 | 0.1805 | 0.1903 | 0.060* | |
| C26 | 0.4469 (7) | 0.141 (1) | 0.1446 (4) | 0.106 (6) | |
| H26A | 0.4438 | 0.1932 | 0.1374 | 0.127* | |
| H26B | 0.4729 | 0.1147 | 0.1237 | 0.127* | |
| C27 | 0.3818 (6) | 0.1113 (9) | 0.1478 (3) | 0.079 (4) | |
| H27A | 0.3780 | 0.0664 | 0.1315 | 0.095* | |
| H27B | 0.3469 | 0.1472 | 0.1385 | 0.095* | |
| C28 | 0.3694 (4) | 0.0931 (5) | 0.1902 (2) | 0.039 (2) | |
| H28A | 0.3319 | 0.1240 | 0.2010 | 0.047* | |
| H28B | 0.3563 | 0.0408 | 0.1931 | 0.047* | |
| O12 | 0.2795 (3) | 0.1498 (4) | 0.2820 (3) | 0.064 (2) | |
| C29 | 0.2461 (4) | 0.0793 (5) | 0.2839 (3) | 0.043 (2) | |
| H29A | 0.2488 | 0.0586 | 0.3108 | 0.052* | |
| H29B | 0.2674 | 0.0442 | 0.2653 | 0.052* | |
| C30 | 0.1722 (4) | 0.0958 (4) | 0.2727 (2) | 0.032 (2) | |
| H30A | 0.1665 | 0.0983 | 0.2438 | 0.039* | |
| H30B | 0.1400 | 0.0586 | 0.2835 | 0.039* | |
| C31 | 0.1614 (4) | 0.1697 (5) | 0.2915 (2) | 0.037 (2) | |
| H31A | 0.1467 | 0.1643 | 0.3192 | 0.044* | |
| H31B | 0.1261 | 0.1984 | 0.2772 | 0.044* | |
| C32 | 0.2314 (4) | 0.2065 (4) | 0.2890 (2) | 0.034 (2) | |
| H32A | 0.2322 | 0.2427 | 0.2673 | 0.040* | |
| H32B | 0.2423 | 0.2321 | 0.3139 | 0.040* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Nb1 | 0.0160 (2) | 0.0185 (3) | 0.0163 (3) | −0.0005 (2) | −0.0003 (2) | −0.0025 (2) |
| Nb2 | 0.0162 (3) | 0.0219 (3) | 0.0134 (2) | −0.0015 (2) | 0.0002 (2) | 0.0005 (2) |
| Nb3 | 0.0174 (3) | 0.0176 (3) | 0.0131 (2) | −0.0013 (2) | −0.0016 (2) | −0.0010 (2) |
| Nb4 | 0.0176 (3) | 0.0184 (3) | 0.0152 (3) | −0.0019 (2) | 0.0025 (2) | −0.0005 (2) |
| Nb5 | 0.0156 (3) | 0.0223 (3) | 0.0150 (3) | −0.0012 (2) | −0.0007 (2) | −0.0043 (2) |
| Nb6 | 0.0163 (2) | 0.0190 (3) | 0.0135 (2) | −0.0008 (2) | −0.0004 (2) | −0.0006 (2) |
| Cl1 | 0.0186 (7) | 0.0289 (8) | 0.0217 (7) | 0.0012 (6) | 0.0042 (6) | −0.0010 (6) |
| Cl2 | 0.0211 (7) | 0.0231 (7) | 0.0212 (7) | 0.0010 (6) | −0.0057 (6) | −0.0016 (6) |
| Cl3 | 0.0259 (8) | 0.0191 (7) | 0.0267 (8) | −0.0008 (6) | 0.0025 (6) | 0.0020 (6) |
| Cl4 | 0.0210 (7) | 0.0245 (8) | 0.0254 (8) | 0.0001 (6) | −0.0024 (6) | −0.0106 (6) |
| Cl5 | 0.0190 (7) | 0.0250 (7) | 0.0214 (7) | −0.0055 (6) | −0.0017 (6) | 0.0001 (6) |
| Cl6 | 0.0286 (8) | 0.0256 (8) | 0.0131 (7) | −0.0015 (6) | 0.0007 (6) | 0.0003 (6) |
| Cl7 | 0.0197 (7) | 0.0237 (7) | 0.0273 (8) | −0.0061 (6) | 0.0012 (6) | −0.0065 (6) |
| Cl8 | 0.0239 (7) | 0.0360 (9) | 0.0133 (7) | −0.0008 (7) | −0.0005 (6) | −0.0024 (6) |
| Cl9 | 0.0226 (7) | 0.0237 (7) | 0.0196 (7) | −0.0007 (6) | 0.0006 (6) | 0.0045 (6) |
| Cl10 | 0.0232 (7) | 0.0190 (7) | 0.0189 (7) | 0.0001 (6) | −0.0029 (6) | −0.0029 (5) |
| Cl11 | 0.0213 (7) | 0.0232 (7) | 0.0203 (7) | 0.0006 (6) | 0.0063 (6) | −0.0009 (6) |
| Cl12 | 0.0188 (7) | 0.0292 (8) | 0.0206 (7) | 0.0017 (6) | −0.0058 (5) | −0.0031 (6) |
| I1 | 0.0253 (2) | 0.0272 (2) | 0.0336 (2) | 0.0051 (2) | −0.0037 (2) | −0.0057 (2) |
| I2 | 0.0281 (2) | 0.0276 (2) | 0.0266 (2) | 0.0062 (2) | −0.0003 (2) | 0.0003 (2) |
| O1 | 0.019 (2) | 0.042 (3) | 0.023 (2) | 0.001 (2) | 0.005 (2) | 0.010 (2) |
| O2 | 0.031 (3) | 0.023 (2) | 0.017 (2) | −0.001 (2) | −0.004 (2) | −0.002 (2) |
| O3 | 0.035 (3) | 0.028 (3) | 0.026 (3) | −0.004 (2) | 0.011 (2) | 0.005 (2) |
| O4 | 0.021 (2) | 0.042 (3) | 0.025 (2) | −0.001 (2) | −0.003 (2) | −0.012 (2) |
| O5 | 0.021 (2) | 0.034 (3) | 0.044 (3) | −0.004 (2) | 0.000 (2) | 0.008 (2) |
| C1 | 0.034 (4) | 0.043 (5) | 0.058 (6) | −0.008 (4) | −0.011 (4) | 0.015 (4) |
| C2 | 0.026 (4) | 0.057 (5) | 0.035 (4) | 0.006 (4) | −0.003 (3) | 0.004 (4) |
| C3 | 0.028 (4) | 0.033 (4) | 0.042 (4) | −0.008 (3) | 0.004 (3) | −0.014 (3) |
| C4 | 0.027 (4) | 0.039 (4) | 0.032 (4) | −0.003 (3) | 0.004 (3) | 0.001 (3) |
| O6A_a | 0.020 (6) | 0.041 (7) | 0.029 (8) | 0.001 (5) | −0.006 (5) | 0.010 (5) |
| C5A_a | 0.04 (2) | 0.065 (7) | 0.05 (2) | 0.02 (1) | 0.021 (9) | 0.00 (1) |
| C6A_a | 0.08 (1) | 0.033 (8) | 0.07 (1) | −0.004 (8) | 0.021 (9) | −0.005 (7) |
| C7A_a | 0.041 (5) | 0.047 (6) | 0.045 (9) | −0.004 (5) | −0.015 (5) | 0.001 (6) |
| C8A_a | 0.036 (4) | 0.049 (5) | 0.039 (5) | 0.011 (4) | −0.002 (4) | 0.004 (4) |
| O6B_b | 0.05 (3) | 0.14 (4) | 0.02 (2) | 0.04 (2) | 0.02 (2) | 0.02 (2) |
| C5B_b | 0.04 (2) | 0.065 (7) | 0.05 (2) | 0.02 (1) | 0.021 (9) | 0.00 (1) |
| C6B_b | 0.06 (2) | 0.04 (1) | 0.02 (1) | −0.01 (1) | 0.007 (9) | −0.009 (8) |
| C7B_b | 0.041 (5) | 0.047 (6) | 0.045 (9) | −0.004 (5) | −0.015 (5) | 0.001 (6) |
| C8B_b | 0.036 (4) | 0.049 (5) | 0.039 (5) | 0.011 (4) | −0.002 (4) | 0.004 (4) |
| O7 | 0.042 (3) | 0.065 (4) | 0.020 (3) | 0.011 (3) | −0.005 (2) | 0.007 (3) |
| C9 | 0.036 (4) | 0.045 (5) | 0.034 (4) | 0.001 (4) | −0.005 (3) | −0.001 (4) |
| C10 | 0.043 (5) | 0.095 (8) | 0.030 (4) | 0.009 (5) | −0.010 (4) | 0.006 (5) |
| C11 | 0.056 (6) | 0.094 (9) | 0.033 (5) | 0.011 (6) | 0.002 (4) | 0.013 (5) |
| C12 | 0.044 (5) | 0.058 (6) | 0.038 (5) | 0.020 (4) | −0.001 (4) | −0.012 (4) |
| O8 | 0.031 (3) | 0.029 (3) | 0.026 (3) | 0.000 (2) | 0.002 (2) | −0.005 (2) |
| C13 | 0.034 (4) | 0.037 (4) | 0.020 (3) | 0.002 (3) | 0.006 (3) | −0.008 (3) |
| C14 | 0.055 (5) | 0.037 (4) | 0.039 (5) | 0.010 (4) | −0.004 (4) | −0.008 (4) |
| C15 | 0.073 (6) | 0.030 (4) | 0.027 (4) | −0.001 (4) | −0.001 (4) | −0.002 (3) |
| C16 | 0.054 (5) | 0.037 (4) | 0.032 (4) | −0.005 (4) | 0.021 (4) | −0.001 (3) |
| O9 | 0.065 (5) | 0.043 (4) | 0.154 (9) | −0.030 (4) | −0.041 (5) | 0.040 (5) |
| C17 | 0.09 (1) | 0.056 (8) | 0.25 (2) | −0.022 (7) | −0.11 (1) | 0.07 (1) |
| C18 | 0.082 (9) | 0.045 (6) | 0.13 (1) | 0.005 (6) | −0.017 (8) | 0.040 (7) |
| C19 | 0.044 (6) | 0.042 (5) | 0.11 (1) | −0.009 (4) | 0.001 (6) | 0.013 (6) |
| C20 | 0.050 (6) | 0.037 (5) | 0.12 (1) | −0.005 (5) | −0.020 (6) | 0.011 (6) |
| O10 | 0.042 (4) | 0.114 (7) | 0.036 (4) | −0.003 (4) | 0.010 (3) | 0.011 (4) |
| C21 | 0.039 (6) | 0.17 (1) | 0.039 (6) | 0.003 (7) | 0.002 (4) | 0.021 (7) |
| C22 | 0.052 (7) | 0.24 (2) | 0.077 (9) | 0.03 (1) | 0.024 (6) | 0.10 (1) |
| C23 | 0.049 (6) | 0.094 (9) | 0.053 (6) | 0.008 (6) | 0.020 (5) | 0.023 (6) |
| C24 | 0.038 (5) | 0.087 (8) | 0.048 (6) | −0.014 (5) | 0.009 (4) | −0.008 (5) |
| O11 | 0.034 (3) | 0.117 (6) | 0.038 (3) | −0.006 (4) | −0.001 (3) | −0.036 (4) |
| C25 | 0.028 (4) | 0.065 (6) | 0.058 (6) | −0.010 (4) | 0.008 (4) | −0.028 (5) |
| C26 | 0.054 (7) | 0.16 (2) | 0.10 (1) | −0.023 (9) | −0.006 (7) | 0.07 (1) |
| C27 | 0.061 (7) | 0.14 (1) | 0.036 (5) | −0.044 (8) | −0.007 (5) | 0.018 (6) |
| C28 | 0.032 (4) | 0.051 (5) | 0.034 (4) | −0.008 (4) | 0.001 (3) | −0.017 (4) |
| O12 | 0.025 (3) | 0.049 (4) | 0.119 (7) | −0.003 (3) | 0.007 (4) | −0.037 (4) |
| C29 | 0.021 (4) | 0.053 (5) | 0.056 (5) | −0.003 (4) | −0.006 (4) | −0.002 (4) |
| C30 | 0.026 (4) | 0.038 (4) | 0.033 (4) | −0.006 (3) | −0.001 (3) | 0.003 (3) |
| C31 | 0.021 (3) | 0.049 (5) | 0.039 (4) | 0.005 (3) | 0.000 (3) | 0.002 (4) |
| C32 | 0.029 (4) | 0.037 (4) | 0.035 (4) | 0.002 (3) | 0.001 (3) | −0.005 (3) |
Geometric parameters (Å, º)
| Nb1—Cl1 | 2.457 (2) | C6B_b—H6D_b | 0.9900 |
| Nb1—Cl4 | 2.457 (2) | C7B_b—H7C_b | 0.9900 |
| Nb1—Cl3 | 2.460 (2) | C7B_b—H7D_b | 0.9900 |
| Nb1—Cl2 | 2.464 (2) | O7—C9 | 1.423 (9) |
| Nb1—I1 | 2.9312 (7) | O7—C12 | 1.43 (1) |
| Nb1—Nb4 | 2.9367 (8) | C9—C10 | 1.50 (1) |
| Nb1—Nb5 | 2.9367 (8) | C9—H9A | 0.9900 |
| Nb1—Nb3 | 2.9394 (8) | C9—H9B | 0.9900 |
| Nb1—Nb2 | 2.9424 (8) | C10—C11 | 1.50 (1) |
| Nb2—O1 | 2.219 (5) | C10—H10A | 0.9900 |
| Nb2—Cl9 | 2.457 (2) | C10—H10B | 0.9900 |
| Nb2—Cl1 | 2.461 (2) | C11—C12 | 1.49 (1) |
| Nb2—Cl5 | 2.461 (2) | C11—H11A | 0.9900 |
| Nb2—Cl8 | 2.473 (2) | C11—H11B | 0.9900 |
| Nb2—Nb3 | 2.8867 (7) | C12—H12A | 0.9900 |
| Nb2—Nb5 | 2.9008 (8) | C12—H12B | 0.9900 |
| Nb2—Nb6 | 2.9381 (8) | O8—C16 | 1.438 (9) |
| Nb3—O2 | 2.233 (4) | O8—C13 | 1.455 (8) |
| Nb3—Cl5 | 2.462 (2) | C13—C14 | 1.51 (1) |
| Nb3—Cl10 | 2.464 (2) | C13—H13A | 0.9900 |
| Nb3—Cl2 | 2.465 (2) | C13—H13B | 0.9900 |
| Nb3—Cl6 | 2.470 (2) | C14—C15 | 1.51 (1) |
| Nb3—Nb4 | 2.9029 (8) | C14—H14A | 0.9900 |
| Nb3—Nb6 | 2.9418 (8) | C14—H14B | 0.9900 |
| Nb4—O3 | 2.232 (5) | C15—C16 | 1.52 (1) |
| Nb4—Cl3 | 2.462 (2) | C15—H15A | 0.9900 |
| Nb4—Cl11 | 2.463 (2) | C15—H15B | 0.9900 |
| Nb4—Cl6 | 2.467 (2) | C16—H16A | 0.9900 |
| Nb4—Cl7 | 2.468 (2) | C16—H16B | 0.9900 |
| Nb4—Nb5 | 2.8923 (8) | O9—C17 | 1.39 (1) |
| Nb4—Nb6 | 2.9366 (8) | O9—C20 | 1.40 (1) |
| Nb5—O4 | 2.209 (5) | C17—C18 | 1.42 (2) |
| Nb5—Cl4 | 2.452 (2) | C17—H17A | 0.9900 |
| Nb5—Cl12 | 2.461 (2) | C17—H17B | 0.9900 |
| Nb5—Cl7 | 2.475 (2) | C18—C19 | 1.49 (2) |
| Nb5—Cl8 | 2.476 (2) | C18—H18A | 0.9900 |
| Nb5—Nb6 | 2.9352 (8) | C18—H18B | 0.9900 |
| Nb6—Cl12 | 2.456 (2) | C19—C20 | 1.49 (1) |
| Nb6—Cl9 | 2.457 (2) | C19—H19A | 0.9900 |
| Nb6—Cl11 | 2.461 (2) | C19—H19B | 0.9900 |
| Nb6—Cl10 | 2.461 (2) | C20—H20A | 0.9900 |
| Nb6—I2 | 2.9563 (7) | C20—H20B | 0.9900 |
| O1—H1A | 0.8498 | O10—C21 | 1.41 (1) |
| O1—H1B | 0.8500 | O10—C24 | 1.46 (1) |
| O2—H2A | 0.8499 | C21—C22 | 1.43 (2) |
| O2—H2B | 0.8501 | C21—H21A | 0.9900 |
| O3—H3A | 0.8505 | C21—H21B | 0.9900 |
| O3—H3B | 0.8501 | C22—C23 | 1.50 (2) |
| O4—H4A | 0.8500 | C22—H22A | 0.9900 |
| O4—H4B | 0.8498 | C22—H22B | 0.9900 |
| O5—C1 | 1.420 (9) | C23—C24 | 1.46 (1) |
| O5—C4 | 1.447 (9) | C23—H23A | 0.9900 |
| C1—C2 | 1.49 (1) | C23—H23B | 0.9900 |
| C1—H1C | 0.9900 | C24—H24A | 0.9900 |
| C1—H1D | 0.9900 | C24—H24B | 0.9900 |
| C2—C3 | 1.51 (1) | O11—C25 | 1.43 (1) |
| C2—H2C | 0.9900 | O11—C28 | 1.432 (9) |
| C2—H2D | 0.9900 | C25—C26 | 1.46 (2) |
| C3—C4 | 1.51 (1) | C25—H25A | 0.9900 |
| C3—H3C | 0.9900 | C25—H25B | 0.9900 |
| C3—H3D | 0.9900 | C26—C27 | 1.37 (2) |
| C4—H4C | 0.9900 | C26—H26A | 0.9900 |
| C4—H4D | 0.9900 | C26—H26B | 0.9900 |
| O6A_a—C8A_a | 1.48 (3) | C27—C28 | 1.50 (1) |
| O6A_a—C5A_a | 1.53 (6) | C27—H27A | 0.9900 |
| C5A_a—C6A_a | 1.55 (7) | C27—H27B | 0.9900 |
| C5A_a—H5A_a | 0.9900 | C28—H28A | 0.9900 |
| C5A_a—H5B_a | 0.9900 | C28—H28B | 0.9900 |
| C6A_a—C7A_a | 1.46 (8) | O12—C32 | 1.409 (9) |
| C6A_a—H6A_a | 0.9900 | O12—C29 | 1.44 (1) |
| C6A_a—H6B_a | 0.9900 | C29—C30 | 1.51 (1) |
| C7A_a—C8A_a | 1.5 (1) | C29—H29A | 0.9900 |
| C7A_a—H7A_a | 0.9900 | C29—H29B | 0.9900 |
| C7A_a—H7B_a | 0.9900 | C30—C31 | 1.50 (1) |
| C8A_a—H8A_a | 0.9900 | C30—H30A | 0.9900 |
| C8A_a—H8B_a | 0.9900 | C30—H30B | 0.9900 |
| O6B_b—C5B_b | 1.2 (1) | C31—C32 | 1.51 (1) |
| C5B_b—C6B_b | 1.6 (1) | C31—H31A | 0.9900 |
| C5B_b—H5C_b | 0.9900 | C31—H31B | 0.9900 |
| C5B_b—H5D_b | 0.9900 | C32—H32A | 0.9900 |
| C6B_b—C7B_b | 1.6 (1) | C32—H32B | 0.9900 |
| C6B_b—H6C_b | 0.9900 | ||
| Cl1—Nb1—Cl4 | 88.15 (6) | Nb2—O1—H1A | 126.4 |
| Cl1—Nb1—Cl3 | 164.99 (6) | Nb2—O1—H1B | 135.5 |
| Cl4—Nb1—Cl3 | 89.96 (6) | H1A—O1—H1B | 91.8 |
| Cl1—Nb1—Cl2 | 88.73 (6) | Nb3—O2—H2A | 109.8 |
| Cl4—Nb1—Cl2 | 165.04 (6) | Nb3—O2—H2B | 127.2 |
| Cl3—Nb1—Cl2 | 89.28 (6) | H2A—O2—H2B | 103.6 |
| Cl1—Nb1—I1 | 82.67 (4) | Nb4—O3—H3A | 109.8 |
| Cl4—Nb1—I1 | 81.57 (4) | Nb4—O3—H3B | 126.8 |
| Cl3—Nb1—I1 | 82.32 (4) | H3A—O3—H3B | 108.6 |
| Cl2—Nb1—I1 | 83.52 (4) | Nb5—O4—H4A | 109.4 |
| Cl1—Nb1—Nb4 | 141.61 (5) | Nb5—O4—H4B | 109.3 |
| Cl4—Nb1—Nb4 | 96.11 (4) | H4A—O4—H4B | 109.5 |
| Cl3—Nb1—Nb4 | 53.40 (4) | C1—O5—C4 | 109.5 (6) |
| Cl2—Nb1—Nb4 | 95.42 (4) | O5—C1—C2 | 107.2 (7) |
| I1—Nb1—Nb4 | 135.71 (2) | O5—C1—H1C | 110.3 |
| Cl1—Nb1—Nb5 | 95.59 (4) | C2—C1—H1C | 110.3 |
| Cl4—Nb1—Nb5 | 53.17 (4) | O5—C1—H1D | 110.3 |
| Cl3—Nb1—Nb5 | 95.17 (4) | C2—C1—H1D | 110.3 |
| Cl2—Nb1—Nb5 | 141.75 (4) | H1C—C1—H1D | 108.5 |
| I1—Nb1—Nb5 | 134.73 (2) | C1—C2—C3 | 103.0 (6) |
| Nb4—Nb1—Nb5 | 59.00 (2) | C1—C2—H2C | 111.2 |
| Cl1—Nb1—Nb3 | 94.90 (4) | C3—C2—H2C | 111.2 |
| Cl4—Nb1—Nb3 | 141.48 (4) | C1—C2—H2D | 111.2 |
| Cl3—Nb1—Nb3 | 95.84 (4) | C3—C2—H2D | 111.2 |
| Cl2—Nb1—Nb3 | 53.40 (4) | H2C—C2—H2D | 109.1 |
| I1—Nb1—Nb3 | 136.92 (2) | C4—C3—C2 | 103.2 (6) |
| Nb4—Nb1—Nb3 | 59.21 (2) | C4—C3—H3C | 111.1 |
| Nb5—Nb1—Nb3 | 88.35 (2) | C2—C3—H3C | 111.1 |
| Cl1—Nb1—Nb2 | 53.30 (4) | C4—C3—H3D | 111.1 |
| Cl4—Nb1—Nb2 | 94.48 (4) | C2—C3—H3D | 111.1 |
| Cl3—Nb1—Nb2 | 141.71 (4) | H3C—C3—H3D | 109.1 |
| Cl2—Nb1—Nb2 | 95.36 (4) | O5—C4—C3 | 106.2 (6) |
| I1—Nb1—Nb2 | 135.96 (2) | O5—C4—H4C | 110.5 |
| Nb4—Nb1—Nb2 | 88.31 (2) | C3—C4—H4C | 110.5 |
| Nb5—Nb1—Nb2 | 59.13 (2) | O5—C4—H4D | 110.5 |
| Nb3—Nb1—Nb2 | 58.78 (2) | C3—C4—H4D | 110.5 |
| O1—Nb2—Cl9 | 80.9 (1) | H4C—C4—H4D | 108.7 |
| O1—Nb2—Cl1 | 81.0 (1) | C8A_a—O6A_a—C5A_a | 107 (3) |
| Cl9—Nb2—Cl1 | 161.91 (6) | O6A_a—C5A_a—C6A_a | 99 (3) |
| O1—Nb2—Cl5 | 79.4 (1) | O6A_a—C5A_a—H5A_a | 112.0 |
| Cl9—Nb2—Cl5 | 89.21 (6) | C6A_a—C5A_a—H5A_a | 112.0 |
| Cl1—Nb2—Cl5 | 88.78 (6) | O6A_a—C5A_a—H5B_a | 112.0 |
| O1—Nb2—Cl8 | 82.3 (1) | C6A_a—C5A_a—H5B_a | 112.0 |
| Cl9—Nb2—Cl8 | 86.65 (6) | H5A_a—C5A_a—H5B_a | 109.7 |
| Cl1—Nb2—Cl8 | 89.63 (6) | C7A_a—C6A_a—C5A_a | 105 (4) |
| Cl5—Nb2—Cl8 | 161.62 (6) | C7A_a—C6A_a—H6A_a | 110.7 |
| O1—Nb2—Nb3 | 133.5 (1) | C5A_a—C6A_a—H6A_a | 110.7 |
| Cl9—Nb2—Nb3 | 97.19 (4) | C7A_a—C6A_a—H6B_a | 110.7 |
| Cl1—Nb2—Nb3 | 96.17 (4) | C5A_a—C6A_a—H6B_a | 110.7 |
| Cl5—Nb2—Nb3 | 54.11 (4) | H6A_a—C6A_a—H6B_a | 108.8 |
| Cl8—Nb2—Nb3 | 144.22 (4) | C6A_a—C7A_a—C8A_a | 102 (5) |
| O1—Nb2—Nb5 | 136.4 (1) | C6A_a—C7A_a—H7A_a | 111.4 |
| Cl9—Nb2—Nb5 | 95.69 (4) | C8A_a—C7A_a—H7A_a | 111.4 |
| Cl1—Nb2—Nb5 | 96.44 (4) | C6A_a—C7A_a—H7B_a | 111.4 |
| Cl5—Nb2—Nb5 | 144.18 (4) | C8A_a—C7A_a—H7B_a | 111.4 |
| Cl8—Nb2—Nb5 | 54.17 (4) | H7A_a—C7A_a—H7B_a | 109.3 |
| Nb3—Nb2—Nb5 | 90.07 (2) | O6A_a—C8A_a—C7A_a | 108 (3) |
| O1—Nb2—Nb6 | 134.2 (1) | O6A_a—C8A_a—H8A_a | 110.2 |
| Cl9—Nb2—Nb6 | 53.29 (4) | C7A_a—C8A_a—H8A_a | 110.2 |
| Cl1—Nb2—Nb6 | 144.78 (4) | O6A_a—C8A_a—H8B_a | 110.2 |
| Cl5—Nb2—Nb6 | 96.26 (4) | C7A_a—C8A_a—H8B_a | 110.2 |
| Cl8—Nb2—Nb6 | 95.58 (4) | H8A_a—C8A_a—H8B_a | 108.5 |
| Nb3—Nb2—Nb6 | 60.66 (2) | O6B_b—C5B_b—C6B_b | 109 (8) |
| Nb5—Nb2—Nb6 | 60.35 (2) | O6B_b—C5B_b—H5C_b | 109.9 |
| O1—Nb2—Nb1 | 134.2 (1) | C6B_b—C5B_b—H5C_b | 109.9 |
| Cl9—Nb2—Nb1 | 144.85 (4) | O6B_b—C5B_b—H5D_b | 109.9 |
| Cl1—Nb2—Nb1 | 53.20 (4) | C6B_b—C5B_b—H5D_b | 109.9 |
| Cl5—Nb2—Nb1 | 96.78 (4) | H5C_b—C5B_b—H5D_b | 108.3 |
| Cl8—Nb2—Nb1 | 96.90 (5) | C7B_b—C6B_b—C5B_b | 91 (7) |
| Nb3—Nb2—Nb1 | 60.56 (2) | C7B_b—C6B_b—H6C_b | 113.5 |
| Nb5—Nb2—Nb1 | 60.34 (2) | C5B_b—C6B_b—H6C_b | 113.5 |
| Nb6—Nb2—Nb1 | 91.58 (2) | C7B_b—C6B_b—H6D_b | 113.5 |
| O2—Nb3—Cl5 | 80.2 (1) | C5B_b—C6B_b—H6D_b | 113.5 |
| O2—Nb3—Cl10 | 81.6 (1) | H6C_b—C6B_b—H6D_b | 110.8 |
| Cl5—Nb3—Cl10 | 87.84 (6) | C6B_b—C7B_b—H7C_b | 111.0 |
| O2—Nb3—Cl2 | 80.1 (1) | C6B_b—C7B_b—H7D_b | 111.0 |
| Cl5—Nb3—Cl2 | 89.33 (6) | H7C_b—C7B_b—H7D_b | 109.0 |
| Cl10—Nb3—Cl2 | 161.77 (5) | C9—O7—C12 | 108.6 (6) |
| O2—Nb3—Cl6 | 81.7 (1) | O7—C9—C10 | 106.2 (7) |
| Cl5—Nb3—Cl6 | 161.93 (6) | O7—C9—H9A | 110.5 |
| Cl10—Nb3—Cl6 | 89.20 (5) | C10—C9—H9A | 110.5 |
| Cl2—Nb3—Cl6 | 87.92 (6) | O7—C9—H9B | 110.5 |
| O2—Nb3—Nb2 | 134.3 (1) | C10—C9—H9B | 110.5 |
| Cl5—Nb3—Nb2 | 54.08 (4) | H9A—C9—H9B | 108.7 |
| Cl10—Nb3—Nb2 | 96.13 (4) | C11—C10—C9 | 104.8 (7) |
| Cl2—Nb3—Nb2 | 96.76 (4) | C11—C10—H10A | 110.8 |
| Cl6—Nb3—Nb2 | 143.99 (4) | C9—C10—H10A | 110.8 |
| O2—Nb3—Nb4 | 135.6 (1) | C11—C10—H10B | 110.8 |
| Cl5—Nb3—Nb4 | 144.11 (4) | C9—C10—H10B | 110.8 |
| Cl10—Nb3—Nb4 | 96.56 (4) | H10A—C10—H10B | 108.9 |
| Cl2—Nb3—Nb4 | 96.26 (4) | C12—C11—C10 | 103.5 (8) |
| Cl6—Nb3—Nb4 | 53.95 (4) | C12—C11—H11A | 111.1 |
| Nb2—Nb3—Nb4 | 90.04 (2) | C10—C11—H11A | 111.1 |
| O2—Nb3—Nb1 | 133.5 (1) | C12—C11—H11B | 111.1 |
| Cl5—Nb3—Nb1 | 96.83 (4) | C10—C11—H11B | 111.1 |
| Cl10—Nb3—Nb1 | 144.85 (4) | H11A—C11—H11B | 109.0 |
| Cl2—Nb3—Nb1 | 53.39 (4) | O7—C12—C11 | 103.1 (7) |
| Cl6—Nb3—Nb1 | 95.83 (4) | O7—C12—H12A | 111.2 |
| Nb2—Nb3—Nb1 | 60.66 (2) | C11—C12—H12A | 111.2 |
| Nb4—Nb3—Nb1 | 60.35 (2) | O7—C12—H12B | 111.2 |
| O2—Nb3—Nb6 | 134.9 (1) | C11—C12—H12B | 111.2 |
| Cl5—Nb3—Nb6 | 96.15 (4) | H12A—C12—H12B | 109.1 |
| Cl10—Nb3—Nb6 | 53.28 (4) | C16—O8—C13 | 109.3 (6) |
| Cl2—Nb3—Nb6 | 144.95 (4) | O8—C13—C14 | 105.6 (6) |
| Cl6—Nb3—Nb6 | 96.33 (4) | O8—C13—H13A | 110.6 |
| Nb2—Nb3—Nb6 | 60.53 (2) | C14—C13—H13A | 110.6 |
| Nb4—Nb3—Nb6 | 60.32 (2) | O8—C13—H13B | 110.6 |
| Nb1—Nb3—Nb6 | 91.56 (2) | C14—C13—H13B | 110.6 |
| O3—Nb4—Cl3 | 81.6 (1) | H13A—C13—H13B | 108.7 |
| O3—Nb4—Cl11 | 80.0 (1) | C13—C14—C15 | 102.3 (7) |
| Cl3—Nb4—Cl11 | 161.58 (6) | C13—C14—H14A | 111.3 |
| O3—Nb4—Cl6 | 81.8 (1) | C15—C14—H14A | 111.3 |
| Cl3—Nb4—Cl6 | 88.54 (6) | C13—C14—H14B | 111.3 |
| Cl11—Nb4—Cl6 | 88.29 (6) | C15—C14—H14B | 111.3 |
| O3—Nb4—Cl7 | 79.9 (1) | H14A—C14—H14B | 109.2 |
| Cl3—Nb4—Cl7 | 88.21 (6) | C14—C15—C16 | 102.1 (7) |
| Cl11—Nb4—Cl7 | 89.13 (6) | C14—C15—H15A | 111.3 |
| Cl6—Nb4—Cl7 | 161.75 (6) | C16—C15—H15A | 111.3 |
| O3—Nb4—Nb5 | 134.2 (1) | C14—C15—H15B | 111.3 |
| Cl3—Nb4—Nb5 | 96.25 (4) | C16—C15—H15B | 111.3 |
| Cl11—Nb4—Nb5 | 96.97 (4) | H15A—C15—H15B | 109.2 |
| Cl6—Nb4—Nb5 | 143.93 (4) | O8—C16—C15 | 105.7 (6) |
| Cl7—Nb4—Nb5 | 54.31 (4) | O8—C16—H16A | 110.6 |
| O3—Nb4—Nb3 | 135.8 (1) | C15—C16—H16A | 110.6 |
| Cl3—Nb4—Nb3 | 96.72 (4) | O8—C16—H16B | 110.6 |
| Cl11—Nb4—Nb3 | 96.07 (4) | C15—C16—H16B | 110.6 |
| Cl6—Nb4—Nb3 | 54.02 (4) | H16A—C16—H16B | 108.7 |
| Cl7—Nb4—Nb3 | 144.23 (4) | C17—O9—C20 | 109.7 (8) |
| Nb5—Nb4—Nb3 | 89.92 (2) | O9—C17—C18 | 110 (1) |
| O3—Nb4—Nb6 | 133.4 (1) | O9—C17—H17A | 109.6 |
| Cl3—Nb4—Nb6 | 145.05 (4) | C18—C17—H17A | 109.6 |
| Cl11—Nb4—Nb6 | 53.36 (4) | O9—C17—H17B | 109.6 |
| Cl6—Nb4—Nb6 | 96.51 (4) | C18—C17—H17B | 109.6 |
| Cl7—Nb4—Nb6 | 96.41 (4) | H17A—C17—H17B | 108.1 |
| Nb5—Nb4—Nb6 | 60.47 (2) | C17—C18—C19 | 106.1 (9) |
| Nb3—Nb4—Nb6 | 60.50 (2) | C17—C18—H18A | 110.5 |
| O3—Nb4—Nb1 | 134.9 (1) | C19—C18—H18A | 110.5 |
| Cl3—Nb4—Nb1 | 53.33 (4) | C17—C18—H18B | 110.5 |
| Cl11—Nb4—Nb1 | 145.08 (4) | C19—C18—H18B | 110.5 |
| Cl6—Nb4—Nb1 | 95.94 (4) | H18A—C18—H18B | 108.7 |
| Cl7—Nb4—Nb1 | 96.49 (4) | C20—C19—C18 | 104.3 (9) |
| Nb5—Nb4—Nb1 | 60.50 (2) | C20—C19—H19A | 110.9 |
| Nb3—Nb4—Nb1 | 60.44 (2) | C18—C19—H19A | 110.9 |
| Nb6—Nb4—Nb1 | 91.72 (2) | C20—C19—H19B | 110.9 |
| O4—Nb5—Cl4 | 80.6 (1) | C18—C19—H19B | 110.9 |
| O4—Nb5—Cl12 | 81.0 (1) | H19A—C19—H19B | 108.9 |
| Cl4—Nb5—Cl12 | 161.67 (6) | O9—C20—C19 | 106.4 (9) |
| O4—Nb5—Cl7 | 81.0 (1) | O9—C20—H20A | 110.5 |
| Cl4—Nb5—Cl7 | 89.82 (6) | C19—C20—H20A | 110.5 |
| Cl12—Nb5—Cl7 | 87.28 (6) | O9—C20—H20B | 110.5 |
| O4—Nb5—Cl8 | 80.9 (1) | C19—C20—H20B | 110.5 |
| Cl4—Nb5—Cl8 | 88.27 (6) | H20A—C20—H20B | 108.7 |
| Cl12—Nb5—Cl8 | 88.88 (6) | C21—O10—C24 | 107.8 (8) |
| Cl7—Nb5—Cl8 | 161.88 (6) | O10—C21—C22 | 108.4 (9) |
| O4—Nb5—Nb4 | 135.1 (1) | O10—C21—H21A | 110.0 |
| Cl4—Nb5—Nb4 | 97.38 (4) | C22—C21—H21A | 110.0 |
| Cl12—Nb5—Nb4 | 95.53 (4) | O10—C21—H21B | 110.0 |
| Cl7—Nb5—Nb4 | 54.06 (4) | C22—C21—H21B | 110.0 |
| Cl8—Nb5—Nb4 | 144.02 (4) | H21A—C21—H21B | 108.4 |
| O4—Nb5—Nb2 | 134.9 (1) | C21—C22—C23 | 103 (1) |
| Cl4—Nb5—Nb2 | 95.66 (4) | C21—C22—H22A | 111.1 |
| Cl12—Nb5—Nb2 | 97.30 (4) | C23—C22—H22A | 111.1 |
| Cl7—Nb5—Nb2 | 144.04 (4) | C21—C22—H22B | 111.1 |
| Cl8—Nb5—Nb2 | 54.06 (4) | C23—C22—H22B | 111.1 |
| Nb4—Nb5—Nb2 | 89.97 (2) | H22A—C22—H22B | 109.1 |
| O4—Nb5—Nb6 | 134.3 (1) | C24—C23—C22 | 102.7 (9) |
| Cl4—Nb5—Nb6 | 145.07 (4) | C24—C23—H23A | 111.2 |
| Cl12—Nb5—Nb6 | 53.26 (4) | C22—C23—H23A | 111.2 |
| Cl7—Nb5—Nb6 | 96.28 (4) | C24—C23—H23B | 111.2 |
| Cl8—Nb5—Nb6 | 95.58 (4) | C22—C23—H23B | 111.2 |
| Nb4—Nb5—Nb6 | 60.51 (2) | H23A—C23—H23B | 109.1 |
| Nb2—Nb5—Nb6 | 60.45 (2) | O10—C24—C23 | 104.3 (8) |
| O4—Nb5—Nb1 | 134.0 (1) | O10—C24—H24A | 110.9 |
| Cl4—Nb5—Nb1 | 53.35 (4) | C23—C24—H24A | 110.9 |
| Cl12—Nb5—Nb1 | 144.97 (4) | O10—C24—H24B | 110.9 |
| Cl7—Nb5—Nb1 | 96.31 (4) | C23—C24—H24B | 110.9 |
| Cl8—Nb5—Nb1 | 96.97 (4) | H24A—C24—H24B | 108.9 |
| Nb4—Nb5—Nb1 | 60.50 (2) | C25—O11—C28 | 109.1 (7) |
| Nb2—Nb5—Nb1 | 60.53 (2) | O11—C25—C26 | 106.8 (8) |
| Nb6—Nb5—Nb1 | 91.75 (2) | O11—C25—H25A | 110.4 |
| Cl12—Nb6—Cl9 | 89.46 (6) | C26—C25—H25A | 110.4 |
| Cl12—Nb6—Cl11 | 88.43 (6) | O11—C25—H25B | 110.4 |
| Cl9—Nb6—Cl11 | 164.93 (6) | C26—C25—H25B | 110.4 |
| Cl12—Nb6—Cl10 | 164.84 (6) | H25A—C25—H25B | 108.6 |
| Cl9—Nb6—Cl10 | 89.32 (5) | C27—C26—C25 | 110 (1) |
| Cl11—Nb6—Cl10 | 88.82 (5) | C27—C26—H26A | 109.8 |
| Cl12—Nb6—Nb5 | 53.44 (4) | C25—C26—H26A | 109.8 |
| Cl9—Nb6—Nb5 | 94.80 (4) | C27—C26—H26B | 109.8 |
| Cl11—Nb6—Nb5 | 95.91 (4) | C25—C26—H26B | 109.8 |
| Cl10—Nb6—Nb5 | 141.71 (4) | H26A—C26—H26B | 108.2 |
| Cl12—Nb6—Nb4 | 94.55 (4) | C26—C27—C28 | 107.9 (9) |
| Cl9—Nb6—Nb4 | 141.64 (4) | C26—C27—H27A | 110.1 |
| Cl11—Nb6—Nb4 | 53.42 (4) | C28—C27—H27A | 110.1 |
| Cl10—Nb6—Nb4 | 95.77 (4) | C26—C27—H27B | 110.1 |
| Nb5—Nb6—Nb4 | 59.02 (2) | C28—C27—H27B | 110.1 |
| Cl12—Nb6—Nb2 | 96.47 (4) | H27A—C27—H27B | 108.4 |
| Cl9—Nb6—Nb2 | 53.27 (4) | O11—C28—C27 | 105.9 (7) |
| Cl11—Nb6—Nb2 | 141.81 (4) | O11—C28—H28A | 110.6 |
| Cl10—Nb6—Nb2 | 94.90 (4) | C27—C28—H28A | 110.6 |
| Nb5—Nb6—Nb2 | 59.19 (2) | O11—C28—H28B | 110.6 |
| Nb4—Nb6—Nb2 | 88.39 (2) | C27—C28—H28B | 110.6 |
| Cl12—Nb6—Nb3 | 141.76 (4) | H28A—C28—H28B | 108.7 |
| Cl9—Nb6—Nb3 | 95.75 (4) | C32—O12—C29 | 110.4 (6) |
| Cl11—Nb6—Nb3 | 95.13 (4) | O12—C29—C30 | 103.7 (7) |
| Cl10—Nb6—Nb3 | 53.38 (4) | O12—C29—H29A | 111.0 |
| Nb5—Nb6—Nb3 | 88.34 (2) | C30—C29—H29A | 111.0 |
| Nb4—Nb6—Nb3 | 59.19 (2) | O12—C29—H29B | 111.0 |
| Nb2—Nb6—Nb3 | 58.81 (2) | C30—C29—H29B | 111.0 |
| Cl12—Nb6—I2 | 81.77 (4) | H29A—C29—H29B | 109.0 |
| Cl9—Nb6—I2 | 82.38 (4) | C31—C30—C29 | 101.6 (6) |
| Cl11—Nb6—I2 | 82.55 (4) | C31—C30—H30A | 111.5 |
| Cl10—Nb6—I2 | 83.09 (4) | C29—C30—H30A | 111.5 |
| Nb5—Nb6—I2 | 135.19 (2) | C31—C30—H30B | 111.5 |
| Nb4—Nb6—I2 | 135.95 (2) | C29—C30—H30B | 111.5 |
| Nb2—Nb6—I2 | 135.65 (2) | H30A—C30—H30B | 109.3 |
| Nb3—Nb6—I2 | 136.47 (2) | C30—C31—C32 | 104.3 (6) |
| Nb1—Cl1—Nb2 | 73.50 (5) | C30—C31—H31A | 110.9 |
| Nb1—Cl2—Nb3 | 73.21 (4) | C32—C31—H31A | 110.9 |
| Nb1—Cl3—Nb4 | 73.26 (5) | C30—C31—H31B | 110.9 |
| Nb5—Cl4—Nb1 | 73.48 (5) | C32—C31—H31B | 110.9 |
| Nb2—Cl5—Nb3 | 71.81 (4) | H31A—C31—H31B | 108.9 |
| Nb4—Cl6—Nb3 | 72.03 (4) | O12—C32—C31 | 106.1 (6) |
| Nb4—Cl7—Nb5 | 71.63 (4) | O12—C32—H32A | 110.5 |
| Nb2—Cl8—Nb5 | 71.77 (4) | C31—C32—H32A | 110.5 |
| Nb2—Cl9—Nb6 | 73.44 (5) | O12—C32—H32B | 110.5 |
| Nb6—Cl10—Nb3 | 73.34 (4) | C31—C32—H32B | 110.5 |
| Nb6—Cl11—Nb4 | 73.21 (4) | H32A—C32—H32B | 108.7 |
| Nb6—Cl12—Nb5 | 73.30 (4) | ||
| C4—O5—C1—C2 | 14.9 (9) | C20—O9—C17—C18 | 10 (2) |
| O5—C1—C2—C3 | −29.6 (9) | O9—C17—C18—C19 | 3 (2) |
| C1—C2—C3—C4 | 32.4 (8) | C17—C18—C19—C20 | −13 (2) |
| C1—O5—C4—C3 | 6.3 (9) | C17—O9—C20—C19 | −18 (2) |
| C2—C3—C4—O5 | −24.3 (8) | C18—C19—C20—O9 | 18 (2) |
| C8A_a—O6A_a—C5A_a—C6A_a | −30 (3) | C24—O10—C21—C22 | 3 (2) |
| O6A_a—C5A_a—C6A_a—C7A_a | 45 (5) | O10—C21—C22—C23 | −24 (2) |
| C5A_a—C6A_a—C7A_a—C8A_a | −41 (5) | C21—C22—C23—C24 | 36 (2) |
| C5A_a—O6A_a—C8A_a—C7A_a | 7 (4) | C21—O10—C24—C23 | 20 (1) |
| C6A_a—C7A_a—C8A_a—O6A_a | 21 (5) | C22—C23—C24—O10 | −34 (1) |
| O6B_b—C5B_b—C6B_b—C7B_b | −32 (10) | C28—O11—C25—C26 | 6 (1) |
| C12—O7—C9—C10 | 20 (1) | O11—C25—C26—C27 | −9 (2) |
| O7—C9—C10—C11 | 4 (1) | C25—C26—C27—C28 | 8 (2) |
| C9—C10—C11—C12 | −25 (1) | C25—O11—C28—C27 | −1 (1) |
| C9—O7—C12—C11 | −35 (1) | C26—C27—C28—O11 | −5 (2) |
| C10—C11—C12—O7 | 36 (1) | C32—O12—C29—C30 | 27 (1) |
| C16—O8—C13—C14 | 12.1 (8) | O12—C29—C30—C31 | −36.7 (8) |
| O8—C13—C14—C15 | −31.5 (8) | C29—C30—C31—C32 | 33.1 (8) |
| C13—C14—C15—C16 | 38.1 (8) | C29—O12—C32—C31 | −6 (1) |
| C13—O8—C16—C15 | 12.5 (9) | C30—C31—C32—O12 | −17.8 (9) |
| C14—C15—C16—O8 | −31.7 (9) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···O5 | 0.85 | 1.79 | 2.642 (7) | 180 |
| O1—H1B···O6A_a | 0.85 | 1.75 | 2.60 (3) | 178 |
| O1—H1B···O6B_b | 0.85 | 1.83 | 2.68 (5) | 173 |
| O2—H2A···O7 | 0.85 | 1.83 | 2.639 (7) | 158 |
| O2—H2B···O8 | 0.85 | 1.78 | 2.634 (7) | 180 |
| O3—H3B···O9 | 0.85 | 1.75 | 2.601 (8) | 179 |
| O3—H3A···O10 | 0.85 | 1.88 | 2.637 (8) | 148 |
| O4—H4B···O11 | 0.85 | 1.92 | 2.613 (8) | 138 |
| O4—H4A···O12 | 0.85 | 2.09 | 2.530 (8) | 112 |
| C3—H3C···Cl4i | 0.99 | 2.94 | 3.930 (8) | 177 |
| C3—H3D···Cl12ii | 0.99 | 2.95 | 3.657 (8) | 130 |
| C9—H9B···Cl11iii | 0.99 | 2.98 | 3.642 (8) | 125 |
| C14—H14A···Cl6iv | 0.99 | 2.97 | 3.931 (9) | 165 |
| C23—H23A···Cl5v | 0.99 | 2.99 | 3.74 (1) | 134 |
| C31—H31B···Cl8vi | 0.99 | 2.79 | 3.778 (8) | 176 |
Symmetry codes: (i) −x+3/2, y+1/2, z; (ii) x+1/2, y, −z+1/2; (iii) x+1/2, −y+1/2, −z+1; (iv) −x+1, −y+1, −z+1; (v) x−1/2, −y+1/2, −z+1; (vi) x−1/2, y, −z+1/2.
Funding Statement
Funding for this research was provided by: Deutsche Forschungsgemeinschaft (grant No. KO1616-8-2 to MK).
References
- Brandenburg, K. & Putz, H. (2019). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Brničević, N., Kojić–Prodić, B. & Plavšić, D. (1981). Z. Anorg. Allg. Chem. 478, 200–204.
- Bruker (2017). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Cotton, F. A. (1964). Inorg. Chem. 3, 1217–1220.
- Koknat, F. W., Parson, J. A. & Vongvusharintra, A. (1974). Inorg. Chem. 13, 1699–1702.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Lemoine, P., Halet, J.-F. & Cordier, S. (2019). In Ligated Transition Metal Clusters in Solid-State Chemistry: The Legacy of Marcel Sergent, edited by J.-F. Halet, pp. 143–190. Berlin, Heidelberg: Springer.
- Schäfer, H., Plautz, B. & Plautz, H. (1972). Z. Anorg. Allg. Chem. 392, 10–22.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Simon, A. (1988). Angew. Chem. 100, 163–188.
- Steiner, T. (2002). Angew. Chem. 114, 50–80.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314622006186/wm4165sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314622006186/wm4165Isup2.hkl
CCDC reference: 2178831
Additional supporting information: crystallographic information; 3D view; checkCIF report




