Abstract
Because prolonged viral replication of SARS-CoV-2 is increasingly being recognized among immunocompromised patients, subacute or chronic COVID-19 pneumonia can cause persistent lung damage and may lead to viral escape phenomena. Highly efficacious antiviral therapies in immunosuppressed hosts with COVID-19 are urgently needed. From February 2022, we introduced novel treatment combining antiviral therapies and neutralizing antibodies with frequent monitoring of spike-specific antibody and RT-PCR cycle threshold (Ct) values as indicators of viral load for immunocompromised patients with persistent COVID-19 infection. We applied this treatment to 10 immunosuppressed patients with COVID-19, and all completed treatment without relapse of infection. This may be a potentially successful treatment strategy that enables us to sustain viral clearance, determine optimal timing to stop treatment, and prevent virus reactivation in immunocompromised patients with persistent COVID-19.
Keywords: COVID-19, SARS-CoV-2
To the Editor,
The coronavirus disease 2019 (COVID-19) pandemic has caused significant morbidity and mortality worldwide. Driven by findings of high-quality randomized trials, management of patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has developed rapidly over the past year [1]. Although these trials justifiably focused on preventing severe disease in general patients, their benefits might not apply to some immunodeficient patients at high risk for recurrence of persistent infection. Prolonged Covid-19 is a developing issue for patients with lymphoma or immune deficiency [2]. Several reports described persistent SARS-CoV-2 replication with severe symptoms in immunocompromised patients, including those with lymphoma [3, 4]. This population is at increased risk of persistent SARS-CoV-2 infection, severe outcomes, and mortality due to COVID-19 [5]. Host genomic evolution and viral escape phenomena may potentially occur in patients with primary immunodeficiency due to prolonged relapse of SARS-CoV-2-related infection [6]. Clark et al. reported that SARS-CoV-2 evolution in an immunocompromised host shows neutralization escape mechanisms [7]. As an underlying defect in the immune response of patients with haematological malignancies, the lack of B-cell precursors is the main reason for continuing viral replication and defective viral clearance [8]. Furthermore, immune system deficiencies occurring following anti-CD20 monoclonal antibody treatment can slow development of neutralizing antibodies after administration of two doses of mRNA vaccines against SARS-CoV-2 [9]. Thus, highly efficacious antiviral therapies are urgently needed for these patients. Furlan et al. noted that therapeutic strategies combining immunotherapy with prolonged antiviral treatment may be decisive in patients with B cell immunodeficiencies [10]. Although rapid viral elimination with combined antiviral and antibody-based therapy might preclude further evolution, no optimal, decisive strategy is currently available for patients with persistent infection that allows clinicians to sustain viral clearance, determine optimal timing to stop treatment, and prevent virus reactivation. Some reports discuss RT-PCR cycle threshold (Ct) values and specific antibodies that indicate prolonged COVID-19 infection and responses to vaccines, but none address use of both indicators for treatment [11, 12].
From February 2022, we introduced a novel treatment combining antiviral and neutralizing antibody-based therapies with monitoring of spike-specific antibody and Ct values as indicators of viral load for immunocompromised patients with persistent COVID-19 infection. Knowledge of specific immune responses to antibody-based therapy in immunosuppressed patients is important, and well-validated quantitative PCR that correlates with both viral culture titres and Ct values may help in clarifying infectious viral shedding, guiding treatments, and assessing outcomes [11]. We examined these titre values at least twice weekly. Monitoring of spike-specific antibody response and Ct values during treatment allowed us to evaluate effects of antivirals and neutralizing antibody-based therapies and determine when to end treatment.
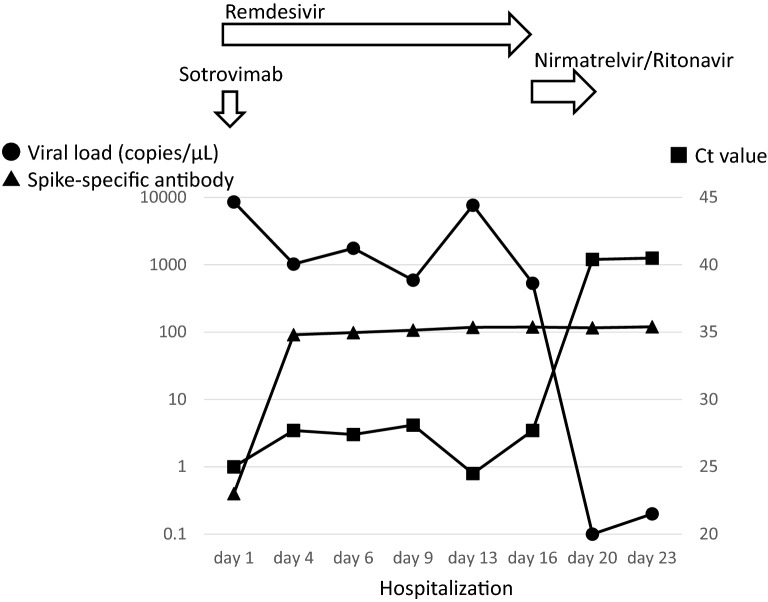
In an immunosuppressed COVID-19 patient taking tacrolimus hydrate and mycophenolate mofetil following kidney transplantation, we administered remdesivir as antiviral therapy on day 1 along with sotrovimab as neutralizing antibody-based therapy (Fig. 1). We continued remdesivir while monitoring spike-specific antibody response, viral load, and Ct values. Because the latter two values did not improve, we changed to nirmatrelvir/ritonavir antiviral therapy, following which these values improved to treatment level. After the patient’s viral load stopped rising without antiviral therapy and antibody response was maintained, the patient was removed from isolation and discharged on day 23. We applied this treatment strategy to 10 immunosuppressed COVID-19 patients who currently or previously received immunosuppressive agents for their disease (Table 1). In 5 patients, we switched from initial remdesivir to other antiviral therapy during treatment because viral load and Ct values remained unchanged or worsened. In the other 5 patients who responded well to remdesivir, we terminated it only after confirming that viral load and Ct values reached target levels. All patients were removed from isolation after confirmation that viral load, Ct values, and antibody titres had not worsened since end of treatment. No patient suffered relapse of the viral infection.
Fig. 1.
The graph shows the clinical course of a COVID-19 patient taking tacrolimus hydrate and mycophenolate mofetil as immunosuppressive therapy following kidney transplantation. The round circle indicates viral load, the square indicates Ct value, and the triangle indicates the spike-specific antibody
Table 1.
Immunosuppressed patients with COVID-19 completing treatment without relapse of the viral infection
| Factors | Patient identification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Age (years) | 51 | 74 | 49 | 94 | 51 | 66 | 72 | 57 | 51 | 84 |
| Sex (male/female) | M | M | F | F | F | F | M | M | M | M |
| Primary disease | Follicular lymphoma | Follicular lymphoma | Myasthenia gravis |
Myasthenia gravis Rheumatoid arthritis |
Kidney transplant | Kidney transplant | Kidney transplant | Liver transplant | Chronic myeloid leukemia | Chronic lymphocytic leukemia |
| Other comorbidity | None | Hypertension | Thyrotoxicosis | Hypertension | Epilepsy | Hypertension |
Hypertension Diabetes mellitus |
Hypertension Diabetes mellitus Nephrotic syndrome |
None |
Diabetes mellitus Coronary heart disease |
| COVID-19 vaccination | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes |
| Anti-CD20 antibody | Obinutuzumab |
Rituximab Obinutuzumab |
None | None | None | None | None | None | None | None |
| Immunosuppressive agents for primary disease |
Cyclophosphamide Prednisolone |
Cyclophosphamide Prednisolone |
Tacrolimus |
Tacrolimus Prednisolone |
Tacrolimus Mycophenolate mofetil Everolimus Prednisolone |
Tacrolimus Mycophenolate mofetil Methylprednisolone |
Tacrolimus Mycophenolate mofetil Methylprednisolone |
Tacrolimus Mycophenolate mofetil Everolimus |
Iguratimod Tocilizumab Prednisolone |
Prednisolone |
|
Initial antiviral therapy Switched antiviral therapy |
Remdesivir |
Remdesivir Nirmatrelvir/Ritonavir |
Remdesivir Nirmatrelvir/Ritonavir |
Remdesivir Molnupiravir |
Remdesivir |
Remdesivir Nirmatrelvir/Ritonavir |
Remdesivir |
Remdesivir Nirmatrelvir/Ritonavir |
Remdesivir | Remdesivir |
| Neutralizing antibody-based therapy |
Sotrovimab Casirivimab/Imdevimab |
Sotrovimab | Casirivimab/Imdevimab | Casirivimab/Imdevimab | Sotrovimab | Sotrovimab | Sotrovimab | Sotrovimab | Casirivimab/Imdevimab | Sotrovimab |
| Initial Ct value | 18.6 | 19.8 | 25.3 | 18.2 | 19.6 | 25 | 17.9 | 22.4 | 15 | 25.6 |
| Initial spike-specific antibody | Negative | Negative | 163 U/mL | Negative | Negative | Negative | Negative | Negative | Negative | 1.41 U/mL |
| Length of antiviral and antibody-based therapy | 10 days | 14 days | 9 days | 10 days | 10 days | 21 days | 20 days | 18 days | 8 days | 7 days |
| Length of hospital stay | 14 days | 19 days | 13 days | 28 days | 13 days | 23 days | 43 days | 18 days | 12 days | 17 days |
As limitations, first, although the progression of SARS-CoV-2 infectious viral shedding and specific immune responses by this treatment are understandable, criteria for frequency of measuring spike-specific antibody and Ct values remain unknown. Second, long-term data on potential viral relapse is unknown. Third, administration of antiviral drugs at above-normal prescription limits based on frequent test results could increase long-term drug and hospitalization costs. Fourth, because the sample size is small, results should be interpreted cautiously. More rigorous research including randomization and larger sample size is needed. Nevertheless, our novel strategy may offer potentially successful treatment for immunocompromised patients with persistent COVID-19.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- Ct
RT-PCR cycle threshold
Authors contributions
DW analysed the patient data and wrote the first draft of this manuscript. YN, SM, HS, FS, and KY helped to draft the manuscript and revise it critically for important intellectual content. YK contributed to final approval of the manuscript to be published. All authors read and approved the final manuscript.
Funding
There are no sources of funding for this case report.
Availability of data and materials
This case report only contains clinical data from the medical records of the patient reported herein. The data will be made available upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We received written consent from the patient reported herein to present this case. The consent form will be provided upon request.
Competing interests
The authors declare that they have competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daiki Wada, Email: dk0116-w@live.jp.
Yasushi Nakamori, Email: 99nakamori@gmail.com.
Shuhei Maruyama, Email: Suugakunotiti@gmail.com.
Haruka Shimazu, Email: kukki.lucky.candy@gmail.com.
Fukuki Saito, Email: saitof@takii.kmu.ac.jp.
Kazuhisa Yoshiya, Email: kyoshiya@msn.com.
Yasuyuki Kuwagata, Email: kuwagata@hirakata.kmu.ac.jp.
References
- 1.Moran E, Cook T, Goodman AL, Gupta RK, Jolles S, Menon DK, et al. Persistent SARS-CoV-2 infection: the urgent need for access to treatment and trials. Lancet Infect Dis. 2021;21:1345–7. doi: 10.1016/S1473-3099(21)00464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duléry R, Lamure S, Delord M, Di Blasi R, Chauchet A, Hueso T, et al. Prolonged in-hospital stay and higher mortality after COVID-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96:934–44. doi: 10.1002/ajh.26209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARSCoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–8. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hueso T, Pouderoux C, Péré H, Beaumont AL, Raillon LA, Ader F, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136:2290–5. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385:562–6. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabañero-Navalon MD, Garcia-Bustos V, Ruiz-Rodriguez P, Comas I, Coscollá M, Martinez-Priego L, et al. Persistent SARS-CoV-2 infection with repeated clinical recurrence in a patient with common variable immunodeficiency. Clin Microbiol Infect. 2022;28:308–10. doi: 10.1016/j.cmi.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark SA, Clark LE, Pan J, Coscia A, McKay LGA, Shankar S, et al. SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralization escape mechanisms. Cell. 2021;184:2605–17.e18. doi: 10.1016/j.cell.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guisado-Vasco P, Carralón-González MM, Aguareles-Gorines J, Martí-Ballesteros EM, Sánchez-Manzano MD, Carnevali-Ruiz D, et al. Plitidepsin as a successful rescue treatment for prolonged viral SARS-CoV-2 replication in a patient with previous anti-CD20 monoclonal antibody-mediated B cell depletion and chronic lymphocytic leukemia. J Hematol Oncol. 2022;15:4. doi: 10.1186/s13045-021-01220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furlan A, Forner G, Cipriani L, Vian E, Rigoli R, Gherlinzoni F, et al. COVID-19 in B cell-depleted patients after rituximab: a diagnostic and therapeutic challenge. Front Immunol. 2021;12:763412. doi: 10.3389/fimmu.2021.763412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton CS, Huntley K, Berenger BM, Bristow M, Evans DH, Fonseca K, et al. Prolonged SARS-CoV-2 infection following rituximab treatment: clinical course and response to therapeutic interventions correlated with quantitative viral cultures and cycle threshold values. Antimicrob Resist Infect Control. 2022;11:28. doi: 10.1186/s13756-022-01067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parry H, McIlroy G, Bruton R, Damery S, Tyson G, Logan N, et al. Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia. J Hematol Oncol. 2022;15:3. doi: 10.1186/s13045-021-01219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This case report only contains clinical data from the medical records of the patient reported herein. The data will be made available upon request.