Abstract
Background
The association between inflammation and major depressive disorder (MDD) remains poorly understood, given the heterogeneity of patients with MDD.
Aims
We investigated inflammatory markers, such as interleukin (IL)-6, high-sensitivity C reactive protein (hsCRP) and tumour necrosis factor-α (TNF-α) in melancholic, atypical and anxious depression and explored whether baseline inflammatory protein levels could indicate prognosis.
Methods
The sample consisted of participants (aged 18–55 years) from a previously reported multicentre randomised controlled trial with a parallel-group design registered with ClinicalTrials.gov, including melancholic (n=44), atypical (n=37) and anxious (n=44) patients with depression and healthy controls (HCs) (n=33). Subtypes of MDD were classified according to the 30-item Inventory of Depressive Symptomatology, Self-Rated Version and the 17-item Hamilton Depression Rating Scale. Blood levels of TNF-α, IL-6 and hsCRP were assessed using antibody array analysis.
Results
Patients with MDD, classified according to melancholic, atypical and anxious depression subtypes, and HCs did not differ significantly in baseline TNF-α, IL-6 and hsCRP levels after adjustment. In patients with anxious depression, hsCRP levels increased significantly if they experienced no pain (adjusted (adj.) p=0.010) or mild to moderate pain (adj. p=0.038) compared with those with severe pain. However, the patients with anxious depression and severe pain showed a lower trend in hsCRP levels than patients with atypical depression who experienced severe pain (p=0.022; adj. p=0.155). Baseline TNF-α (adj. p=0.038) and IL-6 (adj. p=0.006) levels in patients in remission were significantly lower than those in patients with no remission among the participants with the atypical depression subtype at the eighth-week follow-up.
Conclusions
This study provides evidence of differences in inflammatory proteins in patients with varied symptoms among melancholic, atypical and anxious depression subtypes. Further studies on the immunoinflammatory mechanism underlying different subtypes of depression are expected for improved individualised therapy.
Trial registration number
Keywords: major depressive disorder, inflammatory markers, antibody array, somatic symptoms, melancholic, atypical, anxious
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies revealed differences in inflammatory marker levels, such as interleukin (IL)-6, serum C reactive protein (CRP) and tumour necrosis factor-α (TNF-α) between patients with major depressive disorder (MDD) and healthy controls, as well as differences among different subtypes of MDD. For example, higher CRP, IL-6 and TNF-α levels were observed in patients with atypical depression. Moreover, patients had higher levels of CRP and IL-6. However, there are few studies on the association between inflammatory markers and anxious depression, though it accounts for the largest number of patients with MDD with ‘pure’ subtypes (up to 53%–78%). In addition, whether the levels of inflammatory biomarkers at baseline can predict treatment outcomes remains unknown.
WHAT THIS STUDY ADDS
This study employed an antibody array analysis to investigate the inflammatory markers (IL-6, TNF-α and high-sensitivity C reactive protein (hsCRP)) in three MDD subtypes: melancholic, atypical and anxious depression. We also explored whether baseline inflammatory marker levels could indicate the prognosis during acute treatment at the 8th and 12th weeks of follow-up.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides evidence of differences in inflammatory factors in patients with different symptoms among melancholic, atypical and anxious subtypes of depression. It also adds evidence about the immunoinflammatory mechanism underlying different subtypes of depression to further improve individualised therapy.
Introduction
Major depressive disorder (MDD) is generally considered a heterogeneous disease,1 with significant variability in symptom patterns, course trajectories and treatment responses.2 Therefore, studying subgroups or subtypes of patients with MDD that were more homogenous could help reduce research heterogeneity, better identify aetiological mechanisms, and develop more patient-specific diagnostic and therapeutic biomarkers.
Presently, new methods, such as data-driven and latent class analysis,3 are used to identify clinical subtypes of MDD and have received keen attention. However, most of these methods are based on ideal circumstances—effectively collecting sufficient data while feasibly capturing patients with similar backgrounds—which are difficult to achieve in clinical practice.4 Therefore, the classification of MDD subtypes remains mainly based on clinical symptom patterns or symptom clusters.5 The most comprehensively explored subtypes of depression, which entail the largest number of patients with ‘pure’ subtypes, include melancholic, atypical and anxious depression.6 Patients with the three subtypes of MDD differ not only in clinical symptoms but also in biological aspects such as brain structure and function, genetic variation, energy and inflammatory protein levels.7 This study focused on the differences in inflammatory markers.
Contrary to expectations, previous studies failed to reveal significant differences in the inflammatory marker interleukin (IL)-6 levels between healthy participants and patients with MDD8; this may be related to the heterogeneity among patients with MDD. Compared with healthy participants, IL-6 levels in patients with melancholic depression were significantly increased, while there was no significant difference in patients with atypical depression.9 This finding was consistent with another study10 indicating that the IL-6 levels may correlate with different subtypes of MDD. Other inflammation-associated proteins, such as serum C reactive protein (CRP) and tumour necrosis factor-α (TNF-α), also differed in different subtypes of MDD. For example, compared with melancholic depression, patients with atypical depression had higher CRP, IL-6 and TNF-α levels and higher body mass index (BMI), waist circumference and triglyceride levels but lower high-density lipid cholesterol levels. Moreover, patients with melancholic depression had significantly higher salivary cortisol levels than those with atypical depression, suggesting differences in the hypothalamic-pituitary-adrenal (HPA) -axis function, inflammation and biological function of the metabolic syndrome between these two subtypes.11 This is similar to the findings of another study reporting that patients with certain specific clinical characteristics among patients with atypical depression, such as atypical depression patients with increased appetite (n=23), had higher levels of insulin, insulin resistance, leptin, CRP and IL-6 at baseline.12
However, a systematic review study13 did not fully support these findings. This review included eight studies (n=6307). The meta-analysis results indicated that IL-6 and IL-1β increased in patients with melancholic depression and CRP increased in patients with atypical depression, while TNF-α, IL-2 and IL-10 did not differ between these two subtypes.13 A study from the Netherlands Study of Depression in Older Persons14 and a cross-sectional study in a large community cohort8 reported that no inflammatory biomarkers (CRP and IL-6) differed among older patients with MDD with severe atypical, severe melancholic and moderate–severe subtypes of depression. The observed significant increase in high-sensitivity C reactive protein (hsCRP) levels in patients with atypical depression was due to somatic diseases such as abnormal BMI, diabetes and hypertension rather than heterogeneity in MDD subtypes.8 In other words, there is no consistent conclusion on whether the levels of inflammatory markers are significantly different between atypical and melancholic depression subtypes, which needs further exploration. Moreover, there are few reports from Asian populations.
It is worth noting that there are few studies on the association between inflammatory markers and anxious depression, which accounts for the majority of patients with MDD with pure subtypes (up to 53%–78%).15 Two studies reported increased counts of leucocyte subsets (monocytes16 and basophils17 and enhanced glucocorticoid receptor-induced leucocyte responses18 in patients with the anxious depression subtype, indirectly suggesting changes in the immune system and suggesting a role of inflammation in the pathophysiology of anxious depression). The results of the Netherlands Study of Depression and Anxiety Disorders demonstrated that the anxious distress specifier was associated with increased innate cytokine production capacity rather than with basal inflammation (CRP, IL-6 and TNF-α) levels within a large sample of patients with MDD (n=1078).19 Other reports involved only the function of the HPA axis and the association of cortisol with anxious depression. In summary, previous evidence suggests that the association between inflammatory marker levels and patients with anxious depression urgently warrants further investigation.
In addition, whether the levels of inflammatory biomarkers at baseline can predict treatment outcomes remains to be explored. Previous evidence indicated that the severity of depressive symptoms in patients with melancholic depression was positively correlated with IL-6 and cortisol levels. In contrast, the severity of depressive symptoms in patients with atypical depression was negatively correlated with the expression levels of IL-6 and CRP.20 However, a large prospective cohort study (n=3118) revealed that baseline inflammatory marker levels, including IL-6, TNF-α and hsCRP, could not predict treatment outcomes among subtypes (atypical, melancholic, combined atypical and melancholic, and unspecified depression subtypes) at a 5.5-year follow-up.21
Thus, the protein levels of inflammatory factors in melancholic, atypical and anxious subtypes of depression remain ambiguous. However, there are few reports on the expression of inflammatory factors in patients with anxious depression. Additionally, it is unclear whether baseline inflammatory biomarker levels can indicate treatment outcomes at follow-up, and reports about Asian populations are lacking. Therefore, in this study, we employed an antibody array analysis to investigate the inflammatory markers (IL-6, TNF-α and hsCRP) in three different MDD subtypes—melancholic, atypical and anxious depression—and explored whether the baseline inflammatory marker levels could indicate the prognosis during acute treatment and at the 8th and 12th weeks of follow-up. We hypothesised that inflammatory biomarker levels among the three MDD subtypes would differ and that baseline inflammatory factors could indicate treatment outcomes during the follow-up period of acute treatment.
Materials and methods
Participants
All participants were enrolled in a previously reported randomised clinical trial (RCT) with a parallel-group design registered with ClinicalTrials.gov. The protocol of this RCT study has been published elsewhere.22 This trial was performed between August 2017 and December 2020 to enroll participants diagnosed with MDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria.
This study, which enrolled a total of 808 patients with MDD, was performed at eight sites in China.22 However, blood samples were taken only from patients in the Shanghai Mental Health Centre, so all the samples in this study were obtained from this site and the total patients involved numbered 125. All participants were adults (18–55 years old) who met the DSM-5 diagnostic criteria for MDD and were currently experiencing their first, single episode, or recurrent acute non-psychotic major depressive episode. In brief, the inclusion criteria for patients with MDD were as follows: (1) 17-item Hamilton Depression Rating Scale (17-HDRS) score ≥17; (2) not taking any antidepressant medication or undergoing physical or psychological therapy within the past 6 months before recruitment into the study; and (3) meeting criteria for melancholic, atypical or anxious depression subtypes. The exclusion criteria were as follows: (1) a history of another Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition axis I disorder (eg, bipolar disorder, schizophrenia, schizoaffective disorder and autism disorder); (2) apparent suicidal ideation, attempt or behaviour (eg, score for item 3 of 17-HDRS ≥3); (3) difficult to define as melancholic, atypical or anxious depression subtype; (4) a history of treatment-resistant depression; (5) serious physical illness (eg, brain tumour or damage) or condition that may interfere with the study protocol; (6) pregnant or lactating women or women planning to become pregnant; (7) patients with a history of mania or mild mania present during this episode, or patients with mental retardation, personality disorder or anorexia nervosa/bulimia; or (8) patients with secondary depressive disorder caused by organic lesion or drugs.
The healthy control (HC) participants were recruited from the community through advertising. The inclusion criteria were (1) 18–55 years of age; (2) age, sex, and years of education matched with enrolled patients with depression; and (3) voluntary participation in this study and signed informed consent. The exclusion criteria were (1) a history of mental disease, serious physical disease, cerebrovascular disease or brain injury; (2) severe allergic reactions or having suffered from immune system diseases; and (3) pregnant or lactating women or women planning to become pregnant.
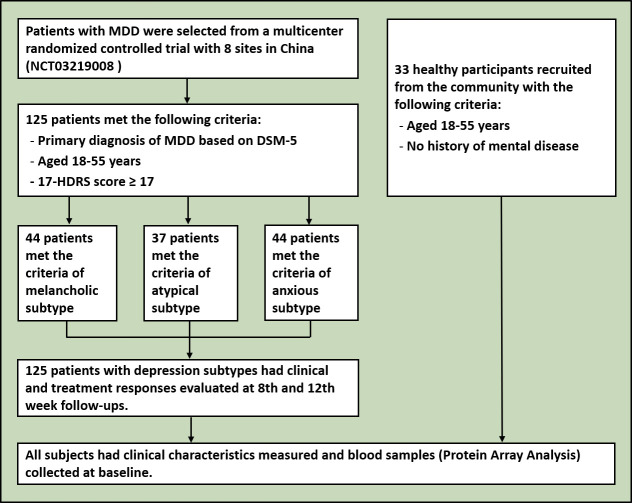
The demographic and clinical characteristics of the participants, including age, sex, BMI, education, occupation, marital status, duration of current episode, symptoms and other clinical characteristics of depression were collected (figure 1).
Figure 1.
Flowchart of the study. DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; 17-HDRS, 17-item Hamilton Depression Rating Scale; MDD, major depressive disorder
Clinical measures and subtype classification
The Mini-International Neuropsychiatric Interview (MINI) was used to confirm the criteria for MDD at screening. The 17-HDRS, the 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR (16)) and the 30-item Inventory of Depressive Symptomatology, Self-Rated Version (IDS-30) were used to evaluate depressive symptoms. Quality of life (QOL) was also included in the clinical assessment. The three subtypes of depression were classified mainly by the IDS-30 and 17-HDRS scores, referring to the assessment methods used in the International Study to Predict Optimised Treatment in Depression trial23 and the Sequenced Treatment Alternatives to Relieve Depression study.24 Briefly, the classification criteria for the melancholic depression subtype included (1) a score of ≥2 for items 9 or 21 of the IDS-30; and (2) at least three of the following characteristics: a pronounced depression characterised by extreme depression, despair or melancholy, or the emotion of emptiness; more severe depressive symptoms in the morning; irritability; agitation or psychomotor slowness; and severe anorexia or weight loss. The classification criteria for the atypical depression subtype included (1) a score of ≥2 for item 21 of the IDS-30, (2) increased appetite or significant weight gain, (3) sensitive interpersonal relationships and (4) leaden paralysis (feeling heaviness in the arms or legs). The classification criteria for the anxious depression subtype included (1) a score of ≥7 for items 10, 11, 12, 13, 15 and 17 of the 17-HDRS; and (2) at least two of the following patient characteristics: feeling nervous or upset, feeling unusually uneasy, difficulty concentrating due to worry, fear of terrible things happening and being afraid of losing control of oneself.
All participants were categorised into melancholic, atypical or anxious depression subtypes and were randomly assigned to different intervention groups using a random cipher. Clinical symptoms and side effects were evaluated at critical decision points, including baseline and at 2, 4, 6, 8 and 12 weeks after treatment. The total score of the 17-HDRS was used to evaluate depression severity, and the treatment outcome was measured by the reduction of the total 17-HDRS score at specific follow-up weeks compared with that at baseline. Remission was defined as a 17-HDRS total score of ≤7. Additionally, five factors were derived from the 17-HDRS: anxiety/somatisation, weight loss, psychomotor retardation, cognitive disturbance and sleep disturbance. Each factor consisted of corresponding items acquired from each patient to obtain more comprehensive clinical information.
Clinical symptoms and subclassifications
Eligible patients were grouped according to items 11, 12, 25 and 28 of the original IDS-30 scale. Patients with gastrointestinal symptoms were divided into two groups according to the items 11, 12 and 28 of the IDS-30: 0, no gastrointestinal symptoms; or 1, gastrointestinal symptoms indicated. Patients with pain symptoms were grouped according to item 25 of the IDS-30, and the severity of pain was divided into three levels: 0, no pain; 1, mild or moderate pain; or 2, severe pain. The patients were grouped by appetite according to items 11 and 12 of the IDS-30: 0, no change in appetite; 1, decreased appetite; or 2, increased appetite.
Plasma collection and laboratory tests
This study extracted 158 plasma samples from 44 patients with melancholic depression, 37 with atypical depression, 44 with anxious depression and 33 HCs at baseline. At baseline, 5 mL of whole blood was collected from each participant at the cubical vein in an EDTA anticoagulation tube between 7:00 AM and 9:00 AM. The blood was centrifuged for plasma separation under anticoagulant conditions at 3000 rounds per min for 10 min at 4°C within 2 hours. The supernatant was collected and evenly subpacked 0.2 mL for each subpackage into 0.5 mL Eppendorf tubes and frozen at −80°C for storage until analysis. Repeated freeze–thaw cycles were avoided.
The inflammatory proteins (IL-6, TNF-α and hsCRP) in plasma were detected using a custom antibody array (QAH-CUSTOM-6; RayBiotech, Peachtree Corners, Georgia, USA). Array analysis was performed according to the manufacturer’s instructions. Briefly, plasma samples were diluted, added to the array pools and incubated with capture antibodies overnight. After washing, the samples were sealed and incubated, followed by fluorescence detection. The signal was scanned using an InnoScan 300 Microarray Scanner (Innopsys, France) with a wavelength of 532 nm and a resolution of 10 µm. Signal values were captured using Mapex software. The data were normalised using positive control values from the array with the RayBiotech analysis tool, specifically designed to analyse the data of a custom antibody array.
Statistical analysis
Variables were reported as frequency (percentage), mean (standard deviation, SD) or median (interquartile range, IQR), as appropriate. No imputation was performed for the missing data. Differences in characteristics among the four diagnostic groups (melancholic, atypical, anxious depression and healthy participants) were tested by χ2 tests (categorical variables), one-way analysis of variance (numerical variables) or Kruskal-Wallis analyses (non-normally distributed variables) of variance using SPSS V.23.0.
The concentrations of inflammatory proteins were subsequently log-transformed (log2) for analyses due to a skewed distribution. The R software package limma from R/Bioconductor was used to analyse differentially expressed proteins (DEPs) of three inflammation markers (TNF-α, IL-6 and hsCRP). This software was specifically used to compare the levels of inflammation factors between two different groups using moderated t-statistics. The results included (log2) fold changes and p values for each factor. Next, the Benjamini-Hochberg (B-H) false discovery method, which was employed to correct for multiple tests for all depressive subtypes, was used to calculate adjusted (adj.) p values. DEPs were defined as those with an adj. p value of <0.05 and a fold change of >1.2 or <0.83 (absolute logFC >0.263). All corresponding figures in this study, including the column diagram and violin diagram of comparison between subtypes or clinical symptom groups, were constructed using GraphPad Prism V.8.3.0 (GraphPad Software, San Diego, California, USA).
Results
Study characteristics
The demographic and clinical characteristics of the 158 participants (mean age: 27.5 (7.5) years, age range: 18–50 years, 62.6% women) at baseline and the follow-ups of 8 and 12 weeks are presented in table 1. Age, sex, marital status and BMI were matched among all three MDD subtypes and for the HCs, while they differed in career, education and 17-HDRS total scores (table 1). There was no difference among the three subtypes of MDD in the first onset, recrudescence, age of first depressive episode, duration of current episode, pain, gastrointestinal symptoms or five-factor scores of 17-HDRS (p>0.05). In contrast, differences were observed in appetite symptoms (p=0.019) at baseline. The three MDD subtypes differed significantly in the total scores of 17-HDRS, IDS-30, QIDS-SR (16) and appetite symptoms at baseline, but there were no differences in the dropout rate or the total scores of 17-HDRS, QOL and QIDS-SR (16) at follow-up (table 1).
Table 1.
Characteristics of melancholic, atypical and anxious subtypes of MDD in the study participants
| Characteristics | Patients with MDD | Healthy participants | P value | ||
| Melancholic | Atypical | Anxious | |||
| n=44 | n=37 | n=44 | n=33 | ||
| Demographic characteristics | |||||
| Age (years), mean (SD) | 27.8 (7.2) | 26.9 (7.1) | 28.2 (7.8) | 27.5 (8.7) | 0.888* |
| Gender, n (%) | |||||
| Female | 29 (65.9) | 21 (56.8) | 30 (68.2) | 19 (57.6) | 0.640† |
| Male | 15 (34.1) | 16 (43.2) | 14 (31.8) | 14 (42.4) | |
| Marital status, n (%) | |||||
| Unmarried | 33 (75.0) | 26 (70.3) | 30 (68.2) | 27 (81.8) | 0.209† |
| Married/cohabitation | 9 (20.5) | 6 (16.2) | 13 (29.5) | 5 (15.2) | |
| Divorced/separated | 2 (4.5) | 5 (13.5) | 1 (2.3) | 1 (3.0) | |
| Career, n (%) | |||||
| Employed | 26 (59.1) | 18 (48.6) | 20 (45.5) | 9 (27.3) | 0.010† |
| Student | 14 (31.8) | 14 (37.8) | 18 (40.9) | 24 (72.7) | |
| Unemployed | 4 (9.1) | 5 (13.5) | 6 (13.6) | 0 (0.0) | |
| Education (years), mean (SD) | 15.6 (1.8) | 14.0 (3.9) | 15.3 (2.4) | 16.2 (2.7) | 0.007* |
| BMI (kg/m2), mean (SD) | 20.5 (3.3) | 20.1 (6.9) | 21.2 (2.8) | 22.2 (3.4) | 0.189* |
| Clinical characteristics | |||||
| 17-HDRS total score, mean (SD) | 23.3 (4.3) | 20.4 (3.1) | 24.5 (4.3) | 0.8 (0.9) | <0.001* |
| Five-factor scores of 17-HDRS, mean (SD) | |||||
| Anxiety/somatisation | 6.7 (2.2) | 6.0 (1.7) | 9.0 (1.5) | 0.3 (0.6) | <0.001‡ |
| Cognitive disturbance | 4.4 (1.4) | 3.9 (1.3) | 4.0 (1.7) | 0.1 (0.1) | <0.001‡ |
| Sleep disturbance | 3.4 (1.8) | 2.8 (1.6) | 3.1 (1.8) | 0.3 (0.5) | <0.001‡ |
| Psychomotor retardation | 7.6 (1.4) | 7.5 (1.6) | 7.6 (1.8) | 0.1 (0.1) | <0.001‡ |
| Weight loss | 0.9 (0.9) | 0.3 (0.6) | 0.6 (0.8) | 0.1 (0.4) | <0.001‡ |
| IDS-30 total score, mean (SD) | 47.3 (10.5) | 40.2 (9.3) | 39.3 (10.1) | / | <0.001* |
| QOL total score, mean (SD) | 15.5 (2.7) | 15.8 (2.3) | 16.3 (2.5) | / | 0.319* |
| QIDS-SR (16) total score, mean (SD) | 21.9 (6.3) | 18.9 (5.5) | 18.2 (5.9) | / | 0.008* |
| First onset, n (%) | 24 (54.5) | 24 (64.9) | 30 (68.2) | / | 0.471† |
| Recrudescence, n (%) | 19 (43.2) | 13 (35.1) | 14 (31.8) | / | |
| Age of first depressive episode, n (%) | 25.2 (8.7) | 24.5 (8.6) | 25.9 (7.6) | / | 0.763* |
| Duration of current episode, mean (SD) | 26.3 (39.7) | 41.1 (128.8) | 26.5 (60.7) | / | 0.664* |
| ≤6 months, n (%) | 14 (31.8) | 13 (35.1) | 17 (38.6) | / | 0.814† |
| 6 to ≤12 months, n (%) | 10 (22.7) | 12 (32.4) | 13 (29.5) | / | |
| 12 to ≤24 months, n (%) | 8 (18.2) | 4 (10.8) | 4 (9.1) | / | |
| 24 to ≤60 months, n (%) | 6 (13.6) | 5 (13.5) | 7 (15.9) | / | |
| 60 to ≤120 months, n (%) | 3 (6.8) | 1 (2.7) | 0 (0.0) | / | |
| >120 months, n (%) | 2 (4.5) | 2 (5.4) | 2 (4.5) | / | |
| NA, n(%) | 1 (2.3) | / | 1 (2.3) | / | |
| Appetite symptoms, n (%) | 0.019† | ||||
| None | 10 (22.7) | 8 (21.6) | 10 (22.7) | / | |
| Decreased | 30 (68.2) | 16 (43.2) | 29 (65.9) | / | |
| Increased | 4 (9.1) | 13 (35.1) | 5 (11.4) | / | |
| Pain symptoms, n (%) | 0.920† | ||||
| None | 14 (31.8) | 13 (35.1) | 15 (34.1) | / | |
| Moderate and mild | 22 (50.0) | 18 (48.6) | 24 (54.5) | / | |
| Severe | 8 (18.2) | 6 (16.2) | 5 (11.4) | / | |
| Gastrointestinal symptoms | 0.932† | ||||
| None | 8 (18.2) | 7 (18.9) | 7 (15.9) | / | |
| Present | 36 (81.8) | 30 (81.1) | 37 (84.1) | / | |
| Follow-up | |||||
| 8-week follow-up, n (%) | 0.080† | ||||
| Remission | 7 (15.9) | 10 (27.0) | 13 (29.5) | / | |
| No remission | 22 (50.0) | 9 (24.3) | 14 (31.8) | / | |
| Dropped out | 15 (34.1) | 18 (48.6) | 17 (38.6) | / | |
| 17-HDRS total score, mean (SD) | 8.7 (4.9) | 9.5 (6.6) | 9.1 (7.3) | / | 0.911* |
| QIDS-SR (16) total score, mean (SD) | 9.4 (6.0) | 9.4 (7.5) | 8.4 (7.6) | / | 0.848* |
| QOL total score, mean (SD) | 20.4 (3.6) | 20.5 (2.9) | 19.4 (3.4) | / | 0.449* |
| 12-week follow-up, n (%) | |||||
| Remission | 10 (22.7) | 5 (13.5) | 13 (29.5) | / | 0.433† |
| No remission | 17 (38.6) | 11 (29.7) | 13 (29.5) | / | |
| Dropped out | 17 (38.6) | 21 (56.8) | 18 (40.9) | / | |
| 17-HDRS total score, mean (SD) | 6.0 (4.3) | 9.2 (6.7) | 8.2 (7.9) | / | 0.2461* |
| QIDS-SR (16) total score, mean (SD) | 6.7 (5.0) | 10.2 (8.9) | 6.4 (5.2) | / | 0.117* |
| QOL total score, mean (SD) | 21.4 (2.5) | 10.8 (3.1) | 20.8 (2.9) | / | 0.193* |
Superscripts represent statistical results analysed by specific statistical methods.
Bold p values indicate <0.05.
The bold values means statistically significant difference.
*One-way analysis of variance.
†χ2 test.
‡Kruskal-Wallis H test.
BMI, body mass index; 17-HDRS, 17-item Hamilton Depression Rating Scale; IDS-30, 30-item Inventory of Depressive Symptomatology, Self-Rated Version; MDD, major depressive disorder; QIDS-SR (16), 16-item Quick Inventory of Depressive Symptomatology–Self-Report; QOL, Quality of Life.
Inflammatory markers and clinical symptoms at baseline
The laboratory test results, related clinical symptoms and statistical results among the groups at baseline are presented in online supplemental tables 1–3, respectively. There was no difference in the levels of TNF-α, IL-6 or hsCRP between MDD and HCs participants. Similarly, there was no difference in TNF-α or IL-6 levels between all three subtypes of MDD and HCs, respectively, or between any two groups among the three MDD subtypes. However, although hsCRP levels differed significantly between groups (p=0.017), especially for the group of atypical (p=0.011) or anxious (p=0.042) depression versus HCs, or the melancholic versus atypical depression (p=0.021), no differences were significant after adjustment (adj. p>0.05) (online supplemental table 1 and figure 2).
Figure 2.
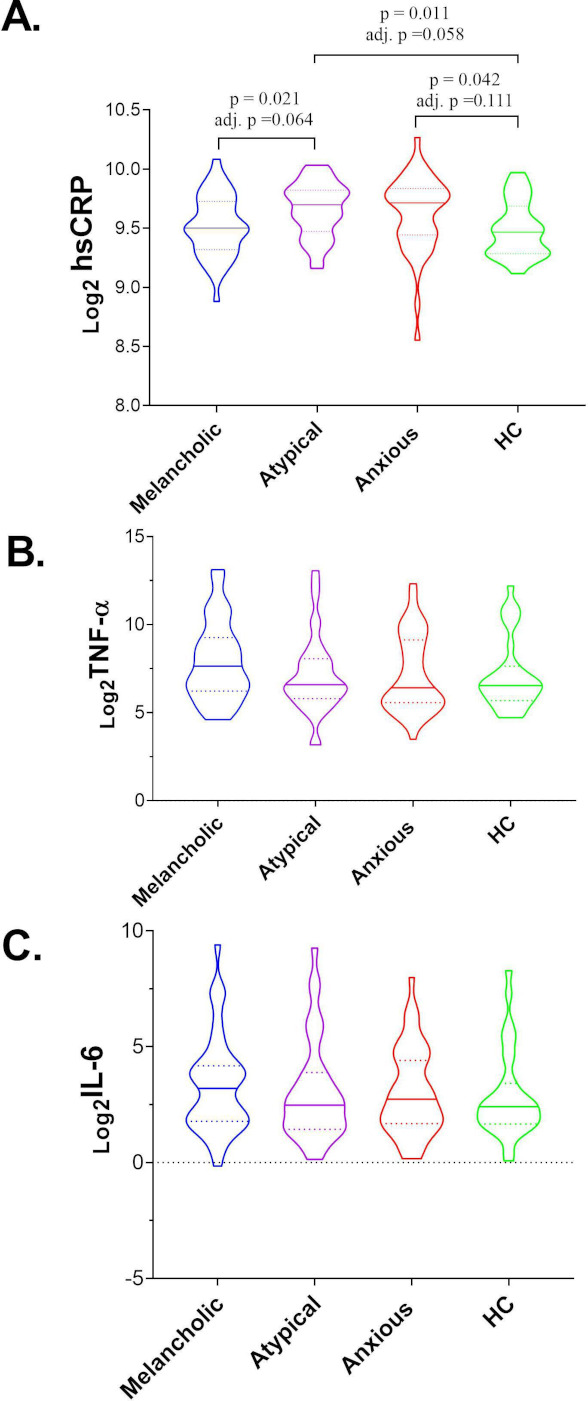
Peripheral levels of TNF-α, IL-6 and hsCRP in melancholic, atypical and anxious depression and HC participants at baseline. (A) hsCRP; (B) TNF-α; (C) IL-6. The horizontal ordinate represents the three subtypes of MDD and HC participants. The longitudinal coordinates represent the baseline inflammatory marker levels after a log2 transformation detected by an antibody array analysis. The solid and dotted lines on the violin diagram indicated the median and IQR of the measured levels of the inflammatory markers that were logarithmically transformed, respectively. hsCRP, high-sensitivity C reactive protein; HC, healthy control; IL, interleukin; IQR, interquartile range; MDD, major depressive disorder; TNF-α, tumour necrosis factor-α.
gpsych-2022-100844supp001.pdf (207.6KB, pdf)
In terms of the clinical symptoms of the three MDD subtypes, there was no difference in any of the three inflammatory biomarkers between any two subtypes with or without gastrointestinal symptoms (online supplemental table 3). However, the results indicated that patients with MDD with decreased or increased appetite differed significantly in TNF-α levels (p=0.018), specifically demonstrating that the levels of TNF-α in patients with MDD with decreased appetite increased 2.482-fold compared with those with increased appetite. Between-group comparisons revealed that the difference mainly originated from the melancholic (p=0.038) rather than the atypical or anxious subtypes of depression (online supplemental table 3). Compared with patients with increased appetite, the levels of TNF-α in patients with decreased appetite in the melancholic subtype were approximately 5.879-fold higher. In addition, the log2 transformation TNF-α differed significantly (p=0.042) between melancholic and anxious depression subtype patients with mild to moderate pain; it was approximately 2.651-fold higher in the melancholic subtype compared with the anxious subtype patients. All adj. p values were >0.05. However, except for the anxious depression subtype, the levels of hsCRP differed between patients without pain and those with severe pain (p=0.001, adj. p=0.010) and between patients with mild to moderate pain and those with severe pain (p=0.005, adj. p=0.038) (table 2).
Table 2.
Inflammatory proteins with significant statistical differences in clinical symptoms among melancholic, atypical and anxious depression subtypes
| Time/symptoms | Subtypes | Clinical symptoms | Protein label | AveExp. group 1 | AveExp. group 2 | LogFC | P value | Adjusted p value | Fold change | Regulation |
| Baseline/appetite | — | Decreased versus increased | TNF-α | 7.728 | 6.417 | 1.311 | 0.018 | 0.063 | 2.482 | Up |
| M | Decreased versus increased | TNF-α | 8.123 | 5.568 | 2.556 | 0.038 | 0.155 | 5.879 | Up | |
| Baseline/pain | M versus AN | Mild to moderate | TNF-α | 8.240 | 6.833 | 1.407 | 0.042 | 0.146 | 2.651 | Up |
| AT versus AN | Severe | hsCRP | 9.644 | 9.243 | 0.401 | 0.022 | 0.155 | 1.321 | Up | |
| AN | None versus severe | hsCRP | 9.725 | 9.243 | 0.483 | 0.001 | 0.010 | 1.397 | Up | |
| AN | Mild to moderate versus severe | hsCRP | 9.640 | 9.243 | 0.398 | 0.005 | 0.038 | 1.317 | Up | |
| 8-week follow-up | M versus AT | No remission | IL-6 | 3.250 | 4.836 | −1.586 | 0.018 | 0.063 | 0.333 | Down |
| AT versus AN | No remission | IL-6 | 4.836 | 3.085 | 1.751 | 0.016 | 0.109 | 3.366 | Up | |
| AT | No remission versus remission | IL-6 | 4.836 | 2.184 | 2.651 | 0.001 | 0.006 | 6.283 | Up | |
| AT | No remission versus remission | TNF-α | 8.793 | 6.220 | 2.573 | 0.011 | 0.038 | 5.953 | Up | |
| 12-week follow-up | AT | No remission versus remission | IL-6 | 4.258 | 2.044 | 2.214 | 0.022 | 0.077 | 4.639 | Up |
AveExp. group 1 and AveExp. group 2 represent the mean logarithmically transformed levels of the two groups involved in the comparison. In this study, the p value was adjusted by the method of Benjamini-Hochberg. Bold p values indicate <0.05.
AN, anxious depression subtype; AT, atypical depression subtype; hsCRP, high-sensitivity C reactive protein; IL, interleukin; M, melancholic depression subtype; TNF-α, tumour necrosis factor-α.
Inflammatory markers at baseline and remission at follow-up
Data for the levels of the inflammatory proteins at baseline and remission at follow-up are summarised in table 2 and online supplemental table 3. None of the inflammatory biomarkers statistically differed in the three general subtypes at the follow-up at 8 or 12 weeks (online supplemental table 2). However, in diverse subtypes, IL-6 and TNF-α significantly differed between remission and non-remission patients (table 2 and online supplemental table 3). Specifically, at the 8-week follow-up, the levels of IL-6 in all patients without remission were significantly higher in patients with an atypical subtype than in patients with melancholic (p=0.018) or anxious subtypes (p=0.016). For patients with atypical subtype depression, the levels of IL-6 (p=0.001, adj. p=0.006) and TNF-α (p=0.011, adj. p=0.038) differed between patients with and without remission, respectively, even after adjustment using the B-H method. At the 12-week follow-up, the level of IL-6 in patients without remission showed a higher trend (increased by 4.639-fold) than in patients with remission in the atypical subtype; however, it was not significant after adjustment (table 2). There was no difference in the levels of other inflammatory markers in specific subtypes or for patients with or without remission.
Discussion
Main findings
This study is the first study to explore and evaluate the expression levels of inflammatory markers in patients with melancholic, atypical and anxious depression. It is also the first study to investigate the relationship between baseline clinical symptoms, baseline levels of inflammatory markers and remission status at follow-up. Our results do not fully support our hypothesis. On the contrary, they generally refuted our hypothesis; except for hsCRP, TNF-α and IL-6 did not differ between the MDD and HC groups at baseline, nor was there any significant difference in the three different MDD subtypes after adjustment. Further, blood levels of inflammatory markers at baseline could not indicate the clinical status of patients at follow-up. However, at the 8-week follow-up, IL-6 and TNF-α levels were significantly higher in non-remitted patients with atypical depression than in remitted patients. The IL-6 level of non-remitted patients with atypical depression was significantly higher than that of non-remitted patients among the melancholic and anxious subtypes. These findings partially support some previous findings that MDD implies elevated inflammation, but we also found the converse was not true: inflammation does not predict MDD.25 Although we overcame the shortcomings of previous studies by grouping patients according to clinical symptom subtypes, we did not observe that the concentration of inflammatory proteins is related to the severity of depressive symptoms.20 In other words, a correlation between the level of inflammatory markers at baseline and clinical remission at follow-up is lacking, suggesting that inflammation may not be a risk factor for the onset of MDD and other related mood disorders, but it is a manifestation of disease onset. These findings are consistent with our results of no differences in TNF-α, IL-6 or hsCRP adjusted between HCs and patients with MDD and its three subtypes (online supplemental table 1).
However, our results did not support the notion that baseline inflammation levels indicate clinical remission at follow-up, consistent with another report.21 However, we observed that at the follow-up of 8 and 12 weeks, IL-6 and TNF-α levels were significantly lower in patients with immediate remission than in those without remission; this phenomenon was detected only in patients with atypical depression. Further, another study assessed serum IL-6, TNF-α and hsCRP levels and observed no difference in hsCRP but some differences in CRP, IL-6 and cortisol concentrations between patients with MDD and HCs, as well as between patients with atypical and melancholic depression.9 However, patients with anxious depression were not included in that study; moreover, the participants’ average age (26–68 years) was older, and the sample size was smaller (32 with melancholic depression characteristics, 23 with atypical characteristics and 18 HCs) than ours. Additionally, the classification basis of depression subtypes (relying only on HRSD-17 and MINI evaluation) and the detection of inflammatory factors (immunoturbidimetry and the enzyme-linked immunosorbent assay (ELISA)) were also different from our methods, potentially contributing to the inconsistency with our observations. In this study, we adopted the antibody array technology that is more sensitive and specific than the conventional ELISA and capable of detecting multiple proteins simultaneously with more precise measurements.26 Another sizeable prospective cohort study with a long-term follow-up of approximately 5.5 years from Switzerland21 also indicated no difference in the baseline TNF-α, IL-6 and hsCRP between patients with atypical and melancholic depression and two other subtypes of depression (a combination of atypical and depression, and unspecified), but there were differences in hsCRP during the 5-year follow-up. However, that study did not include anxious depression. It is well known that higher levels of IL-6 can promote the secretion of hsCRP and inhibit the secretion of TNF-α, resulting in an inverse relationship between IL-6 and TNF-α,27 which may be consistent with our findings that follow-up remission status was significantly associated with only IL-6 and TNF-α. The results also imply that endogenous immune-inflammatory changes in atypical depression might not be consistent with melancholic and anxious-type depression.
Surprisingly, our results revealed that the expression levels of inflammatory factors associated with pain and appetite symptoms varied significantly among the different depression subtypes. For patients in the anxious depression group, even after adjustment, the hsCRP levels of those with severe pain were significantly lower than for those with no pain or mild to moderate pain. In addition, the hsCRP levels of those with severe pain with anxious depression were lower than those with severe pain in the atypical depression group, suggesting that hsCRP may play an essential role in pain symptoms, especially for patients with anxious depression. This finding implies that hsCRP may be an important marker of pain symptoms. In our study, hsCRP showed lower levels for patients with anxious depression and severe pain than those in the same subtype with mild to moderate pain or no pain (table 2). This result seems inconsistent with common sense and may be due to the following reasons. First, this study’s relatively small sample size may result in some bias. Second, depressed patients’ subjective feelings of pain may differ from objective measures of pain, especially during depressive episodes. For example, patients with anxious depression may be more sensitive to feelings of pain than those with melancholic depression. Third, previous studies proved that depressive episodes were associated with higher levels of inflammation, while the reverse situation may not draw the same conclusion. In a sense, hsCRP tends to be more of an indicator of acute inflammation than a long-term chronic inflammation indicator during depressive episodes. Besides, it is known that CRP may be a manifestation of a broader metabolic syndrome, especially in patients who currently have multiple, significant disease symptoms. Patients with the anxious depression subtype tend to have more somatic comorbidities28 and a wider range of physiological stress responses,29 leading to more severe HPA axis activation and oxidative stress, which may be related to anxious depression.21 In addition, pain, like loneliness, is an uncomfortable physical and mental experience and is one of the most common physical symptoms of a depressive disorder, which has a proven relationship to CRP.30 Therefore, our study is a reminder to pay more attention to clinical symptoms of pain in both clinical practice and research. In addition, our findings also demonstrated that TNF-α is associated with appetite symptoms in patients with general MDD, especially for those with melancholic depression. However, in patients with atypical depression, appetite change symptoms were not significantly associated with the three inflammatory proteins, suggesting that the same symptom changes in the three different depression subtypes may be related to other potential biological factors. In future studies, it may be necessary to classify the subtypes of depressive disorders in combination with multiple factors, such as clinical symptoms and neurobiology. This multidimensional classification method may be more specific than classification based on symptom clusters alone.
Strengths and limitations
This study has both strengths and limitations. First, three subtypes of MMD depression were studied simultaneously, and the subtypes considered were relatively comprehensive. This approach was especially informative, given that the expression levels of inflammatory factors in patients with anxious depression have been rarely reported. Second, the enrolled patients were either experiencing their first onset of MDD or had relapsed MDD without drug treatment for at least 6 months, avoiding the confounding effect of drug treatment on the expression levels of inflammatory proteins. Finally, we also investigated the relationship between physical symptoms such as appetite, pain and gastrointestinal symptoms and the expression level of inflammatory factors, as well as their relationship with the remission state during the follow-up period.
However, this study had some unavoidable limitations. First, this study reported the levels of inflammatory factors only at baseline, not during the follow-up period, which may partially miss the dynamic relationship between MDD and inflammatory markers over time. Second, this study included only hsCRP and two proinflammatory cytokines, IL-6 and TNF-α, excluding other proinflammatory and anti-inflammatory cytokines and potential inflammatory biomarkers such as cortisol, leucocytes and platelet cells. Thus, these results may not fully reflect the changes in inflammatory cytokines. Third, the classification of depression subtypes in this study was based on similar clinical characteristics. Other sources of heterogeneity, such as biochemical markers, genetic variation and brain region activity/connectivity, should also be considered for a more rigorous segmentation. Fourth, although we strictly controlled several factors that may affect the differences in inflammatory proteins, such as age, sex, and BMI, other factors may have affected the expression of inflammatory proteins, such as blood lipid levels and living habits, such as smoking, drinking and exercise frequency. Fifth, the expression of some endogenous factors that may affect the levels of inflammatory factors, such as brain-derived neurotrophic factor, leptin and other energy-related proteins, was not evaluated. This should be further explored in future research. Sixth, since our inclusion criteria required a total 17-HDRS score of ≥17, that determined that our study included only patients with moderate or severe depression, excluding those with mild depression. Patients with comorbid mental and physical disorders were also excluded. Therefore, our findings can reflect only the three pure depression subtypes of patients and may not be generalised to the entire population of patients with MDD. Finally, the sample size of this study was relatively small; to better investigate the mechanism of inflammatory factor changes underlying different depression subtypes, studies with larger sample sizes are needed in the future.
Implications
In conclusion, melancholic, atypical and anxious depression subtypes differed in hsCRP levels, and patients with different pain and appetite symptoms displayed significant diversity in TNF-α and hsCRP levels in the melancholic and anxious depression groups at baseline. There was a significant difference in IL-6 and TNF-α among patients with differing remitted statuses and in patients with melancholic, atypical and anxious depression. This study provides evidence of differences in inflammatory proteins among melancholic, atypical and anxious subtypes of depression. Further studies on the immunoinflammatory mechanism underlying different subtypes of depression are expected for improved individualised therapy.
Biography
Hongmei Liu is an assistant research fellow who graduated from Fudan University, China in 2009 with a master’s degree in biochemistry and molecular biology. In September 2017, she was admitted to Shanghai Jiao Tong University School of Medicine in China to pursue her PhD. She is mainly engaged in clinical and basic research on major depressive and bipolar disorders. Her work has been supported by the youth program of the Natural Science Foundation of China project (NSFC), the medical guidance science and technology support program of the Shanghai Science and Technology Commission, and the youth program of the Shanghai Science and Technology Commission, etc. She has published more than ten papers as first author, nine of which were included in the Science Citation Index Expanded.

Footnotes
HL and XW contributed equally.
Contributors: HL conceived and designed the study and wrote the draft of the manuscript. XW contributed to the statistical analysis and helped to revise the manuscript. YWa, XL and DP managed the procedure of the study, controlled the quality of the study and trained all investigators. YWu, JC, YS, JX, XM, YL, JS, XY and HR enrolled the participants. MDF and YF finalised the manuscript. YF is responsible for the overall content as the guarantor. All authors read and approved the final version of the manuscript.
Funding: This study was funded by Key Projects of Clinical Research Center of Shanghai Mental Health Center (grant number CRC2018ZD02), key supporting projects of Clinical Research Center of Shanghai Mental Health Center (grant number SHDC2020CR6023), Research and Development Program of China (grant number 2016YFC1307100), Shanghai Key Project of Science and Technology (grant number 2018SHZDZX05) and Natural Science Foundation of China (grant number 81771465, 81801338 and 81930033).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the institutional review board of Shanghai Mental Health Center (2017-07R) and by the ethics committees of all participating centres. Written informed consent was obtained from all participants in accordance with the Helsinki Declaration of 1975 (as revised in 1983), following a thorough description of the assessments.
References
- 1. Park S-C, Kim Y-K. Challenges and strategies for current classifications of depressive disorders: proposal for future diagnostic standards. In: Kim Y-K, ed. Major depressive disorder: rethinking and understanding recent discoveries. Singapore: Springer Singapore, 2021: 103–16. [DOI] [PubMed] [Google Scholar]
- 2. Simon GE, Perlis RH. Personalized medicine for depression: can we match patients with treatments? Am J Psychiatry 2010;167:1445–55. 10.1176/appi.ajp.2010.09111680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merikangas KR, Wicki W, Angst J. Heterogeneity of depression. classification of depressive subtypes by longitudinal course. Br J Psychiatry 1994;164:342–8. 10.1192/bjp.164.3.342 [DOI] [PubMed] [Google Scholar]
- 4. Beijers L, Wardenaar KJ, van Loo HM, et al. Data-driven biological subtypes of depression: systematic review of biological approaches to depression subtyping. Mol Psychiatry 2019;24:888–900. 10.1038/s41380-019-0385-5 [DOI] [PubMed] [Google Scholar]
- 5. van Loo HM, de Jonge P, Romeijn J-W, et al. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med 2012;10:156. 10.1186/1741-7015-10-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fava M, Rush AJ, Alpert JE, et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry 2008;165:342–51. 10.1176/appi.ajp.2007.06111868 [DOI] [PubMed] [Google Scholar]
- 7. Ionescu DF, Nugent AC, Luckenbaugh DA, et al. Baseline working memory activation deficits in dimensional anxious depression as detected by magnetoencephalography. Acta Neuropsychiatr 2015;27:143–52. 10.1017/neu.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glaus J, Vandeleur CL, von Känel R, et al. Associations between mood, anxiety or substance use disorders and inflammatory markers after adjustment for multiple covariates in a population-based study. J Psychiatr Res 2014;58:36–45. 10.1016/j.jpsychires.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 9. Karlović D, Serretti A, Vrkić N, et al. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res 2012;198:74–80. 10.1016/j.psychres.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 10. Spanemberg L, Caldieraro M, Arrua Vares E, et al. Biological differences between melancholic and nonmelancholic depression subtyped by the core measure. Neuropsychiatr Dis Treat 2014;10:1523–31. 10.2147/NDT.S66504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamers F, Vogelzangs N, Merikangas KR, et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 2013;18:692–9. 10.1038/mp.2012.144 [DOI] [PubMed] [Google Scholar]
- 12. Simmons WK, Burrows K, Avery JA, et al. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol Psychiatry 2020;25:1457–68. 10.1038/s41380-018-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang C, Tiemessen KM, Bosker FJ, et al. Interleukin, tumor necrosis factor-α and C-reactive protein profiles in melancholic and non-melancholic depression: a systematic review. J Psychosom Res 2018;111:58–68. 10.1016/j.jpsychores.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 14. Veltman EM, Lamers F, Comijs HC, et al. Inflammatory markers and cortisol parameters across depressive subtypes in an older cohort. J Affect Disord 2018;234:54–8. 10.1016/j.jad.2018.02.080 [DOI] [PubMed] [Google Scholar]
- 15. Zimmerman M, Clark H, McGonigal P, et al. Reliability and validity of the DSM-5 anxious distress specifier interview. Compr Psychiatry 2017;76:11–17. 10.1016/j.comppsych.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 16. Shim IH, Woo YS, Bahk W-M. Associations between immune activation and the current severity of the "with anxious distress" specifier in patients with depressive disorders. Gen Hosp Psychiatry 2016;42:27–31. 10.1016/j.genhosppsych.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 17. Baek JH, Kim H-J, Fava M, et al. Reduced venous blood basophil count and anxious depression in patients with major depressive disorder. Psychiatry Investig 2016;13:321–6. 10.4306/pi.2016.13.3.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menke A, Lehrieder D, Fietz J, et al. Childhood trauma dependent anxious depression sensitizes HPA axis function. Psychoneuroendocrinology 2018;98:22–9. 10.1016/j.psyneuen.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 19. Gaspersz R, Lamers F, Wittenberg G, et al. The role of anxious distress in immune dysregulation in patients with major depressive disorder. Transl Psychiatry 2017;7:1268. 10.1038/s41398-017-0016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karlović D, Serretti A, Vrkić N, et al. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res 2012;198:74–80. 10.1016/j.psychres.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 21. Glaus J, von Känel R, Lasserre AM, et al. Mood disorders and circulating levels of inflammatory markers in a longitudinal population-based study. Psychol Med 2018;48:961–73. 10.1017/S0033291717002744 [DOI] [PubMed] [Google Scholar]
- 22. Liu X, Wang Y, Peng D, et al. The developmental and translational study on biomarkers and clinical characteristics-based diagnostic and therapeutic identification of major depressive disorder: study protocol for a multicenter randomized controlled trial in China. Neuropsychiatr Dis Treat 2020;16:2343–51. 10.2147/NDT.S271842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnow BA, Blasey C, Williams LM, et al. Depression subtypes in predicting antidepressant response: a report from the iSPOT-D trial. Am J Psychiatry 2015;172:743–50. 10.1176/appi.ajp.2015.14020181 [DOI] [PubMed] [Google Scholar]
- 24. Stewart JW, McGrath PJ, Fava M, et al. Do atypical features affect outcome in depressed outpatients treated with citalopram? Int J Neuropsychopharmacol 2010;13:15–30. 10.1017/S1461145709000182 [DOI] [PubMed] [Google Scholar]
- 25. Duivis HE, de Jonge P, Penninx BW, et al. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: prospective findings from the heart and soul study. Am J Psychiatry 2011;168:913–20. 10.1176/appi.ajp.2011.10081163 [DOI] [PubMed] [Google Scholar]
- 26. Huang W, Luo S, Burgess R, et al. New insights into the tumor microenvironment utilizing protein array technology. Int J Mol Sci 2018;19:559. 10.3390/ijms19020559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodney T, Osier N, Gill J. Pro- and anti-inflammatory biomarkers and traumatic brain injury outcomes: a review. Cytokine 2018;110:248–56. 10.1016/j.cyto.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 28. Chaturvedi SK, Manche Gowda S, Ahmed HU, et al. More anxious than depressed: prevalence and correlates in a 15-nation study of anxiety disorders in people with type 2 diabetes mellitus. Gen Psychiatr 2019;32:e100076. 10.1136/gpsych-2019-100076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohammed AA, Moustafa HA, Nour-Eldein H, et al. Association of anxiety-depressive disorders with irritable bowel syndrome among patients attending a rural family practice center: a comparative cross-sectional study. Gen Psychiatr 2021;34:e100553. 10.1136/gpsych-2021-100553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loeffler A, Steptoe A. Bidirectional longitudinal associations between loneliness and pain, and the role of inflammation. Pain 2021;162:930–7. 10.1097/j.pain.0000000000002082 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2022-100844supp001.pdf (207.6KB, pdf)
Data Availability Statement
No data are available.