Abstract
Introduction
The pathophysiology of Charcot neuroarthropathy (CN) remains unclear. There are a number of hypotheses but these are not exclusive. In its clinical presentation, this complication intersects with the semiology of diabetic-induced neuropathy, such as peripheral hypervascularization and the appearance of arteriovenous shunt. The EPICHAR study is as yet an unpublished cohort of people living with diabetes complicated by CN (in active or chronic phase). Based on the findings of the EPICHAR study, this study aimed to investigate whether a reduction in the rate of hyperglycemia accompanies the onset of an active phase of CN.
Research design and methods
Hemoglobin A1c (HbA1c) levels were assessed 3 months (M3) and 6 months (M6) before the diagnosis of active CN (M0).
Results
103 patients living with diabetes and presenting active CN were included between January and December 2019 from the 31 centers participating in this study (30 in France and 1 in Belgium). The mean age of the participants was 60.2±12.2 years; the vast majority were men (71.8%) living with type 2 diabetes (75.5%). Mean HbA1c levels significantly declined between M6 (median 7.70; Q1, Q3: 7.00, 8.55) and M3 (median 7.65; Q1, Q3: 6.90, 8.50) (p=0.012), as well as between M6 and M0 (median 7.40; Q1, Q3: 6.50, 8.50) (p=0.014). No significant difference was found between M3 and M0 (p=0.072).
Conclusions
A significant reduction in HbA1c levels seems to accompany the onset of the active phase of CN.
Trial registration number
NCM03744039.
Keywords: neuroarthropathy, diagnosis, HbA1c, diabetes complications
WHAT IS ALREADY KNOWN ON THIS TOPIC
Rapid correction of hyperglycaemia has previously been described in the form of treatment-induced neuropathy. However, little is known about the impact of this correction on the development of neuroarthropathy.
WHAT THIS STUDY ADDS
The significant correction of hyperglycaemia based on the onset of neuroarthropathy seems to be the factor inducing inflammation, which is described as a central element in the mosaic of neuroarthropathy pathophysiology.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This link may help us to understand this complication and reduce its prevalence.
Introduction
Neuroarthropathy or Charcot neuroarthropathy (CN) consists of acute osteoarticular destruction in the context of neuropathy, most commonly in the foot but also in the knee in some cases.1 The physiopathology of this disorder is poorly understood and the knowledge of its causality remains at the theoretical stage. The most common explanation is inflammatory arthropathy, according to which CN occurs as an increased inflammatory response to a lesion inducing increased bone lysis, with the involvement of bone remodelling factors,2 especially the receptor activator of nuclear factor B ligand (RANKL) and its natural antagonist osteoprotegerin (OPG). Many studies have confirmed abnormalities in the balance of the RANK/OPG system during the development of CN.3 A recent study examined in a retrospective manner the evolution of glycemic control in patients who have developed an active form of CN and demonstrated a significant drop in hemoglobin A1c (HbA1c) level in the near period of presentation of CN.4 We therefore wanted to know if the same reduction in the rate of HbA1c appears in cases of active CN presented through the descriptive multicentric study ‘EPICHAR’ handled at diabetic foot centers in France and Belgium, the main objective of which is to map patients living with Charcot’s foot (ie, identification of patients with acute and chronic Charcot and the diagnostics and therapeutic methods used).
Study objectives
The primary objective of our study is to investigate if rapid correction of HbA1c levels is accompanied by onset of the active phase of CN. The primary assessment criterion is the comparison of HbA1c levels 6 months (M6) before the diagnosis of the active phase of CN (M0). The secondary objective is to determine whether HbA1c levels rapidly fell between M6 and M3 (3 months) before the diagnosis of the active phase of CN, as well as between M3 and M0. The secondary assessment criterion is the dosage of HbA1c levels between M6 and M3 and between M3 and M0.
Methods
EPICHAR is a prospective, multicenter, observational study conducted from January 1 to December 31, 2019. Thirty-one diabetic foot centers (30 in France and 1 in Belgium) took part in the study. The study population consisted of all people aged ≥18 years living with diabetes who were admitted to hospital or consulted for acute or chronic CN, with or without foot ulcers. Unless the patient was participating in another interventional study or refused to participate, no exclusion criteria were used. No follow-up was required in the protocol. For the active phase, the diagnosis of CN was retained if the clinical examination showed a joint with an inflamed appearance and a temperature that was 3°C higher than the contralateral joint. This was validated by MRI.
In this ancillary evaluation, we analyzed the HbA1c levels (measured by high-performance liquid chromatography) of participants with diabetes and acute CN who had been referred to one of the participating diabetic foot centers during the study period. For each patient living with diabetes who was admitted to one of the participating centers and presenting with active CN (diagnosis performed by MRI and clinical examination), HbA1c levels were assessed (M0). The HbA1c values 3 and 6 months before the discovery of CN (respectively, M3 and M6) were then cross-checked via the patient’s medical file or by contacting his or her family physician.
Data are presented as median and IQR. To compare numerical values, statistical analysis was performed with non-parametric Wilcoxon signed-rank tests. Pairwise comparisons between time points were performed using the Bonferroni-Holm method for p value adjustment. The Benjamini and Hochberg false discovery rate method was used to adjust for multiple comparisons. Categorical variables were compared using the Fisher’s exact test. Linear mixed-effect regression models were applied to investigate the evolution of repeated glycated hemoglobin measures and their association with diabetes type. The fixed-effect variable was diabetes type and the random-effect was the individual subject. Influence and residual diagnostics were performed to ascertain whether all the assumptions of the mixed-effects regression models were met in the analyses. An unstructured covariance was chosen. All statistical analyses were performed using the Statistical Analysis System. This study applied the MIXED procedure with fixed and random effects in SAS V.9.3 to implement the linear mixed-effects regression models. This is an ancillary evaluation of the EPICHAR cohort (unpublished results).
Results
There were 467 people recruited in the EPICHAR study. In brief, 26.55% of the participants were women. The mean age of the population was 61.97 (SD 11.45). Of the patients, 17.13% had type 1 diabetes, 79.66% had type 2 diabetes, and 3% had other types of diabetes. Of the patients, 21.62% had bilateral Charcot foot. Only 103 participants presented an active phase of CN. All individuals with missing HbA1c levels at the three time points were excluded. The demographic characteristics of the participants and their body mass index, diabetes type and duration, and any diabetes-related complications are presented in table 1. The HbA1c levels and values of the patients at M0, M3, and M6, expressed in percentages (DCCT/NGSP), are summarized in table 2.
Table 1.
Demographic data and diabetes-related complications of participants with active CN
| Acute CN | |
| Age, mean years±IC (S1) (A2) | 60.2±12.2 |
| Men, n (%) | 74 (71.8) |
| Body mass index (kg/m²), median (IQR) | 30.11 (25.74–33.14) |
| Missing data, n | 4 |
| Type of diabetes, n (%) | |
| Type 1 | 22 (21.6) |
| Type 2 | 77 (75.5) |
| Other | 3 (2.9) |
| Missing data, n | 1 |
| Diabetes duration, years, n (%) | |
| >20 | 41 (40.2) |
| 10–20 | 43 (42.2) |
| 5–10 | 10 (9.8) |
| <5 | 8 (7.8) |
| Missing data, n | 1 |
| Insulin use, n (%) | 69 (67.6) |
| Missing data, n | 1 |
| Microangiopathy, n (%) | 96 (94.1) |
| Missing data, n | 1 |
| Dialysis, n (%) | 4 (10.3) |
| Missing data, n | 64 |
| GFR (mL/min/1.73 m2), median (IQR) | 64 (46.5–90) |
| Missing data, n | 71 |
| Macroangiopathy, n (%) | 30 (31.3) |
| Missing data, n | 7 |
| DFU history (grade 3 IWGDF), n (%) | 63 (61.8) |
| Missing data, n | 1 |
CN, Charcot neuroarthropathy.
Table 2.
Hb1Ac levels and the results for active Charcot neuroarthropathy cases in the EPICHAR study
| Variable | Type 2 diabetes | Type 1 | Type 2 | Total | P value |
| Age | n | 22 | 77 | 99 | 0.0014 |
| Median | 54.00 | 63.00 | 61.00 | ||
| Q1, Q3 | 44.00, 60.00 | 55.00, 71.00 | 54.00, 68.00 | ||
| Min, max | 30.00, 68.00 | 40.00, 90.00 | 30.00, 90.00 | ||
| HbA1c_t0 | n | 15 | 60 | 75 | 0.4342 |
| Median | 7.50 | 7.30 | 7.40 | ||
| Q1, Q3 | 6.60, 8.70 | 6.40, 8.35 | 6.50, 8.50 | ||
| Min, max | 6.20, 9.70 | 5.00, 12.10 | 5.00, 12.10 | ||
| HbA1c_m_3 | n | 9 | 41 | 50 | |
| Median | 7.80 | 7.50 | 7.65 | 0.2943 | |
| Q1, Q3 | 7.60, 8.40 | 6.90, 8.70 | 6.90, 8.50 | ||
| Min, max | 7.30, 9.10 | 5.20, 11.90 | 5.20, 11.90 | ||
| HbA1c_m_6 | n | 14 | 30 | 44 | |
| Median | 8.05 | 7.60 | 7.70 | 0.2461 | |
| Q1, Q3 | 7.20, 9.40 | 6.80, 8.40 | 7.00, 8.55 | ||
| Min, max | 6.40, 13.00 | 6.00, 12.00 | 6.00, 13.00 |
HbA1c, hemoglobin A1c; max, maximum; min, minimum.
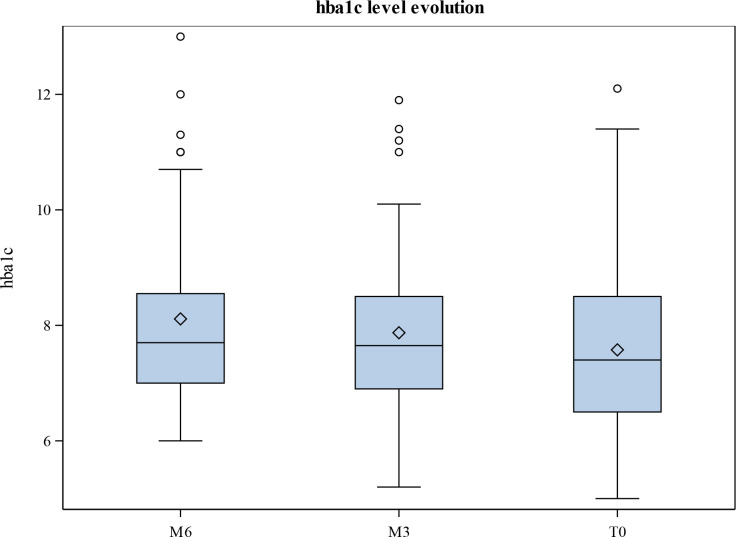
In the total study sample, the paired Wilcoxon test showed a significant difference between M6 (median 7.70; Q1, Q3: 7.00, 8.55) and M3 (median 7.65; Q1, Q3: 6.90, 8.50) (p=0.012), with mean HbA1c levels significantly falling from 62 mmol/mol (7.8%±1.62) at M6 to 58 mmol/mol (7.45%±1.42) at M0. The paired Wilcoxon test likewise showed a significant difference between M6 and M0 (median 7.40; Q1, Q3: 6.50, 8.50) (p=0.014), with mean HbA1c levels decreasing from 62 mmol/mol (7.8%±1.62) at M6 to 60 mmol/mol (7.67%±1.48) at M3. However, the reduction in HbA1c levels was not significant between M3 and M0 as they decreased only slightly from 60 mmol/mol (7.67%±1.48) at M3 to 58 mmol/mol (7.45%±1.42) (p=0.072) at M0 (table 3 and figure 1).
Table 3.
Pairwise comparison of HbA1c levels for all diabetes types and for each group
| Diabetes type | Time 1 | Time 2 | Mean difference | SE | P value | Adjusted p value |
| All | HbA1c_t0 | HbA1c_m_3 | −0.228 | 0.134 | 0.072 | 0.072 |
| All | HbA1c_m_3 | HbA1c_m_6 | −0.627 | 0.234 | 0.012 | 0.021 |
| All | HbA1c_t0 | HbA1c_m_6 | −0.608 | 0.232 | 0.014 | 0.021 |
| Type 1 | HbA1c_t0 | HbA1c_m_3 | −0.325 | 0.384 | 0.844 | – |
| Type 1 | HbA1c_m_3 | HbA1c_m_6 | −0.786 | 0.552 | 0.297 | – |
| Type 1 | HbA1c_t0 | HbA1c_m_6 | −0.667 | 0.485 | 0.210 | – |
| Type 2 | HbA1c_t0 | HbA1c_m_3 | −0.206 | 0.143 | 0.065 | 0.065 |
| Type 2 | HbA1c_m_3 | HbA1c_m_6 | −0.578 | 0.262 | 0.029 | 0.060 |
| Type 2 | HbA1c_t0 | HbA1c_m_6 | −0.579 | 0.258 | 0.04 | 0.060 |
HbA1c, hemoglobin A1c.
Figure 1.
Mean hemoglobin A1c (HbA1c) levels (mmol/mol) and their evolution at month 6 (M6), month 3 (M3), and month 0 (M0).
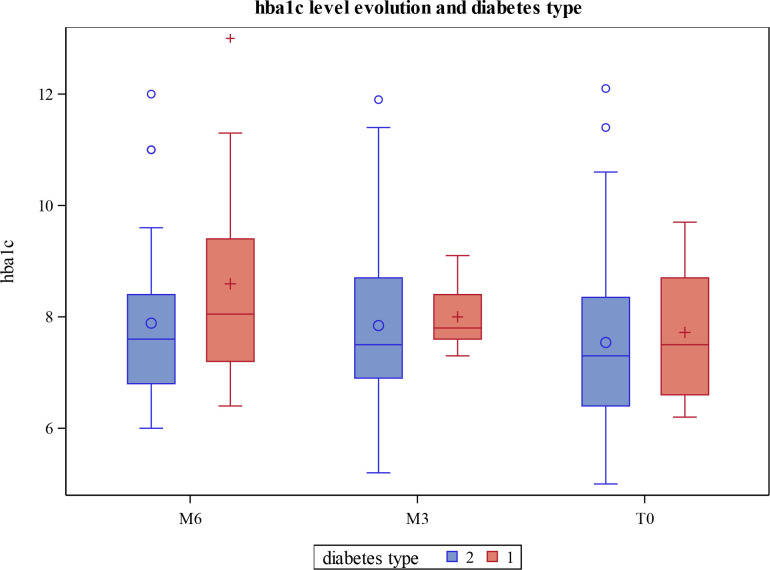
We studied the evolution of HbA1c levels according to diabetes type (mixed model). A similar trend in the evolution of HbA1c was found (figure 2). There was a significant main effect of time between M6 and M0 and between M6 and M3. However, there were no significant main effects for diabetes type. It is important to note that the results of HbA1c adjusted and confounded for age, sex, body mass index, and duration of diabetes did not impact the significance of the reduction in HbA1c (table 4).
Figure 2.
Mean hemoglobin A1c (HbA1c) levels (mmol/mol) and their evolution at month 6 (M6), month 3 (M3), and month 0 (M0) according to diabetes type.
Table 4.
HbA1c values adjusted for selected confounders
| Estimate | SE | Inferior | Superior | P value | ||
| Intercept | 11.2374 | 1.3134 | 8.6223 | 13.8525 | <0.0001 | |
| HbA1c (temporality) | M3 | 0.1876 | 0.1683 | −0.1469 | 0.5222 | 0.268 |
| M6 | 0.6137 | 0.1789 | 0.2581 | 0.9692 | 0.0009 | |
| M0 | (ref) | (ref) | (ref) | (ref) | (ref) | |
| Diabetes type | 1 | −0.3871 | 0.4516 | −1.2878 | 0.5136 | 0.3942 |
| 2 | (ref) | (ref) | (ref) | (ref) | (ref) | |
| Age | −0.05798 | 0.01355 | −0.08496 | −0.031 | <0.0001 | |
| Sex | Female | −0.09947 | 0.3303 | −0.7583 | 0.5594 | 0.7642 |
| Male | (ref) | (ref) | (ref) | (ref) | (ref) | |
| BMI | −0.01083 | 0.02981 | −0.07028 | 0.04862 | 0.7174 | |
| Age of diabetes | ≥20 | 0.5098 | 0.4354 | −0.3579 | 1.3776 | 0.2454 |
| 10–20 | 0.4165 | 0.4161 | −0.4129 | 1.246 | 0.3202 | |
| <10 | (ref) | (ref) | (ref) | (ref) | (ref) |
BMI, body mass index; HbA1c, hemoglobin A1c; M3, month 3; M6, month 6; ref, reference.
Discussion
CN generally evolves in two phases: (1) acute and (2) chronic.5 6 The typical clinical picture of active CN is a red swollen joint with a temperature difference greater than 2°C compared with the unaffected joint. These symptoms may go unnoticed because the pain may be absent or disproportionate depending on the presence or absence of lesions on the foot.7 The pathogenic mechanisms of CN have been subject to a long-running debate with several diverging theories. The inflammatory arthropathy theory described by Jeffcoate2 is the most common theory to explain the development of active CN. A new series of experiments were recently carried out to assess the evolution of bone modelling factors in the appearance of CN by associating RANKL and OPG.3 However, this explanation does not address the link between the appearance of the active phase of CN and the rapid correction of HbA1c levels. The latter element is increasingly present in the pathophysiology of CN, with several cases showing the onset of active CN after the rapid correction of HbA1c levels, as in the context of a double pancreatic kidney transplant8 or significant weight loss after bariatric surgery.9
Our study included 103 people with acute CN. This represents a large sample size for a rare disorder, as its frequency varies between 0.1% and 0.4% of people with diabetes.10 Nevertheless, aggressive antidiabetic therapy and rapid glycemic control may result in diabetic neuropathy, also known as treatment-induced neuropathy of diabetes (TIND).11 It is noteworthy that in the clinical presentation of active CN and TIND, a common symptom is hypervascularization in the extremities.
Taking the above elements into account, we may legitimately consider that the rapid and significant correction of HbA1c levels may accompany the onset of the active phase of CN. An interesting evaluation12 demonstrated that the RANKL antagonist OPG is inhibited by hyperglycemia correction, which may explain the elevated levels of RANKL observed during the active phase of CN.3 Indeed, high RANKL levels will be linked to the inhibition of its antagonist following decrease in HbA1c levels. It is important to mention that osteoblast,2 the cell described as the main actor in the pathophysiology of CN, has insulin receptors on its membranes. We may therefore suppose that the sensitivity of these cells to insulin changes in the event of a significant reduction in HbA1c levels. Therefore, the rapid correction of hyperglycemia seems to be the inflammation-inducing factor, which is described by Jeffcoate2 as a core component in the mosaic of the CN pathophysiology.
In conclusion, the link between the onset of active CN and a significant reduction in HbA1c levels is once again brought to light in this paper. This description can help to understand the physiopathology of CN and potentially to implement monitoring measures, screening, and support for patients living with neuropathy-complicated diabetes who intend to rapidly correct their HbA1c levels.
Acknowledgments
The authors acknowledge the Diabetic Foot Group of the French Society of Diabetology for their support.
Footnotes
Contributors: DD, SS, C-AJ, GHV, JM'B, MB, AS, ML, SG-V, FB, MF, SC, and JM conceived of the presented idea. DD, JV, PM, MMo, AM, VC-M, VR, ID, LK, IS, BB, PB, AL, EDCC, MMBF, MMu, MC, MZZ, and AH developed the theoretical formalism, performed the analytic calculations, and performed the numerical simulations.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. According to French law (Decree No 2016-1872 of December 26, 2016), a file was submitted to INDS (Institut National des Données de Santé) and CEREES (Comité d’Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé) for approval. The EPICHAR study received agreement from the ethical committee for the protection of patients (Barboin hospital authorization number: 18.09.01). Participants gave informed consent to participate in the study before taking part.
References
- 1.Lambert AP, Close CF. Knee neuroarthropathy in a patient with type I diabetes. Diabet Med 1998;15:S12. [Google Scholar]
- 2.Jeffcoate WJ. Theories concerning the pathogenesis of the acute Charcot foot suggest future therapy. Curr Diab Rep 2005;5:430–5. 10.1007/s11892-005-0050-z [DOI] [PubMed] [Google Scholar]
- 3.Mabilleau G, Petrova NL, Edmonds ME, et al. Increased osteoclastic activity in acute Charcot’s osteoarthopathy: the role of receptor activator of nuclear factor-kappaB ligand. Diabetologia 2008;51:1035–40. 10.1007/s00125-008-0992-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dardari D, Van GH, M'Bemba J, et al. Rapid glycemic regulation in poorly controlled patients living with diabetes, a new associated factor in the pathophysiology of Charcot's acute neuroarthropathy. PLoS One 2020;15:e0233168. 10.1371/journal.pone.0233168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edmonds ME. Progress in care of the diabetic foot. Lancet 1999;354:270–2. 10.1016/S0140-6736(99)90012-0 [DOI] [PubMed] [Google Scholar]
- 6.Chantelau EA, Grützner G. Is the Eichenholtz classification still valid for the diabetic Charcot foot? Swiss Med Wkly 2014;144:w13948. 10.4414/smw.2014.13948 [DOI] [PubMed] [Google Scholar]
- 7.Petrova NL, Edmonds ME. Acute Charcot neuro-osteoarthropathy. Diabetes Metab Res Rev 2016;32 Suppl 1:281–6. 10.1002/dmrr.2734 [DOI] [PubMed] [Google Scholar]
- 8.Rangel Érika B, Sá JR, Gomes SA, et al. Charcot neuroarthropathy after simultaneous pancreas-kidney transplant. Transplantation 2012;94:642–5. 10.1097/TP.0b013e31825cadbb [DOI] [PubMed] [Google Scholar]
- 9.Murchison R, Gooday C, Dhatariya K. The development of a Charcot foot after significant weight loss in people with diabetes: three cautionary tales. J Am Podiatr Med Assoc 2014;104:522–5. 10.7547/0003-0538-104.5.522 [DOI] [PubMed] [Google Scholar]
- 10.Sinha S, Munichoodappa CS, Kozak GP. Neuro-arthropathy (Charcot joints) in diabetes mellitus (clinical study of 101 cases). Medicine 1972;51:191–210. 10.1097/00005792-197205000-00006 [DOI] [PubMed] [Google Scholar]
- 11.Gibbons CH. Treatment-Induced neuropathy of diabetes. Curr Diab Rep 2017;17:127. 10.1007/s11892-017-0960-6 [DOI] [PubMed] [Google Scholar]
- 12.Xiang G-da, Sun H-ling, Zhao L-shuang, et al. [Changes of osteoprotegerin before and after insulin therapy in type 1 diabetic patients]. Zhonghua Yi Xue Za Zhi 2007;87:199–206. 10.1016/j.diabres.2006.09.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.