Abstract
The tra gene of Streptomyces lividans plasmid pIJ101 encodes a 621-amino-acid protein that can mediate both plasmid transfer and the interbacterial transfer of chromosomal genes (i.e., chromosome-mobilizing ability [Cma]) during mating. Here we report the results of in-frame insertional mutagenesis studies aimed at defining regions of Tra required for these functions. While hexameric linker insertions throughout the tra gene affected plasmid and chromosomal gene transfer, insertions in a 200-amino-acid region of the Tra protein that contains presumed nucleotide-binding motifs and that is widely conserved among a functionally diverse family of bacterial and plasmid proteins (K. J. Begg, S. J. Dewar, and W. D. Donachie, J. Bacteriol. 177:6211–6222, 1995) had especially prominent effects on both functions. Insertions near the N terminus of Tra reduced Cma for either circular or linear host chromosomes to a much greater extent than pIJ101 plasmid transfer. Our results suggest that Cma involves Tra functions incremental to those needed for plasmid DNA transfer.
During growth in their natural soil habitat or on agar media, bacteria of the gram-positive genus Streptomyces proceed through a morphologically and physiologically complex developmental program (6). Following germination of spores, cells filament and grow vegetatively as a multinucleate substrate mycelium. Upon nutrient limitation, growth within the substratum ceases, initiating a developmental process that involves the emergence of branching airborne hyphae as well as the production of secondary metabolites. Eventually, aerial hyphal growth slows and individual hyphae develop further into spore chains.
During a portion of their life cycle, Streptomyces bacteria are able to interact conjugally to promote the transfer of DNA. Plasmids, which are prevalent throughout Streptomyces species, play an essential role in the ability of these bacteria to efficiently transfer both chromosomal and plasmid genes (10). On agar plates, transfer of conjugative plasmids between streptomycete cells from individual donors to a surrounding recipient lawn produces circular regions known as pocks, which consist of transconjugant cells showing transiently slowed growth (4). Upon replica plating onto agar selective for the plasmid, transconjugants grow at normal rates in regions that approximate the original pocks (4, 14).
A distinguishing feature of conjugation in Streptomyces is that few plasmid genes are required for this process. For example, the high-copy-number circular plasmid pIJ101, which was originally isolated from Streptomyces lividans but which has since been found to have a broad host range among streptomycetes, carries a single gene (tra) that enables the efficient intermycelial transfer of pIJ101 to up to 100% of potential recipients during mating, while also promoting the movement of chromosomal genes between mating cells such that up to 1% of all cells following mating are recombinants (12, 14). The latter phenotype is commonly referred to as chromosome-mobilizing ability (Cma) (8). An additional cis-acting plasmid element, clt, which is requisite for efficient pIJ101 transfer, was shown to be dispensable for Cma associated with tra, a result that raises the possibility that the Tra-mediated processes of pIJ101 transfer and chromosome mobilization occur by distinct mechanisms (22).
While insertions into either the tra gene or the clt locus eliminate pocking and plasmid transfer, insertions into three other genes, spdA, spdB, and kilB, reduce pock size and thus plasmid “spread” but have little or no effect on either plasmid transfer or Cma (10). Because of their mutant phenotype, spdA, spdB, and kilB have been proposed to be involved in the intramycelial movement of plasmids within recipient hyphae (10, 14).
The tra gene product (Tra), which was predicted from sequence analysis to be a 621-amino-acid protein (11), is present as a 70-kDa component in membrane fractions of S. lividans cells (21). Although its function remains to be determined, Tra, like transfer proteins of other Streptomyces plasmids, contains a Walker type A motif (11), as well as a less-conserved version of the Walker type B motif (5, 15) for presumptive ATP binding. Alteration of amino acid positions within these same domains of the TraB protein of Streptomyces nigrifaciens plasmid pSN22 demonstrated that such sequences are vital for the conjugal function of this protein (15). Energy production via binding and subsequent hydrolysis of ATP is believed to be an integral feature of multiple proteins involved in the processing and translocation of DNA across cell membranes during conjugation in other bacteria (7).
The ATP binding motifs of the pIJ101 Tra protein as well as those of most other essential transfer proteins of Streptomyces plasmids occur within a larger conserved region of amino acids that is shared by proteins from both gram-negative and gram-positive sources; included in this group are, interestingly, proteins that appear to direct the movement of double-stranded DNA molecules across cellular membranes (3, 28). This intriguing conservation of sequences thus raises the possibility that conjugation in Streptomyces may not occur by the interbacterial movement of single-stranded DNA, as is characteristic for conjugation in other bacteria (25), but rather potentially may occur by a double-stranded DNA transfer mechanism.
As a first step towards elucidating the precise role of the pIJ101 Tra protein in intermycelial DNA transfer in Streptomyces, we have used in-frame insertional mutagenesis to define regions of the protein that are important for conjugation. Two insertions in particular, which are located within the conserved portion of Tra but at sites other than the nucleotide-binding folds, greatly reduced or eliminated both plasmid transfer and Cma activities and therefore appear to define additional functional motifs that are common to all proteins sharing these sequences. Other amino acid insertions towards the N terminus of Tra greatly reduced or eliminated Tra's Cma function while leaving the protein still capable of promoting plasmid transfer at significant frequencies; these insertions may thus identify Tra domains that are devoted solely to the task of chromosome mobilization.
MATERIALS AND METHODS
Bacterial strains, plasmids, and bacteriological methods.
S. lividans TK23 (spc-1) and TK64 (str-6 pro-2) have been described previously (9), while S. lividans ZX7 (13) is a better-sporulating derivative of ZX1 (str-6 pro-2 rec-46) (30). S. lividans YSC27-14 is derived from strain ZX7 and contains a circularized chromosome (16). Escherichia coli strain BRL2288 (Life Technologies Inc., Gaithersburg, Md.) was the host for cloning. Plasmids used in this study are described in Table 1. Transformation procedures for S. lividans and E. coli (9) were as described elsewhere. S. lividans was grown in liquid YEME (yeast extract-malt extract) medium (9) or on solid media, including Luria-Bertani agar (23), R5 medium, R2 medium containing 0.1% yeast extract, and minimal medium (9). Plasmid DNA was isolated using the DNA-binding columns and other reagents from Qiagen (Santa Clara, Calif.) either as described by the manufacturer for E. coli or as modified for Streptomyces (D. F. Brolle, T. Henzler, S. Stefani, A. Weissenborn, D. Zell, and W. Wohlleben, Qiagen News 5:9–10, 1997).
TABLE 1.
Plasmids used in this study
| Plasmid | Descriptiona | Reference or source |
|---|---|---|
| pGSP218 | 2.2-kb BglII-PvuII fragment of pIJ101 cloned into pSP72 | This study |
| pGSP218-L13 | EcoRI linker inserted after nt 5723 | This study |
| pGSP218-L58 | EcoRI linker inserted after nt 5589 | This study |
| pGSP218-L115 | EcoRI linker inserted after nt 5417 | This study |
| pGSP218-L263 | EcoRI linker inserted after nt 4974 | This study |
| pGSP218-L340 | EcoRI linker inserted after nt 4742 | This study |
| pGSP218-L388 | EcoRI linker inserted after nt 4597 | This study |
| pGSP218-L437 | EcoRI linker inserted after nt 4451 | This study |
| pGSP218-L531 | Double EcoRI linker inserted after nt 4172 | This study |
| pGSP218-L577 | Double EcoRI linker inserted after nt 4031 | This study |
| pGSP196 | Tsrr transfer-defective derivative of pIJ101 | 22 |
| pSP72 | Ampr cloning vector | Promega |
| pSP72X | pSP72 deleted for all sites in polylinker except XhoI | This study |
| pHYG3 | Hygr wild-type pIJ101 derivative | 22 |
| pHYG3-L13 | pHYG3 with L13 insertion | This study |
| pHYG3-L58 | pHYG3 with L58 insertion | This study |
| pHYG3-L115 | pHYG3 with L115 insertion | This study |
| pHYG3-L263 | pHYG3 with L263 insertion | This study |
| pHYG3-L340 | pHYG3 with L340 insertion | This study |
| pHYG3-L388 | pHYG3 with L388 insertion | This study |
| pHYG3-L437 | pHYG3 with L437 insertion | This study |
| pHYG3-L531 | pHYG3 with L531 insertion | This study |
| pHYG3-L577 | pHYG3 with L577 insertion | This study |
| pHYG3-L595 | pHYG3 with L595 insertion | This study |
| pSP72X:pHYG3 | pHYG3 with pSP72X at XhoI | This study |
| pSP72X:pHYG3-L13 | pSP72X:pHYG3 with L13 insertion | This study |
| pSP72X:pHYG3-L58 | pSP72X:pHYG3 with L58 insertion | This study |
| pSP72X:pHYG3-L115 | pSP72X:pHYG3 with L115 insertion | This study |
| pSP72X:pHYG3-L263 | pSP72X:pHYG3 with L263 insertion | This study |
| pSP72X:pHYG3-L340 | pSP72X:pHYG3 with L340 insertion | This study |
| pSP72X:pHYG3-L388 | pSP72X:pHYG3 with L388 insertion | This study |
| pSP72X:pHYG3-L437 | pSP72X:pHYG3 with L437 insertion | This study |
| pSP72X:pHYG3-L531 | pSP72X:pHYG3 with L531 insertion | This study |
| pSP72X:pHYG3-L577 | pSP72X:pHYG3 with L577 insertion | This study |
| pSP72X:pHYG3-L595 | pSP72X:pHYG3 with EcoRI linker inserted after nt 3977 | This study |
| pIJ303 | Tsrr wild-type pIJ101 derivative | 14 |
| pIJ303-K22 | pUC19 inserted into spdB on pIJ303 | 12 |
| pHYG3-K22 | K22 pUC19 insertion on pHYG3 | This study |
| pUC19 | Ampr cloning vector | 29 |
Hygr, Tsrr, and Ampr, resistance to hygromycin, thiostrepton, and ampicillin, respectively. nt, nucleotide. Positions are according to the sequence of Kendall and Cohen (11).
Molecular biology and genetic techniques.
Cloning using standard molecular techniques was performed as described previously (23). Nucleotide sequencing using relevant primers was performed manually using α-35S-dATP and Sequenase, version 2.0 (U. S. Biochemicals, Cleveland, Ohio), or by automated sequencing on a model 310 genetic analyzer (PE Applied Biosystems Inc., Foster City, Calif.).
Replica plate testing for plasmid transmission was based on the method of Kosono et al. (15). Here, spores of TK23 containing individual pHYG3 linker derivatives were pipetted in a thin line onto a lawn of TK64 spores present on R2 containing 0.1% yeast extract plates. Upon sporulation, spores were replica plated to the same agar containing 200 μg of hygromycin and 50 μg of streptomycin/ml, and, following incubation for 42 h, the maximum width of growth from the original donor line was determined. The results shown are the averages from three independent assays.
Matings to determine the effects of linker insertions in the tra gene on plasmid transfer and Cma were performed as previously described (22) by mixing approximately equivalent numbers of spores of the two strains (i.e., TK23 containing pHYG3 linker insertion derivatives and TK64 containing pGSP196) and plating the mixtures on R2 medium containing 0.1% yeast extract. Following growth for one life cycle, donor (hygromycin-resistant), recipient (thiostrepton-resistant), and transconjugant (thiostrepton- and hygromycin-resistant) cell types for plasmid transfer and parental (one is spectinomycin-resistant, and the other is streptomycin-resistant and auxotrophic for proline biosynthesis) and recombinant (streptomycin-resistant and prototrophic) cell types for Cma were quantified as described previously (22) using antibiotics at the previously recommended (10) concentrations: hygromycin, 200 μg/ml; spectinomycin, 20 μg/ml; streptomycin, 10 μg/ml; thiostrepton, 50 μg/ml. Relative plasmid transfer was calculated as the average ratio of transconjugants to recipients for a given plasmid from at least four independent matings, which was then divided by the average ratio of transconjugants to recipients for pHYG3 and multiplied by 100%. Relative Cma was determined as the average ratio of recombinants to the sum of the parental values for a given plasmid (again from at least four independent matings), which was then divided by that same average ratio for pHYG3 and multiplied by 100%.
Matings involving S. lividans ZX7 or YSC27-14 (each containing pHYG3 linker derivatives) and TK23(pGSP196) were performed as described above, and Cma was determined as the average ratio of recombinants to the sum of the parental values for a particular plasmid, which was then divided by this same average for matings involving strain ZX7 containing pHYG3 and then multiplied by 100%. The numbers reported for each plasmid are based on two separate matings, where almost identical Cma results were obtained.
In-frame linker mutagenesis.
Two methods were used to obtain in-frame insertions of the sequence 5′-GAATTC into the pIJ101 tra gene. In the first method, which was based on the protocol of Barany (1, 2), the phosphorylated hexamer 5′-CGAATT was ligated to purified linear pGSP218 DNA that was generated following partial digestion with AhaII and treatment with calf intestinal phosphatase. Sites of insertion for pGSP218 plasmids containing linkers were identified by double digestions using EcoRI and another relevant enzyme. Clones containing linkers in the tra gene were digested to completion with EcoRI, and the purified linear plasmid DNA was religated to create clones that generally contained only a single linker insertion. The number of linkers present was then verified by nucleotide sequencing across the site of insertion. To construct linker-containing pHYG3 plasmids, the 1.8-kb BglII-PvuII fragment of pSP72X:pHYG3 was replaced with the same fragment derived from pGSP218 containing a linker insertion within tra. Digestion of resulting clones with XhoI, followed by ligation and transformation of S. lividans TK23, allowed for isolation of pHYG3 plasmids that contained the various linker insertions.
In the second method, pGSP218 was either partially digested with NaeI (for L115 and L531) or digested to completion with BalI (for L388) or pSP72X:pHYG3 was digested to completion with PvuII (for L595) and, following dephosphorylation, these molecules were ligated to the phosphorylated dodecamer 5′-GAATTCGAATTC. Clones possessing EcoRI sites were digested to completion with EcoRI and then religated as in the first method in order to create pGSP218 or pSP72X:pHYG3 derivatives with single 5′-GAATTC hexameric insertions (the L531 insertion still retained its original sequence despite this EcoRI digestion step). Following nucleotide sequencing across the site of insertion, construction of pHYG3 derivatives containing these linkers was achieved using the relevant steps outlined in the first mutagenesis method.
RESULTS AND DISCUSSION
Effects of amino acid insertions on Tra-dependent conjugal activities.
To identify regions of the pIJ101 Tra protein that are important for conjugation, we added primarily two codons using in-frame insertion of linkers (see Materials and Methods) at individual positions throughout the tra gene. These derivatives of the conjugative pIJ101-derived plasmid pHYG3 were then tested for conjugal activities dependent on proper Tra protein function. Combined plasmid spread and transfer were tested by a previously described replica plate assay (15) in which donor spores were seeded along defined lines within a lawn of recipient spores and, following growth for one life cycle, the distance that plasmids had been transmitted outward from the original donor line was determined by replica plating of spores to plates selective for transconjugant cells. The effects of linker insertions on plasmid transfer alone and on Cma were assessed following assays of mating between large, approximately equivalent numbers of S. lividans strain TK23 cells containing each of the linker-containing pHYG3 plasmids and a suitable mating partner (i.e., S. lividans TK64 containing the nonconjugative plasmid pGSP196) (22).
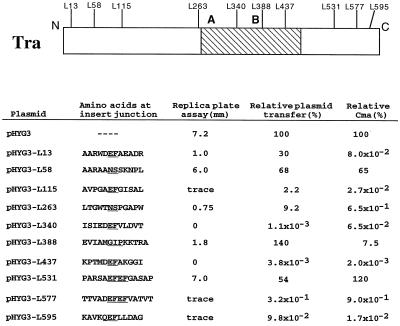
Beginning with the introduction of amino acids towards the C terminus of Tra (Fig. 1), the L577 insertion (linkers are named after their insertion position; for example, L577 is inserted after codon 577 of the tra gene) and the L595 insertion both significantly reduced all Tra-associated conjugal activities. In replica plate assays (Fig. 1), only trace amounts of transconjugant growth were seen around the outer edge of the original donor line for plasmids pHYG3-L577 and pHYG3-L595, which was a substantial reduction in transmission from the 7.2-mm distance seen for the parental plasmid. Likewise, in mating assays, transfer of pHYG3-L577 and pHYG3-L595 was reduced relative to that of the parental plasmid by more than 300-fold and more than 1,000-fold, respectively, while Cma was also reduced by more than 100-fold and nearly 6,000-fold, respectively (Fig. 1).
FIG. 1.
Positions and conjugative effects of amino acid insertions in the pIJ101 Tra protein. Linkers were inserted at individual positions throughout the pIJ101 tra gene (see Materials and Methods), which resulted in the addition of two to four amino acids at the corresponding positions shown (denoted by the linker numbers) in the Tra protein. Hatched area, conserved region of approximately 200 amino acids that shows homology to a family of proteins from both gram-positive and gram-negative sources (see Results and Discussion). Within the conserved region, the positions of sequences that resemble Walker type A and type B nucleotide-binding folds (5, 11, 15) are indicated. The amino acid sequence at each insert junction is shown using one-letter designations, and inserted amino acids are underlined. Insertion of L388 resulted in the loss of the alanine residue at position 389 of Tra (11). Replica plate assays and matings to determine plasmid transfer and Cma capabilities of each of the indicated plasmids were performed and quantified as described in Materials and Methods. Numbers for the replica plate assay are the averages from three independent experiments, while those for plasmid transfer and Cma are averages from at least four independent matings. “Trace” growth in the replica plate test was <0.5 mm and was patchy (i.e., not continuous around the perimeter of the donor line).
In contrast to the results obtained for the L577 and L595 pHYG3 derivatives, pHYG3-L531 demonstrated no significant reduction in transmission of the plasmid in replica plate assays and showed plasmid transfer and Cma rates that were similar to those of the parental plasmid (Fig. 1). Taken together, these results suggest that C-terminal sequences beginning somewhere beyond the L531 insertion point and including (at least) those sequences disrupted by the L577 and L595 insertions are important for normal Tra function in both plasmid transfer and Cma.
Towards the middle of the Tra protein (Fig. 1) is a region of approximately 200 amino acids that shows strong similarity to those of other essential transfer proteins of Streptomyces plasmids, as well as those of proteins from bacterial sources other than Streptomyces that participate in cellular events seemingly related in some unknown manner to the process of streptomycete conjugation (3, 28). The latter group includes SpoIIIE proteins from several gram-positive bacteria (19, 20, 27), including Bacillus subtilis, in which it was demonstrated that SpoIIIE is required for intracellular transfer of one chromosomal copy across the fully formed or nearly fully formed prespore septum during sporulation (27, 28); also included in this group is the FtsK protein of E. coli, which is required for, among other activities, proper resolution and thus partitioning of daughter chromosomes during cell division (18, 24).
Tra activity was largely or entirely eliminated upon introduction of amino acids at certain locations within this conserved region. For example, insertion of L340, which adds 2 residues to the 74-amino-acid stretch that separates the Walker type A motif from the less-conserved type B motif in the Tra protein (11, 15), eliminated transmission in the replica plate assay and severely reduced pHYG3 plasmid transfer and Cma in matings by over 90,000-fold and over 1,500-fold, respectively (Fig. 1). From its position, L340 may disrupt as yet unidentified functional sequences that are located between the putative ATP-binding domains. The extra amino acids in the L340 derivative of Tra are located 21 positions away (in the direction of the C terminus) from a second sequence (i.e., LVVVD) that shows some similarity to the consensus Walker type B motif (11); however, the functional importance of this sequence, which may be suboptimally spaced relative to the type A motif (15), is unknown.
Like the L340 insertion, the L437 insertion, which introduced amino acids near the right end of the conserved region as shown in Fig. 1, dramatically reduced or eliminated all Tra-dependent conjugal activities. No transconjugants were seen in replica plate assays, and, in mating assays, plasmid transfer was reduced by over 26,000-fold while Cma showed a 50,000-fold reduction (Fig. 1). The L437 insertion is located within a particularly well-conserved stretch of approximately 50 amino acids (i.e., positions 413 to 464 of Tra) (11), which begins 30 residues C terminal to the Walker type B motif (15) and which may therefore represent additional functional domains that are important for this family of proteins.
It was also possible to insert amino acids within or near the conserved region that had much less significant effects on overall Tra function. L388, which introduces amino acids five positions following the putative Walker type B motif in Tra (11, 15), reduced plasmid transmission in replica plate assays to 1.8 mm (Fig. 1); interestingly, this distance was very similar to the result obtained for pHGY3-K22 (1.4 mm), a pHYG3 derivative that contains a pUC19 insertion in the spdB gene of pIJ101, which previously resulted in the production of small pocks (12). In matings, transfer of pHYG3-L388 still occurred at frequencies at least as high as those for pHYG3 (a result also similar to that observed previously for pIJ101 spd mutants) (10), while pHYG3-L388 was reduced for Cma by about 13-fold (Fig. 1).
Our finding that the replica plate assay and transfer assay results for pHYG3-L388 phenotypically resemble those of a pIJ101 spd mutant raises the possibility that Tra itself may participate in the proposed intramycelial spread of plasmids and that the L388 insertion therefore affects sequences that are critical for Tra's function in plasmid spread; alternatively, L388 may only moderately reduce Tra's effectiveness in promoting intermycelial plasmid transfer, yielding a defect that is evident when plasmids are transmitted through presumably multiple rounds of transfer from single donor cells outward to a surrounding recipient lawn (as in the replica plate test) but not when plasmids are transferred in a single round between equivalent numbers of donors and recipients (as in the mating assay). A role in plasmid spread has also been proposed recently for the transfer-essential TraB protein of S. nigrifaciens plasmid pSN22 (15).
One additional insertion near the conserved region that was also of less functional consequence than either L340 and L437 was L263, which introduced amino acids 26 residues prior to the Walker type A motif (11). L263 reduced transmission of pHYG3 to 0.75 mm in the replica plate assay and lowered plasmid transfer and Cma in matings by 11-fold and about 150-fold, respectively (Fig. 1).
Perhaps the most intriguing insertion results involved the addition of amino acids at certain positions towards the N terminus of Tra (Fig. 1), since such additions showed dramatically greater effects on Tra-associated Cma than on plasmid transfer. For example, although the L13 insertion reduced transmission of the plasmid to 1 mm in replica plate assays, pHYG3-L13 still transferred in matings at frequencies only threefold lower than those for the parental plasmid (Fig. 1); however, Cma in matings involving pHYG3-L13 was reduced by over 1,200-fold (Fig. 1). Similarly, while the limited pHYG3-L115 transmission observed in the replica plate assay correlated with transfer frequencies during matings that were within 50-fold of the rate observed for pHYG3, Cma was reduced by the L115 insertion by approximately 3,700-fold (Fig. 1). In contrast to the other insertions towards the N terminus of Tra, L58 only slightly reduced transmission in replica plate assays, while transfer and Cma values in matings involving pHYG3-L58 remained comparable to those for the parental plasmid (Fig. 1).
Matings between plasmidless versions of S. lividans strains TK23 and TK64 have previously yielded Cma rates as high as nearly 4.0 × 10−2% of the number seen when conjugative pIJ101 plasmids were present (22), and such rare formation of apparent recombinants in the absence of conjugative plasmids has been speculated to arise solely through reverse mutation of one or the other parent (10) rather than by conjugative transfer and subsequent recombination of chromosomal markers. Therefore, based on their Cma values (Fig. 1), the L13 and L115 Tra derivatives (as well as the L340, L437, and L595 Tra mutants) probably retain little or no actual Cma function; given that the L13 and L115 Tra derivatives still show significant plasmid transfer capability (Fig. 1), our results, particularly for L13, thus implicate N-terminal sequences of Tra as providing a chromosome mobilization function that is not essential for efficient plasmid transfer.
In E. coli, nicking of a single strand of F plasmid DNA within the oriT region and translocation of the nicked strand through the cell membrane result in transfer of either autonomous F plasmids or, in cells where F exists in a chromosomally integrated state, in transfer of additional chromosomal sequences (26); in other words, the same mechanism directs both the transfer of the F plasmid and F-related Cma. Our results for the L13 and L115 Tra mutants, coupled with previous data demonstrating that the pIJ101 clt locus is required for efficient plasmid transfer but is dispensable for Tra-dependent Cma (22), raise the possibility that fundamental differences in the mechanisms by which Tra mediates plasmid transfer and chromosome mobilization in Streptomyces may exist.
Cma deficiencies of L13 and L115 Tra derivatives for linear versus circular host chromosomes.
How might the L13 and L115 insertions specifically reduce the ability of the Tra protein to promote Cma? The precise role of Tra in promoting Cma is still unknown, as is the overall mechanism of how chromosomes are mobilized during streptomycete conjugation. However, given that the chromosomes of many Streptomyces species including S. lividans have been shown to be linear (17), we wondered whether these insertions may somehow differentially reduce Tra's ability to promote the transfer of linear DNA (e.g., the chromosome of TK23) while leaving its function in the transfer of circular DNA (e.g., pIJ101) relatively intact. To test this notion, we took advantage of recently constructed strains of S. lividans that contain stable, artificially circularized chromosomes (16, 17). pHYG3 plasmids containing relevant linker insertions in tra were introduced into strain YSC27-14, which contains a circularized chromosome (16), and into its parental strain, ZX7, whose chromosome is in the natural linear configuration (17), and the efficiencies of Cma following matings of these strains with a suitable partner (i.e., S. lividans TK23 containing plasmid pGSP196) (22) were determined.
If the L13 and L115 linkers indeed diminish Tra's capacity to promote transfer of linear chromosomal DNA, then Cma in matings involving strain ZX7 should be much lower than that seen for matings involving strain YSC27-14. Instead we found that, while Cma mediated by the parental pHYG3 plasmid occurred at comparable rates during matings involving either ZX7 or YSC27-14, Cma values for pHYG3 derivatives containing L13 or L115 were actually lower (i.e., by 300-fold and 6-fold, respectively) when the host strain contained a circular (i.e., YSC27-14) rather than a linear (i.e., ZX7) chromosome (Table 2). The basis for decreased Cma by these linker derivatives when present in strain YSC27-14 compared to that when they are present in the ZX7 host is currently unknown, as is the reason why both plasmid transfer (data not shown) and Cma rates for strain ZX7 containing either the L13 or L115 pHYG3 derivative were noticeably higher than those seen for strain TK23 containing these plasmids (Fig. 1). Strain ZX7 is an S. lividans mutant that contains a large chromosomal deletion that affects multiple and unrelated phenotypes, including DNA modification and phage resistance (30, 31); genes that affect the frequency of conjugal DNA transfer may also be affected by the ZX7 deletion. This speculation aside, our data nevertheless suggest that the reduced capacity of the L13 and L115 mutant Tra proteins to effectively promote Cma is related to some still-undetermined, intrinsic feature of the S. lividans chromosome other than its linearity per se.
TABLE 2.
Cma for pHYG3 linker derivatives in matings involving S. lividans strains with circular versus linear chromosomesa
| Plasmid | Chromosome configuration | Relative Cma (%) |
|---|---|---|
| pHYG3 | Linear | 100 |
| Circular | 220 | |
| pHYG3-L13 | Linear | 1.3 |
| Circular | 4.3 × 10−3 | |
| pHYG3-L115 | Linear | 3.1 × 10−1 |
| Circular | 5.1 × 10−2 |
Matings between either S. lividans ZX7 (linear chromosome) or YSC27-14 (circular chromosome), in which each contained the indicated plasmids, and strain TK23(pGSP196) were performed and quantified for Cma as described in Materials and Methods. Numbers represent the averages for two independent matings, in which nearly identical results were obtained.
ACKNOWLEDGMENTS
This work was supported by grant MCB-9604879 from the National Science Foundation (to G.S.P.) and by NIAID grant AI08619 (to S.N.C). G.S.P. was supported during a preliminary portion of this work by Postdoctoral Fellowship PF-3401 from the American Cancer Society.
We are grateful to David Hopwood and Carton Chen for strains and to Fred A. Rainey for help with automated sequencing. We thank Christine Miller and John Battista for critical reading of the manuscript.
REFERENCES
- 1.Barany F. Single-stranded hexameric linkers: a system for in-phase insertion mutagenesis and protein engineering. Gene. 1985;37:111–123. doi: 10.1016/0378-1119(85)90263-x. [DOI] [PubMed] [Google Scholar]
- 2.Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci USA. 1985;82:4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begg K J, Dewar S J, Donachie W D. A new Escherichia coli cell division gene, ftsK. J Bacteriol. 1995;177:6211–6222. doi: 10.1128/jb.177.21.6211-6222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibb M J, Hopwood D A. Genetic studies of the fertility plasmid SCP2 and its SCP2* variants in Streptomyces coelicolor A3(2) J Gen Microbiol. 1981;126:427–442. [Google Scholar]
- 5.Brolle D-F, Pape H, Hopwood D A, Kieser T. Analysis of the transfer region of the Streptomyces plasmid SCP2*. Mol Microbiol. 1993;10:157–170. doi: 10.1111/j.1365-2958.1993.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 6.Chater K F. Genetics of differentiation in Streptomyces. Annu Rev Microbiol. 1993;47:685–713. doi: 10.1146/annurev.mi.47.100193.003345. [DOI] [PubMed] [Google Scholar]
- 7.Dreiseikelmann B. Translocation of DNA across bacterial membranes. Microbiol Rev. 1994;58:293–316. doi: 10.1128/mr.58.3.293-316.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holloway B W. Plasmids that mobilize bacterial chromosome. Plasmid. 1979;2:1–19. doi: 10.1016/0147-619x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- 9.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, England: John Innes Foundation; 1985. [Google Scholar]
- 10.Hopwood D A, Kieser T. Conjugative plasmids of Streptomyces. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 293–311. [Google Scholar]
- 11.Kendall K J, Cohen S N. Complete nucleotide sequence of the Streptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol. 1988;170:4634–4651. doi: 10.1128/jb.170.10.4634-4651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall K J, Cohen S N. Plasmid transfer in Streptomyces lividans: identification of a kil-kor system associated with the transfer region of pIJ101. J Bacteriol. 1987;169:4177–4183. doi: 10.1128/jb.169.9.4177-4183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieser H M, Kieser T, Hopwood D A. A combined genetic and physical map of the Streptomyces coelicolor A3(2) chromosome. J Bacteriol. 1992;174:5496–5507. doi: 10.1128/jb.174.17.5496-5507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kieser T, Hopwood D A, Wright H M, Thompson C J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185:223–238. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- 15.Kosono S, Kataoka M, Seki T, Yoshida T. The TraB protein, which mediates the intermycelial transfer of the Streptomyces plasmid pSN22, has functional NTP-binding motifs and is localized to the cytoplasmic membrane. Mol Microbiol. 1996;19:397–405. doi: 10.1046/j.1365-2958.1996.379909.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y-S, Chen C W. Instability of artificially circularized chromosomes of Streptomyces lividans. Mol Microbiol. 1997;26:709–719. doi: 10.1046/j.1365-2958.1997.5991975.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y-S, Kieser H M, Hopwood D A, Chen C W. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993;10:923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Draper G C, Donachie W D. FtsK is a bifunctional protein involved in cell division and chromosome localization in Escherichia coli. Mol Microbiol. 1998;29:893–903. doi: 10.1046/j.1365-2958.1998.00986.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller S, Pesci E C, Pickett C L. Genetic organization of the region upstream from the Campylobacter jejuni flagellar gene flhA. Gene. 1994;146:31–38. doi: 10.1016/0378-1119(94)90830-3. [DOI] [PubMed] [Google Scholar]
- 20.Oswald W, Krauss H, Thiele D. A sporulation gene of Coxiella burnetii. J Vet Med Sci. 1993;40:366–370. doi: 10.1111/j.1439-0450.1993.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 21.Pettis G S, Cohen S N. Plasmid transfer and expression of the transfer (tra) gene product of plasmid pIJ101 are temporally regulated during the Streptomyces lividans life cycle. Mol Microbiol. 1996;19:1127–1135. doi: 10.1046/j.1365-2958.1996.493986.x. [DOI] [PubMed] [Google Scholar]
- 22.Pettis G S, Cohen S N. Transfer of the pIJ101 plasmid in Streptomyces lividans requires a cis-acting function dispensable for chromosomal gene transfer. Mol Microbiol. 1994;13:955–964. doi: 10.1111/j.1365-2958.1994.tb00487.x. . (Corrigendum, 16:170, 1995.) [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Steiner W, Liu G, Donachie W D, Kuempel P. The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol. 1999;31:579–583. doi: 10.1046/j.1365-2958.1999.01198.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins B, Lanka E. DNA processing and replication during plasmid transfer between gram-negative bacteria. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 105–136. [Google Scholar]
- 26.Willetts N, Skurray R. Structure and function of the F factor and mechanism of conjugation. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1110–1133. [Google Scholar]
- 27.Wu L J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- 28.Wu L J, Lewis P J, Allmansberger R, Hauser P M, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]
- 29.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Deng Z, Firmin J L, Hopwood D A, Kieser T. Site-specific degradation of Streptomyces lividans DNA during electrophoresis in buffers contaminated with ferrous iron. Nucleic Acids Res. 1988;16:4341–4352. doi: 10.1093/nar/16.10.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Deng Z, Hopwood D A, Kieser T. Streptomyces lividans 66 contains a gene for phage resistance which is similar to the phage λ ea59 endonuclease gene. Mol Microbiol. 1994;12:789–797. doi: 10.1111/j.1365-2958.1994.tb01065.x. [DOI] [PubMed] [Google Scholar]