Abstract
Introduction
Globally, it is estimated that more than three-quarters of people with chronic hepatitis C virus (HCV) are unaware of their HCV status. HCV self-testing (HCVST) may improve access and uptake of HCV testing particularly among key populations such as people who inject drugs (PWID) and men who have sex with men (MSM) where HCV prevalence and incidence are high and barriers to accessing health services due to stigma and discrimination are common.
Methods and analysis
This randomised controlled trial compares an online programme offering oral fluid-based HCVST delivered to the home with referral to standard-of-care HCV testing at HCV testing sites. Eligible participants are adults self-identifying as either MSM or PWID who live in Tbilisi or Batumi, Georgia, and whose current HCV status is unknown. Participants will be recruited through an online platform and randomised to one of three arms for MSM (courier delivery, peer delivery and standard-of-care HCV testing (control)) and two for PWID (peer delivery and standard-of-care HCV testing (control)). Participants in the postal delivery group will receive an HCVST kit delivered by an anonymised courier. Participants in the peer delivery groups will schedule delivery of the HCVST by a peer. Control groups will receive information on how to access standard-of-care testing at a testing site. The primary outcome is the number and proportion of participants who report completion of testing. Secondary outcomes include the number and proportion of participants who (a) receive a positive result and are made aware of their status, (b) are referred to and complete HCV RNA confirmatory testing, and (c) start treatment. Acceptability, feasibility, and attitudes around HCV testing and cost will also be evaluated. The target sample size is 1250 participants (250 per arm).
Ethics and dissemination
Ethical approval has been obtained from the National Centers for Disease Control and Public Health Georgia Institutional Review Board (IRB) (IRB# 2021-049). Study results will be disseminated by presentations at conferences and via peer-reviewed journals. Protocol version 1.1; 14 July 2021.
Trial registration number
ClinicalTrials.gov Registry (NCT04961723).
Keywords: Public health, World Wide Web technology, Hepatology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This will be one of the first clinical trials to assess the impact of, and evidence on optimal service delivery options for, hepatitis C self-testing.
The randomised design allows for comparison of two different hepatitis C self-testing service delivery models compared with the standard of care.
The intervention group employing peer delivery of testing may generate some negative bias if participants wish to remain anonymous.
The control arm uptake rates may be more heavily affected by ongoing COVID-19 movement restrictions than the delivery arms.
The study will reach only people who have access to the internet; therefore, the results may not be generalisable to harder-to-reach populations/settings.
Introduction
The WHO estimates that 58 million people globally have chronic hepatitis C virus (HCV) infection.1 Of these, only 21% are diagnosed, with lack of awareness, poor access to testing services and stigma and discrimination surrounding HCV infection contributing to low uptake of HCV testing services.1 As evidenced by self-testing for HIV, the option to self-test at home can increase access to testing. As such, WHO recently published the first recommendations and guidance for HCV self-testing (HCVST), which highlights HCVST as an additional approach to HCV testing to reduce the gap in diagnosis.1 The recommendations are based on broad evidence with self-testing for HIV, as well as specific studies on HCVST performance, usability, acceptability and user values and preferences.2–6 A number of evidence gaps relating to HCVST remain, however, including a need for data on the impact of HCVST on uptake of HCV testing and linkage to care, the need for better understanding of optimal service delivery options for HCVST, and on the use of HCVST in key populations such as people who inject drugs (PWID) and men who have sex with men (MSM).
Georgia is a middle-income country with a high prevalence of chronic HCV infection (5.4%) in the adult population from a population-based serosurvey conducted in 2015,7 with the burden of infection largely within the PWID population (numbering over 52 250 in 2017).8 9 Prior to the implementation of a national elimination programme in 2015,7 8 the seroprevalence in PWID in Georgia ranged from 50% to 92%, depending on region.10–13 The programme has been successful in identifying and linking people with HCV to care,8 but gaps still remain in hard-to-reach key populations, and so a pilot HCVST programme has been initiated, based on an existing self-testing programme for HIV.14 Here we describe the protocol of a randomised controlled trial (Georgian Institutional Review Board (IRB) ethics approval number: IRB# 2021-049, ClinicalTrials.gov: NCT04961723) that aims to assess the impact and acceptability of an online programme offering home delivery of HCVST to PWID and MSM in Georgia.
Methods and analysis
Study settings and participants
This is a randomised controlled trial comparing home delivery of HCVSTs with referral to standard-of-care community-based HCV testing sites in PWID and MSM in Tbilisi or Batumi, Georgia. Six study HCV sites in Tbilisi and five in Batumi will participate as outlined in table 1.
Table 1.
Study sites
| Tbilisi | Batumi | |
| MSM peer delivery site and community testing site | Tbilisi Tanadgoma Centre | Batumi Tanadgoma Centre |
| MSM courier delivery site and community testing site | Tbilisi Equality Movement Centre | Batumi Identoba Centre |
| PWID peer delivery site and community testing site | ‘Tbilisi New Way’ Harm Reduction Site | ‘Batumi Imedi’ Harm Reduction Site |
| Hepatitis testing and treatment site | Infectious Diseases, AIDS and Clinical Immunology Research Center | Batumi Infectious Diseases Hospital |
| Hepatitis testing and treatment site | ‘Neo-Lab’ Clinic | Batumi Imedi Harm Reduction Site |
| Hepatitis testing and treatment site | ‘Hepa’ Clinic |
MSM, men who have sex with men; PWID, people who inject drugs.
Eligible participants are adults aged ≥18 years living in Tbilisi or Batumi who can access services on the online platform and who self-identify as a PWID or MSM. Participants must be able to read and understand Georgian and have unknown HCV status (defined as never tested for anti-HCV or most recent test for anti-HCV antibodies negative and performed ≥6 months prior to enrolment). People who have a self-reported previously confirmed anti-HCV positive status or who are ineligible for the Georgian National Hepatitis Elimination Programme (ie, do not have a Georgian ID card) will be excluded from the study.
Study participants will be prospectively recruited through an existing HIV self-testing online platform (http://selftest.ge), with community organisations and peers promoting the study. Interested participants will sign up to be contacted for study eligibility screening and to complete online informed consent. All study participants will complete a baseline survey collecting demographics and knowledge and attitudes towards HCV testing. Recruitment is expected to start in October 2021.
Study design
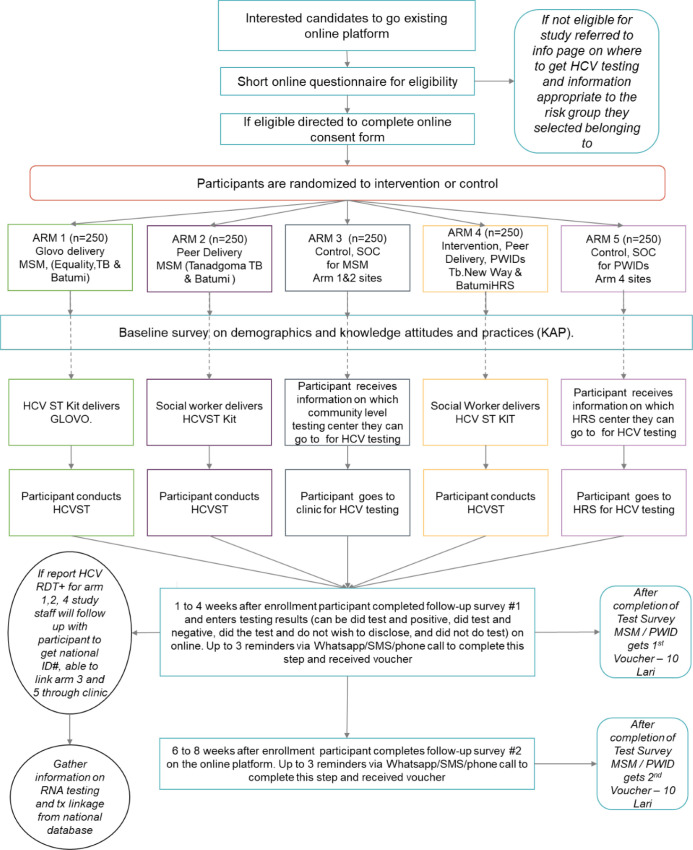
Eligible participants who primarily identify as MSM will be randomised separately from those who primarily identify as PWID (figure 1). Those who primarily identify as MSM will be randomised to one of the following study arms in a 1:1 ratio: (a) courier delivery, (b) peer delivery and (c) control. Participants in the courier delivery group will receive a home-delivered HCVST kit; this test kit package includes the self-test, instructions for use and supporting materials such as details on how to access to live chat and call centre for questions about testing. Participants in the peer delivery group will schedule delivery of the self-test to the location of their choice and instructions for use by a peer worker from the study site. The peer worker is a member of the community who has been trained to engage in HIV prevention services; this peer worker will provide basic information on the test, how to proceed after a positive result, and how to access live chat and call centre. Participants in the control arm will receive information about standard-of-care professionally administered HCV testing at one of the study sites. These participants will also have access to the live chat and call centre facilities. Participants who primarily identify as PWID will be randomised to either peer delivery or control in a 1:1 ratio.
Figure 1.
Study design. HCV, hepatitis C virus; HCVST, hepatitis C virus self-testing; HRS, harm reduction site; MSM, men who have sex with men; PWID, people who inject drugs; RDT+, rapid diagnostic test positive; SMS, Short Message Service; SOC, standard of care; TB, Tbilisi; tx, treatment.
Approximately 2–4 weeks after enrolment, each participant will complete a follow-up survey, which will include the opportunity to upload any test result (online supplemental annex 1). A second follow-up survey will be sent after the closure of the first survey (approximately 6–8 weeks after enrolment) (online supplemental annex 2). Up to three telephone reminders may be sent for each survey if a survey has not been completed. Participants will receive telephone credit (10 Georgian lari, equivalent to ~US$3) for completion of each survey.
bmjopen-2021-056243supp001.pdf (493.9KB, pdf)
Any individual reporting a positive HCVST will be referred to further HCV testing. Those confirmed to have active HCV infection will be linked to HCV treatment and care which is provided for free through the Georgian National Elimination Programme.
Participants may withdraw from the study at any time or be withdrawn at the discretion of the primary investigator. Participants will be considered lost to follow-up to the study if they fail to complete one of the online surveys after receiving three reminders.
Data collection
Participants will complete the baseline, the first and second follow-up surveys on the online platform (online supplemental annex 3). The baseline survey will assess participants’ current knowledge of hepatitis C including risk factors for contracting hepatitis C, as well as gathering information on their current risk-related behaviours.
The purpose of the follow-up surveys is to collect from the participant if they have completed the test, and if completed what the result of the test was, to collect information on risk behaviours to assess if any change in risk behaviours may have taken place during the study, and gather feedback on how the participants felt about the testing process.
The first follow-up survey will be given 2–4 weeks post-enrolment and will ask participants to report if they conducted the HCV test and if so, the results of the test. If the participant reports having taken the test, they will be asked to answer questions relating to their perception of the testing experience and the actions they took following the test. If the participant reports that they did not take the test, they will be asked questions as to why they have not yet taken the test. This survey will also gather information for all participants on their current behaviours that may be related to risk factors for HCV.
The second follow-up survey will be given 4–8 weeks post-enrolment (at least 2 weeks after completion of first survey), will ask the participants to report, if they have not already reported taking the test in the first follow-up survey, if they conducted the HCV test and if so, the results of the test. If the participant reports having taken the test, they will be asked to answer questions relating to their perception of the testing experience and the actions they took following the test. If the participant reports that they did not take the test, they will be asked questions as to why they have not yet taken the test. For those who reported taking the HCV test in the first follow-up survey, this survey will start by gathering information on what actions the person has since taken regarding seeking further HCV care (if their HCV test was positive). This survey will also gather information from all participants on their current behaviours that may be related to risk factors for HCV.
Strategies to improve adherence to interventions
Participants will be provided several supporting tools to minimise the rate of errors in the self-testing process and any possible confusion in interpretation of the test results. Printed instructions for use in Georgian will be delivered with the test kit and contain pictorial guides on how to use the test. In addition, participants will be provided a link to a video guide and have access to live chat and a call centre.
Randomisation and blinding
Prior to study enrolment, a list of study IDs in ascending numerical order for each key population (PWID or MSM) will be generated by an employee of the sponsor who will not be involved in the execution of the study. Study IDs will be randomised by use of an algorithm to a study arm. Enrolment and assignment of study IDs will take place via the online platform. Participants will be assigned via the online platform study IDs in a consecutive fashion, thereby completing assignment to a study group. Due to the nature of the study, there is no blinding as the study sites will know which participant received courier delivery, peer delivery or standard of care.
Interventions
The HCVST used in this study will be the OraQuick HCV Rapid Antibody Test (OraSure Technologies, Bethlehem, Pennsylvania, USA). This test is CE marked and has received WHO prequalification for professional use by healthcare workers. The test has been validated by the manufacturer for self-testing, but use as a self-test is currently for research use only, thus test results are not used for patient management. Instructions for use in Georgian were developed for previous studies and have been optimised based on feedback received.
Outcomes
The primary outcome of the study is the number and proportion of participants who report completion of testing in the postal or peer delivery arms. We hypothesise the intervention arms will show 20% more participants reporting completion of the testing result compared with the control arms (table 2).
Table 2.
Trial objectives, endpoints and statistical analysis methods
| Objectives | Endpoints | Statistical analysis methods |
| Primary | ||
| To assess the impact of HCV self-testing home delivery on HCV antibody testing rates in PWID and MSM | Number and point estimate of the proportion of participants who report completing the HCV antibody testing in the intervention groups. Superiority of the proportion of participants who report completing the HCV antibody testing in the intervention groups compared with the control groups (margin 20%) |
The primary outcome 1.2 will be evaluated in the MITT population (primary analysis) and will be repeated for the PP population. The difference pfo, I–pfo, C will be assessed in a one-sided test with a margin of 20% by applying the following hypothesis: Intervention types (arm 1, 2, 4) as well as the control groups (arm 3, 5) will be considered. The proportion of individuals reporting HCV completing the test in the following intervention and control groups will be compared (three comparisons):
|
| Secondary | ||
| To assess the impact of HCV self-testing on the number of HCV antibody-positive individuals who are aware of their status | Number and estimate of the proportion of HCV antibody-positive participants made aware of their status in the intervention vs control groups | The outcome (patient has a positive test result y/n) is defined overall (as primary analysis) and for visit 1 (as additional analysis). The proportion of test positives ppos will be calculated among all patients with test results (=favourable outcome) as well as among all MITT and PP patients. These proportions will be investigated in the comparison via hypothesis testing. |
| To assess the impact of HCV self-testing on linkage and completion of HCV RNA confirmatory testing in HCV antibody-positive individuals | Number and estimate of the proportion of HCV antibody-positive participants who are referred to and complete HCV RNA confirmatory testing in the intervention vs control groups | The outcome (patient is referred to and complete HCV RNA confirmatory testing: y/n) is defined overall (as primary analysis) and for visit 1 (as additional analysis). The proportion of patients referred pref will be calculated among all patients with positive test results as well as among all MITT and PP patients. These proportions will be investigated in the comparison via hypothesis testing. |
| To assess the impact of HCV self-testing on treatment initiation in HCV RNA-positive individuals eligible to start treatment | Number and estimate of the proportion of HCV RNA-positive participants who start treatment in the intervention vs control groups | Here the outcome (patient has started treatment y/n) is defined overall (as primary analysis) and for visit 1 (as additional analysis). The proportion of patients treated ptrt will be calculated among all patients with positive test results as well as among all MITT and PP patients. The comparisons will refer to proportion with number with patients with a positive test result in the denominators (a+b, f+g). |
| To assess the acceptability and feasibility of HCV self-testing at baseline and after study participation. Information about knowledge, attitudes and practices related to HCV and risk-taking behaviours may also be collected | Analysis of survey responses using proportions and means | The secondary outcome 2.4 will be evaluated for the PP and MITT population. Intervention types (arm 1, 2, 4) as well as the control groups (arm 3, 5) will be considered separately. Descriptive statistics for survey responses will be reported either in absolute numbers and proportions or summarised by mean, median, SD, minimum, maximum and quartiles by arm and visit. |
| To assess the cost of HCV self-testing | Cost per test completed, cost per person diagnosed (serology, RNA) in the intervention vs control groups | |
MITT: all participants in ITT who were randomised to HCV self-testing (arm 1–5). PP: all participants in ITT who fully complied with the protocol (ie, primary endpoint variable is available).
HCV, hepatitis C virus; MITT, modified intention-to-treat; MSM, men who have sex with men; PP, per-protocol; PWID, people who inject drugs.
Secondary outcomes include the number and proportion of HCV antibody-positive participants who are made aware of their HCV status, who are referred to and complete HCV RNA confirmatory testing, and who receive a positive HCV RNA result and start treatment, in each study arm (table 2). Acceptability and feasibility of HCVST, along with knowledge, attitudes, and practices around HCV testing and care, will be assessed by analysis of survey responses at baseline and post-testing. The cost of HCVST will be evaluated by comparing costs in the intervention arms versus the control arm.
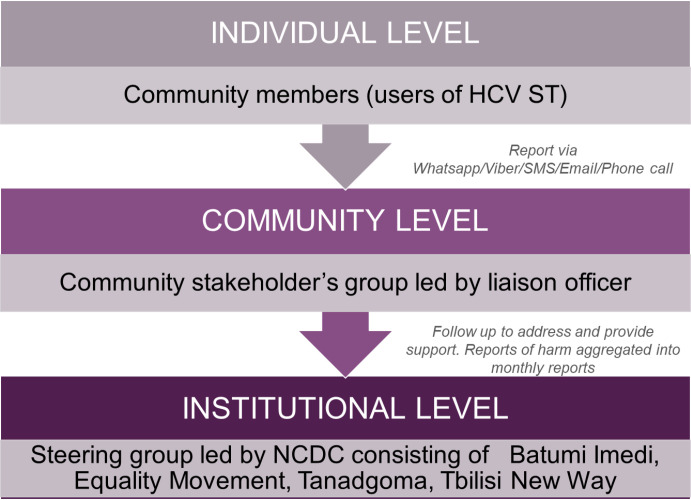
Safety analyses will not be performed, as the HCVST used in this study is a low-risk test already approved for professional use by a stringent regulatory authority. Social harms relating to self-testing will be evaluated by a community stakeholder group (figure 2).
Figure 2.
Social harms monitoring structure. HCVST, hepatitis C virus self-testing; SMS, Short Message Service; NCDC, National Center for Disease Control
Sample size and statistical analyses
The target sample size is a minimum of 1250 participants (250 per study arm). The sample size was calculated using G*Power V.3.1 software (University of Dusseldorf, Germany) using a one-tailed test, 80% power and a 5% significance level in order to detect a significant change in the primary outcome between the control and intervention groups. With up to a 20% loss to follow-up rate, we conservatively estimate that 250 participants in each group will be sufficient to detect differences between the control and each intervention group.
As the estimated proportion of anti-HCV positive results among study participants is estimated to be ≤10%, the study is not powered to detect statistical differences between study arms in the secondary endpoints.
Statistical analyses will be performed in the per-protocol population (all participants who fully comply with the protocol). A 20% difference between intervention and control arms for the primary endpoint will be considered as demonstrating superiority of HCVST compared with referral to standard of care. In our settings, the superiority test is a (one-sided) hypothesis test where the null hypothesis is that the outcome in the intervention arm is not better than in the control arm, so rejecting the null hypothesis will support the evidence of the anticipated superiority of the intervention arm.
Secondary outcomes will be analysed using descriptive statistics including proportions and means, with the exception of cost of HCVST, for which a cost-effectiveness analysis will be performed.
Building off the lessons learnt from the HIV self-testing (HIVST) pilot study, the sample size will be reached using social media to promote the study to the target population. The promotional strategies will be tailored to the clientele of each site. For Tanadgoma and Equality Movement, posts and social media advertisements will be generated using Facebook and online dating sites and mobile applications Hornet, PlanetRomeo and Tinder; advertisements will also be placed in the gay video section of pornography sites. For Imedi Batumi and Tbilisi New Way, promotions will be done through posts and advertisements on Facebook as well as flyers distributed at the harm reduction sites. Promotional materials will include digital fliers and posters (approved by the National Ethics Board), as well as online talk shows and videos which will provide basic information on hepatitis C and why testing is important and explain about the HCVST study providing information on where to enrol.
Data management
Data recorded in the online platform will be protected with multilayer security and each study personnel will have individualised access rights appropriate to their role in the study. Any participant records that are transferred from the online platform for analysis will contain the study ID only; no information that would allow identification of participants will be transferred. The Foundation for Innovative New Diagnostics (FIND) is responsible for data management, including quality control checks and assessment of protocol compliance. FIND or a designee may conduct audits of investigational sites as part of routine quality assurance.
There is only one study database with no direct links with any other databases. In terms of following participants along the continuation of care offered by the National Elimination Programme, the National Center for Disease Control (NCDC) study team will, with consent from participants, attain the ID numbers of individuals who test positive in the control group, as well as those in the intervention groups who attend to a clinic for a professional use of rapid diagnostic test after completion of a self-test. This ID number will allow NCDC study staff to follow their progress in the national HCV database which captures all diagnostic and treatment data of the National Elimination Programme.
Study oversight and monitoring
The support for this study is provided by:
The principle investigator who has overall responsibility for the supervision of the study and medical responsibility of the participants.
Batumi Imedi, Equality Movement, Tanadgoma and Tbilisi New Way which each have a study coordinator who ensures the online platform is functioning correctly and that study procedures are followed as needed in terms of the arm of the study they are responsible for.
Study team members send out reminders to participants to complete surveys, and organise payment of incentives to participants who have completed the surveys.
Study peer support team provides support to participants if they have questions or concerns regarding the testing process, assists those participants who have an HCV positive antibody result, and are interested, in linkage to further care (both intervention and control group).
FIND is the study sponsor and has written the protocol, maintains the data collection tools, will oversee the data analysis and have final decision to submit the study report for publication.
The study team meets weekly. While there is no study steering committee, there is a social harms monitoring structure (figure 2). This structure is comprised of the individual, community, and instructional partners and is designed to capture any potential harms that may arise related to the use of HCVST.
There is no data monitoring committee for this study due in large part to the lack of serious adverse events in the previous feasibility and acceptability studies on HCVST completed in Georgia as well as six other countries as well the fact that many large-scale HIVST studies and pilots have been conducted without such committees.
Patient and public involvement
Several of the organisations involved in this trial are community-based organisations which include people with experience of living with HCV, living with HIV and injection drug use. They have contributed their input into the trial from the conceptualisation phase and are included as authors in this paper.
Representatives and target end users from the MSM and PWID organisations have reviewed and commented on an information overview sheet that is provided with the self-tests. Prior to finalisation of the data collection forms and website interface, we piloted the forms and interface with 41 potential end users from MSM community and 19 potential end users from PWID community. We incorporated the feedback into the final design of the data collection tools and website interface.
Members of the public will be engaged in the social harms monitoring structure throughout the trial. The trial partners have several dissemination events planned which will be open to the public.
Ethics and dissemination
Ethical approval of the study protocol has been obtained from the National Centers for Disease Control and Public Health Georgia IRB (IRB# 2021-049) and any protocol amendment that may arise will be submitted to the same. The trial will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines (ICH GCP E6 (R2)) and applicable laws and regulations. All participants will be informed that their participation is voluntary and will be required to sign and date a statement of informed consent meeting Georgian regulations. The consent form will be available on the online platform and will include information on the nature of the trial in Georgian, and details on access to a hotline for questions about the trial.
A variety of methods and forums will be used to disseminate the results of the study including presentation at scientific conferences, peer-reviewed publications and advocacy-based literature. Special efforts will be put into sharing the results with organisations representing PWID and MSM at the national, regional and global level. Dependent on the outcomes of the trial, dissemination work may entail working with stakeholders to facilitate the national programming for scale up of HCVST.
Discussion
To our knowledge, this will be the first study to assess the acceptability and impact of using an online platform, which was developed initially for HIVST, for providing home delivery of HCVSTs.
Limitations of this study design include the use of an online platform for enrolment, limiting the study population to people who have access to the internet and have internet literacy. This may exclude people who could also benefit from HCVST but are not able to access the internet. There could be operator errors while participants conduct the test and false reporting of results. Uptake of testing in the control arm may be affected by the geographical location of the participant and the distance to a nearest testing centre. Moreover, the ongoing COVID-19 pandemic may affect participants’ willingness to visit a healthcare facility and therefore may negatively impact the uptake of testing in the control arm and the uptake of treatment in both intervention and control arms. The survey questionnaires have a multiple-choice design and may not capture some important context-specific aspects. Finally, the context of Georgia, which has an advanced elimination programme, can both have an advantage and limitation. An advantage is that people are more aware of HCV and could be more motivated to seek testing. However, as most of Georgia’s population has been tested at least once already, this may result in challenges in recruiting the needed sample size (mitigated by including those previously tested anti-HCV negative).
Understanding how integration of HCVST into self-testing platforms for HIV can leverage existing mechanisms to maximise investments that global funders have made in other areas is critical for HCV, as there is very limited funding available, of which most is domestic.15 The findings of this study will inform the Georgian National Centers for Disease Control and Public Health on scale-up of HCVST to reach last mile service delivery for HCV. Additionally, these findings will have global importance as this will provide some of the first ever evidence about implementation of HCVST in key populations that could be relevant to other settings and countries which are advancing in their hepatitis response.
Supplementary Material
Acknowledgments
The information described herein is based on version 1 of the study protocol, dated 31 May 2021. Medical writing services, funded by FIND, were provided by Rachel Wright, in accordance with Good Publication Practice (GPP3).
Footnotes
Contributors: SS, KS and ER conceptualised the study. SS designed and wrote the protocol. SS, KS, ER, MJg, NT, DU, SP and MJ finalised the protocol. AM, NL, CJ and PN provided technical input on the trial design. CJ provided guidance on the social harms monitoring structure. SS wrote the first draft of the manuscript. SO developed the statistical component of the protocol. KS, ER, MJg, NT, DU, JM, SP, MJ, AM, NL, PN and AG reviewed the manuscript. All authors have read and approved the manuscript.
Funding: This work is funded by the Government of the Netherlands.
Disclaimer: The opinions expressed herein are the author's own and do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated. Where the authors are identified as personnel of the WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the WHO.
Competing interests: SS, MJ, PN, JM, SO and ER declare that they are employees of the Foundation for Innovative New Diagnostics (FIND). The other authors have no conflicting or competing interests to declare.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.World Health Organization (WHO) . Recommendations and guidance on hepatitis C virus self-testing, 2021. Available: https://www.who.int/publications/i/item/9789240031128 [Accessed 20 Jul 2021]. [PubMed]
- 2.Guise A, Witzel TC, Mandal S, et al. A qualitative assessment of the acceptability of hepatitis C remote self-testing and self-sampling amongst people who use drugs in London, UK. BMC Infect Dis 2018;18:281. 10.1186/s12879-018-3185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majam M, Fischer A, Ivanova Reipold E, et al. A lay-user assessment of hepatitis C virus self-testing device usability and interpretation in Johannesburg, South Africa. Diagnostics 2021;11:463. 10.3390/diagnostics11030463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez-Pérez GZ, Nikitin DS, Bessonova A, et al. Values and preferences for hepatitis C self-testing among people who inject drugs in Kyrgyzstan. BMC Infect Dis 2021;21:609. 10.1186/s12879-021-06332-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen LT, Nguyen VTT, Le Ai KA, et al. Acceptability and usability of HCV self-testing in high risk populations in Vietnam. Diagnostics 2021;11:377. 10.3390/diagnostics11020377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reipold EI, Farahat A, Elbeeh A, et al. Usability and acceptability of self-testing for hepatitis C virus infection among the general population in the Nile delta region of Egypt. BMC Public Health 2021;21:1188. 10.1186/s12889-021-11169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gvinjilia L, Nasrullah M, Sergeenko D, et al. National progress toward hepatitis C elimination — Georgia, 2015–2016. MMWR Morb Mortal Wkly Rep 2016;65:1132–5. 10.15585/mmwr.mm6541a2 [DOI] [PubMed] [Google Scholar]
- 8.Mitruka K, Tsertsvadze T, Butsashvili M, et al. Launch of a nationwide hepatitis C elimination program — Georgia, April 2015. MMWR Morb Mortal Wkly Rep 2015;64:753–7. 10.15585/mmwr.mm6428a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chikovani I, Shengelia N, Sulaberidze L. HIV risk and prevention behaviors among people who inject drugs in seven cities of Georgia, 2017. Available: http://curatiofoundation.org/wp-content/uploads/2018/02/PWID-IBBS-Report-2017-ENG.pdf [Accessed 20 Jul 2021]. [DOI] [PubMed]
- 10.Stvilia K, Tsertsvadze T, Sharvadze L, et al. Prevalence of hepatitis C, HIV, and risk behaviors for blood-borne infections: a population-based survey of the adult population of T'bilisi, Republic of Georgia. J Urban Health 2006;83:289–98. 10.1007/s11524-006-9032-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karchava M, Sharvadze L, Gatserelia L, et al. Prevailing HCV genotypes and subtypes among HIV infected patients in Georgia. Georgian Med News 2009;177:51–5. [PubMed] [Google Scholar]
- 12.Bouscaillou J, Champagnat J, Luhmann N, et al. Hepatitis C among people who inject drugs in Tbilisi, Georgia: an urgent need for prevention and treatment. Int J Drug Policy 2014;25:871–8. 10.1016/j.drugpo.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 13.Dershem L, Tabatadze M, Sirbiladze T. Characteristics, high-risk behaviors and knowledge of STI/HIV/AIDS and prevalence of HIV, syphilis and hepatitis among injecting drug users in Kutaisi, Georgia: 2007-2009 USAID Report; 2009. [Accessed 20 Jul 2021]. [Google Scholar]
- 14.Georgia Country Coordinating Mechanism, Global Fund . Georgia HIV/AIDS national strategic plan. Available: http://www.georgia-ccm.ge/wp-content/uploads/Georgia-HIV-AIDS-National-Strategic-Plan-2019-20222.pdf [Accessed 20 Jul 2021].
- 15.Wingrove C, Hicks J, Regan S, et al. Investment cases for hepatitis C: never more important. Lancet Gastroenterol Hepatol 2021;6:340–1. 10.1016/S2468-1253(21)00060-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-056243supp001.pdf (493.9KB, pdf)