Abstract
Purpose
The Norwegian Registry of Persons Assessed for Cognitive Symptoms (NorCog) was established to harmonise and improve the quality of diagnostic practice across clinics assessing persons with cognitive symptoms in Norwegian specialist healthcare units and to establish a large research cohort with extensive clinical data.
Participants
The registry recruits patients who are referred for assessment of cognitive symptoms and suspected dementia at outpatient clinics in Norwegian specialist healthcare units. In total, 18 120 patients have been included in NorCog during the period of 2009–2021. The average age at inclusion was 73.7 years. About half of the patients (46%) were diagnosed with dementia at the baseline assessment, 35% with mild cognitive impairment and 13% with no or subjective cognitive impairment; 7% received other specified diagnoses such as mood disorders.
Findings to date
All patients have a detailed baseline characterisation involving lifestyle and demographic variables; activities of daily living; caregiver situation; medical history; medication; psychiatric, physical and neurological examinations; neurocognitive testing; blood laboratory work-up; and structural or functional brain imaging. Diagnoses are set according to standardised diagnostic criteria. The research biobank stores DNA and blood samples from 4000 patients as well as cerebrospinal fluid from 800 patients. Data from NorCog have been used in a wide range of research projects evaluating and validating dementia-related assessment tools, and identifying patient characteristics, symptoms, functioning and needs, as well as caregiver burden and requirement of available resources.
Future plans
The finish date of NorCog was originally in 2029. In 2021, the registry’s legal basis was reformalised and NorCog got approval to collect and keep data for as long as is necessary to achieve the purpose of the registry. In 2022, the registry underwent major changes. Paper-based data collection was replaced with digital registration, and the number of variables collected was reduced. Future plans involve expanding the registry to include patients from primary care centres.
Keywords: dementia, delirium & cognitive disorders, geriatric medicine, old age psychiatry
Strengths and limitations of this study.
A key strength of the Norwegian Registry of Persons Assessed for Cognitive Symptoms (NorCog) is the large sample size with extensive clinical data combined with a research biobank. Few, if any, national registries collect a similarly broad spectrum of variables as NorCog, allowing for exploration of the main domains of dementia and cognitive impairment.
Patients consent to linkage of data with medical records from later admissions to hospitals and nursing homes, as well as to national health registries, making it possible to investigate predictors of real-world outcomes.
NorCog only recruits patients who are referred for assessment in a specialist setting and cannot be used to generalise to the general dementia population in Norway.
In contrast to the restricted setting of a clinical trial, NorCog is a clinical registry collecting data as part of routine clinical practice and may, accordingly, be limited by lower data quality, lack of adjudication and missing data.
Introduction
Cognitive symptoms, such as problems with memory, reasoning and language skills, as well as emotional and behavioural changes, may signal pathological changes in the brain that can cause dementia. Dementia is the deterioration of cognitive functions to an extent that impedes a person’s ability to perform functions of daily living and, ultimately, resulting in premature death.1
In Norway, the municipalities are responsible for primary care, including the assessment of cognitive impairment in older patients with uncomplicated dementia symptoms. In more complicated cases, the recommendation of the Norwegian national guideline on dementia is to refer patients to specialist healthcare units for an extended assessment.2 In Norway, specialist care is administered by four regional health authorities. According to the national guideline on dementia, cases that are appropriate to refer to specialist healthcare units may include younger patients with cognitive decline, patients with coexisting psychiatric, neurological or somatic problems, and atypical dementia disorders and patient groups with complex disorders.
Recently, research including real-world outcome data has been requested.3 Although consensus on the definition of real-world data is lacking, the term is most often defined as data used for coverage and payment decisions collected outside the constraints of conventional randomised controlled trials (RCTs).4 Observational registries represent one type of sources for real-world data, typically including a larger and more diverse group of patients than generally studied in RCTs and, thereby, better reflecting populations that are representative of routine clinical practice.5
The purpose of this cohort profile is to describe the background, methods, baseline data and future plans of the Norwegian Registry of Persons Assessed for Cognitive Symptoms (NorCog). NorCog was established in 2008 with two main aims:
To harmonise and improve the quality of diagnostic practice across outpatient clinics assessing persons with cognitive symptoms in specialist care units.
To establish a large research cohort with extensive clinical data and provide an opportunity to link baseline data to important real-world outcomes in regional and national registries.
Oslo University Hospital has the overall responsibility for the registry data, and the Norwegian National Centre for Ageing and Health is managing the registry.
Cohort description
NorCog has an observational design and recruits patients who are referred for assessment of cognitive symptoms and suspected dementia at outpatient clinics in Norwegian specialist healthcare units. Patients are encouraged to bring their next of kin or another person who knows them well, referred to hereafter as the informant.
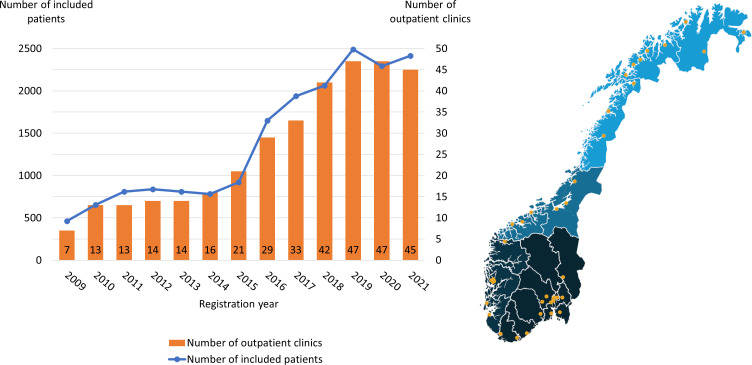
NorCog was originally established as a regional quality and research registry in 2008. During the first year of data collection, in 2009, seven outpatient clinics from the South-Eastern Norway Regional Health Authority participated. Since then, outpatient clinics from all four regional health authorities in Norway have joined, and the registry received status as a national quality registry in 2013. Most of the clinics are referred to as memory clinics or outpatient clinics in old-age psychiatric and geriatric units.6 There is no clear consensus for which type of outpatient clinics should be assessing dementia in Norway and there are variations between regions. To be eligible for data collection in NorCog, the outpatient clinics need to have a specialist as part of their interdisciplinary staff. Some clinics have had staffing problems and have stopped or paused data collection in NorCog until specialists are rehired. In December 2021, 45 out of 49 eligible outpatient clinics (memory clinics or outpatient clinics in old-age psychiatric and geriatric units) participated with data collection in NorCog.
The number of included patients per year has increased in line with the recruitment of new clinics, from 462 patients in 2009 to 2414 in 2021 (figure 1). Because of the COVID-19 pandemic, the number of included patients in 2020 (n=2293) was somewhat lower than could be expected under normal circumstances. In total, 18 120 patients have been included in NorCog during the period of 2009–2021. Table 1 shows some of the demographic, clinical and social characteristics of the included patients at baseline. The average age at inclusion was 73.7 years, with an age range of 26–99 years. Reports from 13 615 informants showed that memory problems were, by far, the most frequently reported first symptoms of the patient, as stated by 63% of the informants.
Figure 1.
Number of outpatient clinics and number of included patients at baseline in Norwegian Registry of Persons Assessed for Cognitive Symptoms per year during 2009–2021 (n=18 120). In December 2021, 45 clinics from all four regional health authorities in Norway participated.
Table 1.
Selection of demographic, social and clinical characteristics of patients included in the NorCog cohort at baseline
| Patients included in NorCog during 2009–2021 | Missing data total cohort, n (%) | |||||
| Total cohort | NCI/SCI | MCI | Dementia | Other diagnoses | ||
| n (%) | 18 120 (100) | 2258 (12.5) | 6307 (34.8) | 8368 (46.2) | 1187 (6.6) | |
| Age (years), mean (SD) | 73.7 (9.9) | 67.3 (11.7) | 73.1 (9.7) | 76.3 (8.2) | 70.5 (10.9) | 0 |
| Sex (female), n (%) | 9443 (52.1) | 1167 (51.7) | 3097 (49.1) | 4599 (55.0) | 580 (48.9) | 0 |
| Education (years), mean (SD) | 11.3 (3.8) | 12.5 (3.9) | 11.5 (3.8) | 10.8 (3.6) | 11.3 (3.9) | 1521 (8.4) |
| Married/cohabiting, n (%) | 10 709 (61.6) | 1402 (65.0) | 3800 (62.7) | 4812 (59.9) | 695 (61.2) | 729 (4.0) |
| Living alone, n (%) | 6589 (36.4) | 737 (32.6) | 2284 (36.2) | 3135 (37.5) | 433 (36.5) | 687 (3.8) |
| Public care, n (%) | 5639 (32.1) | 388 (18.1) | 1626 (26.6) | 3260 (40.0) | 356 (31.6) | 597 (3.3) |
| MMSE score, mean (SD) | 23.6 (4.6) | 27.1 (3.3) | 25.2 (3.4) | 21.0 (4.4) | 26.2 (3.8) | 478 (2.6) |
| Information from informant obtained, n (%) | 17 234 (95.1) | 1983 (87.8) | 5907 (93.7) | 8275 (98.9) | 1069 (90.1) | |
Numbers of patients with missing data are shown in the far right column.
MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NCI, no cognitive impairment; NorCog, Norwegian Registry of Persons Assessed for Cognitive Symptoms; SCI, subjective cognitive impairment.
During 2009–2021, about half of the patients (46%) were diagnosed with dementia at the baseline assessment, 35% with mild cognitive impairment (MCI), 13% with no cognitive impairment (NCI) or subjective cognitive impairment (SCI), and 7% received other specified diagnoses such as mood disorders. The diagnostic work-up and criteria will be explained further in a separate paragraph. Table 2 shows the frequency of the different aetiological dementia diagnoses and a selection of characteristics by dementia diagnoses. The frequency of dementia subtypes is similar to the distributions in the Swedish Dementia Registry (SveDem) and the Danish Dementia Registry (DanDem),7 although these two registries recruit patients from both specialist units and primary care centres. Since NorCog only recruits from specialist units, one might have expected a higher share of the less frequent dementia subtypes such as dementia with Lewy bodies (DLB) and frontotemporal dementia (FTD). The distribution of dementia subtypes in NorCog is also similar to the findings in a recent Norwegian population-based prevalence study.8 The subtype of unspecified dementia is often used as a pending diagnosis when the aetiological diagnosis is not yet confirmed at the time of baseline registration. It is possible that rarer or more complicated dementia subtypes are over-represented in this diagnostic group. Better registry follow-up data in the future may reveal the aetiological diagnoses in the unspecified dementia group.
Table 2.
Demographic characteristics by etiological dementia diagnoses registered in NorCog at baseline during 2009–2021
| AD dementia | Mixed AD and VaD | VaD | DLB | PDD | FTD | Unspecified dementia | Other dementia types | |
| n (%) | 4481 (53.5) | 1047 (12.5) | 925 (11.1) | 343 (4.1) | 199 (2.4) | 131 (1.6) | 1154 (13.8) | 88 (1.1) |
| Age (years), mean (SD) | 75.9 (8.3) | 79.0 (6.7) | 78.4 (7.2) | 74.3 (8.2) | 75.0 (6.5) | 66.8 (10.9) | 75.9 (8.1) | 70.6 (9.1) |
| Sex (female), n (%) | 2750 (61.4) | 552 (52.7) | 411 (44.4) | 140 (40.8) | 70 (35.2) | 61 (46.6) | 574 (49.7) | 40 (45.5) |
| Education (years), mean (SD) | 10.9 (3.5) | 10.6 (4.1) | 10.3 (3.4) | 11.3 (3.6) | 11.4 (3.8) | 12.3 (3.6) | 10.5 (3.5) | 11.5 (3.3) |
| Married/cohabiting, n (%) | 2606 (60.3) | 569 (57.1) | 505 (56.7) | 223 (67.4) | 151 (77.8) | 97 (77.6) | 610 (55.7) | 51 (61.4) |
| Living alone, n (%) | 1676 (38.8) | 428 (41.9) | 371 (41.5) | 98 (29.4) | 42 (22.0) | 30 (23.8) | 461 (41.4) | 28 (32.9) |
| Public care, n (%) | 1429 (32.7) | 516 (50.4) | 510 (56.5) | 123 (37.3) | 96 (50.0) | 32 (25.4) | 517 (46.0) | 36 (43.4) |
| MMSE score, mean (SD) | 20.8 (4.4) | 20.7 (4.2) | 21.4 (4.3) | 21.5 (4.5) | 21.7 (4.3) | 24.1 (3.7) | 20.9 (4.6) | 23.7 (4.0) |
AD, Alzheimer’s disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; MMSE, Mini-Mental State Examination; NorCog, Norwegian Registry of Persons Assessed for Cognitive Symptoms; PDD, Parkinson’s disease dementia; VaD, vascular dementia.
Dementia severity was assessed with the Clinical Dementia Rating (CDR) scale, a global rating scale covering six domains of cognitive and functional performance.9 The CDR is scored according to an algorithm that gives precedence to the memory domain, with a total score of 0 (NCI), 0.5 (questionable dementia), 1 (mild dementia), 2 (moderate dementia) and 3 (severe dementia). In NorCog, 81% of patients who were diagnosed with dementia at baseline during 2009–2021 had a score of 0.5 or 1.0, corresponding to a very mild or mild stage of dementia. Furthermore, 17% had a score of 2 (moderate dementia), and 2% had a score of 3 (severe dementia).
Informed consent
Participation in NorCog is voluntary and enrolment requires written informed consent. To give informed consent, a person must have the ability to fully understand what the participation in the registry means. During 2009–2021, NorCog has recruited only patients who have the capacity to give informed consent. However, from January 2022, patients who are unable, or have reduced capacity, to provide informed consent can also be included in NorCog based on proxy consent.
Patients not included in NorCog
The number of non-included patients during 2009–2015 has not been registered. Beginning in 2016, all participating clinics have recorded the age and sex of patients who were not included in NorCog. During 2016–2021, a total of 613 patients lacked capacity to give consent and were, therefore, not eligible for inclusion in NorCog. The total number of patients eligible for inclusion at the participating clinics during 2016–2021 was 17 597, whereof 73% of the patients (n=12 845) were included and 27% (n=4752) were not included. The average age and the proportion of women were higher among the non-included patients (76 years and 55% women) compared with the included patients (74 years and 51% women). Among the patients who were not included, some were not asked to join the registry due to capacity considerations at the clinic (22%) or because the clinician perceived that the situation/condition of the patient did not allow it (31%). In other cases, patients were asked to participate but declined (21%). In 26% of cases, the cause of non-inclusion was unknown, or another test battery was used due to language issues.
Follow-up data
No predetermined follow-up schedule has been established for the registry. However, a registration form for assessment at follow-up according to clinical indication was implemented in 2015 (table 3). Thus far, the clinics are encouraged but not obliged to deliver follow-up data to the registry. In several substudies, selected patients have been invited to receive additional examinations or follow-ups in compliance with specific research protocols. In 2017, a large research project began collecting follow-up data from patients in the registry. The project is called ‘Trajectories and Risk Factors of Dementia (TRAIL-DEM)’ and involves linking data from the patients in NorCog with medical records in hospitals and nursing homes as well as national health registries (table 3). The project investigates trajectories from dementia onset until nursing home admission and death, providing a unique opportunity to investigate dementia aetiology and progression.1 10–12 TRAIL-DEM will provide an extensive database that will form the basis for future projects.
Table 3.
Overview of the samples/phases, measurements, biomaterial collection and linkages in the registry
| Sample/phase | Measurements | Biomaterial collection/linkage |
| Baseline assessment NorCog 2009–2021 n=18 120 |
A comprehensive clinical assessment including lifestyle and demographic variables; activities of daily living; caregiver situation; medical history; current medication; psychiatric, physical and neurological examinations; neurocognitive testing; blood laboratory work-up; and routine MRI; when indicated: lumbar puncture for the measurement of Aβ and tau proteins in CSF and/or functional brain imaging | EDTA—whole blood, n=4342 Serum, n=4509 Plasma, n=4293 CSF, n=800 PAXgene RNA, n=3544 DNA, n=3714 |
| Follow-up NorCog patients by clinical indication 2015–2021 n=3656* |
A shorter set of registration forms including living situation, care resource use, sum score on neurocognitive tests, Clinical Dementia Rating scale, Neuropsychiatric Inventory Questionnaire, blood pressure and pulse, clinical evaluation of change from baseline, clinical diagnosis and recommended follow-up | |
| The Trajectories and Risk Factors of Dementia study conducted during 2017–2022 Follow-up of patients in NorCog included during 2009–2016 New linkage in 2022 for patients included during 2017–2021 |
Baseline data from NorCog and follow-up data from medical records of NorCog patients have been merged with clinical data from the NPR (any hospital admittance during lifetime) prior to and after the baseline investigation. Furthermore, patients from NorCog have been traced by the CoDR to examine time and cause of death and by the NPoR to learn which patients have been admitted to nursing homes. Clinical data on level of functioning, level of dementia, neuropsychiatric symptoms and drug use have been retrieved from nursing home records or from interviews with nursing home staff, by visiting the nursing homes or by phone. | Linkage to NPR, CoDR, NPoR and Norwegian Prescription Database |
*Number of patients with data from at least one registered follow-up using the short registration form.
CoDR, Cause of Death Registry; CSF, cerebrospinal fluid; NorCog, Norwegian Registry of Persons Assessed for Cognitive Symptoms; NPoR, National Population Registry; NPR, Norwegian Patient Registry.
Standardised dementia assessment
All patients have a detailed baseline characterisation. The baseline data were collected using a standardised dementia assessment protocol developed in an interdisciplinary collaboration among geriatricians, psychiatrists, neurologists, other clinicians and researchers. The assessment protocol is designed as a paper-based case report form that can be optically scanned and includes questionnaires, a battery of tests and forms on which relevant measures can be added. The scales are in accordance with the recommendations of the Norwegian National Guidelines on Dementia.2 Usually, the patient is followed by the person who is defined as the closest proxy and serves as the key informant. As a rule, the patient and informant are interviewed separately. Some of the variables, depending on the level of cognitive impairment, are collected from both the patient and the informant. If an informant is not able to accompany the patient to the assessment, information is usually collected by phone interviews.
The interviews include demographic and lifestyle variables, measures of care resource use, physical and social activities, activities of daily living (ADLs), caregiver distress, medical history, current medications and neuropsychiatric symptoms. Further examination of the patient includes an evaluation and testing of cognitive function, a psychiatric evaluation with an emphasis on comorbid depressive disorder, and an assessment of somatic status including a physical and neurological examination, blood laboratory work-up, and structural brain imaging using CT or MRI. A spinal fluid examination and/or functional brain imaging such as positron emission tomography are performed in subgroups, according to clinical indication. See table 4 for a summary of the measures and scales registered in NorCog at baseline. The assessment protocol is a valuable tool for the participating clinics. To optimise and update the protocol, revisions have been made over the years. The revision process is comprehensive and carefully considers variables that should be added, modified or removed. All revisions must be approved by the steering committee for NorCog.
Table 4.
Measures and scales registered in NorCog at baseline and source of information
| Main test domain | Measures and scales registered in NorCog | Source |
| Lifestyle/background | ||
| Demographic | Age, gender, education, occupational activity, marital status, children, living condition, informant (yes/no), relation to informant | I, P |
| Care resource use | Care provided by the municipality (hours per week) Private or family care (hours per week) |
I, P |
| Activities | Physical activity (hours per week and intensity) Social and cultural activity (hours per week) |
I, P |
| Stimulant use | Tobacco, alcohol, other substances | I, P |
| Safety | Driving, access to weapons, falls | I, P |
| Nutrition and natural functions | Involuntary weight loss? Unsatisfactory food intake? Incontinence? | I, P |
| ADLs | ||
| Personal ADL | Physical Self-Maintenance Scale | I |
| Instrumental ADL | Lawton Instrumental Activities of Daily Living Scale (instrumental activities of daily living) | I |
| Patient-reported outcome measures and caregiver situation | ||
| Caregiver distress | Relatives’ Stress Scale | I |
| Patient experience | Do you think your memory is worse than before? If yes, does this worry you? | P |
| Fatigue | Do you mostly feel strong and rested or tired? | P |
| Medical history | ||
| Present symptoms | Onset, course, type of symptoms Informant Questionnaire on Cognitive Decline in the Elderly |
I, P I |
| Family history | Dementia, other disorders of the CNS | I, P |
| Present or previous physical and/or psychiatric disease | Cerebrovascular, other CNS disorders, cancer, arthritis, cardiovascular, endocrine, kidney, chronic obstructive pulmonary disease, psychiatric disease | I, P |
| Medication | ||
| Current medication | ATC code and defined daily dose of regular medication | I, P |
| NPS | ||
| Overall NPS | Neuropsychiatric Inventory Questionnaire | I |
| Depression | Cornell Scale for Depression in Dementia | I |
| Montgomery and Aasberg Depression Rating Scale | P | |
| Neurocognitive tests | ||
| Global cognition | Mini-Mental State Examination | P |
| Clock Drawing Test | P | |
| Clinical Dementia Rating | C | |
| Memory | Ten-word recall test | P |
| Attention | Trail Making Test A and B | P |
| Fluency | Controlled Oral Word Association Test | P |
| Constructional praxis | CERAD figure copying (including recall) | P |
| Word retrieval | Boston Naming Test | P |
| Insight | Reed Scale for evaluation of anosognosia (lack of insight) | C |
| Somatic status | ||
| Physical and neurological examination | Blood pressure, pulse, weight, height, gait speed, chair stand, balance test, vision, hearing, auscultation of neck, central facial paresis, plantar reflex, gait, rigidity, spasticity, hypokinesia, tremor, Romberg’s test | D |
| Blood test results | Haemoglobin, thrombocytes, CRP, s-cholesterol, Na, K, Ca, S-folate, homocysteine, vitamin B12, TSH, T4, ALP, SR, ALAT, G-GT, albumin, creatinine, vitamin D, HbA1C | D |
| CSF test results | Beta-amyloid, total tau, phosphorylated tau | D |
| Brain imaging | Structural and functional (yes/no): CT, MRI, PET, DAT, SPECT, EEG | D |
| Clinical evaluation | ||
| Baseline diagnosis | ICD-10 codes/SCI, MCI, dementia (AD, VaD, FTD, DLB, PDD, unspecified), other | C |
| Recommended follow-up | Specialist healthcare, primary healthcare, support for caregivers | C |
AD, Alzheimer’s disease; ADLs, activities of daily living; ALAT, alanine aminotransferase; ALP, alkaline phosphatase; ATC, Anatomical Therapeutic Chemical; C, clinical evaluation; Ca, calcium; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CNS, central nervous system; CRP, C reactive protein; CSF, cerebrospinal fluid; D, direct test; DAT, dopamine transporter; DLB, dementia with Lewy bodies; EEG, electroencephalogram; FTD, frontotemporal dementia; G-GT, gamma-glutamyl transferase; HbA1C, haemoglobin A1c; I, informant; K, potassium; MCI, mild cognitive impairment; Na, sodium; NorCog, Norwegian Registry of Persons Assessed for Cognitive Symptoms; NPS, Neuropsychiatric symptom; P, patient; PDD, Parkinson’s disease dementia; PET, positron emission tomography; SCI, subjective cognitive impairment; SPECT, single-photon emission CT; SR, sedimentation rate; T4, thyroxine; TSH, thyroid-stimulating hormone; VaD, vascular dementia.
Diagnostic work-up and criteria
The diagnostic work-ups are conducted by specialists (eg, geriatricians, psychiatrists, neurologists or psychologists) at the outpatient clinics. Diagnoses are discussed in interdisciplinary consensus meetings and are based on all available data from the assessment, including biological markers such as imaging, blood tests and cerebrospinal fluid (CSF) biomarkers. The specialists at the outpatient clinics conclude on a diagnosis according to standardised diagnostic criteria. ICD-10 is the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD), a medical classification list by the WHO. In Norway, ICD-10 is established in clinical practice. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) is widely used in clinical practice in the USA and internationally, wherein the terms mild neurocognitive disorder and major neurocognitive disorder have largely supplanted the term dementia.13 DSM-5 is not widely used in Norwegian clinical practice, but ICD-11, which is more similar to DSM-5 than ICD-10, will be implemented in the near future. Clinical diagnoses in NorCog are registered according to the ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic criteria for research.14 The clinicians also draw a conclusion on one of the following categories for each patient: dementia (ICD-10), MCI according to the Winblad criteria,15 SCI defined as a subjective experience of cognitive problems in the absence of objectively measured cognitive deficits, or the category ‘other’ for patients who do not fulfil the criteria for the previous categories. If dementia is present, an aetiological diagnosis according to ICD-10 is registered in NorCog.
Furthermore, the specialists at the outpatient clinics are encouraged, but not obliged, to make a more specific subclassification according to a number of research diagnostic criteria. Here, the National Institute on Aging and Alzheimer's Association (NIA/AA) criteria are used for Alzheimer’s disease (AD),16 the Vascular Behavioral and Cognitive Disorders (VASCOG) criteria for vascular dementia,17 the McKeith criteria for DLB,18 the Emre criteria for Parkinson’s disease dementia,19 the Rascovsky criteria for FTD behavioural variant20 and the Gorno-Tempini criteria for the language variants of FTD.21 In addition, the NIA/AA criteria are used for the subclassification of MCI.22 Since the specialists at the outpatient clinics are not obliged to register diagnoses according to research criteria other than ICD-10, research diagnoses are determined retrospectively in some research projects.
Biomaterial collection
A subsample of the participating clinics collects blood and CSF for a general research biobank for NorCog. Blood samples include serum, EDTA plasma, EDTA whole blood, as well as whole blood in PAXgene RNA tubes. Genomic DNA has been isolated from EDTA whole blood. The samples are collected, processed and temporarily stored at the respective units according to a standard operating protocol before shipment to the central biobank for NorCog in Oslo. The central research biobank for NorCog currently stores DNA and blood samples from over 4000 patients as well as CSF from 800 patients. The samples have been stored for up to 12 years at −80°C. Samples are collected once, at baseline, but follow-up projects have collected follow-up samples from subgroups. Approximately 400–500 patients are recruited to the biobank yearly, and collection is ongoing.
Quality indicators and patient-reported measures
As a national quality registry, a principal aim of NorCog is to contribute to better quality of, and reduce unwarranted variation in, diagnostic practice in the specialist health services in Norway. Indicators to evaluate the quality of the dementia assessments across different hospitals were developed in 2017 and are reported annually in a national, publicly available report (written in Norwegian and can be found at www.kvalitetsregistre.no). Moreover, patient-reported outcome measures are registered in NorCog, and patient-reported experience measures (PREM) will be implemented during 2022.
Patient and public involvement
Patient involvement is essential to ensure that NorCog is relevant to the population it will impact. Therefore, it is stipulated in the articles of association for NorCog that users shall be represented in the registry’s steering committee. The user representation is attended by The Norwegian Health Association, a voluntary, humanitarian organisation promoting the interests of people with dementia and their carers. The members of the steering committee further include clinicians and managers from all health regions in Norway with backgrounds from memory clinics or outpatient clinics in old-age psychiatric and geriatric units, of which many have research competence. Furthermore, a patient advisory group, consisting of people with dementia and/or their proxies, has been involved in several processes, such as developing and pilot testing a PREM questionnaire, evaluating documents and information sheets, as well as involvement in strategies for presenting studies based on NorCog data.
Findings to date
Data from NorCog have been used in a wide range of research projects within the field of cognitive impairment and dementia, incorporating geriatric medicine, neurology, psychiatry, psychology, pharmacy, nursing, occupational therapy and basic research. Up to December 2021, more than 100 scientific papers, 22 PhD dissertations, and 18 postdoctoral studies were fully or partially based on data from NorCog. A complete list of research projects using data from NorCog and their publications can be found on the website (www.aldringoghelse.no/forskning/norkog). A short description of results from a few of the published studies based on data from NorCog follows.
Studies have been conducted to evaluate and validate dementia-related assessment tools. One study compared the validity of the Cornell Scale for Depression in Dementia (CSDD) and the Montgomery-Aasberg Depression Rating Scale among memory clinic patients and concluded that both scales are suitable as screening tools. The prevalence of depressive symptoms was shown to be high among memory clinic patients, as measured by the CSDD.23 Caregiver burden and the patients’ neuropsychiatric symptoms have been shown to be important biasing factors when caregivers report on patients’ cognitive functioning and instrumental ADLs.24 Caregiver distress as measured by the Relatives’ Stress Scale was shown to be higher in people caring for someone with DLB compared with people caring for someone with AD.25
Biomarkers that can aid in the diagnostic work-up of patients suspected of having a dementia disorder have become increasingly important in research, clinical trials and clinical practice. A number of studies have shown that the CSF biomarkers amyloid-β 42 (Aβ42), total tau and phosphorylated tau can be used to distinguish patients with AD from healthy controls.26 However, when analysed in a heterogeneous memory clinic population of patients enrolled in NorCog, the authors found a much lower discriminating power for CSF biomarkers than previously reported.27 In June 2017, the Aβ42 cut-off level for a pathological test result was revised at the Norwegian laboratory analysing the CSF markers, while the methodological routines remained unchanged. The change in the Aβ42 cut-off for the diagnosis of AD nearly doubled the sensitivity of the CSF biomarkers, from 31.9% to 60.9%.28 Another study evaluated the clinical usefulness of automatic MRI assessment using NeuroQuant (NQ) and found that NQ measures could distinguish AD dementia from non-dementia fairly well but were generally poorer in regard to distinguishing AD dementia from non-AD dementia.29 Furthermore, biological material from the research biobank has been used in studies identifying previously unknown genetic variants conferring a risk of AD.30–33
A recent study described patients assessed for cognitive decline in primary healthcare, compared with patients assessed in specialist healthcare that have been included in NorCog. The study found that patients assessed in primary healthcare were older, less educated, had poorer cognitive functioning and activity limitations, more often lived alone, and had more behavioural and psychological symptoms of dementia and depression.34
Strengths and limitations
The main strengths of NorCog are the large sample size with extensive clinical data combined with a research biobank, inclusion on a national level, and the opportunity to link variables covering the most relevant domains of cognitive impairment or dementia with real-world outcomes from national health registries, claims databases and chart records in hospitals and nursing homes.
The number of national registries specifically directed at persons with cognitive symptoms or dementia worldwide is scarce, but such registries now exist in all three Scandinavian countries.35 The SveDem has included a considerably larger number of patients than NorCog and recruits patients from both specialist units and primary care centres.36 The DanDem has a similar profile as SveDem.37 SveDem and DanDem are quality registries for patients with dementia disorders, and enrolment does not require written consent. NorCog is a combined quality and research registry focusing on the extended assessment of cognitive symptoms in a specialist setting that may or may not lead to a diagnosis of dementia, and from 2009 to 2021, it included only those patients who were able to give their informed consent. NorCog is, therefore, not a dementia registry in the same sense as SveDem and DanDem, and it cannot be used to generalise to the general dementia population in Norway. However, unlike SveDem and DanDem, NorCog collects extensive data from all parts of the assessment with detailed information about test results on cognition and neuropsychiatric symptoms, caregiver situation, medications, medical history and somatic status, including results from blood laboratory work-up and lumbar puncture. Few, if any, national registries have a similarly broad spectrum of variables as NorCog, allowing for exploration of the main domains of dementia and cognitive impairment. A number of people are referred for assessment because of subjective memory complaints or mild cognitive impairment but do not fulfil the criteria for dementia. By following these patients over time, we have a unique possibility to identify risk factors for the development of dementia. The opportunity to link data with medical records from later admissions to hospitals and nursing homes, as well as to national registries, makes it possible to investigate predictors of real-world outcomes that are also highly relevant to decision makers. Moreover, the collection of biomaterials in a general research biobank can be used in research that aims to understand the aetiology of different dementia disorders and to explore fluid biomarkers for prediction, diagnosis and disease progression.
In contrast to the restricted setting of a clinical trial, NorCog is a clinical registry collecting data as part of routine clinical practice and may, accordingly, be limited by lower data quality, lack of adjudication and missing data. Data not missing at random introduce bias and confounding that complicate statistical analyses. Nevertheless, the fact that the clinical registries reflect populations that are representative of routine clinical practice is a strength, increasing generalisability and external validity. The broad inclusion criteria for NorCog make the sample representative for patients being referred for evaluation of cognitive symptoms in a specialist setting. However, there are differences in the patient population depending on the profile of the outpatient clinic. NorCog enrols patients from memory clinics and outpatient clinics in old-age psychiatry and geriatric medicine, but thus far, no neurological departments have joined the registry. The representativeness is, thereby, somewhat uncertain.
Future plans
Originally, the finish date of NorCog was set to the year of 2029. However, the legal basis of NorCog was reformalised in 2021 according to the Norwegian Regulations relating to medical quality registries (FOR-2019-06-21-789), and the registry got approval to collect and keep data as long as is necessary to achieve the purpose of the registry. Thus, data collection in NorCog will continue in the coming years without a specific finish date. In the spring of 2022, the registry underwent major changes. Paper-based data collection was replaced with digital registration, and the number of variables collected were reduced. To ensure the quality of the assessment and data collection, the registry will continue to provide the participating clinics with validated screening and diagnostic tools in accordance with the recommendations of the Norwegian national guidelines on dementia. In the paper-based version of NorCog, the registry has provided and collected data on a fixed set of measures and scales, shown in table 4. The new digital version of NorCog, starting in 2022, will allow for a more individualised assessment to be able to include all groups of patients in NorCog regardless of linguistic and cultural background as well as degree and type of dementia. A core set of variables will be collected for each patient, but beyond this, the clinicians may choose the screening tools that are most appropriate based on the background and symptoms of the individual patient. Therefore, the participating clinics will be provided with a selection of additional validated tools in line with national and international guidelines. All follow-up assessments will be registered in the digital platform. Future plans also involve expanding the registry to include patients from additional Norwegian specialist clinics as well as including patients from primary care centres. Moreover, patients who have reduced or lost their capacity to provide informed consent will be recruited based on proxy consent. The registry will continue to serve as a combined quality and research registry with biomaterial collection for years to come.
Collaboration and access to data and biomaterial
The information and biomaterial collected in NorCog can be made available to researchers if access is permitted under certain regulations. Applicants from outside Norway are advised to identify a Norwegian collaborator. Enquiries can be submitted to the corresponding author, Geir Selbæk. An application form in Norwegian and in English may be found online (https://www.aldringoghelse.no/forskning/norkog). Oslo University Hospital has the overall responsibility for the data, and the Norwegian National Centre for Ageing and Health is managing the registry.
Supplementary Material
Acknowledgments
The authors thank all patients and informants for providing information. We are grateful for the efforts done by the reporting outpatient clinics and the steering committee for the Norwegian Registry of Persons Assessed for Cognitive Symptoms.
Footnotes
Contributors: ITM prepared the initial draft of the manuscript in collaboration with GS, KP, THT, SB, MNå and CST. A-BK, AB, ARØ, POH, IS, ALL, AHR, DBS, MNa, JZS and BJ contributed with data resources. ARØ is also the user representative from The Norwegian Health Association. IU and KE were responsible for the initial design and creation of the registry. All authors provided critical review of the manuscript and approved the final version. GS is responsible for the overall content as guarantor.
Funding: Norwegian Registry of Persons Assessed for Cognitive Symptoms is funded by the South-Eastern Norway Regional Health Authority and the Norwegian National Centre for Ageing and Health (grant number not applicable).
Competing interests: A-BK was the principal investigator of three drug trials (ROCHE BN29553, Boehringer-Ingelheim 1346.23 and Novo Nordisk NN6535-4730). KP was rater in the ROCHE BN29553 and Novo Nordisk NN6535-4730 trials, and IS was investigator in the Boehringer-Ingelheim 1346.23 trial.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Cohort description section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The information and biomaterial collected from the Norwegian Registry of Persons Assessed for Cognitive Symptoms can be made available to researchers if access is permitted under certain regulations. Details are described in the section 'Collaboration and access to data and biomaterial'.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the regional committees for Medical and Health Research Ethics in Norway (REK Sør-Øst A, S-08143a). The participants gave informed consent to participate in the study before taking part. The use of data and biological material from the Norwegian Registry of Persons Assessed for Cognitive Symptoms (NorCog) is subject to ethical and legal regulations, including the General Data Protection Regulation, the Health Register Act, the Health Research Act and the Register Regulations. All research projects must be approved by the Regional Committees for Medical and Health Research Ethics in Norway and by the Steering Committee for NorCog.
References
- 1.Strand BH, Knapskog A-B, Persson K, et al. Survival and years of life lost in various aetiologies of dementia, mild cognitive impairment (MCI) and subjective cognitive decline (SCD) in Norway. PLoS One 2018;13:e0204436. 10.1371/journal.pone.0204436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norwegian Directorate of Health . The Norwegian national guideline on dementia, IS-2658: Helsedirektoratet, 2017. Available: https://www.helsedirektoratet.no/retningslinjer/demens [Accessed 16 Aug 2017].
- 3.Gallacher J, de Reydet de Vulpillieres F, Amzal B, et al. Challenges for Optimizing Real-World Evidence in Alzheimer’s Disease: The ROADMAP Project. Journal of Alzheimer's Disease 2019;67:495–501. 10.3233/JAD-180370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makady A, de Boer A, Hillege H, et al. What is real-world data? A review of definitions based on literature and Stakeholder interviews. Value Health 2017;20:858–65. 10.1016/j.jval.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 5.Garrison LP, Neumann PJ, Erickson P, et al. Using real-world data for coverage and payment decisions: the ISPOR real-world data Task force report. Value Health 2007;10:326–35. 10.1111/j.1524-4733.2007.00186.x [DOI] [PubMed] [Google Scholar]
- 6.Brækhus A, Ulstein I, Wyller TB, et al. The Memory Clinic--outpatient assessment when dementia is suspected. Tidsskr Nor Laegeforen 2011;131:2254–7. 10.4045/tidsskr.11.0786 [DOI] [PubMed] [Google Scholar]
- 7.Fereshtehnejad S-M, Johannsen P, Waldemar G, et al. Dementia diagnosis, treatment, and care in specialist clinics in two Scandinavian countries: a data comparison between the Swedish dementia registry (SveDem) and the Danish dementia registry. J Alzheimers Dis 2015;48:229–39. 10.3233/JAD-150144 [DOI] [PubMed] [Google Scholar]
- 8.GjØra L, Strand BH, Bergh S, et al. Current and future prevalence estimates of mild cognitive impairment, dementia, and its subtypes in a population-based sample of people 70 years and older in Norway: the HUNT study. J Alzheimers Dis 2021;79:1213–26. 10.3233/JAD-201275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–72. 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- 10.Edwin TH, Henjum K, Nilsson LNG, et al. A high cerebrospinal fluid soluble TREM2 level is associated with slow clinical progression of Alzheimer's disease. Alzheimers Dement 2020;12:e12128. 10.1002/dad2.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwin TH, Strand BH, Persson K, et al. Trajectories and risk factors of dementia progression: a memory clinic cohort followed up to 3 years from diagnosis. Int Psychogeriatr 2021;33:779–89. 10.1017/S1041610220003270 [DOI] [PubMed] [Google Scholar]
- 12.Mjørud M, Selbæk G, Bjertness E, et al. Time from dementia diagnosis to nursing-home admission and death among persons with dementia: a multistate survival analysis. PLoS One 2020;15:e0243513. 10.1371/journal.pone.0243513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. 5th edn. Washington, D.C: American Psychiatric Publishing, 2013. [Google Scholar]
- 14.World Health Organization . The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva, 1993. [Google Scholar]
- 15.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–6. 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 16.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's & Dementia 2011;7:263–9. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sachdev P, Kalaria R, O'Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014;28:206–18. 10.1097/WAD.0000000000000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–707. quiz 837. 10.1002/mds.21507 [DOI] [PubMed] [Google Scholar]
- 20.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–77. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–14. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's & Dementia 2011;7:270–9. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knapskog A-B, Barca ML, Engedal K. A comparison of the validity of the Cornell scale and the MADRS in detecting depression among memory clinic patients. Dement Geriatr Cogn Disord 2011;32:287–94. 10.1159/000334983 [DOI] [PubMed] [Google Scholar]
- 24.Persson K, Brækhus A, Selbæk G, et al. Burden of care and patient's neuropsychiatric symptoms influence carer's evaluation of cognitive impairment. Dement Geriatr Cogn Disord 2015;40:256–67. 10.1159/000437298 [DOI] [PubMed] [Google Scholar]
- 25.Svendsboe E, Terum T, Testad I, et al. Caregiver burden in family carers of people with dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry 2016;31:1075–83. 10.1002/gps.4433 [DOI] [PubMed] [Google Scholar]
- 26.Olsson B, Lautner R, Andreasson U, et al. Csf and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol 2016;15:673–84. 10.1016/S1474-4422(16)00070-3 [DOI] [PubMed] [Google Scholar]
- 27.Knapskog A-B, Engedal K, Braekhus A. Performance of cerebrospinal fluid biomarkers of Alzheimer disease in a memory clinic in Norway. Alzheimer Dis Assoc Disord 2016;30:8–14. 10.1097/WAD.0000000000000126 [DOI] [PubMed] [Google Scholar]
- 28.Knapskog A-B, Braekhus A, Engedal K. The effect of changing the amyloid β42 cut-off of cerebrospinal fluid biomarkers on Alzheimer disease diagnosis in a memory clinic population in Norway. Alzheimer Dis Assoc Disord 2019;33:72–4. 10.1097/WAD.0000000000000268 [DOI] [PubMed] [Google Scholar]
- 29.Persson K, Selbæk G, Brækhus A, et al. Fully automated structural MRI of the brain in clinical dementia workup. Acta Radiol 2017;58:740–7. 10.1177/0284185116669874 [DOI] [PubMed] [Google Scholar]
- 30.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 2013;368:107–16. 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg S, Stefansson H, Jonsson T, et al. Loss-Of-Function variants in ABCA7 confer risk of Alzheimer's disease. Nat Genet 2015;47:445–7. 10.1038/ng.3246 [DOI] [PubMed] [Google Scholar]
- 32.Witoelar A, Rongve A, Almdahl IS, et al. Meta-Analysis of Alzheimer's disease on 9,751 samples from Norway and IGAP study identifies four risk loci. Sci Rep 2018;8:18088. 10.1038/s41598-018-36429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansen IE, Savage JE, Watanabe K, et al. Genome-Wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet 2019;51:404–13. 10.1038/s41588-018-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michelet M, Lund A, Strand BH, et al. Characteristics of patients assessed for cognitive decline in primary healthcare, compared to patients assessed in specialist healthcare. Scand J Prim Health Care 2020;38:107–16. 10.1080/02813432.2020.1753334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krysinska K, Sachdev PS, Breitner J, et al. Dementia registries around the globe and their applications: a systematic review. Alzheimers Dement 2017;13:1031–47. 10.1016/j.jalz.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Religa D, Fereshtehnejad S-M, Cermakova P, et al. SveDem, the Swedish Dementia Registry - a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PLoS One 2015;10:e0116538. 10.1371/journal.pone.0116538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johannsen P, Jørgensen K, Kørner A, et al. Development of a dementia assessment quality database. Aging Ment Health 2011;15:40–6. 10.1080/13607863.2010.508769 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The information and biomaterial collected from the Norwegian Registry of Persons Assessed for Cognitive Symptoms can be made available to researchers if access is permitted under certain regulations. Details are described in the section 'Collaboration and access to data and biomaterial'.