Abstract
Objectives
Rapid changes in glucocorticoid (GC) levels and adrenal insufficiency are related to the development of post-cardiac arrest (CA) syndrome. However, GC receptor (GR) expression changes have not been studied. Hence, this study aimed to investigate the association of early changes in GR expression and prognosis and immune response in patients who experienced CA.
Design
Prospective observational study.
Setting
Emergency department.
Participants
Patients (85) in the early period of return of spontaneous circulation (ROSC) after CA were admitted between October 2018 and October 2019. After a physical examination, age-matched and sex-matched healthy individuals (40) were recruited for the control group.
Primary and secondary outcome measures
GR expression and cell counts of circulatory T and B lymphocytes, natural killer cells and regulatory T (Treg) cells were assessed. Plasma total cortisol and adrenocorticotrophic hormone (ACTH) levels were also tested.
Results
All cell counts were lower, and plasma total cortisol levels were higher (p<0.001) in patients who experienced CA than in the healthy control group. GR expression in Treg cells and CD3+CD4+ T lymphocytes were not significantly different, but the mean fluorescence intensity and GR expression in other cells were lower in patients who experienced CA (p<0.05) than in the healthy control group. ACTH levels were not different. There were no significant differences between survivors and non-survivors.
Conclusions
This study revealed that GR expression and cell counts rapidly decreased, whereas plasma total cortisol levels increased in the early period after ROSC among patients who experienced CA. Our findings provide important information about GR level and function, and immunosuppressive status in these patients. Assessing GR expression in patients who experienced CA may help screening for those who are more sensitive to GC therapy.
Keywords: accident & emergency medicine, intensive & critical care, adult intensive & critical care
Strengths and limitations of this study.
The study was designed as single-centre, prospective study.
This is the first study to evaluate the glucocorticoid receptor (GR) expression in the early period following return of spontaneous circulation (ROSC) among patients who experienced cardiac arrest (CA).
We only studied the GR expression of patients who experienced CA in the early period following ROSC; therefore, our results cannot be extrapolated to time points beyond 24 hours.
Decreased GR expression may affect the sensitivity of patients who experienced CA to glucocorticoids.
Decreased GR expression may affect potential immune consequences of patients who experienced CA.
Introduction
Cardiac arrest (CA) is a significant health problem globally; about 356 500 people experience medical emergencies due to CA in the USA, and over 544 000 people die from sudden CA in China annually.1 2 The systemic ischaemia–reperfusion response in patients who have experienced CA can present as post-cardiac arrest syndrome (PCAS) or systematic inflammatory response syndrome (SIRS), which increases the risk of multiple organ failure and infection and affects the inflammatory response and prognosis of patients after the return of spontaneous circulation (ROSC).3–6
CA is the most intense among acute stress events, which seriously affect the pituitary and adrenal axis function.7 Studies have shown that abnormal cortisol levels and relative adrenocortical insufficiency after ROSC in patients who experienced CA are related to their prognosis.8–11 However, the clinical application of glucocorticoids (GCs) is controversial. In the 2015 International Cardiopulmonary Resuscitation Guidelines, the routine use of GCs is not recommended for the resuscitation of patients with in-hospital or out-of-hospital CA.12 Recent clinical studies have shown that early administration of corticosteroids after CA can improve the success rate of ROSC, nervous system functional outcome and prognosis, which is speculated to be related to its influence on haemodynamics, and SIRS response, and other mechanisms.12–17 Therefore, the role of GCs in the occurrence and development of PCAS needs to be studied further.
GCs combine with intracellular GC receptors (GRs) to exert anti-inflammatory and immunosuppressive effects and reduce the production and the release of inflammatory cytokines.18 19 The affinity of GRs to GCs in circulating monocytes is decreased in patients with acquired immune deficiency syndrome (AIDS).20 The expression of GR alpha and beta in peripheral polymorphonuclear cells is decreased in patients with critical illness,21 paediatric septic shock, and high serum cortisol levels.22 However, no study has reported the GR expression after ROSC in patients who experienced CA. Previous studies have found that the counts of circulating B and T lymphocytes, regulatory T (Treg) cells and monocytes and expression of human leucocyte antigen DR on circulatory monocytes and B and T lymphocytes are reduced.23 24 Hence, this study aimed to investigate the relationship between GR expression and immune alteration in the early period after ROSC in patients who experienced CA by observing GR expression in circulatory T and B lymphocytes, natural killer (NK) cells and Treg cells, their cell counts, and total plasma cortisol and adrenocorticotrophic hormone (ACTH) levels.
Materials and methods
Study participants
This was an observational study conducted in the Emergency Department (ED). According to the 2015 International Cardiopulmonary Resuscitation Guidelines,25 we enrolled patients in the early ROSC period after CA (both in-hospital and out-of-hospital CA) and were admitted to the ED between October 2018 and October 2019. The inclusion criteria were patients with CA >6 and <24 hours after ROSC, with a Glasgow coma score (GCS) <8. The exclusion criteria were (a) <18 years of age, (b) terminal stage of disease (such as cancer of any type, AIDS), (c) corticosteroid treatment within the past 3 months, (d) administration of corticosteroids and (e) adrenal insufficiency. All patients were treated according to the 2015 International Cardiopulmonary Resuscitation Consensus.13 After a physical examination, age-matched and sex-matched healthy individuals were recruited for the control group.
Data collection
Data collection was performed according to the 2004 guidelines of the Utstein Style template.26 We collected data on demographics, resuscitation (initial heart rhythm, ROSC time and cumulative epinephrine (epinephrine) dose, and laboratory findings routine blood cell counts, blood gas analysis and blood biochemical tests performed >6 hours and <24 hours after ROSC). Acute Physiology and Chronic Health Evaluation (APACHE) II and the Sequential Organ Failure Assessment (SOFA) were used to determine disease severity. Residual blood samples from routine clinical tests or physical health examinations in the morning were collected, maintained at 4°C during transport and storage and used to determine GR expression in circulatory T and B lymphocytes, NK cells and Treg cells and their cell counts. The plasma was maintained at −80°C during storage and used to determine total cortisol and ACTH levels. During follow-up, 28-day survival data were also collected. Online supplemental figure 1 shows the workflow of this study.
bmjopen-2021-060246supp001.pdf (1.6MB, pdf)
Outcome measures
The primary outcomes of this study were GR expression and cell counts of T and B cells, NK cells and Treg cells, measured by flow cytometry. Venous blood samples collected in ethylenediaminetetraacetic acid tubes, then used to measure GR expression in T and B lymphocytes, NK cells and Treg cells. Briefly, a 100 µL peripheral blood sample was stained for 20 min with surface antibodies (CD3, CD4, CD8, CD19, CD16, CD56, CD25 and CD127) in a dark place. Erythrocytes were lysed for 15 min, and the debris was washed away. Before staining of the intracellular GR antibody and its isotype control (Bio-Rad AbD Serotec, Oxford, UK), surface-stained cells were fixed and permeabilised using the BD Transcription Factor Buffer Set (BD Pharmingen, San Diego, USA, Catalogue No. 562574). Monoclonal antibodies and their isotype controls were all purchased from BD Biosciences (San Jose, California, USA). Details of all antibodies are shown in online supplemental table 1. According to the manufacturer’s recommendations, all antibodies and their isotype controls were used at a concentration of 1 µL per 100 µL of whole blood. Samples were measured using the Gallios flow cytometer (Beckman Coulter, Brea, California, USA) and analysed using Gallios Software V.1.0 (Beckman Coulter). The flow cytometer was periodically calibrated by an engineer. Cells were stained for 20 min; thresholds were defined using the manufacturer’s recommended isotype controls. Representative plots and gating strategy from a single sample are shown in online supplemental figure 2. T cells were gated by CD3+CD4+ or CD3+CD8+, B cells were gated by CD3−CD19+, NK cells were gated by CD16+CD56+ and Tregs were gated by CD4+CD25highCD127low. At least 10 000 events were collected in the lymphocyte cell gate for each sample. Results are expressed as percentages and mean fluorescence intensity (MFI) values.
Absolute CD3+ and CD4+ lymphocyte, NK cell and Treg cell counts were obtained using Flow-Count fluorospheres (Beckman Coulter, Catalogue No. 7547053), according to the manufacturer’s instructions. B, CD3+CD4+T, CD3+CD8+T and Treg cell counts were calculated by their percentages in CD3+ or CD4+ lymphocytes multiplied by CD3+ or CD4+ lymphocyte counts.
The secondary outcomes of this study were plasma total cortisol and ACTH levels after ROSC. Venous blood samples were collected in heparin anticoagulant tubes, centrifuged 10 min at 3000 rpm and then stored at −80°C. Plasma total cortisol (IMMULITE 2000 Cortisol, L2KCO2, UK) and ACTH (IMMULITE 2000 ACTH, L2KAC2, UK) levels were assayed using a chemiluminescent immunoassay on a Siemens automated analyzer (IMMULITE 2000 XPi; Siemens Healthcare Diagnostics, Erlangen, Germany). The equipment and reagents were calibrated by engineers before use. The lower detection limit of total cortisol was 2.00 ng/mL, and that of ACTH was 5.00 pg/mL.
Sample size calculation and statistical analysis
The sample size was calculated using the PASS V.15.0 software (NCSS, LLC, Kaysville, Utah, USA) and the non-parametric test method. The median GR expression was 0.93 and 0.80 in the healthy and CA groups, respectively, and the interquartile spacing was 0.1 and 0.3. According to the ratio of 1:2 between the two groups, with a test level of 0.05 and a CI of 0.90, a total of 105 samples were required, comprising at least 35 in the healthy group and 70 in the CA group. The number of people included in the two groups in this study was 40 and 85, respectively, which met our research requirements. Data analysis was done using in SPSS V.22.0 (IBM Corp, Armonk, New York, USA). For normally distributed data, continuous variables are expressed as means with SD. Since the data for total cortisol and ACTH levels had a skewed distribution, we compared our results with the natural logarithmic conversion values after adding 1 (ln (total cortiso+1), ln (ACTH+1)). Measurement data with a skewed distribution are expressed as medians (25th and 75th percentiles). The Mann-Whitney U test was used to compare variables between groups. The qualitative parameters in the 2×2 contingency table were used for analysis. All statistical tests were two-tailed, and a p value of <0.05 was considered statistically significant.
Follow-up
Patients were classified into survivor and non-survivor groups according to the 28-day survival endpoint. Those with all-cause mortality within the follow-up period were considered non-survivors. If data were lost, the corresponding candidate was excluded.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Patient characteristics
Forty healthy individuals and 85 patients who experienced CA were analysed. The demographics and clinical characteristics of both groups are shown in table 1. In this study, acute cardiac and brain events were the main causes of CA, with those in the latter category emanating from strokes. Other causes of CA included poisoning (including carbon monoxide poisoning) and hypokalaemia. Sex and age were not significantly different between the CA and healthy control groups. The comparisons of clinical characteristics of the survivor and non-survivor groups based on 28-day survival are shown in online supplemental table 2. The APACHE II and SOFA scores were significantly different between the CA and healthy control groups (p<0.001 for all) and survivor and non-survivor groups (p<0.001 and p=0.011, respectively).
Table 1.
Patientcharacteristics at admission
| Characteristics | Healthy control group (n=40) | Successful resuscitation group (n=85) |
| Age (years), median (IQR) | 64.0 (54.3 to 69.8) | 65.0 (55.0 to 74.0) |
| Male/female (n) | 23/17 | 58/27 |
| Previous medical history, n (%) | ||
| Hypertension | 5 (12.5%) | 38 (44.7%) |
| Diabetes | 3 (7.5%) | 27 (31.8%) |
| Coronary heart disease | 2 (5.0%) | 29 (34.1%) |
| Chronic lung disease | 1 (2.5%) | 9 (10.6%) |
| Chronic kidney disease | 0 | 9 (10.6%) |
| Cardiac arrest cause (n, %) | ||
| Cardiac | 34 (40.0%) | |
| Respiratory | 20 (23.5%) | |
| Cerebral | 23 (27.1%) | |
| Others | 7 (8.2%) | |
| Unknown | 1 (1.2%) | |
| Initial resuscitation | ||
| Time to ROSC (min), median (IQR) | 20.0 (10.0 to 30.0) | |
| Epinephrine (mg), median (IQR) | 2.0 (0.0 to 5.0) | |
| Initial rhythm VF/VT, n (%) | 30 (35.3%) | |
| MAP (mm Hg), median (IQR) | 95.7 (86.0 to 103.2) | 74.3 (56.2 to 97.2) |
| White cell count (×109 /L), median (IQR) | 5.81 (4.85 to 6.53) | 13.56 (10.84 to 18.29) |
| APACHE Ⅱ score, mean±SD | 0 | 32.9±6.5 |
| SOFA score, median (IQR) | 0 | 11.5 (8.5 to 14.0) |
| 28-day mortality, n (%) | 65 (76.5%) | |
| 28-day CPC 1–2, n (%) | 14 (16.5%) |
APACHE II, acute physiology and chronic health evaluation; CPC, cerebral performance category; MAP, mean arterial pressure; ROSC, return of spontaneous circulation; SOFA, sequential organ failure assessment; VF, ventricular fibrillation; VT, ventricular tachycardia.
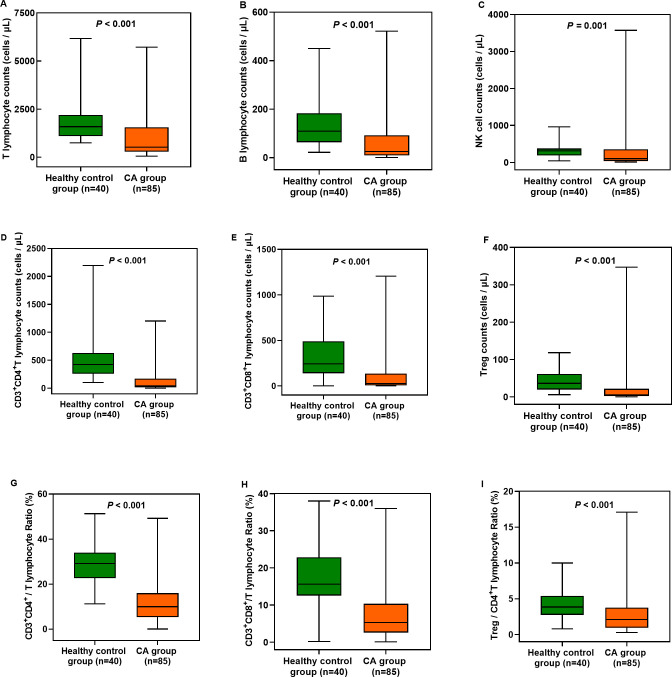
Changes in circulatory T and B lymphocyte, NK cell, and Treg cell counts after ROSC
The T and B lymphocyte, NK cell and Treg cell counts were significantly lower after ROSC in patients who experienced CA than in healthy controls (p<0.001 for all). Additionally, the CD3+CD4+/T lymphocyte, CD3+CD8+/T lymphocyte and Treg cell/CD4+ T lymphocyte ratios were significantly lower after ROSC in patients who experienced CA than in healthy controls (p<0.001 for all) (figure 1; online supplemental table 3). However, there were no significant differences in these cell counts and ratios between survivors (n=20) and non-survivors (n=65) (p>0.05 for all) (online supplemental table 4).
Figure 1.
Changes in circulatory T and B lymphocyte, NK cell and Treg cell counts, CD3+CD4+/T, CD3+CD8+/T and Treg/CD4+T lymphocyte ratios between the healthy control group and CA group. The CA group showed significant differences compared with the healthy control group (p<0.001). CA, cardiac arrest; CD, Cluster-of-Differentiation; NK, natural killer; Treg, regulatory T cells.
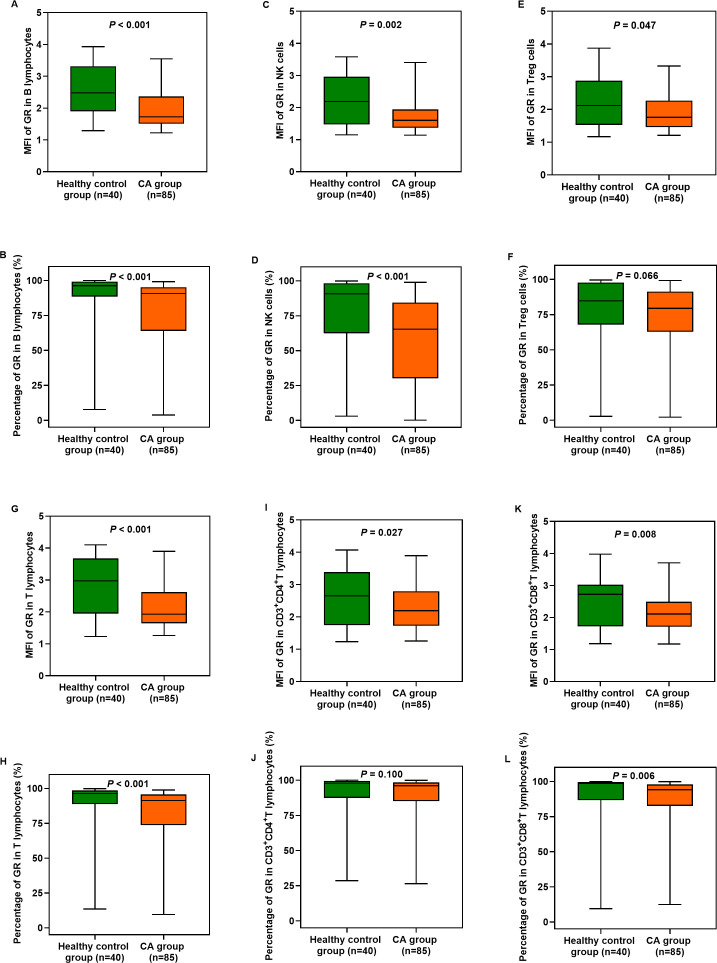
GR expression in circulatory T and B lymphocytes, NK cells and Treg cells after ROSC
The MFI and percentages of GR expression in B and T lymphocytes, NK cells and CD3+CD8+ T lymphocytes were significantly lower after ROSC in patients who experienced CA than in healthy individuals (p<0.01 for all) (figure 2A–D, G, H, K and L). There were also significant reductions in the MFI in Treg cells and CD3+CD4+ T lymphocytes (p<0.05 for all) (figure 2E, I) but not in the percentages of GR expression (p>0.05 for all) (figure 2F and J; online supplemental table 5). However, there were no significant differences in the MFI and percentages of GR expression in these cells between survivors and non-survivors (p>0.05 for all) (online supplemental table 6).
Figure 2.
Expression of GRs in circulatory T and B lymphocytes, NK cells and Treg cells in the healthy control group and CA group. The CA group showed significant differences compared with the healthy control group (p<0.05). CA, cardiac arrest; CD, Cluster-of-Differentiation; GR, glucocorticoid receptor; NK, natural killer; ROSC, return of spontaneous circulation; Treg, regulatory T cells.
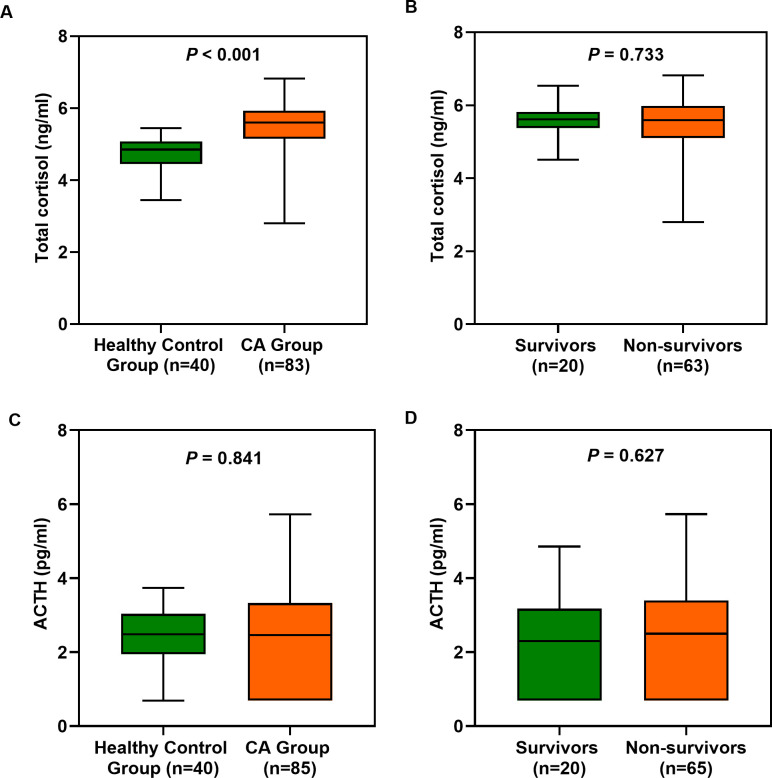
Changes in plasma total cortisol and ACTH levels after ROSC
We measured the plasma total cortisol and ACTH levels of the 40 healthy individuals and 85 patients who experienced CA (two samples were excluded because their total cortisol levels were not measured). Plasma total cortisol levels were significantly higher in patients who experienced CA than in healthy controls (p<0.001), but ACTH levels were not (figure 3A and C). No significant differences in ln (total cortisol+1) and ln (ACTH+1) values were observed between survivors and non-survivors (p>0.05 for all) (figure 3B and D).
Figure 3.
(A, C) Plasma total cortisol and ACTH levels (the natural logarithmic conversion values after adding 1) after ROSC in the healthy control group and CA group. (B, D) Plasma total cortisol and ACTH levels in survivors and non-survivors after ROSC. The CA group showed significant differences compared with the healthy control group (p<0.05). ACTH, adrenocorticotrophic hormone; CA, cardiac arrest; ROSC, return of spontaneous circulation.
Discussion
In this study, we examined the levels of GR expression and plasma corticosteroids in patients who experienced CA in the early period after ROSC. We found that GR expression in circulatory T and B lymphocytes, NK cells and Treg cells, cell counts and ratios in patients who experienced CA was significantly lower compared with that in controls. Furthermore, plasma total cortisol levels in patients who experienced CA were significantly higher compared with the controls.
The ischaemia–reperfusion response initiates an acute inflammatory response that contributes to postresuscitation shock after CA.27 The immune response of patients who experience CA is impaired, and the systemic inflammatory response increases.6 28 The T and B lymphocyte, NK cell and Treg cell counts and CD3+CD4+/T, CD3+CD8+/T and Treg cell/CD4+ T lymphocyte ratios were significantly reduced after ROSC. NK cells, which are special innate immune cells with cytotoxic functions similar to CD3+CD8+ T lymphocytes, mainly distinguish infected and stressed cells from healthy cells and eliminate intracellular infection and dysfunctional cells.29 30 T lymphocytes are also crucial because they function as adaptive immune cells to control and eliminate the infection.29 Moreover, B and T lymphocytes mediate humoral and cellular immunity, respectively. This study was performed earlier and involved a more comprehensive assessment of the immune system of patients who experienced CA. Our findings more substantially supported the rapid emergence of immune dysfunction in these patients after ROSC than in previous reports.
The binding of GCs to GR inside different peripheral blood mononuclear cells leads to changes in the ability of cells to regulate apoptosis, proliferation and activity, and GC–GR complexes limit the transcription (trans-repression) of inflammatory genes, including those encoding for proinflammatory cytokines.31 32 This study is the first to explore GR expression in circulating immune cells in patients who experienced CA after ROSC. We observed that GR expression in B and T lymphocytes, NK cells and CD3+CD8+ T lymphocytes decreased significantly in patients who experienced CA, whereas the percentage of GR+ Treg cells and CD3+CD4+ T lymphocytes decreased slightly. Moreover, we observed a more significant decrease in the MFI of GR expression in Treg cells and CD3+CD4+ T lymphocytes but not in the percentage of GR expression. Previous studies have found decreased expression of GRs in peripheral polymorphonuclear cells in critically ill patients,21 and antagonism to GRs aggravates viral and bacterial infections.33 GCs induced on infections help to maintain homeostasis and mitigate the life-threatening impact of sepsis on the host.31 Although studies have reported that the use of GCs during and after cardiopulmonary resuscitation (CPR) seems to confer benefits concerning ROSC rates and long-term survival, the evidence is scant.13 18 34 35 Since cortisol signalling is mediated by GRs, we hypothesised that the differential responses of patients who experienced CA to GC may be related to their levels of GR expression. This study suggests that the decrease in intracellular GR expression in patients who experienced CA is one of the causes of GC resistance due to insufficient binding of GRs and GCs, GC insensitivity and the inability of GCs to exert anti-inflammatory and immunosuppressive effects effectively. These findings may also explain why different results regarding the clinical application of GCs have been reported previously. Furthermore, it is vital to measure GR levels as sufficient expression of GR is essential for mediating adequate GC effects during and after CPR.
We also found that the total plasma cortisol levels were significantly higher in patients who experienced CA, but ACTH levels were not. High levels of inflammatory cytokines inhibit ACTH release.18 During critical illness, the body does not sufficiently metabolise cortisol.36 In addition, the continuous increase in plasma cortisol levels may trigger the negative feedback pathway of the hypothalamic–pituitary–adrenal axis, inhibiting the release of ACTH and cortisol and eventually leading to adrenal insufficiency.37 These factors may explain the opposite trends of plasma ACTH and cortisol levels in the patients included in this study and who experienced CA. Notably, this result suggests that low GR expression levels are not matched by high plasma total cortisol levels in patients who experienced CA. The dissociation between low GR expression and high cortisol implies an abnormal stress response.38 Previous studies have reported that GR-action was clearly suppressed throughout critical illness; GR resistance could not be overcome by further increasing GC availability.21 39 40 Adequate GR levels and function are also required for normal GC function, which may explain differences in the responsiveness of patients who experienced CA to exogenous steroid administration or endogenous cortisol secretion. Thus, actual GR levels cannot be reflected by measuring total cortisol levels alone. Therefore, the GR level should be considered when applying personalised GC therapy. The determination of GR expression might help to screen those who might respond better to GC prescription.
Limitations
Our study has several limitations. First, to assess changes, we only enrolled patients who experienced CA and had signs of systemic ischaemic hypoxia, such as GCS <8 after ROSC. The patients were not stratified by age, sex, the occurrence of comorbidities or mild systemic ischaemic hypoxia. Second, since this was a preliminary observational study, we observed only early changes. A more relevant control group and dynamic observations obtained over a longer duration would be helpful to understand the significance of GR expression in evolving immunity during the clinical course of CA after ROSC. Third, the samples used in this study were from clinical laboratories; thus, plasma total cortisol and ACTH in the samples were at risk of degradation before we collected the samples. Finally, we did not discuss the changes in and roles of GR isoforms, free cortisol and corticosteroid-binding globulin. Therefore, future studies on these aspects are warranted to better understand the immunosuppressive effects of ROSC among patients who experienced CA.
In conclusion, this study revealed that GR expression, cell counts and ratios rapidly decreased, whereas plasma total cortisol levels increased, in the early period after ROSC among patients who experienced CA. These findings may provide important information about GR expression levels and function, and immunosuppressive status in these patients.
Supplementary Material
Acknowledgments
We thank all the patients and their families who were enrolled in this study and colleagues from the emergency department who provided support. And we are grateful for the efforts of the staff for ongoing resuscitation in hospitals.
Footnotes
Contributors: CL is the guarantor. CL designed the study and reviewed the manuscript. YY searched the literature and contributed to the experimental studies, data analysis and manuscript writing. ZT, C-CH and LA collected and analysed data. JL and MX helped with the statistical analyses. All authors have read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Medical Ethics Committee of Beijing Chaoyang Hospital. Ethics name ID is 2013-KE-1. Participants gave informed consent to participate in the study before taking part.
References
- 1.Myat A, Song K-J, Rea T. Out-Of-Hospital cardiac arrest: current concepts. Lancet 2018;391:970–9. 10.1016/S0140-6736(18)30472-0 [DOI] [PubMed] [Google Scholar]
- 2.Zhang S. Sudden cardiac death in China: current status and future perspectives. Europace 2015;17 Suppl 2:ii14–18. 10.1093/europace/euv143 [DOI] [PubMed] [Google Scholar]
- 3.Nolan JP, Neumar RW, Adrie C. Post-Cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A scientific statement from the International liaison Committee on resuscitation; the American heart association emergency cardiovascular care Committee; the Council on cardiovascular surgery and anesthesia; the Council on cardiopulmonary, perioperative, and critical care; the Council on clinical cardiology; the Council on stroke. Resuscitation 2008. 10.1016/j.resuscitation.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 4.Su C-P, Wu J-H, Yang M-C, et al. Demographics and clinical features of postresuscitation comorbidities in long-term survivors of out-of-hospital cardiac arrest: a national follow-up study. Biomed Res Int 2017;2017:9259182. 10.1155/2017/9259182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsai M-S, Chiang W-C, Lee C-C, et al. Infections in the survivors of out-of-hospital cardiac arrest in the first 7 days. Intensive Care Med 2005;31:621–6. 10.1007/s00134-005-2612-6 [DOI] [PubMed] [Google Scholar]
- 6.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation 2002;106:562–8. 10.1161/01.CIR.0000023891.80661.AD [DOI] [PubMed] [Google Scholar]
- 7.Hall ED. Neuroprotective actions of glucocorticoid and nonglucocorticoid steroids in acute neuronal injury. Cell Mol Neurobiol 1993;13:415–32. 10.1007/BF00711581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong MFC, Beishuizen A, de Jong MJ, et al. The pituitary-adrenal axis is activated more in non-survivors than in survivors of cardiac arrest, irrespective of therapeutic hypothermia. Resuscitation 2008;78:281–8. 10.1016/j.resuscitation.2008.03.227 [DOI] [PubMed] [Google Scholar]
- 9.Mosaddegh R, Kianmehr N, Mahshidfar B, et al. Serum cortisol level and adrenal reserve as a predictor of patients' outcome after successful cardiopulmonary resuscitation. J Cardiovasc Thorac Res 2016;8:61–4. 10.15171/jcvtr.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hékimian G, Baugnon T, Thuong M, et al. Cortisol levels and adrenal reserve after successful cardiac arrest resuscitation. Shock 2004;22:116–9. 10.1097/01.shk.0000132489.79498.c7 [DOI] [PubMed] [Google Scholar]
- 11.Tavakoli N, Bidari A, Shams Vahdati S. Serum cortisol levels as a predictor of neurologic survival inSuccessfully resuscitated victims of cardiopulmonary arrest. J Cardiovasc Thorac Res 2012;4:107–11. 10.5681/jcvtr.2012.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soar J, Callaway CW, Aibiki M. Resuscitation- part 4: advanced life support: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2015. 10.1016/j.resuscitation.2015.07.042 [DOI] [PubMed] [Google Scholar]
- 13.Mentzelopoulos SD, Malachias S, Chamos C, et al. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA 2013;310:270. 10.1001/jama.2013.7832 [DOI] [PubMed] [Google Scholar]
- 14.Tsai M-S, Chuang P-Y, Yu P-H, et al. Glucocorticoid use during cardiopulmonary resuscitation may be beneficial for cardiac arrest. Int J Cardiol 2016;222:629–35. 10.1016/j.ijcard.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 15.Niimura T, Zamami Y, Koyama T, et al. Hydrocortisone administration was associated with improved survival in Japanese patients with cardiac arrest. Sci Rep 2017;7:17919. 10.1038/s41598-017-17686-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalkias A, Xanthos T. Post-Cardiac arrest syndrome: mechanisms and evaluation of adrenal insufficiency. World J Crit Care Med 2012;1:4. 10.5492/wjccm.v1.i1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buddineni JP, Callaway C, Huang DT. Epinephrine, vasopressin and steroids for in-hospital cardiac arrest: the right cocktail therapy? Crit Care 2014;18:308. 10.1186/cc13903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varvarousi G, Stefaniotou A, Varvaroussis D, et al. Glucocorticoids as an emerging pharmacologic agent for cardiopulmonary resuscitation. Cardiovasc Drugs Ther 2014;28:477–88. 10.1007/s10557-014-6547-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci 2013;34:518–30. 10.1016/j.tips.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norbiato G, Bevilacqua M, Vago T, et al. Cortisol resistance in acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1992;74:608–13. 10.1210/jcem.74.3.1740494 [DOI] [PubMed] [Google Scholar]
- 21.Vassiliou AG, Floros G, Jahaj E, et al. Decreased glucocorticoid receptor expression during critical illness. Eur J Clin Invest 2019;49:e13073. 10.1111/eci.13073 [DOI] [PubMed] [Google Scholar]
- 22.Alder MN, Opoka AM, Wong HR. The glucocorticoid receptor and cortisol levels in pediatric septic shock. Crit Care 2018;22:244. 10.1186/s13054-018-2177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Z, Liu Q, Zhang Q, et al. Overexpression of programmed cell death-1 and human leucocyte antigen-DR on circulatory regulatory T cells in out-of-hospital cardiac arrest patients in the early period after return of spontaneous circulation. Resuscitation 2018;130:13–20. 10.1016/j.resuscitation.2018.06.023 [DOI] [PubMed] [Google Scholar]
- 24.Qi Z, An L, Liu B, et al. Patients with out-of-hospital cardiac arrest show decreased human leucocyte antigen-DR expression on monocytes and B and T lymphocytes after return of spontaneous circulation. Scand J Immunol 2018;88:e12707. 10.1111/sji.12707 [DOI] [PubMed] [Google Scholar]
- 25.Perkins GD, Travers AH, Berg RA. Resuscitation-Part 3: adult basic life support and automated external defibrillation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015. 10.1161/CIR.0000000000000272 [DOI] [PubMed] [Google Scholar]
- 26.Jacobs I, Nadkarni V, Bahr J, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein templates for resuscitation registries. A statement for healthcare professionals from a task force of the International liaison Committee on resuscitation (American heart association, European resuscitation Council, Australian resuscitation Council, New Zealand resuscitation Council, heart and stroke Foundation of Canada, InterAmerican heart Foundation, resuscitation Council of southern Africa). Resuscitation 2004;63:233–49. 10.1016/j.resuscitation.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 27.Lindner KH, Strohmenger HU, Ensinger H, et al. Stress hormone response during and after cardiopulmonary resuscitation. Anesthesiology 1992;77:662–8. 10.1097/00000542-199210000-00008 [DOI] [PubMed] [Google Scholar]
- 28.Beurskens CJ, Horn J, de Boer AMT, et al. Cardiac arrest patients have an impaired immune response, which is not influenced by induced hypothermia. Crit Care 2014;18:R162. 10.1186/cc14002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanier LL. Nk cell recognition. Annu Rev Immunol 2005;23:225–74. 10.1146/annurev.immunol.23.021704.115526 [DOI] [PubMed] [Google Scholar]
- 30.Vivier E, Tomasello E, Baratin M. Functions of natural killer cells. Nat Immunol 2008. 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- 31.Zen M, Canova M, Campana C, et al. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev 2011;10:305–10. 10.1016/j.autrev.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 32.Vandewalle J, Libert C. Glucocorticoids in sepsis: to be or not to be. Front Immunol 2020;11:1318. 10.3389/fimmu.2020.01318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol 2004;181:207–21. 10.1677/joe.0.1810207 [DOI] [PubMed] [Google Scholar]
- 34.Andersen LW, Isbye D, Kjærgaard J, et al. Effect of vasopressin and methylprednisolone vs placebo on return of spontaneous circulation in patients with in-hospital cardiac arrest: a randomized clinical trial. JAMA 2021;326:1586. 10.1001/jama.2021.16628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smithline H, Rivers E, Appleton T, et al. Corticosteroid supplementation during cardiac arrest in rats. Resuscitation 1993;25:257–64. 10.1016/0300-9572(93)90123-8 [DOI] [PubMed] [Google Scholar]
- 36.Boonen E, Vervenne H, Meersseman P. Reduced cortisol metabolism during critical illness. N Engl J Med 2013. 10.1056/NEJMoa1214969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peeters B, Langouche L, Van den Berghe G. Adrenocortical stress response during the course of critical illness. Compr Physiol 2017;8:283–98. 10.1002/cphy.c170022 [DOI] [PubMed] [Google Scholar]
- 38.Vassiliou AG, Stamogiannos G, Jahaj E, et al. Longitudinal evaluation of glucocorticoid receptor alpha/beta expression and signalling, adrenocortical function and cytokines in critically ill steroid-free patients. Mol Cell Endocrinol 2020;501:110656. 10.1016/j.mce.2019.110656 [DOI] [PubMed] [Google Scholar]
- 39.Indyk JA, Candido-Vitto C, Wolf IM, et al. Reduced glucocorticoid receptor protein expression in children with critical illness. Horm Res Paediatr 2013;79:169–78. 10.1159/000348290 [DOI] [PubMed] [Google Scholar]
- 40.Téblick A, Van Dyck L, Van Aerde N, et al. Impact of duration of critical illness and level of systemic glucocorticoid availability on tissue-specific glucocorticoid receptor expression and actions: a prospective, observational, cross-sectional human and two translational mouse studies. EBioMedicine 2022;80:104057. 10.1016/j.ebiom.2022.104057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060246supp001.pdf (1.6MB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable.