Abstract
Introduction
Subchondral and intra-articular injections of bone marrow aspirate concentrate (BMAC) showed promising results for knee osteoarthritis (OA) patients. To date, there is no evidence to demonstrate whether the combination of these treatments provides higher benefits than the intra-articular injection alone.
Methods and analysis
Eighty-six patients with symptomatic knee OA (aged between 40 and 70 years) are randomised to BMAC intra-articular injection combined with subchondral BMAC injection or BMAC intra-articular injection alone in a ratio of 1:1. The primary outcome is the total Western Ontario and McMaster Universities Osteoarthritis Index, the secondary outcomes are the International Knee Documentation Committee Subjective and Objective Knee Evaluation Form, the Tegner activity scale, the EuroQol-Visual Analogue Scale, and the health questionnaire European Quality of Life Five Dimension score. Additional CT and MRI evaluations are performed at the baseline assessment and at the final 12-month follow-up. The hypothesis is that the combined injections provide higher knee pain and function improvement compared with BMAC intra-articular injection alone. The primary analysis follows an intention to treat principle.
Ethics and dissemination
The study protocol has been approved by the Emilia Wide Area Ethical Committee of the Emilia-Romagna Region (CE-AVEC), Bologna, Italy. Written informed consent is obtained from all the participants. Findings of this study will be disseminated through peer-reviewed publications and conference presentations.
Protocol version
Version 1 (14 May 2018).
Trial registration number
Keywords: ORTHOPAEDIC & TRAUMA SURGERY, Adult orthopaedics, Orthopaedic & trauma surgery, Knee
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first prospective, randomised, double-blind, and controlled trial evaluating results of bone marrow aspirate concentrate (BMAC) intra-articular injection combined with subchondral injection compared with BMAC intra-articular injection alone in knee osteoarthritis.
Patients are analysed using Patient Reported Outcome Measures (PROMs), objective measures, MRI and CT examination, and biomarker evaluation.
Patient base-line characteristics and disease-related factors can help to better define the aspects that make different individuals more or less responsive to this type of treatments.
The uncontrolled pain medication use by patients (although being discouraged) could influence the primary outcome and this is a relevant limitation of the study.
This study can clarify the benefits and limitations of the newly proposed combination of intra-articular and subchondral BMAC injections, providing clear indications for the clinical practice.
Introduction
Knee osteoarthritis (OA) is a chronic, degenerative disease leading to irreversible structural and functional changes in the entire joint, including subchondral bone sclerosis and cartilage loss, and progressively determines debilitating pain and loss of function.1 2 It affects a large part of the ageing population with a high impact on patients and healthcare costs.3 Total knee arthroplasty represents a definitive solution to address knee OA, but it is also encumbered by several complications.4 Conservative approaches, such as physical therapy and anti-inflammatory drugs, should be pursued, but their benefits are generally temporary with short-term relief, and they are not able to affect the natural course of the disease progression.5 Thus, to delay or avoid the need for arthroplasty, research efforts have been made to find new minimally invasive and more effective procedures to address knee OA.
In this light, the use of orthobiologics is gaining increasing interest due to the availability of several promising products, ranging from blood-derivatives (platelet-rich plasma—PRP) to minimally manipulated mesenchymal stromal cells (MSCs) harvested from bone marrow or adipose tissue. Although the intra-articular use of these products for the treatment of knee OA provided overall positive results, the improvement in terms of pain relief and function remains partial and not always satisfactory.6 7 Thus, a new approach has been recently proposed to further exploit the potential of biological products by targeting the subchondral bone.8 This strategy is supported by the evidence revealing that subchondral bone alterations may play a critical role in both the pathophysiology and progression of knee OA.9 10 It has been suggested that with age and knee OA the number and functionality of MSCs present in the subchondral bone of the knee may decrease. Therefore, MSCs subchondral injections could address this deficiency underlying the pathophysiology by providing many bioactive mediators which have been shown to exert positive effects on joint tissues.11 MSCs subchondral bone injections showed to be safe and may provide even better results than MSCs intra-articular injections addressing knee OA in terms of survival to knee arthroplasty.12 Moreover, the combination of subchondral and intra-articular injections of bone marrow aspirate concentrate (BMAC) already showed promising results in terms of safety and clinical outcomes.13 However, beside promising early findings and the increasing use of this approach in the clinical practice, there is only limited and low-level evidence, and it would be clinically relevant to evaluate with a high-level study design the real benefit provided by the addition of these subchondral injections to improve the results of BMAC intra-articular injections for knee OA.
Objectives and trial design
A double-blinded randomised controlled trial (RCT) was designed to compare the efficacy of a combination of intra-articular and subchondral injections of BMAC (treatment group) versus BMAC intra-articular injection alone (control group) to treat knee OA, with a 1:1 allocation ratio. The aim of this superiority trial is to evaluate the safety and the clinical potential of this new treatment approach up to 1 year of follow-up, and to verify the hypothesis that the combination of subchondral and intra-articular injections provides higher knee pain and function improvement compared with BMAC intra-articular injection alone in knee OA.
Methods and analysis
Study setting
The study is a single-centre, double-blind RCT, with all activities related to the study performed in a single site, the IRCCS Rizzoli Orthopaedic Institute, Bologna, Italy.
This trial protocol is produced according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) reporting guidelines.14
Patient and public involvement
Patients are not involved in planning of research questions, outcome measures, or design of the study.
Eligibility criteria
Patients are recruited according to the following criteria.
Inclusion criteria:
Male or female patients, aged between 40 and 70 years.
OA of the medial compartment of the knee (grade II or III according to the Kellgren-Lawrence classification).
Failure after at least 6 months of conservative treatment (drug therapy with non-steroidal anti-inflammatory drugs and painkillers, hyaluronic acid injection, corticosteroid injection, PRP injection).
Patients' ability and consent to participate in clinical and radiological follow-up.
Signature of informed consent.
Exclusion criteria:
Patients with trauma in the 6 months prior to surgery.
Patients with malignancy.
Patients suffering from rheumatic diseases.
Patients suffering from uncompensated diabetes.
Patients suffering from uncompensated thyroid metabolic disorders.
Patients abusing alcoholic beverages or drugs.
Patients with axial deviations >5°.
Body mass index >35.
Patients treated with joint injections in the previous 6 months.
Patients treated with surgery at the same knee in the previous 12 months.
Intervention
All patients are treated by orthopaedic surgeons with established experience in cartilage and OA orthobiologic procedures. The procedure is performed in a single step in the operating room with patients in supine position under spinal locoregional anaesthesia. The ipsilateral hip is sterilely prepared and draped for anterior iliac crest bone marrow aspiration. The anterior superior iliac spine is the anatomical landmark for a small surgical incision. A diamond tip trocar is inserted in this point and then advanced into the bone marrow using a drill. Bone marrow is collected using two 30 mL syringes coated with heparin for a total of 60 mL. The harvested bone marrow is filtered with a heparin washed filter and then centrifuged through the Magellan® centrifuge (Arteriocyte Medical Systems, Massachusetts, USA) at a rate of 3600 RPM for approximately 15 min, thus obtaining 10 mL of BMAC. The BMAC procedure involved a kit available in the clinical practice. In fact, the purpose of the study was not to evaluate a new product, but rather to explore the potential of applying BMAC also at the subchondral bone level, to give indications on the potential of this approach for physicians considering this technique for their clinical practice.
For each patient, BMAC samples that are not used for surgical treatment are sent to the laboratory for the count of mononuclear cells, cell clonogenic ability by colony forming unit-fibroblast test, and phenotypical characterisation by flow-cytometry evaluation.
Concomitantly with the bone marrow concentration process, all patients undergo an arthroscopic evaluation to confirm the location on both medial femoral condyle and medial tibial plateau involved by osteoarthritic lesions. Arthroscopy is done using the standard anterolateral, anteromedial, and superomedial portals. The same portals are used to access the subchondral bone in the experimental group in order to maintain blinding. If the arthroscopic examination reveals intra-articular problems (excluding minor arthroscopic shaving) requiring surgical intervention which may affect the results of the procedure, the patient is excluded from the study.
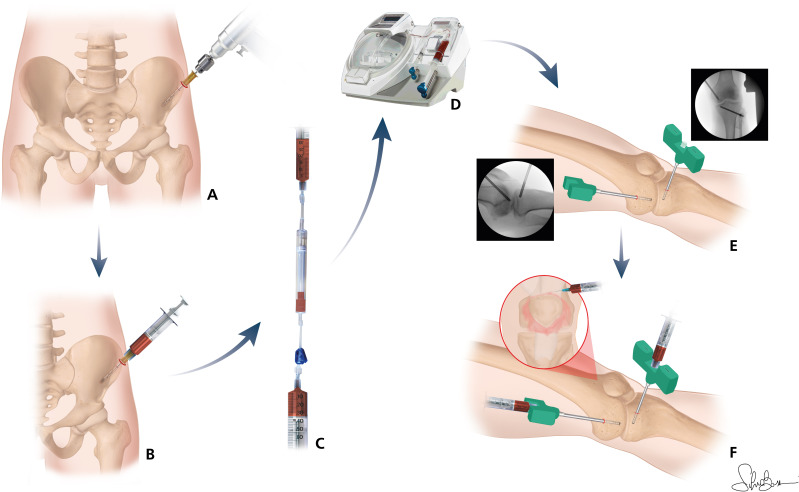
Once the arthroscopy and the BMAC procedure are completed, the injections are performed. The treatment group receives two 2.5 mL subchondral BMAC injections, that are performed inserting two 8-Gauge trocars through the supero-medial and antero-medial arthroscopic portals and are manually introduced with clockwise and anticlockwise movements, under fluoroscopic control, into the bone of both medial femoral condyle and tibial plateau. Following arthroscopic portals suture, both groups of treatment receive a 3 mL intra-articular injection of BMAC using a lateral suprapatellar approach. An elastic bandage is made after wounds medication. The whole procedure is presented in figure 1.
Figure 1.
Anterior iliac crest trocar insertion (A); bone marrow (BM) harvesting (B); BM filtration (C); BM concentration (D); trocar positioning under fluoroscopic control (E); intra-articular and subchondral BM aspirate concentrate injections (F).
Postoperatively, patients are discharged on the same day of the procedure or the day after, based on patient condition. Pain control is prescribed as needed with analgesics only in the immediate period after treatment and thromboembolic prophylaxis is prescribed for 2 weeks. During the same time, patients are taught to walk with the support of two crutches to allow a partial weight-bearing on the operated limb. Cryotherapy is started within the first 24 hours. Passive mobilisation and quadriceps isometric exercises are started at the second postoperative day. Patients are permitted to return to most of their daily activities as tolerated once they reach full weight-bearing. No other conservative treatments are prescribed during the study period. Joint impacting sport activities are discouraged within the first month after treatment.
Outcomes
The primary outcome is the total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 12 months, a 24-item self-administered questionnaire taking into account articular pain and stiffness and physical function limitations due to knee OA. It ranges from 0 to 96 points and higher WOMAC scores indicate worse pain, stiffness and functional limitations. The total WOMAC score was chosen as primary outcome aiming at capturing a more comprehensive assessment of symptoms and function benefits offered by the treatments.
The secondary outcomes include the total WOMAC score at other follow-ups, the WOMAC subscales (pain, stiffness and physical function), as well as the International Knee Documentation Committee Subjective and Objective Knee Evaluation Form (a patient-completed tool taking into account knee symptoms, knee function and sport activity), the Tegner Activity Scale (a one-item score based on work and sports activities), the EuroQol-Visual Analogue Scale (EQ-VAS) that provides an assessment of patients global health, and the health questionnaire European Quality of Life Five Dimension score (a five-level self-assessed, health-related, quality-of-life questionnaire).
Patients also undergo MRI and CT assessments. MRI scans are obtained with a high-resolution 3 Tesla MRI scanner with PD-weighted Turbo Spin Echo 3D sequences with and without fat saturation (FS), 3D T2* Gradient Echo (MERGE) with FS, axial PD-weighted Fast Spin Echo sequences with FS and Multi-Echo T2 Mapping on the sagittal plane with eight different Echo Times.
The Whole-Organ MRI Score is used to assess seven features of the treated knees: articular cartilage morphology, bone marrow oedema, subchondral cysts, articular profile, marginal osteophytes, meniscal integrity, and synovitis.
Articular cartilage morphology is examined with the 3D MERGE and the T2 mapping; bone marrow oedema and synovitis with the PD fat sat sequences, the articular profiles with the PD and MERGE sequences, and the meniscal integrity with the DP sequences.
CT knee scans are obtained with a 64-channel CT scanner to better assess the structural resolution of bone trabeculae as well as to assess the presence of osteophytes, calcifications, and cancellous bone microcysts. The images are acquired using a slice thickness of 1.25 mm and an interval of 0.625 mm at 120kV with 250 mA, postprocessed with the ‘Bone’ filter, and reformatted in the coronal and sagittal plane.
Blood samples are obtained from participants before treatment and at 2, 6, and 12 months of follow-up. Samples are analysed for inflammatory (Interleukin-1 beta, Tumor necrosis factor-alpha) and OA progression markers (Cleavage of type II Collagen, Serum C-telopeptide fragments of type II collagen).
Participant timeline
Research assistants first conduct a screening of potential candidates over the telephone. If early checks of study eligibility are favourable, participants are booked in for a face-to-face screening visit with an orthopaedic specialist to confirm eligibility and explain the study protocol. After the screening visit, patients complete the questionnaires, undergo a knee MRI and CT, and sign the informed written consent. Patient enrolment started on November 2019. The first patient was treated in December 2019. Follow-up assessments is performed at 2, 6 and 12 months postoperatively with patient questionnaires and blood samples. At the final 12-month follow-up patients undergo knee MRI and CT scans. Due to operational delays caused by the COVID-19 pandemic, patient treatment is still ongoing; the study conclusion is foreseen before the end of 2023. Participant timeline is outlined in table 1.
Table 1.
The study procedures schedule
| Before treatment | Treatment | 2-month follow-up | 6-month follow-up | 12-month follow-up | |
| Patient eligibility | X | ||||
| Informed consent | X | ||||
| WOMAC (total and subscale) | X | X | X | X | |
| IKDC score | X | X | X | X | |
| Tegner activity score | X | X | X | X | |
| EQ-5D and EQ-VAS | X | X | X | X | |
| Blood sample | X | X | X | X | |
| BMAC sample | X | ||||
| MRI | X | X | |||
| CT | X | X | |||
| AE reporting | X | X | X | X |
AE, adverse event; BMAC, bone marrow aspirate concentrate; EQ-5D, European Quality of Life Five Dimension; EQ-VAS, European Quality-Visual Analogue Scale; IKDC, International Knee Documentation Committee; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Recruitment
Patients undergo an outpatient visit conducted by properly trained medical staff belonging to the team of orthopaedic surgeons of the Rizzoli Orthopaedic Institute, which assess patients’ eligibility and take care of patient education.
Blinding
This is a double-blind RCT with both participants and physicians assessing outcomes being blinded to treatment allocation. Only after the evaluation at the 12-month follow-up the blinding is opened and it is revealed to the patient which one of the two treatments was administered.
The blindness of treated patients is further guaranteed by the same number of surgical accesses and by the same length of the surgical incisions for both treatments. For ethical reason, no bone puncturing and injection was performed in the control group. This, however, did not compromise blinding since patients presented the same number and type of surgical incisions. Early unblinding occurs in case of premature patients drop-out. The level of blinding prevents from the enhanced placebo effect that a subchondral injection could add to the placebo effect of the intra-articular injection alone.15 16
Imaging evaluation is provided by experienced radiologists which are blinded as well to the type of treatment that the patients have received and evaluation time.
Allocation
A total of 86 eligible patients are allocated to receive either a combination of intra-articular and subchondral BMAC injections or BMAC intra articular injection alone, in a 1:1 ratio (43 patients for each group of treatment) based on a computer-generated random numbers randomisation. This is conducted by research staff members dedicated to study organisation and monitoring with no direct involvement in the study procedures. The randomisation list is covered by password and accessible only by staff members with no direct involvement in the study.
Adverse events and assessment process
Adverse events are monitored throughout the study, intraoperatively and at clinical follow-up evaluations. Standard safety and efficacy monitoring is performed through regular face-to-face visits and phone calls between visits. The patients are also requested to report any adverse events to the research staff spontaneously. Every adverse event is recorded in the patient case report form (CRF). Serious adverse events are considered those resulting in death or being life-threatening, requiring hospitalisation or intervention to prevent permanent impairment or damage; they are reported in accordance with the requirements of the ethical committee. Use of rescue pain medication is recorded at all visits without a diary and without homogenising the type of medication, which is decided by patients autonomously (although discouraged for study purposes).
To ensure high-quality execution of the trial in accordance with the protocol, all trial staff is trained by the chief investigators and provided with a standard protocol book which contains details of standard operating procedures, trial contacts, visits, measurements, monitoring, and CRFs.
Data collection methods
Data are first collected on paper-based CRFs, with the help of research trained orthopaedic residents blinded to treatment allocation and subsequently trained data analysts process data into electronic form for statistical analysis. Baseline and final MRI and CT knee scans are coded and stored at the Rizzoli Orthopaedic Institute to ensure data quality control. Operative data are collected electronically by the respective surgeons shortly after surgery.
Data management
Study data are stored in a password-protected spreadsheet on a server that is hosted at the Rizzoli Orthopaedical Institute. Data transfer is encrypted with all data de-identified. Only trained research personnel specifically dedicated to the data handling can access the database and ensures the correspondence of the electronic data with the original paper-based questionnaires and medical charts.
Statistical methods
A power analysis (G*Power V.3.1.9.2) was conducted using assumptions of 90% of power and 5% of probability of type 1 error (alpha=0.05), with a SD of 18.2 points based on a pilot study and a hypothesised 10-point difference in total WOMAC score at 12 months between treatments. Accordingly, 76 participants are needed. This leads to a moderate size effect (0.55) as per the Cohen convention (effects: small ≥0.20, medium ≥0.50 and large ≥0.80), and is in line with other effect sizes and SD reported in the literature. We increased the number of participants to a total of 86 patients (43 in each arm) to account for a possible 10% lost to follow-up. The primary analyses are intention to treat of primary and secondary outcomes. Per-protocol analyses will be performed as the secondary analyses. All those who have started the treatment are considered part of the research, regardless of whether they will complete it. For the missing data, they will be analysed using the multiple imputation analysis, performed by filling the missing data with random values from the distribution of the variable.
Continuous variables are expressed as means and SD if normally distributed, as medians and range if not. Categorical variables are expressed as frequencies and percentage. Normality of the distribution is assessed by using the Shapiro-Wilk test. The Levene test is used to assess the homoscedasticity of the data. The repeated measures of the analysis of variance (ANOVA), followed by the post hoc Sidak pairwise test, is performed to compare the scores at different follow-up times. The one-way ANOVA test is performed to assess the between group differences of continuous and normally distributed and homoscedastic data; the Mann-Whitney test is used otherwise. The ANOVA test, followed by the Scheffè post hoc pairwise comparison, is used also to assess the among groups differences of continuous, normally distributed and homoscedastic data; the Kruskal-Wallis, test followed by the Mann-Whitney test with the Bonferroni correction for multiple comparison, is used otherwise. The Monte-Carlo method is used to evaluate the non-parametric tests in case of small size of the subgroups. Pearson χ2 exact test is performed to investigate relationships between grouping variables. The Spearman’s rank correlation is used to assess correlations between the numerical scores and continuous data. The general linear model, or the generalised linear model in case of not normal distribution, is used as multivariate analysis to compare the group’s outcomes corrected by the influencing factors. The Kaplan-Meier analysis is performed to assess survival to major adverse events. For all tests, a p<0.05 is considered significant. SPSS V.19.0 (IBM) is applied for the analyses.
Data monitoring
A central project data manager is tasked to perform data quality control on all collected data. An interim report and a final report are foreseen, to be submitted to the Ministry of Health who funded the project. The monitoring personnel belongs to a research structure of the Scientific Direction of the Institution, the Applied and Translational Research Center, and it is independent from the Clinic and the medical personnel performing the study procedures. A further project auditing is performed by another independent entity of the Institution, the Clinical Trial Center. The final study report is also sent to the ethic committee.
Ethics and dissemination
Research ethics approval
Ethical approval was obtained on 5 May 2018 from the central Emilia Wide Area Ethical Committee of the Emilia-Romagna Region (CE-AVEC) settled at the University General Hospital Sant’Orsola-Malpighi of Bologna.
Protocol amendments
Minor protocol amendments, for example, database production changes to facilitate monitoring processes or improve outcome assessment by questionnaire, are fully documented. In case of major amendments, for example, changes to the patient information sheet and consent form, change of a local project leader or the inclusion of a new project site, they are submitted for approval by the lead ethics committee as required.
Consent or assent
All participants will provide informed written consent in Italian and they may drop-out the trial at any time during the study course.
Confidentiality
Data are recorded using CRFs and processed centrally at the Rizzoli Orthopaedics Institute, Bologna, Italy. The hard copies of CRFs are stored in a locked area with secured and restricted access. The electronic data are stored on password protected servers with restricted access. All data collected are kept strictly confidential. Daily backups of all electronic data occur to minimise any risk of lost data. After study completion, paper copies of data are archived in secure storage. Identifiers are kept separately and accessible only to restricted study personnel in case follow-up of study patients is necessary; however, electronic data continue to be kept in a secure electronic database. This remains password protected and with access given only to the study investigators unless otherwise authorised by the study team.
Access to data
Only members of the research team who need to contact study patients, enter data or perform data quality control have access to patient information.
Dissemination policy
This trial is produced according to the SPIRIT international standards. Results will be disseminated through peer-reviewed publications and will be submitted for presentation at national and international conferences. The authorship is based on International Committee of Medical Journal Editors 2018 Recommendations.
Scientific relevance and broader impact
This study provides a detailed method of treatment for knee OA and can offer clear indications on the potential and limitations of the combined use of intra-articular and subchondral bone injections of BMAC. The BMAC analysis provides characterisation of this product to shed greater light on the properties ensuring its effectiveness. Baseline patient-related and disease-related factors analysis can allow to better define those characteristics that make different subjects more or less responsive to this type of treatment.
Supplementary Material
Acknowledgments
Thanks to Silvia Bassini for the contribution to the graphical representation of the technique and to Elettra Pignotti for her help with the statistical analysis.
Footnotes
Contributors: ADM is the principal investigator of this study. SS, LA, AB, and DR wrote the manuscript and will conduct the trial. GV and MM are responsible of imaging evaluation. CC and BG are involved in products and patients’ characterisation. ADM, SZ, and GF applied for funding and supervise the trial. All authors read and approved the final protocol.
Funding: The study is funded by Italian Health Ministry in the Project 'Giovane Ricercatore' (GR-2016-02361990).
Disclaimer: The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Competing interests: SZ reports non-financial support from personal fees from I+SRL, grants from FidiaFarmaceutici S.p.A., Cartiheal, IGEA clinical biophysics, BIOMET and Kensey Nash, outside the submitted work. The principal investigator and other authors declare no financial and other competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med 2016;59:333–9. 10.1016/j.rehab.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 2.Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev 2019;4:221–9. 10.1302/2058-5241.4.180102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitton R. The economic burden of osteoarthritis. Am J Manag Care 2009;15:S230–5. [PubMed] [Google Scholar]
- 4.Delanois RE, Mistry JB, Gwam CU, et al. Current epidemiology of revision total knee arthroplasty in the United States. J Arthroplasty 2017;32:2663–8. 10.1016/j.arth.2017.03.066 [DOI] [PubMed] [Google Scholar]
- 5.Filardo G, Kon E, Longo UG, et al. Non-Surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2016;24:1775–85. 10.1007/s00167-016-4089-y [DOI] [PubMed] [Google Scholar]
- 6.Cavallo C, Boffa A, Andriolo L, et al. Bone marrow concentrate injections for the treatment of osteoarthritis: evidence from preclinical findings to the clinical application. Int Orthop 2021;45:525–38. 10.1007/s00264-020-04703-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boffa A, Di Martino A, Andriolo L, et al. Bone marrow aspirate concentrate injections provide similar results versus viscosupplementation up to 24 months of follow-up in patients with symptomatic knee osteoarthritis. A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2021. 10.1007/s00167-021-06793-4. [Epub ahead of print: 12 Nov 2021]. [DOI] [PubMed] [Google Scholar]
- 8.Hernigou P, Auregan JC, Dubory A, et al. Subchondral stem cell therapy versus contralateral total knee arthroplasty for osteoarthritis following secondary osteonecrosis of the knee. Int Orthop 2018;42:2563–71. 10.1007/s00264-018-3916-9 [DOI] [PubMed] [Google Scholar]
- 9.Singh V, Oliashirazi A, Tan T, et al. Clinical and pathophysiologic significance of MRI identified bone marrow lesions associated with knee osteoarthritis. Arch Bone Jt Surg 2019;7:211–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Yusup A, Kaneko H, Liu L, et al. Bone marrow lesions, subchondral bone cysts and subchondral bone attrition are associated with histological synovitis in patients with end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 2015;23:1858–64. 10.1016/j.joca.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 11.Hernigou J, Vertongen P, Rasschaert J, et al. Role of scaffolds, Subchondral, intra-articular injections of fresh autologous bone marrow concentrate regenerative cells in treating human knee cartilage lesions: different approaches and different results. Int J Mol Sci 2021;22:3844. 10.3390/ijms22083844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernigou P, Bouthors C, Bastard C, et al. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better Postpone knee arthroplasty at fifteen years? A randomized study. Int Orthop 2021;45:391–9. 10.1007/s00264-020-04687-7 [DOI] [PubMed] [Google Scholar]
- 13.Kon E, Boffa A, Andriolo L, et al. Subchondral and intra-articular injections of bone marrow concentrate are a safe and effective treatment for knee osteoarthritis: a prospective, multi-center pilot study. Knee Surg Sports Traumatol Arthrosc 2021;29:4232–40. 10.1007/s00167-021-06530-x [DOI] [PubMed] [Google Scholar]
- 14.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazeli MS, McIntyre L, Huang Y, et al. Intra-Articular placebo effect in the treatment of knee osteoarthritis: a survey of the current clinical evidence. Ther Adv Musculoskelet Dis 2022;14:X211066689. 10.1177/1759720X211066689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Previtali D, Merli G, Di Laura Frattura G, et al. The Long-Lasting Effects of "Placebo Injections" in Knee Osteoarthritis: A Meta-Analysis. Cartilage 2021;13:185S–96. 10.1177/1947603520906597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.