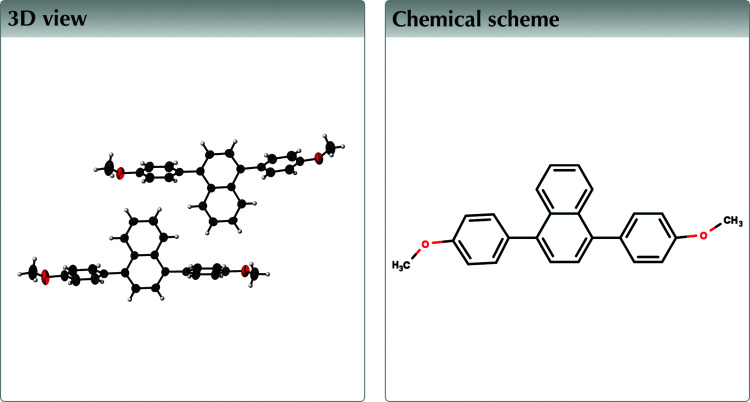
Two independent molecules comprise the asymmetric unit of the title naphthalene derivative, which exhibit very similar conformations. The pendant 4-methyoxybenzene rings are splayed out of the plane through the naphthalene ring system.
Keywords: crystal structure, naphthalene derivative, C—H⋯π interactions
Abstract
The title naphthalene derivative, C24H20O2, features 4-methyoxy-substituted benzene rings in the 1 and 4 positions of the naphthalene ring system. There are two crystallographically independent molecules (A and B) in asymmetric unit. The independent molecules have very similar conformations in which the naphthalene ring systems are only slightly bent, exhibiting dihedral angles between the constituent benzene rings of 3.76 (15) and 3.39 (15)° for A and B, respectively. The pendent 4-methyoxybenzene rings are splayed out of the plane through the naphthalene ring system to which they are connected [range of dihedral angles = 59.63 (13) to 67.09 (13)°]. In the crystal, the molecular packing is consolidated by intermolecular C—H⋯π interactions, leading to supramolecular chains along the b axis. The chains assemble without directional interactions between them.
Structure description
Molecules related to the title compound are of interest in the field of organic electronics. A closely related structure is available whereby a perfluorinated phenyl ring is fused to the naphthalene ring system which is also perfluorinated (Tannaci et al., 2008 ▸). Here, the effects of fluorination are apparent in that the pendant 4-methoxybenzene rings are effectively perpendicular to the central plane.
The molecular structures of the two crystallographically independent molecules comprising the asymmetric unit in the title compound are shown in Fig. 1 ▸. The molecules exhibit very similar conformations, as illustrated in the overlay diagram of Fig. 2 ▸. The r.m.s deviation between the bond lengths in the two molecules is 0.419 Å (Spek, 2020 ▸).
Figure 1.
The molecular structures of the title compound showing atom-numbering scheme and displacement ellipsoids at the 30% probability level. The H atoms are shown as spheres of arbitrary radius.
Figure 2.
An overlay diagram of the first (red image) and inverted-second (black) independent molecules of the title compound.
Within the naphthalene ring system, the dihedral angles between the least-squares planes through the constituent rings are 3.76 (15) and 3.39 (15)° for the two independent molecules. The best plane of the (C1–C10) naphthalene ring system forms dihedral angles of 67.09 (13) and 60.71 (13)°, respectively, with the appended (C11–C16) and (C18–C23) rings of the methoxy-substituted benzene rings indicating splayed dispositions. The corresponding values for the second independent molecule are 59.63 (13) and 63.75 (13)°. The dihedral angle between the peripheral rings, i.e. between the (C11–C16)/(C18–C23) benzene rings is 6.91 (16)° while that for the corresponding rings in the second independent molecule, i.e. (C35–C40)/(C42–C47), is 8.82 (16)°.
In the crystal, C—H⋯π interactions, Table 1 ▸, link molecules into a supramolecular chain along the b-axis direction, i.e. with a helical topology. The chains assemble in the crystal without directional interactions between them.
Table 1. Hydrogen-bond geometry (Å, °).
Cg1–Cg3 are the centroids of the (C42–C47), (C25—C34) and (C11–C16) rings, respectively.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯Cg1 | 0.93 | 2.76 | 3.531 (4) | 141 |
| C15—H15⋯Cg2i | 0.93 | 2.96 | 3.737 (4) | 142 |
| C27—H27⋯Cg3 | 0.93 | 2.81 | 3.610 (4) | 145 |
Symmetry code: (i)
 .
.
Synthesis and crystallization
Tetrathiafulvalene [2-(1,3-dithiolan-2-ylidene)-2H-1,3-dithiole; 0.204 g, 1.0 mmol] was added to a solution of 1,3-bis(4-methoxyphenyl)isobenzofuran (0.33 g, 1.0 mmol) in dry xylenes (15 ml). The solution was refluxed until the benzo[c]furan was consumed, i.e. after ca 6 h, as indicated by the disappearance of fluorescence from the solution. After removal of xylenes in vacuo, the crude product was dissolved in dry dichloromethane (DCM, 15 ml) and kept at 273 K. To this solution, triflic acid (0.075 g, 0.50 mmol) was added followed by stirring at room temperature for 10 min. After the completion of reaction (as monitored by TLC), the solution was poured into ice–water (20 ml) and then extracted with DCM (2 × 10 ml). The combined organic layer was washed with aq. NaHCO3 (2 × 10 ml) and then dried over Na2SO4. The removal of solvent was followed by column chromatographic purification (silica gel, 10% ethyl acetate in hexane) to afford 1,4-bis(4-methoxyphenyl)naphthalene (0.288 g, 85%) as a yellow solid. Single crystals suitable for X-ray diffraction were prepared by slow evaporation of an ethyl acetate solution of the compound held at room temperature; m.p. 421–423 K.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C24H20O2 |
| M r | 340.40 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 296 |
| a, b, c (Å) | 21.5500 (8), 6.0366 (2), 27.4915 (9) |
| β (°) | 92.111 (1) |
| V (Å3) | 3573.9 (2) |
| Z | 8 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.08 |
| Crystal size (mm) | 0.20 × 0.20 × 0.15 |
| Data collection | |
| Diffractometer | Bruker Kappa APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2008 ▸) |
| T min, T max | 0.984, 0.988 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 124530, 7894, 4154 |
| R int | 0.078 |
| (sin θ/λ)max (Å−1) | 0.641 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.077, 0.281, 1.02 |
| No. of reflections | 7894 |
| No. of parameters | 473 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.26, −0.28 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314620002126/tk4061sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620002126/tk4061Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620002126/tk4061Isup3.cml
CCDC reference: 1984009
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank Dr Jagan and Dr Babu Varghese, Senior Scientific Officers, SAIF, IIT Madras, Chennai, India, for the data collection.
full crystallographic data
Crystal data
| C24H20O2 | F(000) = 1440 |
| Mr = 340.40 | Dx = 1.265 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -p 2yn | Cell parameters from 7894 reflections |
| a = 21.5500 (8) Å | θ = 1.2–27.1° |
| b = 6.0366 (2) Å | µ = 0.08 mm−1 |
| c = 27.4915 (9) Å | T = 296 K |
| β = 92.111 (1)° | BLOCK, yellow |
| V = 3573.9 (2) Å3 | 0.20 × 0.20 × 0.15 mm |
| Z = 8 |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 7894 independent reflections |
| Radiation source: fine-focus sealed tube | 4154 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.078 |
| φ & ω scans | θmax = 27.1°, θmin = 1.2° |
| Absorption correction: multi-scan (SADABS; Bruker, 2008) | h = −27→27 |
| Tmin = 0.984, Tmax = 0.988 | k = −7→7 |
| 124530 measured reflections | l = −35→35 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.077 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.281 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.1238P)2 + 3.8143P] where P = (Fo2 + 2Fc2)/3 |
| 7894 reflections | (Δ/σ)max < 0.001 |
| 473 parameters | Δρmax = 0.26 e Å−3 |
| 0 restraints | Δρmin = −0.28 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. The C-bound H-atoms were included in calculated positions and treated as riding with C—H = 0.93–0.96 Å, and with Uiso(H) = 1.5Ueq(C-methyl) and 1.2Ueq(C) for the other H-atoms. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.42322 (15) | 0.6450 (5) | 0.76704 (11) | 0.0430 (8) | |
| H1 | 0.4453 | 0.6431 | 0.7386 | 0.052* | |
| C2 | 0.43579 (16) | 0.4887 (6) | 0.80184 (11) | 0.0468 (8) | |
| H2 | 0.4656 | 0.3804 | 0.7969 | 0.056* | |
| C3 | 0.40344 (16) | 0.4927 (6) | 0.84511 (12) | 0.0485 (8) | |
| H3 | 0.4122 | 0.3874 | 0.8691 | 0.058* | |
| C4 | 0.35946 (15) | 0.6486 (5) | 0.85236 (11) | 0.0442 (8) | |
| H4 | 0.3389 | 0.6492 | 0.8815 | 0.053* | |
| C5 | 0.34414 (14) | 0.8104 (5) | 0.81670 (10) | 0.0357 (7) | |
| C6 | 0.29864 (14) | 0.9775 (5) | 0.82446 (10) | 0.0379 (7) | |
| C7 | 0.29017 (16) | 1.1416 (5) | 0.78998 (11) | 0.0457 (8) | |
| H7 | 0.2614 | 1.2534 | 0.7950 | 0.055* | |
| C8 | 0.32445 (15) | 1.1425 (5) | 0.74734 (11) | 0.0443 (8) | |
| H8 | 0.3182 | 1.2574 | 0.7252 | 0.053* | |
| C9 | 0.36667 (14) | 0.9803 (5) | 0.73731 (10) | 0.0378 (7) | |
| C10 | 0.37770 (14) | 0.8092 (5) | 0.77290 (10) | 0.0360 (7) | |
| C11 | 0.40148 (14) | 0.9863 (5) | 0.69146 (10) | 0.0392 (7) | |
| C12 | 0.39530 (16) | 0.8223 (6) | 0.65583 (11) | 0.0456 (8) | |
| H12 | 0.3686 | 0.7039 | 0.6605 | 0.055* | |
| C13 | 0.42824 (16) | 0.8328 (6) | 0.61361 (11) | 0.0469 (8) | |
| H13 | 0.4231 | 0.7231 | 0.5901 | 0.056* | |
| C14 | 0.46885 (15) | 1.0066 (6) | 0.60642 (10) | 0.0419 (8) | |
| C15 | 0.47614 (17) | 1.1689 (6) | 0.64097 (11) | 0.0495 (9) | |
| H15 | 0.5035 | 1.2856 | 0.6364 | 0.059* | |
| C16 | 0.44226 (17) | 1.1576 (5) | 0.68292 (11) | 0.0478 (8) | |
| H16 | 0.4472 | 1.2692 | 0.7061 | 0.057* | |
| C17 | 0.5414 (2) | 1.1779 (7) | 0.55437 (13) | 0.0680 (11) | |
| H17A | 0.5197 | 1.3165 | 0.5561 | 0.102* | |
| H17B | 0.5577 | 1.1606 | 0.5226 | 0.102* | |
| H17C | 0.5749 | 1.1758 | 0.5784 | 0.102* | |
| C18 | 0.26124 (14) | 0.9741 (5) | 0.86889 (10) | 0.0386 (7) | |
| C19 | 0.22387 (15) | 0.7959 (6) | 0.87984 (11) | 0.0463 (8) | |
| H19 | 0.2223 | 0.6748 | 0.8589 | 0.056* | |
| C20 | 0.18858 (16) | 0.7924 (6) | 0.92110 (11) | 0.0494 (9) | |
| H20 | 0.1637 | 0.6709 | 0.9276 | 0.059* | |
| C21 | 0.19088 (16) | 0.9707 (6) | 0.95229 (11) | 0.0506 (9) | |
| C22 | 0.22778 (18) | 1.1493 (6) | 0.94256 (13) | 0.0584 (10) | |
| H22 | 0.2296 | 1.2691 | 0.9638 | 0.070* | |
| C23 | 0.26234 (17) | 1.1513 (6) | 0.90108 (12) | 0.0526 (9) | |
| H23 | 0.2868 | 1.2740 | 0.8946 | 0.063* | |
| C24 | 0.1212 (2) | 0.8005 (9) | 1.00620 (15) | 0.0839 (15) | |
| H24A | 0.0918 | 0.7689 | 0.9800 | 0.126* | |
| H24B | 0.0994 | 0.8323 | 1.0352 | 0.126* | |
| H24C | 0.1477 | 0.6745 | 1.0117 | 0.126* | |
| C25 | 0.64533 (16) | 0.5039 (5) | 0.64015 (11) | 0.0449 (8) | |
| H25 | 0.6665 | 0.4996 | 0.6113 | 0.054* | |
| C26 | 0.60133 (16) | 0.6621 (5) | 0.64606 (11) | 0.0462 (8) | |
| H26 | 0.5934 | 0.7659 | 0.6216 | 0.055* | |
| C27 | 0.56793 (16) | 0.6694 (6) | 0.68881 (11) | 0.0461 (8) | |
| H27 | 0.5381 | 0.7785 | 0.6928 | 0.055* | |
| C28 | 0.57920 (15) | 0.5166 (5) | 0.72438 (11) | 0.0424 (8) | |
| H28 | 0.5562 | 0.5220 | 0.7523 | 0.051* | |
| C29 | 0.62471 (14) | 0.3499 (5) | 0.72018 (10) | 0.0362 (7) | |
| C30 | 0.63511 (14) | 0.1831 (5) | 0.75665 (10) | 0.0371 (7) | |
| C31 | 0.67742 (16) | 0.0207 (5) | 0.74832 (11) | 0.0452 (8) | |
| H31 | 0.6829 | −0.0925 | 0.7710 | 0.054* | |
| C32 | 0.71299 (16) | 0.0197 (6) | 0.70637 (11) | 0.0462 (8) | |
| H32 | 0.7421 | −0.0921 | 0.7026 | 0.055* | |
| C33 | 0.70595 (14) | 0.1781 (5) | 0.67099 (10) | 0.0378 (7) | |
| C34 | 0.65981 (14) | 0.3453 (5) | 0.67696 (10) | 0.0366 (7) | |
| C35 | 0.74535 (15) | 0.1737 (5) | 0.62787 (11) | 0.0403 (7) | |
| C36 | 0.74759 (17) | −0.0110 (6) | 0.59848 (12) | 0.0491 (8) | |
| H36 | 0.7223 | −0.1314 | 0.6051 | 0.059* | |
| C37 | 0.78635 (18) | −0.0229 (6) | 0.55932 (13) | 0.0561 (9) | |
| H37 | 0.7863 | −0.1480 | 0.5396 | 0.067* | |
| C38 | 0.82476 (17) | 0.1528 (6) | 0.55015 (12) | 0.0517 (9) | |
| C39 | 0.82314 (17) | 0.3408 (6) | 0.57853 (12) | 0.0523 (9) | |
| H39 | 0.8483 | 0.4610 | 0.5717 | 0.063* | |
| C40 | 0.78406 (16) | 0.3503 (6) | 0.61715 (12) | 0.0473 (8) | |
| H40 | 0.7836 | 0.4771 | 0.6364 | 0.057* | |
| C41 | 0.8757 (3) | −0.0372 (9) | 0.48697 (16) | 0.0996 (18) | |
| H41A | 0.8867 | −0.1565 | 0.5087 | 0.149* | |
| H41B | 0.9083 | −0.0150 | 0.4646 | 0.149* | |
| H41C | 0.8379 | −0.0733 | 0.4691 | 0.149* | |
| C42 | 0.59868 (14) | 0.1807 (5) | 0.80161 (10) | 0.0382 (7) | |
| C43 | 0.56057 (17) | 0.0038 (5) | 0.81173 (12) | 0.0481 (8) | |
| H43 | 0.5577 | −0.1130 | 0.7897 | 0.058* | |
| C44 | 0.52641 (17) | −0.0064 (6) | 0.85351 (11) | 0.0492 (8) | |
| H44 | 0.5008 | −0.1269 | 0.8591 | 0.059* | |
| C45 | 0.53109 (15) | 0.1648 (5) | 0.88663 (10) | 0.0423 (8) | |
| C46 | 0.56849 (16) | 0.3437 (6) | 0.87743 (11) | 0.0449 (8) | |
| H46 | 0.5711 | 0.4602 | 0.8995 | 0.054* | |
| C47 | 0.60218 (15) | 0.3520 (5) | 0.83579 (11) | 0.0429 (8) | |
| H47 | 0.6276 | 0.4734 | 0.8304 | 0.051* | |
| C48 | 0.4601 (2) | −0.0070 (8) | 0.93981 (16) | 0.0840 (14) | |
| H48A | 0.4298 | −0.0267 | 0.9137 | 0.126* | |
| H48B | 0.4394 | 0.0220 | 0.9695 | 0.126* | |
| H48C | 0.4847 | −0.1390 | 0.9435 | 0.126* | |
| O1 | 0.49974 (12) | 1.0013 (4) | 0.56346 (8) | 0.0566 (7) | |
| O2 | 0.15737 (14) | 0.9849 (5) | 0.99390 (9) | 0.0790 (9) | |
| O3 | 0.86713 (14) | 0.1572 (5) | 0.51385 (10) | 0.0776 (9) | |
| O4 | 0.49915 (12) | 0.1742 (4) | 0.92913 (8) | 0.0601 (7) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0435 (19) | 0.0480 (19) | 0.0377 (16) | 0.0002 (16) | 0.0024 (14) | −0.0006 (14) |
| C2 | 0.046 (2) | 0.0478 (19) | 0.0462 (18) | 0.0056 (16) | 0.0030 (15) | 0.0003 (15) |
| C3 | 0.052 (2) | 0.049 (2) | 0.0444 (18) | 0.0091 (17) | −0.0002 (15) | 0.0116 (15) |
| C4 | 0.0435 (19) | 0.0504 (19) | 0.0387 (17) | 0.0019 (16) | 0.0013 (14) | 0.0047 (15) |
| C5 | 0.0354 (16) | 0.0401 (16) | 0.0315 (15) | −0.0007 (14) | 0.0003 (12) | 0.0005 (12) |
| C6 | 0.0361 (17) | 0.0430 (17) | 0.0345 (15) | −0.0030 (14) | 0.0012 (13) | −0.0061 (13) |
| C7 | 0.049 (2) | 0.0444 (18) | 0.0445 (18) | 0.0045 (16) | 0.0057 (15) | 0.0011 (15) |
| C8 | 0.050 (2) | 0.0441 (18) | 0.0386 (17) | 0.0036 (16) | 0.0013 (14) | 0.0074 (14) |
| C9 | 0.0399 (17) | 0.0438 (17) | 0.0298 (15) | −0.0032 (15) | 0.0001 (13) | −0.0003 (13) |
| C10 | 0.0377 (17) | 0.0385 (16) | 0.0316 (15) | −0.0035 (14) | −0.0012 (12) | −0.0008 (12) |
| C11 | 0.0422 (18) | 0.0429 (18) | 0.0322 (15) | 0.0007 (15) | 0.0000 (13) | 0.0025 (13) |
| C12 | 0.049 (2) | 0.0512 (19) | 0.0364 (16) | −0.0076 (16) | −0.0023 (14) | −0.0017 (15) |
| C13 | 0.054 (2) | 0.053 (2) | 0.0334 (16) | −0.0058 (17) | −0.0008 (14) | −0.0100 (15) |
| C14 | 0.0452 (19) | 0.0519 (19) | 0.0288 (15) | 0.0021 (16) | 0.0032 (13) | 0.0017 (14) |
| C15 | 0.058 (2) | 0.049 (2) | 0.0422 (18) | −0.0116 (17) | 0.0083 (16) | −0.0018 (15) |
| C16 | 0.065 (2) | 0.0427 (18) | 0.0357 (16) | −0.0100 (17) | 0.0066 (15) | −0.0064 (14) |
| C17 | 0.074 (3) | 0.081 (3) | 0.050 (2) | −0.015 (2) | 0.019 (2) | 0.003 (2) |
| C18 | 0.0351 (17) | 0.0437 (17) | 0.0370 (16) | 0.0006 (14) | 0.0012 (13) | −0.0016 (13) |
| C19 | 0.047 (2) | 0.0501 (19) | 0.0420 (17) | −0.0064 (16) | 0.0039 (15) | −0.0079 (15) |
| C20 | 0.047 (2) | 0.059 (2) | 0.0424 (18) | −0.0118 (17) | 0.0060 (15) | −0.0031 (16) |
| C21 | 0.049 (2) | 0.066 (2) | 0.0374 (17) | −0.0028 (19) | 0.0113 (15) | −0.0043 (16) |
| C22 | 0.066 (2) | 0.059 (2) | 0.051 (2) | −0.013 (2) | 0.0132 (18) | −0.0207 (18) |
| C23 | 0.057 (2) | 0.052 (2) | 0.0493 (19) | −0.0100 (18) | 0.0128 (17) | −0.0088 (16) |
| C24 | 0.078 (3) | 0.116 (4) | 0.059 (3) | −0.024 (3) | 0.026 (2) | 0.001 (3) |
| C25 | 0.0458 (19) | 0.0479 (19) | 0.0409 (17) | 0.0011 (16) | 0.0035 (14) | 0.0043 (15) |
| C26 | 0.050 (2) | 0.0440 (18) | 0.0443 (18) | 0.0094 (16) | −0.0009 (15) | 0.0122 (15) |
| C27 | 0.046 (2) | 0.0485 (19) | 0.0440 (18) | 0.0077 (16) | 0.0039 (15) | 0.0007 (15) |
| C28 | 0.0443 (19) | 0.0447 (18) | 0.0382 (16) | 0.0018 (16) | 0.0031 (14) | −0.0010 (14) |
| C29 | 0.0359 (17) | 0.0356 (16) | 0.0368 (16) | −0.0027 (14) | 0.0007 (13) | −0.0039 (13) |
| C30 | 0.0376 (17) | 0.0391 (17) | 0.0343 (15) | −0.0002 (14) | −0.0017 (13) | −0.0005 (13) |
| C31 | 0.052 (2) | 0.0430 (18) | 0.0411 (17) | 0.0063 (16) | 0.0026 (15) | 0.0075 (14) |
| C32 | 0.045 (2) | 0.0456 (19) | 0.0484 (19) | 0.0078 (16) | 0.0032 (15) | −0.0010 (15) |
| C33 | 0.0375 (17) | 0.0402 (17) | 0.0358 (15) | −0.0003 (14) | 0.0003 (13) | −0.0026 (13) |
| C34 | 0.0351 (16) | 0.0387 (16) | 0.0357 (15) | −0.0008 (14) | −0.0008 (12) | −0.0025 (13) |
| C35 | 0.0400 (18) | 0.0430 (18) | 0.0378 (16) | 0.0041 (15) | 0.0013 (13) | 0.0002 (14) |
| C36 | 0.052 (2) | 0.0454 (19) | 0.0501 (19) | 0.0012 (17) | 0.0084 (16) | −0.0033 (16) |
| C37 | 0.066 (3) | 0.054 (2) | 0.048 (2) | 0.009 (2) | 0.0048 (18) | −0.0102 (17) |
| C38 | 0.052 (2) | 0.064 (2) | 0.0405 (18) | 0.0125 (19) | 0.0116 (16) | 0.0080 (17) |
| C39 | 0.052 (2) | 0.053 (2) | 0.052 (2) | −0.0006 (18) | 0.0096 (16) | 0.0072 (17) |
| C40 | 0.048 (2) | 0.0461 (19) | 0.0477 (19) | −0.0004 (16) | 0.0043 (15) | −0.0035 (15) |
| C41 | 0.126 (5) | 0.119 (4) | 0.056 (3) | 0.029 (4) | 0.033 (3) | −0.009 (3) |
| C42 | 0.0393 (18) | 0.0422 (17) | 0.0327 (15) | 0.0039 (15) | −0.0022 (13) | 0.0007 (13) |
| C43 | 0.062 (2) | 0.0400 (18) | 0.0430 (18) | −0.0083 (17) | 0.0067 (16) | −0.0051 (14) |
| C44 | 0.059 (2) | 0.0448 (19) | 0.0444 (18) | −0.0086 (17) | 0.0072 (16) | 0.0038 (15) |
| C45 | 0.0447 (19) | 0.0513 (19) | 0.0309 (15) | −0.0027 (16) | 0.0003 (13) | 0.0015 (14) |
| C46 | 0.050 (2) | 0.0495 (19) | 0.0345 (16) | −0.0049 (17) | −0.0035 (14) | −0.0091 (14) |
| C47 | 0.0418 (19) | 0.0453 (18) | 0.0412 (17) | −0.0054 (15) | −0.0034 (14) | −0.0024 (14) |
| C48 | 0.100 (4) | 0.088 (3) | 0.066 (3) | −0.027 (3) | 0.033 (3) | 0.004 (2) |
| O1 | 0.0658 (17) | 0.0693 (16) | 0.0355 (12) | −0.0087 (14) | 0.0114 (11) | −0.0048 (11) |
| O2 | 0.087 (2) | 0.096 (2) | 0.0561 (16) | −0.0194 (18) | 0.0347 (15) | −0.0180 (15) |
| O3 | 0.083 (2) | 0.090 (2) | 0.0618 (17) | 0.0176 (17) | 0.0337 (15) | 0.0066 (16) |
| O4 | 0.0695 (17) | 0.0706 (17) | 0.0412 (13) | −0.0107 (14) | 0.0150 (12) | −0.0020 (12) |
Geometric parameters (Å, º)
| C1—C2 | 1.364 (4) | C25—C26 | 1.360 (4) |
| C1—C10 | 1.408 (4) | C25—C34 | 1.419 (4) |
| C1—H1 | 0.9300 | C25—H25 | 0.9300 |
| C2—C3 | 1.401 (4) | C26—C27 | 1.401 (4) |
| C2—H2 | 0.9300 | C26—H26 | 0.9300 |
| C3—C4 | 1.356 (4) | C27—C28 | 1.360 (4) |
| C3—H3 | 0.9300 | C27—H27 | 0.9300 |
| C4—C5 | 1.414 (4) | C28—C29 | 1.413 (4) |
| C4—H4 | 0.9300 | C28—H28 | 0.9300 |
| C5—C10 | 1.427 (4) | C29—C34 | 1.432 (4) |
| C5—C6 | 1.428 (4) | C29—C30 | 1.433 (4) |
| C6—C7 | 1.379 (4) | C30—C31 | 1.364 (4) |
| C6—C18 | 1.488 (4) | C30—C42 | 1.488 (4) |
| C7—C8 | 1.409 (4) | C31—C32 | 1.408 (4) |
| C7—H7 | 0.9300 | C31—H31 | 0.9300 |
| C8—C9 | 1.372 (4) | C32—C33 | 1.369 (4) |
| C8—H8 | 0.9300 | C32—H32 | 0.9300 |
| C9—C10 | 1.437 (4) | C33—C34 | 1.431 (4) |
| C9—C11 | 1.491 (4) | C33—C35 | 1.484 (4) |
| C11—C16 | 1.383 (4) | C35—C36 | 1.379 (4) |
| C11—C12 | 1.395 (4) | C35—C40 | 1.392 (4) |
| C12—C13 | 1.384 (4) | C36—C37 | 1.389 (5) |
| C12—H12 | 0.9300 | C36—H36 | 0.9300 |
| C13—C14 | 1.385 (5) | C37—C38 | 1.375 (5) |
| C13—H13 | 0.9300 | C37—H37 | 0.9300 |
| C14—C15 | 1.369 (4) | C38—O3 | 1.378 (4) |
| C14—O1 | 1.377 (3) | C38—C39 | 1.378 (5) |
| C15—C16 | 1.389 (4) | C39—C40 | 1.380 (4) |
| C15—H15 | 0.9300 | C39—H39 | 0.9300 |
| C16—H16 | 0.9300 | C40—H40 | 0.9300 |
| C17—O1 | 1.422 (4) | C41—O3 | 1.403 (5) |
| C17—H17A | 0.9600 | C41—H41A | 0.9600 |
| C17—H17B | 0.9600 | C41—H41B | 0.9600 |
| C17—H17C | 0.9600 | C41—H41C | 0.9600 |
| C18—C19 | 1.383 (4) | C42—C43 | 1.382 (4) |
| C18—C23 | 1.388 (4) | C42—C47 | 1.398 (4) |
| C19—C20 | 1.389 (4) | C43—C44 | 1.388 (4) |
| C19—H19 | 0.9300 | C43—H43 | 0.9300 |
| C20—C21 | 1.375 (5) | C44—C45 | 1.379 (4) |
| C20—H20 | 0.9300 | C44—H44 | 0.9300 |
| C21—C22 | 1.372 (5) | C45—C46 | 1.377 (4) |
| C21—O2 | 1.378 (4) | C45—O4 | 1.379 (3) |
| C22—C23 | 1.385 (5) | C46—C47 | 1.379 (4) |
| C22—H22 | 0.9300 | C46—H46 | 0.9300 |
| C23—H23 | 0.9300 | C47—H47 | 0.9300 |
| C24—O2 | 1.407 (5) | C48—O4 | 1.418 (5) |
| C24—H24A | 0.9600 | C48—H48A | 0.9600 |
| C24—H24B | 0.9600 | C48—H48B | 0.9600 |
| C24—H24C | 0.9600 | C48—H48C | 0.9600 |
| C2—C1—C10 | 121.9 (3) | C34—C25—H25 | 119.2 |
| C2—C1—H1 | 119.1 | C25—C26—C27 | 120.3 (3) |
| C10—C1—H1 | 119.1 | C25—C26—H26 | 119.9 |
| C1—C2—C3 | 119.4 (3) | C27—C26—H26 | 119.9 |
| C1—C2—H2 | 120.3 | C28—C27—C26 | 119.9 (3) |
| C3—C2—H2 | 120.3 | C28—C27—H27 | 120.1 |
| C4—C3—C2 | 120.6 (3) | C26—C27—H27 | 120.1 |
| C4—C3—H3 | 119.7 | C27—C28—C29 | 122.1 (3) |
| C2—C3—H3 | 119.7 | C27—C28—H28 | 119.0 |
| C3—C4—C5 | 121.6 (3) | C29—C28—H28 | 119.0 |
| C3—C4—H4 | 119.2 | C28—C29—C34 | 118.1 (3) |
| C5—C4—H4 | 119.2 | C28—C29—C30 | 122.3 (3) |
| C4—C5—C10 | 117.9 (3) | C34—C29—C30 | 119.6 (3) |
| C4—C5—C6 | 121.9 (3) | C31—C30—C29 | 118.4 (3) |
| C10—C5—C6 | 120.1 (3) | C31—C30—C42 | 120.6 (3) |
| C7—C6—C5 | 118.6 (3) | C29—C30—C42 | 121.0 (3) |
| C7—C6—C18 | 120.8 (3) | C30—C31—C32 | 121.9 (3) |
| C5—C6—C18 | 120.6 (3) | C30—C31—H31 | 119.1 |
| C6—C7—C8 | 121.0 (3) | C32—C31—H31 | 119.1 |
| C6—C7—H7 | 119.5 | C33—C32—C31 | 122.0 (3) |
| C8—C7—H7 | 119.5 | C33—C32—H32 | 119.0 |
| C9—C8—C7 | 122.5 (3) | C31—C32—H32 | 119.0 |
| C9—C8—H8 | 118.8 | C32—C33—C34 | 118.1 (3) |
| C7—C8—H8 | 118.8 | C32—C33—C35 | 120.2 (3) |
| C8—C9—C10 | 118.1 (3) | C34—C33—C35 | 121.7 (3) |
| C8—C9—C11 | 120.7 (3) | C25—C34—C33 | 122.0 (3) |
| C10—C9—C11 | 121.2 (3) | C25—C34—C29 | 118.0 (3) |
| C1—C10—C5 | 118.5 (3) | C33—C34—C29 | 119.9 (3) |
| C1—C10—C9 | 121.8 (3) | C36—C35—C40 | 117.5 (3) |
| C5—C10—C9 | 119.6 (3) | C36—C35—C33 | 121.1 (3) |
| C16—C11—C12 | 117.2 (3) | C40—C35—C33 | 121.4 (3) |
| C16—C11—C9 | 120.3 (3) | C35—C36—C37 | 122.1 (3) |
| C12—C11—C9 | 122.6 (3) | C35—C36—H36 | 119.0 |
| C13—C12—C11 | 121.2 (3) | C37—C36—H36 | 119.0 |
| C13—C12—H12 | 119.4 | C38—C37—C36 | 119.1 (3) |
| C11—C12—H12 | 119.4 | C38—C37—H37 | 120.5 |
| C12—C13—C14 | 120.0 (3) | C36—C37—H37 | 120.5 |
| C12—C13—H13 | 120.0 | C37—C38—O3 | 124.6 (3) |
| C14—C13—H13 | 120.0 | C37—C38—C39 | 120.2 (3) |
| C15—C14—O1 | 124.4 (3) | O3—C38—C39 | 115.2 (3) |
| C15—C14—C13 | 120.0 (3) | C38—C39—C40 | 119.9 (3) |
| O1—C14—C13 | 115.6 (3) | C38—C39—H39 | 120.0 |
| C14—C15—C16 | 119.4 (3) | C40—C39—H39 | 120.0 |
| C14—C15—H15 | 120.3 | C39—C40—C35 | 121.2 (3) |
| C16—C15—H15 | 120.3 | C39—C40—H40 | 119.4 |
| C11—C16—C15 | 122.3 (3) | C35—C40—H40 | 119.4 |
| C11—C16—H16 | 118.9 | O3—C41—H41A | 109.5 |
| C15—C16—H16 | 118.9 | O3—C41—H41B | 109.5 |
| O1—C17—H17A | 109.5 | H41A—C41—H41B | 109.5 |
| O1—C17—H17B | 109.5 | O3—C41—H41C | 109.5 |
| H17A—C17—H17B | 109.5 | H41A—C41—H41C | 109.5 |
| O1—C17—H17C | 109.5 | H41B—C41—H41C | 109.5 |
| H17A—C17—H17C | 109.5 | C43—C42—C47 | 117.0 (3) |
| H17B—C17—H17C | 109.5 | C43—C42—C30 | 120.7 (3) |
| C19—C18—C23 | 117.2 (3) | C47—C42—C30 | 122.3 (3) |
| C19—C18—C6 | 121.8 (3) | C42—C43—C44 | 122.6 (3) |
| C23—C18—C6 | 121.0 (3) | C42—C43—H43 | 118.7 |
| C18—C19—C20 | 122.0 (3) | C44—C43—H43 | 118.7 |
| C18—C19—H19 | 119.0 | C45—C44—C43 | 119.0 (3) |
| C20—C19—H19 | 119.0 | C45—C44—H44 | 120.5 |
| C21—C20—C19 | 119.3 (3) | C43—C44—H44 | 120.5 |
| C21—C20—H20 | 120.4 | C46—C45—O4 | 115.9 (3) |
| C19—C20—H20 | 120.4 | C46—C45—C44 | 119.7 (3) |
| C22—C21—C20 | 120.1 (3) | O4—C45—C44 | 124.3 (3) |
| C22—C21—O2 | 116.0 (3) | C45—C46—C47 | 120.7 (3) |
| C20—C21—O2 | 124.0 (3) | C45—C46—H46 | 119.6 |
| C21—C22—C23 | 120.0 (3) | C47—C46—H46 | 119.6 |
| C21—C22—H22 | 120.0 | C46—C47—C42 | 120.9 (3) |
| C23—C22—H22 | 120.0 | C46—C47—H47 | 119.5 |
| C22—C23—C18 | 121.4 (3) | C42—C47—H47 | 119.5 |
| C22—C23—H23 | 119.3 | O4—C48—H48A | 109.5 |
| C18—C23—H23 | 119.3 | O4—C48—H48B | 109.5 |
| O2—C24—H24A | 109.5 | H48A—C48—H48B | 109.5 |
| O2—C24—H24B | 109.5 | O4—C48—H48C | 109.5 |
| H24A—C24—H24B | 109.5 | H48A—C48—H48C | 109.5 |
| O2—C24—H24C | 109.5 | H48B—C48—H48C | 109.5 |
| H24A—C24—H24C | 109.5 | C14—O1—C17 | 117.5 (3) |
| H24B—C24—H24C | 109.5 | C21—O2—C24 | 117.5 (3) |
| C26—C25—C34 | 121.6 (3) | C38—O3—C41 | 118.0 (4) |
| C26—C25—H25 | 119.2 | C45—O4—C48 | 117.5 (3) |
| C10—C1—C2—C3 | −1.1 (5) | C27—C28—C29—C30 | −177.5 (3) |
| C1—C2—C3—C4 | 0.7 (5) | C28—C29—C30—C31 | 176.2 (3) |
| C2—C3—C4—C5 | 0.7 (5) | C34—C29—C30—C31 | −1.6 (4) |
| C3—C4—C5—C10 | −1.7 (5) | C28—C29—C30—C42 | −1.2 (4) |
| C3—C4—C5—C6 | −178.9 (3) | C34—C29—C30—C42 | −179.0 (3) |
| C4—C5—C6—C7 | 174.2 (3) | C29—C30—C31—C32 | 3.3 (5) |
| C10—C5—C6—C7 | −3.0 (4) | C42—C30—C31—C32 | −179.4 (3) |
| C4—C5—C6—C18 | −5.8 (4) | C30—C31—C32—C33 | −1.8 (5) |
| C10—C5—C6—C18 | 177.1 (3) | C31—C32—C33—C34 | −1.5 (5) |
| C5—C6—C7—C8 | 1.5 (5) | C31—C32—C33—C35 | 178.6 (3) |
| C18—C6—C7—C8 | −178.5 (3) | C26—C25—C34—C33 | 179.9 (3) |
| C6—C7—C8—C9 | 1.4 (5) | C26—C25—C34—C29 | 2.6 (5) |
| C7—C8—C9—C10 | −2.6 (5) | C32—C33—C34—C25 | −174.1 (3) |
| C7—C8—C9—C11 | 179.3 (3) | C35—C33—C34—C25 | 5.7 (5) |
| C2—C1—C10—C5 | 0.1 (5) | C32—C33—C34—C29 | 3.0 (4) |
| C2—C1—C10—C9 | 176.8 (3) | C35—C33—C34—C29 | −177.1 (3) |
| C4—C5—C10—C1 | 1.3 (4) | C28—C29—C34—C25 | −2.1 (4) |
| C6—C5—C10—C1 | 178.5 (3) | C30—C29—C34—C25 | 175.8 (3) |
| C4—C5—C10—C9 | −175.5 (3) | C28—C29—C34—C33 | −179.4 (3) |
| C6—C5—C10—C9 | 1.7 (4) | C30—C29—C34—C33 | −1.5 (4) |
| C8—C9—C10—C1 | −175.6 (3) | C32—C33—C35—C36 | 56.0 (4) |
| C11—C9—C10—C1 | 2.4 (4) | C34—C33—C35—C36 | −123.9 (3) |
| C8—C9—C10—C5 | 1.1 (4) | C32—C33—C35—C40 | −120.3 (4) |
| C11—C9—C10—C5 | 179.1 (3) | C34—C33—C35—C40 | 59.8 (4) |
| C8—C9—C11—C16 | 63.9 (4) | C40—C35—C36—C37 | −0.4 (5) |
| C10—C9—C11—C16 | −114.1 (4) | C33—C35—C36—C37 | −176.8 (3) |
| C8—C9—C11—C12 | −116.7 (4) | C35—C36—C37—C38 | 1.5 (5) |
| C10—C9—C11—C12 | 65.3 (4) | C36—C37—C38—O3 | 177.0 (3) |
| C16—C11—C12—C13 | −0.6 (5) | C36—C37—C38—C39 | −2.2 (5) |
| C9—C11—C12—C13 | 179.9 (3) | C37—C38—C39—C40 | 1.8 (5) |
| C11—C12—C13—C14 | 0.9 (5) | O3—C38—C39—C40 | −177.5 (3) |
| C12—C13—C14—C15 | −0.4 (5) | C38—C39—C40—C35 | −0.8 (5) |
| C12—C13—C14—O1 | 179.5 (3) | C36—C35—C40—C39 | 0.1 (5) |
| O1—C14—C15—C16 | 179.7 (3) | C33—C35—C40—C39 | 176.5 (3) |
| C13—C14—C15—C16 | −0.3 (5) | C31—C30—C42—C43 | −59.6 (4) |
| C12—C11—C16—C15 | −0.1 (5) | C29—C30—C42—C43 | 117.7 (3) |
| C9—C11—C16—C15 | 179.3 (3) | C31—C30—C42—C47 | 119.0 (3) |
| C14—C15—C16—C11 | 0.6 (5) | C29—C30—C42—C47 | −63.7 (4) |
| C7—C6—C18—C19 | 120.9 (4) | C47—C42—C43—C44 | 0.5 (5) |
| C5—C6—C18—C19 | −59.1 (4) | C30—C42—C43—C44 | 179.2 (3) |
| C7—C6—C18—C23 | −58.8 (4) | C42—C43—C44—C45 | −0.8 (5) |
| C5—C6—C18—C23 | 121.2 (3) | C43—C44—C45—C46 | 1.1 (5) |
| C23—C18—C19—C20 | 0.1 (5) | C43—C44—C45—O4 | 179.4 (3) |
| C6—C18—C19—C20 | −179.6 (3) | O4—C45—C46—C47 | −179.6 (3) |
| C18—C19—C20—C21 | −0.3 (5) | C44—C45—C46—C47 | −1.1 (5) |
| C19—C20—C21—C22 | −0.1 (6) | C45—C46—C47—C42 | 0.8 (5) |
| C19—C20—C21—O2 | 179.4 (3) | C43—C42—C47—C46 | −0.5 (5) |
| C20—C21—C22—C23 | 0.6 (6) | C30—C42—C47—C46 | −179.1 (3) |
| O2—C21—C22—C23 | −178.9 (3) | C15—C14—O1—C17 | −0.7 (5) |
| C21—C22—C23—C18 | −0.8 (6) | C13—C14—O1—C17 | 179.4 (3) |
| C19—C18—C23—C22 | 0.4 (5) | C22—C21—O2—C24 | −177.2 (4) |
| C6—C18—C23—C22 | −179.9 (3) | C20—C21—O2—C24 | 3.4 (6) |
| C34—C25—C26—C27 | −1.3 (5) | C37—C38—O3—C41 | −5.7 (6) |
| C25—C26—C27—C28 | −0.5 (5) | C39—C38—O3—C41 | 173.5 (4) |
| C26—C27—C28—C29 | 1.0 (5) | C46—C45—O4—C48 | 179.9 (3) |
| C27—C28—C29—C34 | 0.4 (5) | C44—C45—O4—C48 | 1.5 (5) |
Hydrogen-bond geometry (Å, º)
Cg1–Cg3 are the centroids of the (C42–C47), (C25—C34) and (C11–C16) rings, respectively.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···Cg1 | 0.93 | 2.76 | 3.531 (4) | 141 |
| C15—H15···Cg2i | 0.93 | 2.96 | 3.737 (4) | 142 |
| C27—H27···Cg3 | 0.93 | 2.81 | 3.610 (4) | 145 |
Symmetry code: (i) x, y+1, z.
References
- Bruker (2008). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2020). Acta Cryst. E76, 1–11. [DOI] [PMC free article] [PubMed]
- Tannaci, J. F., Noji, M., McBee, J. L. & Tilley, T. D. (2008). J. Org. Chem. 73, 7895–7900. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314620002126/tk4061sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620002126/tk4061Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620002126/tk4061Isup3.cml
CCDC reference: 1984009
Additional supporting information: crystallographic information; 3D view; checkCIF report




