An aluminium-deficient γ-brass phase, Al7.85Cr5.16, was synthesized by high-pressure sintering and its crystal structure determined.
Keywords: crystal structure, high-pressure sintering, intermetallic, γ-brass phase
Abstract
An aluminium-deficient Al8Cr5-type intermetallic with formula Al7.85Cr5.16 (octaaluminium pentachromium) was uncovered when high-pressure sintering of a mixture with composition Al11Cr4 was carried out. Structure analysis reveals that there are three co-occupied positions with refined occupancy factors for Al atoms being 0.958, 0.772 and 1/2. The present phase is confirmed to be isotypic with the previously reported rhombohedral Al8Cr5 ordered phase [Bradley & Lu (1937). Z. Kristallogr.
96, 20–37] and structurally closely related to the disordered phases of rhombohedral Al16Cr9.5 and cubic Al8Cr5.
Structure description
The γ2-Al8Cr5 phase (hereafter named as the γ2 phase) was determined to have a γ-brass-like structure by powder diffraction photographs. This phase was found in slowly cooled chromium–aluminium alloys (Bradley & Lu, 1937 ▸). Although the same clusters of 26 atoms are found in the γ2 phase, the atomic arrangement in the γ2 phase is much more complex than that of the γ-brass, and results in a rhombohedral rather than cubic symmetry (Bradley & Lu, 1937 ▸). A high-temperature γ1 phase was also reported to be stable between 1350 and 980°C at the same composition (Bradley & Lu, 1937 ▸) and its structure has been redetermined by single-crystal methods for a sample sintered at 1000°C for 6 h and re-annealed at 1215°C for 287 h (Brandon et al., 1977 ▸). As a result of the close agreement of Brandon’s analysis with that of Bradley & Lu, it was suggested that either the structure of γ1 and γ2 are very similar, or that in the former case the crystals decomposed to γ2 on quenching. In another work, the high-temperature γ1 phase prepared by splat cooling was reported to be of the same type as Cu5Zn8, by using power diffraction data combined with electron diffraction patterns (Braun et al., 1992 ▸). When comparing the three aforementioned models (see Table S1 of the supporting information), it was found that there are one vacancy position and three co-occupied positions in the Brandon model, while all atomic sites are fully occupied in Bradley & Lu’s model. For the convenience of comparison, the cubic Braun model was transformed to the rhombohedral description, and it was found that there are two co-occupied positions. In the study reported herein, the crystal structure of a third type of Al8Cr5 phase, with the refined chemical composition Al7.85Cr5.16 and hereafter named as γ2′-Al8Cr5 phase, was determined by single-crystal X-ray diffraction measurements.
Fig. 1 ▸ shows the crystal structure of γ2′-Al8Cr5 based on the standardized crystal data in the primitive trigonal setting (see Tables S2 and S3 of the supporting information). There are 78 atoms in the unit cell (a = b = 12.8717 Å, c = 7.8408 Å, α = β = 90°, γ = 120°), whose volume is three times that of the refined model (trigonal cell, rhombohedral axes, see Table 1 ▸). For simplicity, only two distorted icosahedra centred at Wyckoff sites 3a (Cr4, with coordinates 0, 0, z) are illustrated in Fig. 1 ▸, and the environment of the Cr4 atoms is shown in Fig. 2 ▸. The twelve vertices include three Al atoms (Al5), three Cr atoms (Cr6) along with six co-occupied Al/Cr sites (Al1/Cr1 and Al3/Cr3), for which the refined site occupancies converged to 0.772 (4) and 0.958 (4) for Al atoms Al1 and Al3.
Figure 1.
The crystal structure of Al7.85Cr5.16. The icosahedra centred on Cr4 are emphasized.
Table 1. Experimental details.
| Crystal data | |
| Chemical formula | Al7.85Cr5.16 |
| M r | 479.72 |
| Crystal system, space group | Trigonal, R3m:R |
| Temperature (K) | 296 |
| a (Å) | 7.8777 (5), 7.8777 (5) |
| α (°) | 109.566 (2) |
| V (Å3) | 375.01 (7) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 8.05 |
| Crystal size (mm) | 0.13 × 0.06 × 0.05 |
| Data collection | |
| Diffractometer | Bruker D8 Venture Photon 100 CMOS |
| Absorption correction | Multi-scan (SADABS; Bruker, 2015 ▸) |
| T min, T max | 0.496, 0.523 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 7330, 576, 547 |
| R int | 0.090 |
| (sin θ/λ)max (Å−1) | 0.634 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.068, 0.178, 1.16 |
| No. of reflections | 576 |
| No. of parameters | 53 |
| No. of restraints | 37 |
| Δρmax, Δρmin (e Å−3) | 1.02, −1.27 |
| Absolute structure | Refined as an inversion twin. |
| Absolute structure parameter | 0.3 (2) |
Figure 2.
The environment of the Cr4 atom. Displacement ellipsoids are given at the 99% probability level. [Symmetry codes: (i) y, z, x; (v) y, z, x.]
The principle building blocks in the structure can also be represented by four interpenetrating distorted icosahedra centred at one Cr4 and three Al3/Cr3 atomic sites, as shown in Fig. 3 ▸, similarly to the building blocks of the I-cell (space group I
 3m) of the γ-brass phase (Pankova et al., 2013 ▸). According to the topological analysis of the structure model with the ‘nanocluster’ method available in the ToposPro package (Akhmetshina & Blatov, 2017 ▸), these one Cr4 and three Al3/Cr3 sites form an inner tetrahedron (IT), followed by an outer tetrahedron (OT), an octahedron (OH), whose vertices are projected onto the edges of the outer tetrahedron, and finally a distorted cuboctahedron (CO) with vertices located above the edges of the octahedron, as illustrated in Fig. 4 ▸.
3m) of the γ-brass phase (Pankova et al., 2013 ▸). According to the topological analysis of the structure model with the ‘nanocluster’ method available in the ToposPro package (Akhmetshina & Blatov, 2017 ▸), these one Cr4 and three Al3/Cr3 sites form an inner tetrahedron (IT), followed by an outer tetrahedron (OT), an octahedron (OH), whose vertices are projected onto the edges of the outer tetrahedron, and finally a distorted cuboctahedron (CO) with vertices located above the edges of the octahedron, as illustrated in Fig. 4 ▸.
Figure 3.
26-atom γ-brass-type cluster represented as four interpenetrating distorted icosahedra centred at one Cr4 and three Al3/Cr3 sites.
Figure 4.
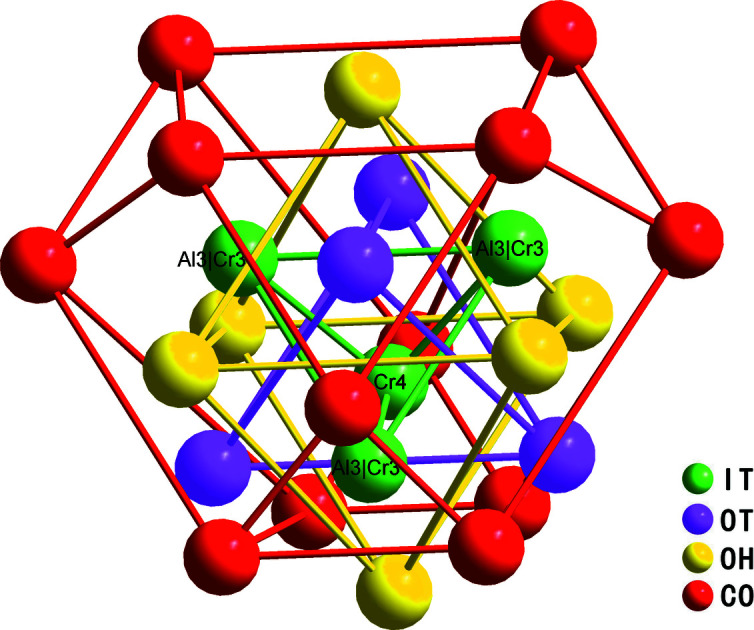
26-atom γ-brass-type cluster represented as a sequence of polyhedral shells.
The present rhombohedral γ2′-Al8Cr5 phase is thus confirmed to be isotypic to the previously reported ordered Al8Cr5 phase (Bradley & Lu, 1937 ▸), and closely related to the the disordered rhombohedral Al16Cr9.5 phase (Brandon et al., 1977 ▸) and the disordered cubic Al8Cr5 phase (Braun et al., 1992 ▸).
Synthesis and crystallization
The high-purity elements Al (indicated purity 99.8%, 0.588 g) and Cr (indicated purity 99.95%, 0.539 g) were mixed uniformly in the stoichiometric ratio 11:4 and thoroughly ground in an agate mortar. The blended powders were then placed in a cemented carbide grinding mould of 5 mm diameter, and pressed into a tablet at about 4 MPa for 5 min. A cylindrical block (5 mm in diameter and 3 mm in height) was obtained without deformations or cracks. Details of the high-pressure sintering experiment using a six-anvil high-temperature high-pressure apparatus can be found elsewhere (Liu & Fan, 2018 ▸). The samples were pressurized up to 5 GPa and heated to 1400°C for 30 minutes, slowly cooled to 660°C and held at this temperature for 2 h, and then rapidly cooled to room temperature by turning off the furnace power. Subsequently, a small amount of powder sample was uniformly placed on the inner wall of a quartz tube, annealed in a vacuum environment, heated to 300°C for 24 h, and then cooled within the furnace. A piece of a single crystal (0.13 × 0.06 × 0.05 mm3) was selected and mounted on a glass fibre for single-crystal X-ray diffraction measurements.
Refinement
Table 1 ▸ shows the details of data collection and structural refinement. Three sites are co-occupied by Al and Cr atoms (Al1/Cr1, Al2/Cr2, Al3/Cr3). Site occupancies were refined, and then fixed to their as-found values, 0.772, 0.5 and 0.958 for Al1, Al2 and Al3, respectively, assuming full occupancy for each site. Atoms sharing the same site were constrained to have the same coordinates and displacement parameters. Moreover, disordered atoms were restrained to be isotropic, with standard deviations of 0.01 Å2 (Sheldrick, 2015b ▸). The maximum and minimum residual electron densities in the last difference map are located 1.68 Å from atom Cr3 and 0.36 Å from atom Cr4, respectively. The crystal was considered as a sample twinned by inversion (Parsons et al., 2013 ▸), and the batch scale factor converged to x = 0.3 (2).
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314620004228/bh4049sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620004228/bh4049Isup2.hkl
supporting information. DOI: 10.1107/S2414314620004228/bh4049sup3.pdf
CCDC reference: 1993113
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| Al7.85Cr5.16 | Dx = 4.248 Mg m−3 |
| Mr = 479.72 | Mo Kα radiation, λ = 0.71073 Å |
| Trigonal, R3m:R | Cell parameters from 2208 reflections |
| a = 7.8777 (5) Å | θ = 3.2–26.3° |
| α = 109.566 (2)° | µ = 8.05 mm−1 |
| V = 375.01 (7) Å3 | T = 296 K |
| Z = 2 | Graininess, metallic silver |
| F(000) = 451 | 0.13 × 0.06 × 0.05 mm |
Data collection
| Bruker D8 Venture Photon 100 CMOS diffractometer | 547 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.090 |
| Absorption correction: multi-scan (SADABS; Bruker, 2015) | θmax = 26.8°, θmin = 3.2° |
| Tmin = 0.496, Tmax = 0.523 | h = −9→9 |
| 7330 measured reflections | k = −9→9 |
| 576 independent reflections | l = −9→9 |
Refinement
| Refinement on F2 | 37 restraints |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0652P)2 + 19.1482P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.068 | (Δ/σ)max < 0.001 |
| wR(F2) = 0.178 | Δρmax = 1.02 e Å−3 |
| S = 1.16 | Δρmin = −1.27 e Å−3 |
| 576 reflections | Absolute structure: Refined as an inversion twin. |
| 53 parameters | Absolute structure parameter: 0.3 (2) |
Special details
| Refinement. Refined as a two-component inversion twin |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Al1 | 0.2998 (14) | 0.2998 (14) | 0.0928 (15) | 0.0108 (19) | 0.772 |
| Cr1 | 0.2998 (14) | 0.2998 (14) | 0.0928 (15) | 0.0108 (19) | 0.228 |
| Al2 | 1.3096 (13) | 0.6646 (11) | 0.6646 (11) | 0.0117 (19) | 0.5 |
| Cr2 | 1.3096 (13) | 0.6646 (11) | 0.6646 (11) | 0.0117 (19) | 0.5 |
| Al3 | 0.9238 (18) | 0.6488 (14) | 0.6488 (14) | 0.012 (2) | 0.958 |
| Cr3 | 0.9238 (18) | 0.6488 (14) | 0.6488 (14) | 0.012 (2) | 0.042 |
| Cr4 | −0.0434 (12) | −0.0434 (12) | −0.0434 (12) | 0.006 (2) | |
| Cr5 | 0.6391 (8) | 0.3060 (8) | 0.3060 (8) | 0.0028 (10) | |
| Cr6 | 1.3001 (10) | 0.9464 (8) | 0.9464 (8) | 0.0108 (14) | |
| Cr7 | 0.5198 (13) | 0.5198 (13) | 0.5198 (13) | 0.008 (2) | |
| Al4 | 0.5500 (17) | −0.0607 (15) | 0.2804 (16) | 0.018 (2) | |
| Al5 | 0.0366 (18) | 0.0366 (18) | −0.313 (2) | 0.015 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Al1 | 0.007 (3) | 0.007 (3) | 0.019 (5) | 0.005 (3) | 0.006 (3) | 0.006 (3) |
| Cr1 | 0.007 (3) | 0.007 (3) | 0.019 (5) | 0.005 (3) | 0.006 (3) | 0.006 (3) |
| Al2 | 0.008 (4) | 0.008 (3) | 0.008 (3) | 0.000 (3) | 0.000 (3) | 0.001 (3) |
| Cr2 | 0.008 (4) | 0.008 (3) | 0.008 (3) | 0.000 (3) | 0.000 (3) | 0.001 (3) |
| Al3 | 0.013 (5) | 0.010 (3) | 0.010 (3) | 0.007 (3) | 0.007 (3) | −0.001 (3) |
| Cr3 | 0.013 (5) | 0.010 (3) | 0.010 (3) | 0.007 (3) | 0.007 (3) | −0.001 (3) |
| Cr4 | 0.007 (4) | 0.007 (4) | 0.007 (4) | 0.005 (4) | 0.005 (4) | 0.005 (4) |
| Cr5 | 0.001 (2) | 0.0034 (18) | 0.0034 (18) | 0.0001 (17) | 0.0001 (17) | 0.002 (2) |
| Cr6 | 0.016 (3) | 0.008 (3) | 0.008 (3) | 0.006 (3) | 0.006 (3) | 0.003 (3) |
| Cr7 | 0.006 (3) | 0.006 (3) | 0.006 (3) | 0.000 (4) | 0.000 (4) | 0.000 (4) |
| Al4 | 0.024 (5) | 0.021 (4) | 0.031 (5) | 0.016 (3) | 0.021 (4) | 0.021 (4) |
| Al5 | 0.015 (4) | 0.015 (4) | 0.017 (5) | 0.004 (5) | 0.011 (4) | 0.011 (4) |
Geometric parameters (Å, º)
| Al1—Cr5 | 2.611 (7) | Cr2—Al5xi | 2.930 (16) |
| Al1—Cr5i | 2.611 (7) | Al3—Cr5 | 2.572 (12) |
| Al1—Cr6ii | 2.634 (7) | Al3—Cr6 | 2.606 (11) |
| Al1—Cr6iii | 2.634 (7) | Al3—Cr4xiii | 2.655 (12) |
| Al1—Cr4 | 2.644 (11) | Al3—Cr7 | 2.678 (13) |
| Al1—Al4i | 2.654 (11) | Al3—Al5xiii | 2.758 (11) |
| Al1—Al4iv | 2.654 (11) | Al3—Al5xiv | 2.758 (11) |
| Al1—Al5 | 2.659 (17) | Al3—Al4xii | 2.798 (12) |
| Al1—Al1i | 2.665 (16) | Al3—Al4ix | 2.798 (12) |
| Al1—Al1v | 2.665 (16) | Cr3—Cr5 | 2.572 (12) |
| Al1—Cr7 | 2.739 (11) | Cr3—Cr6 | 2.606 (11) |
| Cr1—Cr5 | 2.611 (7) | Cr3—Cr4xiii | 2.655 (12) |
| Cr1—Cr5i | 2.611 (7) | Cr3—Cr7 | 2.678 (13) |
| Cr1—Cr6ii | 2.634 (7) | Cr3—Al5xiii | 2.758 (11) |
| Cr1—Cr6iii | 2.634 (7) | Cr3—Al5xiv | 2.758 (11) |
| Cr1—Cr4 | 2.644 (11) | Cr3—Al4xii | 2.798 (12) |
| Cr1—Al4i | 2.654 (11) | Cr3—Al4ix | 2.798 (12) |
| Cr1—Al4iv | 2.654 (11) | Cr4—Al5 | 2.616 (10) |
| Cr1—Al5 | 2.659 (17) | Cr4—Al5v | 2.616 (10) |
| Cr1—Cr7 | 2.739 (11) | Cr4—Al5i | 2.616 (10) |
| Al2—Cr6 | 2.604 (9) | Cr4—Cr6iii | 2.761 (7) |
| Al2—Cr7vi | 2.647 (9) | Cr4—Cr6ii | 2.761 (7) |
| Al2—Al4vii | 2.656 (9) | Cr5—Cr7 | 2.603 (7) |
| Al2—Al4viii | 2.656 (9) | Cr5—Al4xii | 2.620 (10) |
| Al2—Cr5ix | 2.754 (6) | Cr5—Al4ix | 2.620 (10) |
| Al2—Cr5x | 2.754 (6) | Cr5—Al4iv | 2.652 (9) |
| Al2—Al5xi | 2.930 (16) | Cr5—Al4 | 2.652 (9) |
| Al2—Al4xii | 2.996 (10) | Cr5—Al5x | 2.683 (12) |
| Al2—Al4ix | 2.996 (10) | Cr6—Al5xi | 2.654 (12) |
| Cr2—Cr6 | 2.604 (9) | Cr6—Al4xv | 2.739 (11) |
| Cr2—Cr7vi | 2.647 (9) | Cr6—Al4xiii | 2.739 (11) |
| Cr2—Al4vii | 2.656 (9) | Cr6—Al5xiv | 2.849 (8) |
| Cr2—Al4viii | 2.656 (9) | Al4—Al5x | 2.693 (12) |
| Cr2—Cr5ix | 2.754 (6) | Al4—Al4ix | 2.726 (5) |
| Cr2—Cr5x | 2.754 (6) | Al4—Al4xvi | 2.726 (5) |
| Cr5—Al1—Cr5i | 110.4 (5) | Cr3—Cr5—Cr7 | 62.3 (4) |
| Cr5—Al1—Cr6ii | 63.75 (14) | Al3—Cr5—Cr7 | 62.3 (4) |
| Cr5i—Al1—Cr6ii | 167.7 (4) | Cr3—Cr5—Al1v | 116.2 (4) |
| Cr5—Al1—Cr6iii | 167.7 (4) | Al3—Cr5—Al1v | 116.2 (4) |
| Cr5i—Al1—Cr6iii | 63.75 (14) | Cr7—Cr5—Al1v | 63.4 (3) |
| Cr6ii—Al1—Cr6iii | 119.6 (5) | Cr7—Cr5—Al1 | 63.4 (3) |
| Cr5—Al1—Cr4 | 112.8 (3) | Al1v—Cr5—Al1 | 61.4 (4) |
| Cr5i—Al1—Cr4 | 112.8 (3) | Cr7—Cr5—Cr1 | 63.4 (3) |
| Cr6ii—Al1—Cr4 | 63.1 (2) | Cr3—Cr5—Al4xii | 65.2 (3) |
| Cr6iii—Al1—Cr4 | 63.1 (2) | Al3—Cr5—Al4xii | 65.2 (3) |
| Cr5—Al1—Al4i | 125.5 (4) | Cr7—Cr5—Al4xii | 110.8 (3) |
| Cr5i—Al1—Al4i | 60.5 (2) | Al1v—Cr5—Al4xii | 168.9 (3) |
| Cr6ii—Al1—Al4i | 131.8 (4) | Al1—Cr5—Al4xii | 107.8 (3) |
| Cr6iii—Al1—Al4i | 62.4 (2) | Cr1—Cr5—Al4xii | 107.8 (3) |
| Cr4—Al1—Al4i | 120.0 (3) | Cr3—Cr5—Al4ix | 65.2 (3) |
| Cr5—Al1—Al4iv | 60.5 (2) | Al3—Cr5—Al4ix | 65.2 (3) |
| Cr5i—Al1—Al4iv | 125.5 (4) | Cr7—Cr5—Al4ix | 110.8 (3) |
| Cr6ii—Al1—Al4iv | 62.4 (2) | Al1v—Cr5—Al4ix | 107.8 (3) |
| Cr6iii—Al1—Al4iv | 131.8 (4) | Al1—Cr5—Al4ix | 168.9 (3) |
| Cr4—Al1—Al4iv | 120.0 (3) | Cr1—Cr5—Al4ix | 168.9 (3) |
| Al4i—Al1—Al4iv | 81.7 (5) | Al4xii—Cr5—Al4ix | 83.0 (5) |
| Cr5—Al1—Al5 | 124.0 (2) | Cr3—Cr5—Al4iv | 121.2 (3) |
| Cr5i—Al1—Al5 | 124.0 (2) | Al3—Cr5—Al4iv | 121.2 (3) |
| Cr6ii—Al1—Al5 | 65.1 (3) | Cr7—Cr5—Al4iv | 115.6 (3) |
| Cr6iii—Al1—Al5 | 65.1 (3) | Al1v—Cr5—Al4iv | 110.7 (4) |
| Cr4—Al1—Al5 | 59.1 (3) | Al1—Cr5—Al4iv | 60.5 (3) |
| Al4i—Al1—Al5 | 76.7 (3) | Cr1—Cr5—Al4iv | 60.5 (3) |
| Al4iv—Al1—Al5 | 76.7 (3) | Al4xii—Cr5—Al4iv | 62.3 (2) |
| Cr5—Al1—Al1i | 108.1 (2) | Al4ix—Cr5—Al4iv | 129.1 (2) |
| Cr5i—Al1—Al1i | 59.3 (2) | Cr3—Cr5—Al4 | 121.2 (3) |
| Cr6ii—Al1—Al1i | 111.0 (2) | Al3—Cr5—Al4 | 121.2 (3) |
| Cr6iii—Al1—Al1i | 59.6 (2) | Cr7—Cr5—Al4 | 115.6 (3) |
| Cr4—Al1—Al1i | 59.73 (18) | Al1v—Cr5—Al4 | 60.5 (3) |
| Al4i—Al1—Al1i | 109.0 (2) | Al1—Cr5—Al4 | 110.7 (4) |
| Al4iv—Al1—Al1i | 168.2 (3) | Cr1—Cr5—Al4 | 110.7 (4) |
| Al5—Al1—Al1i | 110.2 (3) | Al4xii—Cr5—Al4 | 129.1 (2) |
| Cr5—Al1—Al1v | 59.3 (2) | Al4ix—Cr5—Al4 | 62.3 (2) |
| Cr5i—Al1—Al1v | 108.1 (2) | Al4iv—Cr5—Al4 | 111.7 (5) |
| Cr6ii—Al1—Al1v | 59.6 (2) | Cr3—Cr5—Al5x | 128.3 (4) |
| Cr6iii—Al1—Al1v | 111.0 (2) | Al3—Cr5—Al5x | 128.3 (4) |
| Cr4—Al1—Al1v | 59.73 (18) | Cr7—Cr5—Al5x | 169.4 (4) |
| Al4i—Al1—Al1v | 168.2 (3) | Al1v—Cr5—Al5x | 107.8 (3) |
| Al4iv—Al1—Al1v | 109.0 (2) | Al1—Cr5—Al5x | 107.8 (3) |
| Al5—Al1—Al1v | 110.2 (3) | Cr1—Cr5—Al5x | 107.8 (3) |
| Al1i—Al1—Al1v | 60.000 (1) | Al4xii—Cr5—Al5x | 76.8 (3) |
| Cr5—Al1—Cr7 | 58.2 (2) | Al4ix—Cr5—Al5x | 76.8 (3) |
| Cr5i—Al1—Cr7 | 58.2 (2) | Al4iv—Cr5—Al5x | 60.6 (3) |
| Cr6ii—Al1—Cr7 | 111.2 (3) | Al4—Cr5—Al5x | 60.6 (3) |
| Cr6iii—Al1—Cr7 | 111.2 (3) | Cr3—Cr5—Al2xvi | 64.3 (2) |
| Cr4—Al1—Cr7 | 110.2 (4) | Al3—Cr5—Al2xvi | 64.3 (2) |
| Al4i—Al1—Cr7 | 111.1 (3) | Cr7—Cr5—Al2xvi | 59.1 (2) |
| Al4iv—Al1—Cr7 | 111.1 (3) | Al1v—Cr5—Al2xvi | 60.5 (3) |
| Al5—Al1—Cr7 | 169.4 (5) | Al1—Cr5—Al2xvi | 110.8 (3) |
| Al1i—Al1—Cr7 | 60.89 (18) | Cr1—Cr5—Al2xvi | 110.8 (3) |
| Al1v—Al1—Cr7 | 60.89 (18) | Al4xii—Cr5—Al2xvi | 125.9 (4) |
| Cr5—Cr1—Cr5i | 110.4 (5) | Al4ix—Cr5—Al2xvi | 59.2 (3) |
| Cr5—Cr1—Cr6ii | 63.75 (14) | Al4iv—Cr5—Al2xvi | 170.8 (4) |
| Cr5i—Cr1—Cr6ii | 167.7 (4) | Al4—Cr5—Al2xvi | 67.3 (2) |
| Cr5—Cr1—Cr6iii | 167.7 (4) | Al5x—Cr5—Al2xvi | 123.0 (2) |
| Cr5i—Cr1—Cr6iii | 63.75 (14) | Cr2—Cr6—Al1xiii | 149.6 (2) |
| Cr6ii—Cr1—Cr6iii | 119.6 (5) | Al2—Cr6—Al1xiii | 149.6 (2) |
| Cr5—Cr1—Cr4 | 112.8 (3) | Cr3—Cr6—Al1xiii | 108.9 (3) |
| Cr5i—Cr1—Cr4 | 112.8 (3) | Al3—Cr6—Al1xiii | 108.9 (3) |
| Cr6ii—Cr1—Cr4 | 63.1 (2) | Al1xiii—Cr6—Al1xiv | 60.8 (4) |
| Cr6iii—Cr1—Cr4 | 63.1 (2) | Cr2—Cr6—Al5xi | 67.7 (4) |
| Cr5—Cr1—Al4i | 125.5 (4) | Al2—Cr6—Al5xi | 67.7 (4) |
| Cr5i—Cr1—Al4i | 60.5 (2) | Cr3—Cr6—Al5xi | 136.9 (4) |
| Cr6ii—Cr1—Al4i | 131.8 (4) | Al3—Cr6—Al5xi | 136.9 (4) |
| Cr6iii—Cr1—Al4i | 62.4 (2) | Al1xiii—Cr6—Al5xi | 108.0 (4) |
| Cr4—Cr1—Al4i | 120.0 (3) | Al1xiv—Cr6—Al5xi | 108.0 (4) |
| Cr5—Cr1—Al4iv | 60.5 (2) | Al1xiii—Cr6—Al4xv | 59.2 (3) |
| Cr5i—Cr1—Al4iv | 125.5 (4) | Al1xiv—Cr6—Al4xv | 107.4 (4) |
| Cr6ii—Cr1—Al4iv | 62.4 (2) | Al5xi—Cr6—Al4xv | 59.9 (3) |
| Cr6iii—Cr1—Al4iv | 131.8 (4) | Cr2—Cr6—Al4xiii | 96.4 (3) |
| Cr4—Cr1—Al4iv | 120.0 (3) | Al2—Cr6—Al4xiii | 96.4 (3) |
| Al4i—Cr1—Al4iv | 81.7 (5) | Cr3—Cr6—Al4xiii | 126.1 (2) |
| Cr5—Cr1—Al5 | 124.0 (2) | Al3—Cr6—Al4xiii | 126.1 (2) |
| Cr5i—Cr1—Al5 | 124.0 (2) | Al1xiii—Cr6—Al4xiii | 107.4 (4) |
| Cr6ii—Cr1—Al5 | 65.1 (3) | Al1xiv—Cr6—Al4xiii | 59.2 (3) |
| Cr6iii—Cr1—Al5 | 65.1 (3) | Al5xi—Cr6—Al4xiii | 59.9 (3) |
| Cr4—Cr1—Al5 | 59.1 (3) | Al4xv—Cr6—Al4xiii | 106.5 (5) |
| Al4i—Cr1—Al5 | 76.7 (3) | Al1xiii—Cr6—Cr4xiii | 58.6 (2) |
| Al4iv—Cr1—Al5 | 76.7 (3) | Al1xiv—Cr6—Cr4xiii | 58.6 (2) |
| Cr5—Cr1—Cr7 | 58.2 (2) | Al5xi—Cr6—Cr4xiii | 163.9 (4) |
| Cr5i—Cr1—Cr7 | 58.2 (2) | Al4xv—Cr6—Cr4xiii | 113.1 (3) |
| Cr6ii—Cr1—Cr7 | 111.2 (3) | Al4xiii—Cr6—Cr4xiii | 113.1 (3) |
| Cr6iii—Cr1—Cr7 | 111.2 (3) | Cr2—Cr6—Cr5xiii | 127.0 (4) |
| Cr4—Cr1—Cr7 | 110.2 (4) | Al2—Cr6—Cr5xiii | 127.0 (4) |
| Al4i—Cr1—Cr7 | 111.1 (3) | Cr3—Cr6—Cr5xiii | 163.9 (3) |
| Al4iv—Cr1—Cr7 | 111.1 (3) | Al3—Cr6—Cr5xiii | 163.9 (3) |
| Al5—Cr1—Cr7 | 169.4 (5) | Al1xiii—Cr6—Cr5xiii | 57.7 (2) |
| Cr6—Al2—Cr7vi | 150.7 (4) | Al1xiv—Cr6—Cr5xiii | 57.7 (2) |
| Cr6—Al2—Al4vii | 71.0 (2) | Al5xi—Cr6—Cr5xiii | 59.2 (3) |
| Cr7vi—Al2—Al4vii | 108.3 (2) | Al4xv—Cr6—Cr5xiii | 57.6 (2) |
| Cr6—Al2—Al4viii | 71.0 (2) | Al4xiii—Cr6—Cr5xiii | 57.6 (3) |
| Cr7vi—Al2—Al4viii | 108.3 (2) | Cr4xiii—Cr6—Cr5xiii | 104.7 (3) |
| Al4vii—Al2—Al4viii | 141.3 (4) | Cr2—Cr6—Al5xiv | 99.6 (3) |
| Cr6—Al2—Cr5ix | 128.85 (18) | Al2—Cr6—Al5xiv | 99.6 (3) |
| Cr7vi—Al2—Cr5ix | 57.6 (2) | Cr3—Cr6—Al5xiv | 60.5 (3) |
| Al4vii—Al2—Cr5ix | 159.6 (4) | Al3—Cr6—Al5xiv | 60.5 (3) |
| Al4viii—Al2—Cr5ix | 57.9 (2) | Al1xiii—Cr6—Al5xiv | 105.5 (3) |
| Cr6—Al2—Cr5x | 128.85 (18) | Al1xiv—Cr6—Al5xiv | 57.8 (3) |
| Cr7vi—Al2—Cr5x | 57.6 (2) | Al5xi—Cr6—Al5xiv | 127.5 (2) |
| Al4vii—Al2—Cr5x | 57.9 (2) | Al4xv—Cr6—Al5xiv | 164.0 (4) |
| Al4viii—Al2—Cr5x | 159.6 (4) | Al4xiii—Cr6—Al5xiv | 72.2 (3) |
| Cr5ix—Al2—Cr5x | 102.2 (4) | Cr4xiii—Cr6—Al5xiv | 55.6 (2) |
| Cr6—Al2—Al5xi | 56.9 (3) | Cr5xiii—Cr6—Al5xiv | 111.8 (3) |
| Cr7vi—Al2—Al5xi | 93.8 (4) | Cr5—Cr7—Cr5v | 110.9 (2) |
| Al4vii—Al2—Al5xi | 82.8 (3) | Cr5—Cr7—Cr5i | 110.9 (2) |
| Al4viii—Al2—Al5xi | 82.8 (3) | Cr5v—Cr7—Cr5i | 110.9 (2) |
| Cr5ix—Al2—Al5xi | 111.2 (3) | Cr5—Cr7—Al2xviii | 166.5 (5) |
| Cr5x—Al2—Al5xi | 111.2 (3) | Cr5v—Cr7—Al2xviii | 63.27 (8) |
| Cr6—Al2—Al4xii | 99.9 (3) | Cr5i—Cr7—Al2xviii | 63.27 (8) |
| Cr7vi—Al2—Al4xii | 103.8 (3) | Cr5—Cr7—Al2xix | 63.27 (8) |
| Al4vii—Al2—Al4xii | 57.30 (18) | Cr5v—Cr7—Al2xix | 166.5 (5) |
| Al4viii—Al2—Al4xii | 123.5 (4) | Cr5i—Cr7—Al2xix | 63.27 (8) |
| Cr5ix—Al2—Al4xii | 108.9 (3) | Cr5—Cr7—Al2xvi | 63.27 (8) |
| Cr5x—Al2—Al4xii | 54.7 (2) | Cr5v—Cr7—Al2xvi | 63.27 (8) |
| Al5xi—Al2—Al4xii | 139.6 (3) | Cr5i—Cr7—Al2xvi | 166.5 (5) |
| Cr6—Al2—Al4ix | 99.9 (3) | Al2xviii—Cr7—Al2xvi | 119.39 (7) |
| Cr7vi—Al2—Al4ix | 103.8 (3) | Al2xix—Cr7—Al2xvi | 119.39 (7) |
| Al4vii—Al2—Al4ix | 123.5 (4) | Cr5—Cr7—Al3 | 58.3 (2) |
| Al4viii—Al2—Al4ix | 57.30 (18) | Cr5v—Cr7—Al3 | 124.25 (17) |
| Cr5ix—Al2—Al4ix | 54.7 (2) | Cr5i—Cr7—Al3 | 124.25 (17) |
| Cr5x—Al2—Al4ix | 108.9 (3) | Cr5—Cr7—Cr3 | 58.3 (2) |
| Al5xi—Al2—Al4ix | 139.6 (3) | Cr5v—Cr7—Cr3 | 124.25 (17) |
| Al4xii—Al2—Al4ix | 70.8 (4) | Cr5i—Cr7—Cr3 | 124.25 (17) |
| Cr6—Cr2—Cr7vi | 150.7 (4) | Cr5—Cr7—Al3i | 124.25 (17) |
| Cr6—Cr2—Al4vii | 71.0 (2) | Cr5v—Cr7—Al3i | 124.25 (17) |
| Cr7vi—Cr2—Al4vii | 108.3 (2) | Cr5i—Cr7—Al3i | 58.3 (2) |
| Cr6—Cr2—Al4viii | 71.0 (2) | Al2xviii—Cr7—Al3i | 64.46 (17) |
| Cr7vi—Cr2—Al4viii | 108.3 (2) | Al2xix—Cr7—Al3i | 64.46 (17) |
| Al4vii—Cr2—Al4viii | 141.3 (4) | Al2xvi—Cr7—Al3i | 135.3 (5) |
| Cr6—Cr2—Cr5ix | 128.85 (18) | Al3—Cr7—Al3i | 82.8 (4) |
| Cr7vi—Cr2—Cr5ix | 57.6 (2) | Cr3—Cr7—Al3i | 82.8 (4) |
| Al4vii—Cr2—Cr5ix | 159.6 (4) | Cr5—Cr7—Al3v | 124.25 (17) |
| Al4viii—Cr2—Cr5ix | 57.9 (2) | Cr5v—Cr7—Al3v | 58.3 (2) |
| Cr6—Cr2—Cr5x | 128.85 (18) | Cr5i—Cr7—Al3v | 124.25 (17) |
| Cr7vi—Cr2—Cr5x | 57.6 (2) | Cr5—Cr7—Al1v | 58.5 (2) |
| Al4vii—Cr2—Cr5x | 57.9 (2) | Cr5v—Cr7—Al1v | 58.5 (2) |
| Al4viii—Cr2—Cr5x | 159.6 (4) | Cr5i—Cr7—Al1v | 106.2 (4) |
| Cr5ix—Cr2—Cr5x | 102.2 (4) | Al2xviii—Cr7—Al1v | 110.2 (3) |
| Cr6—Cr2—Al5xi | 56.9 (3) | Al2xix—Cr7—Al1v | 110.2 (3) |
| Cr7vi—Cr2—Al5xi | 93.8 (4) | Al2xvi—Cr7—Al1v | 60.3 (3) |
| Al4vii—Cr2—Al5xi | 82.8 (3) | Al3—Cr7—Al1v | 108.7 (3) |
| Al4viii—Cr2—Al5xi | 82.8 (3) | Cr3—Cr7—Al1v | 108.7 (3) |
| Cr5ix—Cr2—Al5xi | 111.2 (3) | Al3i—Cr7—Al1v | 164.4 (4) |
| Cr5x—Cr2—Al5xi | 111.2 (3) | Al3v—Cr7—Al1v | 108.7 (3) |
| Cr5—Al3—Cr6 | 157.4 (4) | Cr5—Cr7—Al1i | 106.2 (4) |
| Cr5—Al3—Cr4xiii | 139.3 (5) | Cr5v—Cr7—Al1i | 58.5 (2) |
| Cr6—Al3—Cr4xiii | 63.3 (3) | Cr5i—Cr7—Al1i | 58.5 (2) |
| Cr5—Al3—Cr7 | 59.4 (3) | Al2xviii—Cr7—Al1i | 60.3 (3) |
| Cr6—Al3—Cr7 | 143.2 (4) | Al2xix—Cr7—Al1i | 110.2 (3) |
| Cr4xiii—Al3—Cr7 | 79.9 (4) | Al2xvi—Cr7—Al1i | 110.2 (3) |
| Cr5—Al3—Al5xiii | 123.2 (3) | Al3—Cr7—Al1i | 164.4 (4) |
| Cr6—Al3—Al5xiii | 64.1 (3) | Cr3—Cr7—Al1i | 164.4 (4) |
| Cr4xiii—Al3—Al5xiii | 57.8 (3) | Al3i—Cr7—Al1i | 108.7 (3) |
| Cr7—Al3—Al5xiii | 97.1 (3) | Al3v—Cr7—Al1i | 108.7 (3) |
| Cr5—Al3—Al5xiv | 123.2 (3) | Al1v—Cr7—Al1i | 58.2 (4) |
| Cr6—Al3—Al5xiv | 64.1 (3) | Cr5xvi—Al4—Cr5 | 144.8 (4) |
| Cr4xiii—Al3—Al5xiv | 57.8 (3) | Cr5xvi—Al4—Al1v | 86.3 (4) |
| Cr7—Al3—Al5xiv | 97.1 (3) | Cr5—Al4—Al1v | 59.0 (3) |
| Al5xiii—Al3—Al5xiv | 109.5 (6) | Cr5xvi—Al4—Al2xx | 62.9 (2) |
| Cr5—Al3—Al4xii | 58.2 (3) | Cr5—Al4—Al2xx | 150.4 (5) |
| Cr6—Al3—Al4xii | 105.2 (4) | Al1v—Al4—Al2xx | 148.8 (4) |
| Cr4xiii—Al3—Al4xii | 141.1 (3) | Cr5xvi—Al4—Al5x | 145.4 (6) |
| Cr7—Al3—Al4xii | 103.4 (4) | Cr5—Al4—Al5x | 60.2 (3) |
| Al5xiii—Al3—Al4xii | 83.5 (3) | Al1v—Al4—Al5x | 106.3 (3) |
| Al5xiv—Al3—Al4xii | 154.2 (5) | Al2xx—Al4—Al5x | 102.4 (4) |
| Cr5—Al3—Al4ix | 58.2 (3) | Cr5xvi—Al4—Al4ix | 134.5 (5) |
| Cr6—Al3—Al4ix | 105.2 (4) | Cr5—Al4—Al4ix | 58.3 (4) |
| Cr4xiii—Al3—Al4ix | 141.1 (3) | Al1v—Al4—Al4ix | 103.5 (4) |
| Cr7—Al3—Al4ix | 103.4 (4) | Al2xx—Al4—Al4ix | 95.5 (4) |
| Al5xiii—Al3—Al4ix | 154.2 (5) | Al5x—Al4—Al4ix | 74.9 (5) |
| Al5xiv—Al3—Al4ix | 83.5 (3) | Cr5xvi—Al4—Al4xvi | 59.4 (3) |
| Al4xii—Al3—Al4ix | 76.6 (5) | Cr5—Al4—Al4xvi | 128.9 (4) |
| Cr5—Cr3—Cr6 | 157.4 (4) | Al1v—Al4—Al4xvi | 102.1 (5) |
| Cr5—Cr3—Cr4xiii | 139.3 (5) | Al2xx—Al4—Al4xvi | 67.6 (4) |
| Cr6—Cr3—Cr4xiii | 63.3 (3) | Al5x—Al4—Al4xvi | 86.1 (5) |
| Cr5—Cr3—Cr7 | 59.4 (3) | Al4ix—Al4—Al4xvi | 151.5 (6) |
| Cr6—Cr3—Cr7 | 143.2 (4) | Cr5xvi—Al4—Cr6ii | 106.6 (5) |
| Cr4xiii—Cr3—Cr7 | 79.9 (4) | Cr5—Al4—Cr6ii | 61.8 (2) |
| Cr5—Cr3—Al5xiii | 123.2 (3) | Al1v—Al4—Cr6ii | 58.4 (3) |
| Cr6—Cr3—Al5xiii | 64.1 (3) | Al2xx—Al4—Cr6ii | 132.5 (4) |
| Cr4xiii—Cr3—Al5xiii | 57.8 (3) | Al5x—Al4—Cr6ii | 58.5 (3) |
| Cr7—Cr3—Al5xiii | 97.1 (3) | Al4ix—Al4—Cr6ii | 116.5 (5) |
| Cr5—Cr3—Al5xiv | 123.2 (3) | Al4xvi—Al4—Cr6ii | 68.0 (3) |
| Cr6—Cr3—Al5xiv | 64.1 (3) | Cr5xvi—Al4—Al3xvi | 56.6 (3) |
| Cr4xiii—Cr3—Al5xiv | 57.8 (3) | Cr5—Al4—Al3xvi | 118.0 (5) |
| Cr7—Cr3—Al5xiv | 97.1 (3) | Al1v—Al4—Al3xvi | 97.2 (4) |
| Al5xiii—Cr3—Al5xiv | 109.5 (6) | Al2xx—Al4—Al3xvi | 62.7 (3) |
| Cr5—Cr3—Al4xii | 58.2 (3) | Al5x—Al4—Al3xvi | 147.5 (5) |
| Cr6—Cr3—Al4xii | 105.2 (4) | Al4ix—Al4—Al3xvi | 78.0 (4) |
| Cr4xiii—Cr3—Al4xii | 141.1 (3) | Al4xvi—Al4—Al3xvi | 110.9 (4) |
| Cr7—Cr3—Al4xii | 103.4 (4) | Cr6ii—Al4—Al3xvi | 153.0 (5) |
| Al5xiii—Cr3—Al4xii | 83.5 (3) | Cr5xvi—Al4—Al2xvi | 99.8 (4) |
| Al5xiv—Cr3—Al4xii | 154.2 (5) | Cr5—Al4—Al2xvi | 58.0 (2) |
| Cr5—Cr3—Al4ix | 58.2 (3) | Al1v—Al4—Al2xvi | 56.9 (3) |
| Cr6—Cr3—Al4ix | 105.2 (4) | Al2xx—Al4—Al2xvi | 120.5 (4) |
| Cr4xiii—Cr3—Al4ix | 141.1 (3) | Al5x—Al4—Al2xvi | 114.1 (4) |
| Cr7—Cr3—Al4ix | 103.4 (4) | Al4ix—Al4—Al2xvi | 55.1 (2) |
| Al5xiii—Cr3—Al4ix | 154.2 (5) | Al4xvi—Al4—Al2xvi | 153.3 (5) |
| Al5xiv—Cr3—Al4ix | 83.5 (3) | Cr6ii—Al4—Al2xvi | 106.8 (3) |
| Al4xii—Cr3—Al4ix | 76.6 (5) | Al3xvi—Al4—Al2xvi | 61.3 (4) |
| Al5—Cr4—Al5v | 118.81 (14) | Cr4—Al5—Cr6xxi | 152.4 (6) |
| Al5—Cr4—Al5i | 118.81 (14) | Cr4—Al5—Cr1 | 60.2 (4) |
| Al5v—Cr4—Al5i | 118.81 (14) | Cr6xxi—Al5—Cr1 | 147.5 (5) |
| Al5—Cr4—Cr1 | 60.7 (4) | Cr4—Al5—Al1 | 60.2 (4) |
| Al5v—Cr4—Cr1 | 112.2 (3) | Cr6xxi—Al5—Al1 | 147.5 (5) |
| Al5i—Cr4—Cr1 | 112.2 (3) | Cr4—Al5—Cr5xix | 145.1 (6) |
| Al5—Cr4—Al1 | 60.7 (4) | Cr6xxi—Al5—Cr5xix | 62.5 (3) |
| Al5v—Cr4—Al1 | 112.2 (3) | Cr1—Al5—Cr5xix | 84.9 (5) |
| Al5i—Cr4—Al1 | 112.2 (3) | Al1—Al5—Cr5xix | 84.9 (5) |
| Al5—Cr4—Al1i | 112.2 (3) | Cr4—Al5—Al4xxii | 125.1 (3) |
| Al5v—Cr4—Al1i | 112.2 (3) | Cr6xxi—Al5—Al4xxii | 61.6 (3) |
| Al5i—Cr4—Al1i | 60.7 (4) | Al1—Al5—Al4xxii | 102.9 (4) |
| Cr1—Cr4—Al1i | 60.5 (4) | Cr5xix—Al5—Al4xxii | 59.1 (3) |
| Al1—Cr4—Al1i | 60.5 (4) | Cr4—Al5—Al4xix | 125.1 (3) |
| Al5—Cr4—Al1v | 112.2 (3) | Cr6xxi—Al5—Al4xix | 61.6 (3) |
| Al5v—Cr4—Al1v | 60.7 (4) | Cr1—Al5—Al4xix | 102.9 (4) |
| Al5i—Cr4—Al1v | 112.2 (3) | Al1—Al5—Al4xix | 102.9 (4) |
| Cr1—Cr4—Al1v | 60.5 (4) | Cr5xix—Al5—Al4xix | 59.1 (3) |
| Al1—Cr4—Al1v | 60.5 (4) | Al4xxii—Al5—Al4xix | 109.2 (5) |
| Al1i—Cr4—Al1v | 60.5 (4) | Cr4—Al5—Al3iii | 59.1 (3) |
| Al5—Cr4—Al3iii | 63.1 (2) | Cr6xxi—Al5—Al3iii | 101.0 (4) |
| Al5v—Cr4—Al3iii | 134.0 (6) | Cr1—Al5—Al3iii | 103.8 (4) |
| Al5i—Cr4—Al3iii | 63.1 (2) | Al1—Al5—Al3iii | 103.8 (4) |
| Cr1—Cr4—Al3iii | 107.2 (3) | Cr5xix—Al5—Al3iii | 138.3 (3) |
| Al1—Cr4—Al3iii | 107.2 (3) | Al4xxii—Al5—Al3iii | 149.3 (6) |
| Al1i—Cr4—Al3iii | 107.2 (3) | Al4xix—Al5—Al3iii | 79.2 (3) |
| Al1v—Cr4—Al3iii | 165.2 (4) | Cr4—Al5—Al3ii | 59.1 (3) |
| Al5—Cr4—Al3xvii | 134.0 (6) | Cr6xxi—Al5—Al3ii | 101.0 (4) |
| Al5v—Cr4—Al3xvii | 63.1 (2) | Cr1—Al5—Al3ii | 103.8 (4) |
| Al5i—Cr4—Al3xvii | 63.1 (2) | Al1—Al5—Al3ii | 103.8 (4) |
| Cr1—Cr4—Al3xvii | 165.2 (4) | Cr5xix—Al5—Al3ii | 138.3 (3) |
| Al1—Cr4—Al3xvii | 165.2 (4) | Al4xxii—Al5—Al3ii | 79.2 (3) |
| Al1i—Cr4—Al3xvii | 107.2 (3) | Al4xix—Al5—Al3ii | 149.3 (6) |
| Al1v—Cr4—Al3xvii | 107.2 (3) | Al3iii—Al5—Al3ii | 79.9 (6) |
| Al3iii—Cr4—Al3xvii | 83.6 (4) | Cr4—Al5—Cr6ii | 60.5 (3) |
| Al5—Cr4—Al3ii | 63.1 (2) | Cr6xxi—Al5—Cr6ii | 126.6 (2) |
| Al5v—Cr4—Al3ii | 63.1 (2) | Cr1—Al5—Cr6ii | 57.0 (2) |
| Al5i—Cr4—Al3ii | 134.0 (6) | Al1—Al5—Cr6ii | 57.0 (2) |
| Cr1—Cr4—Al3ii | 107.2 (3) | Cr5xix—Al5—Cr6ii | 101.9 (3) |
| Al1—Cr4—Al3ii | 107.2 (3) | Al4xxii—Al5—Cr6ii | 66.8 (2) |
| Al1i—Cr4—Al3ii | 165.2 (4) | Al4xix—Al5—Cr6ii | 155.4 (6) |
| Al1v—Cr4—Al3ii | 107.2 (3) | Al3iii—Al5—Cr6ii | 117.2 (4) |
| Al3iii—Cr4—Al3ii | 83.6 (4) | Al3ii—Al5—Cr6ii | 55.4 (2) |
| Al3xvii—Cr4—Al3ii | 83.6 (4) | Cr4—Al5—Cr6iii | 60.5 (3) |
| Al5—Cr4—Cr6iii | 63.93 (12) | Cr6xxi—Al5—Cr6iii | 126.6 (2) |
| Al5v—Cr4—Cr6iii | 168.5 (6) | Cr1—Al5—Cr6iii | 57.0 (2) |
| Al5i—Cr4—Cr6iii | 63.93 (12) | Al1—Al5—Cr6iii | 57.0 (2) |
| Cr1—Cr4—Cr6iii | 58.27 (18) | Cr5xix—Al5—Cr6iii | 101.9 (3) |
| Al1—Cr4—Cr6iii | 58.27 (18) | Al4xxii—Al5—Cr6iii | 155.4 (6) |
| Al1i—Cr4—Cr6iii | 58.27 (18) | Al4xix—Al5—Cr6iii | 66.8 (2) |
| Al1v—Cr4—Cr6iii | 107.8 (4) | Al3iii—Al5—Cr6iii | 55.4 (2) |
| Al3iii—Cr4—Cr6iii | 57.5 (3) | Al3ii—Al5—Cr6iii | 117.2 (4) |
| Al3xvii—Cr4—Cr6iii | 124.18 (16) | Cr6ii—Al5—Cr6iii | 106.1 (4) |
| Al3ii—Cr4—Cr6iii | 124.18 (16) | Cr4—Al5—Al2xxi | 97.0 (4) |
| Al5—Cr4—Cr6ii | 63.93 (12) | Cr6xxi—Al5—Al2xxi | 55.3 (3) |
| Al5v—Cr4—Cr6ii | 63.93 (12) | Cr1—Al5—Al2xxi | 157.2 (5) |
| Al5i—Cr4—Cr6ii | 168.5 (6) | Al1—Al5—Al2xxi | 157.2 (5) |
| Cr1—Cr4—Cr6ii | 58.27 (18) | Cr5xix—Al5—Al2xxi | 117.9 (4) |
| Al1—Cr4—Cr6ii | 58.27 (18) | Al4xxii—Al5—Al2xxi | 90.1 (4) |
| Al1i—Cr4—Cr6ii | 107.8 (4) | Al4xix—Al5—Al2xxi | 90.1 (4) |
| Al1v—Cr4—Cr6ii | 58.27 (18) | Al3iii—Al5—Al2xxi | 59.8 (3) |
| Al3iii—Cr4—Cr6ii | 124.18 (16) | Al3ii—Al5—Al2xxi | 59.8 (3) |
| Al3xvii—Cr4—Cr6ii | 124.18 (16) | Cr6ii—Al5—Al2xxi | 113.8 (3) |
| Al3ii—Cr4—Cr6ii | 57.5 (3) | Cr6iii—Al5—Al2xxi | 113.8 (3) |
| Cr6iii—Cr4—Cr6ii | 111.1 (2) |
Symmetry codes: (i) z, x, y; (ii) x−1, y−1, z−1; (iii) z−1, x−1, y−1; (iv) x, z, y; (v) y, z, x; (vi) x+1, y, z; (vii) z+1, y+1, x; (viii) z+1, x, y+1; (ix) y+1, z, x; (x) z+1, x, y; (xi) z+2, x+1, y+1; (xii) y+1, x, z; (xiii) x+1, y+1, z+1; (xiv) y+1, z+1, x+1; (xv) x+1, z+1, y+1; (xvi) z, x−1, y; (xvii) y−1, z−1, x−1; (xviii) x−1, y, z; (xix) y, z, x−1; (xx) y, z−1, x−1; (xxi) y−1, z−1, x−2; (xxii) z, y, x−1.
Funding Statement
Funding for this research was provided by: Research Foundation of Education Bureau of Hebei Province (grant No. ZD2018069); The National Natural Science Foundation of China (grant No. 51771165).
References
- Akhmetshina, T. G. & Blatov, V. A. (2017). Z. Kristallogr. 232, 497–506.
- Bradley, A. J. & Lu, S. S. (1937). Z. Kristallogr. 96, 20–37.
- Brandenburg, K. & Putz, H. (2017). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Brandon, J. K., Pearson, W. B., Riley, P. W., Chieh, C. & Stokhuyzen, R. (1977). Acta Cryst. B33, 1088–1095.
- Braun, J., Ellner, M. & Predel, B. (1992). J. Alloys Compd. 183, 444–448.
- Bruker (2015). APEX3, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Liu, C. & Fan, C. (2018). IUCrData, 3, x180363.
- Pankova, A. A., Blatov, V. A., Ilyushin, G. D. & Proserpio, D. M. (2013). Inorg. Chem. 52, 13094–13107. [DOI] [PubMed]
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314620004228/bh4049sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620004228/bh4049Isup2.hkl
supporting information. DOI: 10.1107/S2414314620004228/bh4049sup3.pdf
CCDC reference: 1993113
Additional supporting information: crystallographic information; 3D view; checkCIF report





