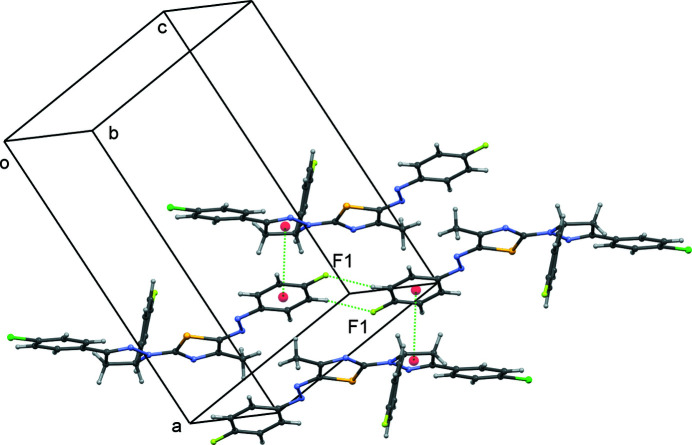
Pairs of molecules related by inversion symmetry are linked by intermolecular C—H⋯F contacts with R(8)2 2 geometry.
Keywords: crystal structure, pyrazole, thiazole, heterocycles
Abstract
The molecule of the title compound, C25H18ClF2N5S, comprises almost co-planar fluorophenyl, methylthiazolyl, pyrazolyl and chlorophenyl rings with the second fluorophenyl ring almost perpendicular to this plane. One fluorophenyl group is disordered over two components of occupancy ratio 0.767 (10):0.233 (10) related by a 24.2 (8)° twist. In the crystal, two molecules related by inversion symmetry are linked by a pair of C—H⋯F contacts in an R(8)2
2 geometry.
Structure description
Various pyrazolinyl thiazoles have pharmacological and biological applications (Abdel-Wahab et al., 2017 ▸; Abd-Rabou et al., 2018 ▸; Saeed et al., 2017 ▸). In addition, heterocycles containing both pyrazole and thiazole moieties have been used as versatile intermediates in organic synthesis of biologically active compounds (Secrieru et al., 2019 ▸; Shaabani et al., 2019 ▸; Sharma et al., 2020 ▸). Recently, we have published the X-ray crystal structures for related heterocycles (El-Hiti, Abdel-Wahab, Alqahtani et al., 2019 ▸; El-Hiti, Abdel-Wahab, Yousif et al., 2019 ▸; El-Hiti et al., 2018 ▸).
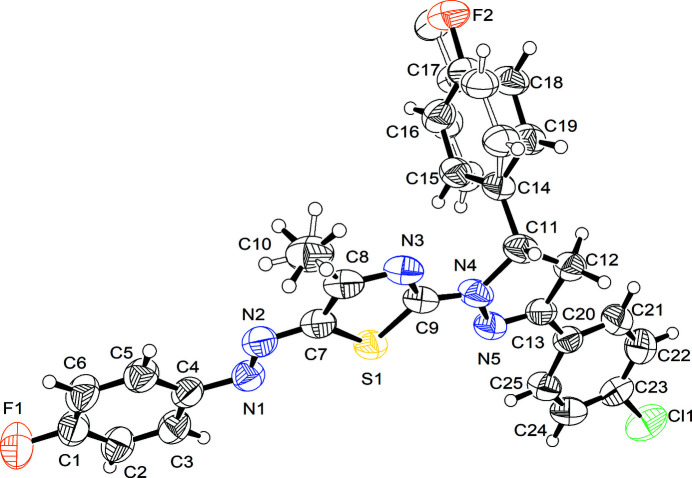
The molecule of the title compound (Fig. 1 ▸) includes fluorophenyl (A, F1/C1–C6), methylthiazolyl (B, S1/N3,C7–C18), pyrazolyl (C, N4/N5/C11–C13), chlorophenyl (D, Cl1/C20–C25) and fluorophenyl (E, F2/C14–C19) rings. Fluorophenyl group E is disordered over two components with an occupancy ratio of 0.767 (10):0.233 (10) and related by a twist of 24.2 (8)°.
Figure 1.
ORTEP representation of the title molecule showing 50% probability ellipsoids.
Rings A–D are close to coplanar with twist angles A/B, B/C and C/D of 4.76 (10)°, 6.51 (11)° and 10.46 (11)° respectively. Ring E is almost perpendicular to A–D with a C/E twist angle of 72.66 (3)° for the major component of E.
In the crystal structure, two molecules related by inversion symmetry are linked by a pair of C—H⋯F contacts (Table 1 ▸, Fig. 2 ▸) with R(8)2 2 geometry to form a dimer. The pyrazolyl and fluorophenyl rings of neighbouring molecules are almost parallel with a centroid-to-centroid distance of 3.6510 (13) Å.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯S1i | 0.93 | 3.00 | 3.699 (2) | 133 |
| C6—H6⋯F1ii | 0.93 | 2.52 | 3.441 (3) | 169 |
Symmetry codes: (i)
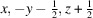 ; (ii)
; (ii)
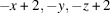 .
.
Figure 2.
A segment of the crystal structure showing intermolecular contacts for the major component of the disordered structure.
Synthesis and crystallization
A mixture of 3-(4-chlorophenyl)-5-(4-fluorophenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (0.67 g, 2.0 mmol), N′-(4-fluorophenyl)-2-oxopropanehydrazonoyl bromide (0.52 g, 2.0 mmol), and triethylamine (0.20 g, 2.0 mmol) in anhydrous ethanol (20 ml) was stirred for 2 h under reflux. The solid obtained on cooling was collected by filtration, washed with ethanol, dried and recrystallized from dimethylformamide solution to give colourless crystals of the title compound in 86% yield (0.85 g; 1.7 mmol), m.p. 243°C, IR (KBr; cm−1): 1590 (N=N), 1625 (C=C), 1650 (C=N).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. One fluorophenyl group is disordered. The two components were restrained to have similar geometries as the other ordered fluorophenyl group (SAME command of SHELXL, e.s.d. = 0.01 and 0.02 Å) and U ij components of disordered atoms’ ADPs were restrained to be similar to each other if within 2.0 Å distance (SIMU restraint of SHELXL, e.s.d. = 0.01 Å2). Refinement gave an occupancy ratio of 0.767 (10):0.233 (10) for the two components related by a twist of 24.2 (8)°.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C25H18ClF2N5S |
| M r | 493.95 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 293 |
| a, b, c (Å) | 16.9376 (6), 13.1440 (4), 10.6399 (4) |
| β (°) | 92.891 (4) |
| V (Å3) | 2365.72 (14) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.29 |
| Crystal size (mm) | 0.32 × 0.19 × 0.04 |
| Data collection | |
| Diffractometer | Rigaku Oxford Diffraction SuperNova, Dual, Cu at zero, Atlas |
| Absorption correction | Gaussian (CrysAlis PRO; Rigaku OD, 2015 ▸) |
| T min, T max | 0.727, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 21848, 5930, 3549 |
| R int | 0.027 |
| (sin θ/λ)max (Å−1) | 0.700 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.048, 0.125, 1.02 |
| No. of reflections | 5930 |
| No. of parameters | 364 |
| No. of restraints | 255 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.14, −0.21 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314620007002/zl4041sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620007002/zl4041Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620007002/zl4041Isup3.cml
CCDC reference: 2005280
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Cardiff University for ongoing support.
full crystallographic data
Crystal data
| C25H18ClF2N5S | F(000) = 1016 |
| Mr = 493.95 | Dx = 1.387 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 16.9376 (6) Å | Cell parameters from 6505 reflections |
| b = 13.1440 (4) Å | θ = 3.5–26.8° |
| c = 10.6399 (4) Å | µ = 0.29 mm−1 |
| β = 92.891 (4)° | T = 293 K |
| V = 2365.72 (14) Å3 | Plate, colourless |
| Z = 4 | 0.32 × 0.19 × 0.04 mm |
Data collection
| Rigaku Oxford Diffraction SuperNova, Dual, Cu at zero, Atlas diffractometer | 3549 reflections with I > 2σ(I) |
| ω scans | Rint = 0.027 |
| Absorption correction: gaussian (CrysAlisPro; Rigaku OD, 2015) | θmax = 29.9°, θmin = 3.1° |
| Tmin = 0.727, Tmax = 1.000 | h = −17→23 |
| 21848 measured reflections | k = −18→17 |
| 5930 independent reflections | l = −14→14 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.125 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0445P)2 + 0.6409P] where P = (Fo2 + 2Fc2)/3 |
| 5930 reflections | (Δ/σ)max = 0.001 |
| 364 parameters | Δρmax = 0.14 e Å−3 |
| 255 restraints | Δρmin = −0.21 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Non-hydrogen atoms were refined with anisotropic diaplacement parameters. All hydrogen atoms were placed in calculated positions and refined using a riding model. Difference Fourier maps showed that the methyl hydrogen atoms were disordered. The methyl group was therefore modelled as two components related by a 60° rotation about the C-C bond and the C-H bond distances were fixed at 0.96 Å, with displacement parameters 1.5 times Ueq(C). The hydrogen atoms were allowed to rotate freely and the occupancy ratio for the two components refined to 57 (3):43 (3)%. C-H distances for sp2 hybridized groups were set to 0.93Å and their Uiso(H) set to 1.2 times the Ueq(C). Methine and methylene C-H bond distances were fixed at 0.98 Å and 0.97 Å with displacement parameters 1.2 times Ueq(C). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.91410 (14) | −0.11438 (18) | 0.8797 (2) | 0.0725 (6) | |
| C2 | 0.87189 (13) | −0.18539 (17) | 0.8117 (2) | 0.0713 (6) | |
| H2 | 0.855169 | −0.245141 | 0.848925 | 0.086* | |
| C3 | 0.85468 (13) | −0.16585 (16) | 0.6857 (2) | 0.0671 (6) | |
| H3 | 0.826052 | −0.213282 | 0.637229 | 0.081* | |
| C4 | 0.87930 (12) | −0.07710 (16) | 0.6308 (2) | 0.0621 (5) | |
| C5 | 0.92300 (13) | −0.00652 (17) | 0.7036 (2) | 0.0704 (6) | |
| H5 | 0.940622 | 0.053154 | 0.667359 | 0.085* | |
| C6 | 0.93986 (14) | −0.02567 (18) | 0.8292 (2) | 0.0774 (6) | |
| H6 | 0.968353 | 0.021092 | 0.878928 | 0.093* | |
| C7 | 0.85842 (12) | 0.03407 (15) | 0.3320 (2) | 0.0626 (5) | |
| C8 | 0.87394 (12) | 0.11841 (15) | 0.2618 (2) | 0.0656 (6) | |
| C9 | 0.80263 (12) | 0.03318 (14) | 0.1218 (2) | 0.0601 (5) | |
| C10 | 0.92100 (14) | 0.20868 (18) | 0.3087 (3) | 0.0885 (8) | |
| H10A | 0.932863 | 0.201734 | 0.397511 | 0.133* | 0.57 (3) |
| H10B | 0.890877 | 0.269636 | 0.293062 | 0.133* | 0.57 (3) |
| H10C | 0.969369 | 0.212399 | 0.265659 | 0.133* | 0.57 (3) |
| H10D | 0.929210 | 0.254112 | 0.239977 | 0.133* | 0.43 (3) |
| H10E | 0.971196 | 0.186210 | 0.344426 | 0.133* | 0.43 (3) |
| H10F | 0.892703 | 0.243447 | 0.371829 | 0.133* | 0.43 (3) |
| C11 | 0.74684 (12) | 0.08582 (15) | −0.09277 (19) | 0.0619 (5) | |
| H11 | 0.796880 | 0.108379 | −0.125855 | 0.074* | |
| C12 | 0.70269 (14) | 0.01744 (15) | −0.1895 (2) | 0.0663 (6) | |
| H12A | 0.652423 | 0.047161 | −0.217543 | 0.080* | |
| H12B | 0.733981 | 0.005413 | −0.261916 | 0.080* | |
| C13 | 0.69096 (12) | −0.07930 (14) | −0.11686 (19) | 0.0575 (5) | |
| C14 | 0.69975 (11) | 0.17716 (14) | −0.05306 (19) | 0.0558 (5) | 0.767 (10) |
| C15 | 0.6495 (3) | 0.1762 (4) | 0.0439 (5) | 0.0655 (13) | 0.767 (10) |
| H15 | 0.643982 | 0.116965 | 0.090367 | 0.079* | 0.767 (10) |
| C16 | 0.6071 (3) | 0.2618 (4) | 0.0739 (6) | 0.0750 (14) | 0.767 (10) |
| H16 | 0.573373 | 0.260671 | 0.140280 | 0.090* | 0.767 (10) |
| C17 | 0.6154 (4) | 0.3473 (4) | 0.0048 (6) | 0.0683 (12) | 0.767 (10) |
| C18 | 0.6614 (2) | 0.3505 (3) | −0.0949 (5) | 0.0728 (11) | 0.767 (10) |
| H18 | 0.664560 | 0.409543 | −0.142531 | 0.087* | 0.767 (10) |
| C19 | 0.7038 (2) | 0.2650 (3) | −0.1254 (5) | 0.0640 (10) | 0.767 (10) |
| H19 | 0.735132 | 0.266155 | −0.194617 | 0.077* | 0.767 (10) |
| F2 | 0.5728 (5) | 0.4315 (5) | 0.0333 (6) | 0.1030 (16) | 0.767 (10) |
| C14B | 0.69975 (11) | 0.17716 (14) | −0.05306 (19) | 0.0558 (5) | 0.233 (10) |
| C15B | 0.6368 (10) | 0.1597 (14) | 0.0220 (18) | 0.062 (2) | 0.233 (10) |
| H15B | 0.624555 | 0.093644 | 0.045623 | 0.074* | 0.233 (10) |
| C16B | 0.5927 (11) | 0.2392 (12) | 0.061 (2) | 0.066 (2) | 0.233 (10) |
| H16B | 0.549429 | 0.227761 | 0.109998 | 0.079* | 0.233 (10) |
| C17B | 0.6127 (14) | 0.3346 (14) | 0.029 (3) | 0.072 (2) | 0.233 (10) |
| C18B | 0.6792 (8) | 0.3578 (9) | −0.0333 (16) | 0.070 (2) | 0.233 (10) |
| H18B | 0.693605 | 0.424663 | −0.049414 | 0.084* | 0.233 (10) |
| C19B | 0.7238 (8) | 0.2759 (8) | −0.0711 (16) | 0.068 (2) | 0.233 (10) |
| H19B | 0.771021 | 0.287906 | −0.109519 | 0.081* | 0.233 (10) |
| F2B | 0.5692 (13) | 0.4152 (14) | 0.0696 (19) | 0.089 (4) | 0.233 (10) |
| C20 | 0.64915 (12) | −0.16914 (15) | −0.16629 (18) | 0.0575 (5) | |
| C21 | 0.60277 (15) | −0.16523 (18) | −0.2771 (2) | 0.0760 (6) | |
| H21 | 0.596607 | −0.103776 | −0.319834 | 0.091* | |
| C22 | 0.56563 (16) | −0.2511 (2) | −0.3250 (2) | 0.0843 (7) | |
| H22 | 0.535116 | −0.247636 | −0.400041 | 0.101* | |
| C23 | 0.57369 (13) | −0.34122 (17) | −0.2621 (2) | 0.0705 (6) | |
| C24 | 0.61747 (14) | −0.34719 (17) | −0.1505 (2) | 0.0766 (6) | |
| H24 | 0.621560 | −0.408430 | −0.106855 | 0.092* | |
| C25 | 0.65523 (13) | −0.26174 (16) | −0.1037 (2) | 0.0688 (6) | |
| H25 | 0.685526 | −0.265981 | −0.028541 | 0.083* | |
| N1 | 0.85765 (10) | −0.06409 (13) | 0.50112 (17) | 0.0643 (4) | |
| N2 | 0.88125 (10) | 0.01956 (14) | 0.45499 (17) | 0.0657 (5) | |
| N3 | 0.84226 (10) | 0.11839 (12) | 0.14170 (18) | 0.0656 (5) | |
| N4 | 0.76260 (10) | 0.01344 (12) | 0.01117 (17) | 0.0649 (5) | |
| N5 | 0.72506 (10) | −0.07906 (12) | −0.00653 (16) | 0.0606 (4) | |
| S1 | 0.80048 (3) | −0.05346 (4) | 0.24354 (5) | 0.06286 (17) | |
| F1 | 0.93157 (10) | −0.13275 (12) | 1.00396 (13) | 0.1024 (5) | |
| Cl1 | 0.52834 (4) | −0.44993 (5) | −0.32473 (8) | 0.1043 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0781 (15) | 0.0764 (15) | 0.0627 (14) | −0.0006 (12) | 0.0011 (11) | −0.0113 (12) |
| C2 | 0.0760 (14) | 0.0675 (14) | 0.0706 (14) | −0.0109 (11) | 0.0055 (11) | −0.0077 (11) |
| C3 | 0.0659 (13) | 0.0632 (13) | 0.0721 (14) | −0.0078 (10) | 0.0020 (11) | −0.0137 (11) |
| C4 | 0.0600 (12) | 0.0591 (12) | 0.0669 (13) | 0.0036 (10) | 0.0004 (10) | −0.0137 (10) |
| C5 | 0.0745 (14) | 0.0599 (13) | 0.0765 (15) | −0.0037 (11) | −0.0006 (12) | −0.0102 (11) |
| C6 | 0.0830 (16) | 0.0709 (15) | 0.0772 (16) | −0.0102 (12) | −0.0073 (12) | −0.0216 (12) |
| C7 | 0.0584 (12) | 0.0511 (11) | 0.0779 (14) | 0.0062 (9) | 0.0003 (10) | −0.0033 (10) |
| C8 | 0.0533 (12) | 0.0491 (11) | 0.0945 (17) | 0.0045 (9) | 0.0032 (11) | −0.0020 (11) |
| C9 | 0.0572 (12) | 0.0472 (11) | 0.0760 (14) | 0.0085 (9) | 0.0030 (10) | 0.0070 (10) |
| C10 | 0.0753 (15) | 0.0601 (13) | 0.129 (2) | −0.0057 (12) | −0.0048 (15) | −0.0040 (14) |
| C11 | 0.0638 (12) | 0.0512 (11) | 0.0717 (13) | 0.0044 (9) | 0.0127 (10) | 0.0152 (10) |
| C12 | 0.0831 (15) | 0.0529 (11) | 0.0639 (12) | 0.0094 (11) | 0.0130 (11) | 0.0085 (10) |
| C13 | 0.0618 (12) | 0.0502 (11) | 0.0614 (12) | 0.0103 (9) | 0.0120 (10) | 0.0059 (9) |
| C14 | 0.0557 (11) | 0.0470 (10) | 0.0648 (11) | 0.0003 (8) | 0.0053 (9) | 0.0079 (9) |
| C15 | 0.079 (2) | 0.056 (2) | 0.063 (2) | 0.0039 (17) | 0.013 (2) | 0.0137 (18) |
| C16 | 0.084 (3) | 0.074 (3) | 0.069 (2) | 0.010 (2) | 0.020 (2) | −0.003 (2) |
| C17 | 0.076 (2) | 0.0528 (19) | 0.075 (3) | 0.0150 (17) | −0.0015 (19) | −0.0016 (17) |
| C18 | 0.089 (2) | 0.0475 (15) | 0.083 (3) | 0.0061 (15) | 0.007 (2) | 0.0131 (17) |
| C19 | 0.069 (2) | 0.0531 (16) | 0.071 (2) | −0.0001 (14) | 0.0117 (18) | 0.0134 (16) |
| F2 | 0.130 (2) | 0.0725 (19) | 0.108 (4) | 0.0422 (17) | 0.018 (2) | −0.0035 (19) |
| C14B | 0.0557 (11) | 0.0470 (10) | 0.0648 (11) | 0.0003 (8) | 0.0053 (9) | 0.0079 (9) |
| C15B | 0.068 (4) | 0.051 (4) | 0.066 (4) | −0.003 (4) | 0.010 (4) | −0.002 (4) |
| C16B | 0.072 (4) | 0.058 (4) | 0.067 (4) | −0.006 (4) | 0.011 (4) | 0.001 (4) |
| C17B | 0.082 (4) | 0.059 (4) | 0.075 (5) | 0.012 (4) | 0.011 (4) | −0.005 (4) |
| C18B | 0.082 (4) | 0.050 (4) | 0.080 (5) | 0.005 (4) | 0.010 (4) | 0.006 (4) |
| C19B | 0.070 (4) | 0.055 (4) | 0.078 (4) | 0.000 (3) | 0.015 (4) | 0.007 (4) |
| F2B | 0.100 (6) | 0.078 (7) | 0.087 (8) | 0.024 (5) | 0.005 (6) | −0.016 (6) |
| C20 | 0.0609 (12) | 0.0531 (11) | 0.0594 (12) | 0.0084 (9) | 0.0115 (9) | 0.0009 (9) |
| C21 | 0.0975 (18) | 0.0627 (14) | 0.0674 (14) | 0.0056 (12) | −0.0016 (13) | 0.0049 (11) |
| C22 | 0.0931 (18) | 0.0861 (18) | 0.0726 (15) | 0.0002 (14) | −0.0064 (13) | −0.0081 (14) |
| C23 | 0.0642 (13) | 0.0632 (13) | 0.0855 (16) | 0.0021 (11) | 0.0166 (12) | −0.0146 (12) |
| C24 | 0.0768 (15) | 0.0545 (13) | 0.0985 (18) | 0.0049 (11) | 0.0053 (14) | 0.0052 (12) |
| C25 | 0.0711 (14) | 0.0565 (12) | 0.0780 (14) | 0.0037 (10) | −0.0028 (11) | 0.0074 (11) |
| N1 | 0.0658 (11) | 0.0576 (10) | 0.0690 (11) | 0.0032 (8) | −0.0017 (9) | −0.0066 (9) |
| N2 | 0.0623 (11) | 0.0583 (10) | 0.0761 (12) | 0.0066 (8) | 0.0001 (9) | −0.0066 (9) |
| N3 | 0.0594 (10) | 0.0475 (9) | 0.0895 (13) | 0.0030 (8) | 0.0009 (9) | 0.0075 (9) |
| N4 | 0.0748 (11) | 0.0442 (9) | 0.0750 (12) | 0.0024 (8) | −0.0038 (9) | 0.0108 (8) |
| N5 | 0.0678 (11) | 0.0465 (9) | 0.0675 (11) | 0.0032 (8) | 0.0030 (9) | 0.0073 (8) |
| S1 | 0.0725 (3) | 0.0461 (3) | 0.0696 (3) | 0.0013 (2) | 0.0004 (3) | 0.0034 (2) |
| F1 | 0.1286 (13) | 0.1099 (11) | 0.0673 (9) | −0.0177 (9) | −0.0094 (8) | −0.0066 (8) |
| Cl1 | 0.0951 (5) | 0.0835 (5) | 0.1349 (6) | −0.0123 (4) | 0.0109 (4) | −0.0357 (4) |
Geometric parameters (Å, º)
| C1—C2 | 1.361 (3) | C14—C15 | 1.370 (5) |
| C1—F1 | 1.362 (3) | C14—C19 | 1.391 (4) |
| C1—C6 | 1.364 (3) | C15—C16 | 1.381 (5) |
| C2—C3 | 1.381 (3) | C15—H15 | 0.9300 |
| C2—H2 | 0.9300 | C16—C17 | 1.354 (6) |
| C3—C4 | 1.379 (3) | C16—H16 | 0.9300 |
| C3—H3 | 0.9300 | C17—C18 | 1.348 (5) |
| C4—C5 | 1.397 (3) | C17—F2 | 1.364 (5) |
| C4—N1 | 1.420 (3) | C18—C19 | 1.380 (4) |
| C5—C6 | 1.375 (3) | C18—H18 | 0.9300 |
| C5—H5 | 0.9300 | C19—H19 | 0.9300 |
| C6—H6 | 0.9300 | C14B—C19B | 1.376 (10) |
| C7—N2 | 1.359 (3) | C14B—C15B | 1.383 (13) |
| C7—C8 | 1.370 (3) | C15B—C16B | 1.363 (13) |
| C7—S1 | 1.755 (2) | C15B—H15B | 0.9300 |
| C8—N3 | 1.361 (3) | C16B—C17B | 1.348 (13) |
| C8—C10 | 1.501 (3) | C16B—H16B | 0.9300 |
| C9—N3 | 1.317 (2) | C17B—C18B | 1.371 (14) |
| C9—N4 | 1.354 (3) | C17B—F2B | 1.371 (13) |
| C9—S1 | 1.726 (2) | C18B—C19B | 1.386 (11) |
| C10—H10A | 0.9600 | C18B—H18B | 0.9300 |
| C10—H10B | 0.9600 | C19B—H19B | 0.9300 |
| C10—H10C | 0.9600 | C20—C21 | 1.385 (3) |
| C10—H10D | 0.9600 | C20—C25 | 1.389 (3) |
| C10—H10E | 0.9600 | C21—C22 | 1.377 (3) |
| C10—H10F | 0.9600 | C21—H21 | 0.9300 |
| C11—N4 | 1.473 (2) | C22—C23 | 1.364 (3) |
| C11—C14B | 1.513 (3) | C22—H22 | 0.9300 |
| C11—C14 | 1.513 (3) | C23—C24 | 1.370 (3) |
| C11—C12 | 1.532 (3) | C23—Cl1 | 1.739 (2) |
| C11—H11 | 0.9800 | C24—C25 | 1.373 (3) |
| C12—C13 | 1.506 (3) | C24—H24 | 0.9300 |
| C12—H12A | 0.9700 | C25—H25 | 0.9300 |
| C12—H12B | 0.9700 | N1—N2 | 1.276 (2) |
| C13—N5 | 1.282 (2) | N4—N5 | 1.380 (2) |
| C13—C20 | 1.461 (3) | ||
| C2—C1—F1 | 118.5 (2) | C20—C13—C12 | 124.86 (18) |
| C2—C1—C6 | 123.2 (2) | C15—C14—C19 | 118.3 (3) |
| F1—C1—C6 | 118.4 (2) | C15—C14—C11 | 124.0 (3) |
| C1—C2—C3 | 117.9 (2) | C19—C14—C11 | 117.6 (2) |
| C1—C2—H2 | 121.1 | C14—C15—C16 | 121.2 (4) |
| C3—C2—H2 | 121.1 | C14—C15—H15 | 119.4 |
| C4—C3—C2 | 121.0 (2) | C16—C15—H15 | 119.4 |
| C4—C3—H3 | 119.5 | C17—C16—C15 | 118.7 (5) |
| C2—C3—H3 | 119.5 | C17—C16—H16 | 120.7 |
| C3—C4—C5 | 119.3 (2) | C15—C16—H16 | 120.7 |
| C3—C4—N1 | 116.42 (18) | C18—C17—C16 | 122.2 (4) |
| C5—C4—N1 | 124.2 (2) | C18—C17—F2 | 118.8 (4) |
| C6—C5—C4 | 119.7 (2) | C16—C17—F2 | 118.8 (4) |
| C6—C5—H5 | 120.1 | C17—C18—C19 | 119.1 (3) |
| C4—C5—H5 | 120.1 | C17—C18—H18 | 120.4 |
| C1—C6—C5 | 118.9 (2) | C19—C18—H18 | 120.4 |
| C1—C6—H6 | 120.5 | C18—C19—C14 | 120.3 (3) |
| C5—C6—H6 | 120.5 | C18—C19—H19 | 119.8 |
| N2—C7—C8 | 125.8 (2) | C14—C19—H19 | 119.8 |
| N2—C7—S1 | 123.29 (16) | C19B—C14B—C15B | 118.6 (9) |
| C8—C7—S1 | 110.82 (17) | C19B—C14B—C11 | 123.0 (5) |
| N3—C8—C7 | 115.76 (19) | C15B—C14B—C11 | 117.5 (8) |
| N3—C8—C10 | 119.3 (2) | C16B—C15B—C14B | 120.1 (13) |
| C7—C8—C10 | 124.9 (2) | C16B—C15B—H15B | 120.0 |
| N3—C9—N4 | 122.10 (19) | C14B—C15B—H15B | 120.0 |
| N3—C9—S1 | 118.06 (17) | C17B—C16B—C15B | 119.1 (15) |
| N4—C9—S1 | 119.83 (15) | C17B—C16B—H16B | 120.4 |
| C8—C10—H10A | 109.5 | C15B—C16B—H16B | 120.4 |
| C8—C10—H10B | 109.5 | C16B—C17B—C18B | 123.6 (13) |
| H10A—C10—H10B | 109.5 | C16B—C17B—F2B | 119.5 (14) |
| C8—C10—H10C | 109.5 | C18B—C17B—F2B | 116.6 (15) |
| H10A—C10—H10C | 109.5 | C17B—C18B—C19B | 116.2 (11) |
| H10B—C10—H10C | 109.5 | C17B—C18B—H18B | 121.9 |
| C8—C10—H10D | 109.5 | C19B—C18B—H18B | 121.9 |
| H10A—C10—H10D | 141.1 | C14B—C19B—C18B | 121.5 (9) |
| H10B—C10—H10D | 56.3 | C14B—C19B—H19B | 119.2 |
| H10C—C10—H10D | 56.3 | C18B—C19B—H19B | 119.2 |
| C8—C10—H10E | 109.5 | C21—C20—C25 | 117.8 (2) |
| H10A—C10—H10E | 56.3 | C21—C20—C13 | 121.36 (18) |
| H10B—C10—H10E | 141.1 | C25—C20—C13 | 120.86 (19) |
| H10C—C10—H10E | 56.3 | C22—C21—C20 | 120.9 (2) |
| H10D—C10—H10E | 109.5 | C22—C21—H21 | 119.6 |
| C8—C10—H10F | 109.5 | C20—C21—H21 | 119.6 |
| H10A—C10—H10F | 56.3 | C23—C22—C21 | 119.8 (2) |
| H10B—C10—H10F | 56.3 | C23—C22—H22 | 120.1 |
| H10C—C10—H10F | 141.1 | C21—C22—H22 | 120.1 |
| H10D—C10—H10F | 109.5 | C22—C23—C24 | 120.8 (2) |
| H10E—C10—H10F | 109.5 | C22—C23—Cl1 | 119.6 (2) |
| N4—C11—C14B | 112.39 (17) | C24—C23—Cl1 | 119.62 (19) |
| N4—C11—C14 | 112.39 (17) | C23—C24—C25 | 119.3 (2) |
| N4—C11—C12 | 100.91 (15) | C23—C24—H24 | 120.4 |
| C14B—C11—C12 | 113.99 (17) | C25—C24—H24 | 120.4 |
| C14—C11—C12 | 113.99 (17) | C24—C25—C20 | 121.4 (2) |
| N4—C11—H11 | 109.7 | C24—C25—H25 | 119.3 |
| C14—C11—H11 | 109.7 | C20—C25—H25 | 119.3 |
| C12—C11—H11 | 109.7 | N2—N1—C4 | 114.00 (17) |
| C13—C12—C11 | 102.89 (16) | N1—N2—C7 | 114.31 (18) |
| C13—C12—H12A | 111.2 | C9—N3—C8 | 108.94 (17) |
| C11—C12—H12A | 111.2 | C9—N4—N5 | 119.63 (16) |
| C13—C12—H12B | 111.2 | C9—N4—C11 | 126.37 (17) |
| C11—C12—H12B | 111.2 | N5—N4—C11 | 113.68 (16) |
| H12A—C12—H12B | 109.1 | C13—N5—N4 | 108.07 (16) |
| N5—C13—C20 | 121.30 (18) | C9—S1—C7 | 86.43 (10) |
| N5—C13—C12 | 113.75 (18) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···S1i | 0.93 | 3.00 | 3.699 (2) | 133 |
| C6—H6···F1ii | 0.93 | 2.52 | 3.441 (3) | 169 |
Symmetry codes: (i) x, −y−1/2, z+1/2; (ii) −x+2, −y, −z+2.
Funding Statement
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through the Vice Deanship of Scientific Research Chairs.
References
- Abdel-Wahab, B. F., Khidre, R. E., Mohamed, H. A. & El-Hiti, G. A. (2017). Arab. J. Sci. Eng. 42, 2441–2448.
- Abd-Rabou, A. A., Abdel-Wahab, B. F. & Bekheit, M. S. (2018). Chem. Pap. 72, 2225–2237.
- Cambridge Soft (2001). CHEMDRAW Ultra. Cambridge Soft Corporation, Cambridge, Massachusetts, USA.
- El-Hiti, G. A., Abdel-Wahab, B. F., Alqahtani, A., Hegazy, A. S. & Kariuki, B. M. (2019). IUCrData, 4, x190218.
- El-Hiti, G. A., Abdel-Wahab, B. F., Yousif, E., Alotaibi, M. H., Hegazy, A. S. & Kariuki, B. M. (2019). IUCrData, 4, x190211.
- El-Hiti, G. A., Mohamed, H. A., Abdel-Wahab, B. F., Alotaibi, M. H., Hegazy, A. S. & Kariuki, B. M. (2018). IUCrData, 3, x180036.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Rigaku OD (2015). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Saeed, A., Mahesar, P. A., Channar, P. A., Abbas, Q., Larik, F. A., Hassan, M., Raza, H. & Seo, S. Y. (2017). Bioorg. Chem. 74, 187–196. [DOI] [PubMed]
- Secrieru, A., O’Neill, P. M. & Cristiano, M. L. S. (2019). Molecules, 25, 42. [DOI] [PMC free article] [PubMed]
- Shaabani, A., Nazeri, M. T. & Afshari, R. (2019). Mol. Divers. 23, 751–807. [DOI] [PubMed]
- Sharma, P. C., Bansal, K. K., Sharma, A., Sharma, D. & Deep, A. (2020). Eur. J. Med. Chem. 188, 112016. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314620007002/zl4041sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620007002/zl4041Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620007002/zl4041Isup3.cml
CCDC reference: 2005280
Additional supporting information: crystallographic information; 3D view; checkCIF report