The title compound was prepared via a Claisen-Schmidt condensation through a solventless, green synthesis technique. The resulting crystals formed in the monoclinic space group P21/c, and adopting the common E configuration.
Keywords: crystal structure, green synthesis, indanone, chalcone
Abstract
The title chalcone, C18H16O3, was prepared by a solventless base-promoted Claisen–Schmidt condensation and, upon recrystallization from ethanol, obtained in 56% yield. The dihedral angle between the indanone ring system and the benzene ring is 2.54 (4) ° and the C atoms of the methoxy groups deviate from the benzene ring by 0.087 (1) and 0.114 (1) Å. In the crystal, π-stacking is the predominant intermolecular force, with the molecules stacking into columns running parallel to the b axis of the unit cell.
Structure description
The chalcone family of compounds possess an aromatic α,β-unsaturated ketone functionality and can readily be formed by base-promoted condensation–dehydrations of an aromatic aldehyde and an aromatic ketone. They are important pharmacophore scaffolds and can possess anti-inflammatory, anti-fungal, anti-cancer, and anti-malarial biological activities (Singh et al., 2015 ▸, 2014 ▸; Berthelette et al., 1997 ▸). Additionally, the aromatic groups can be functionalized so as to produce other biological effects. The indanone family of compounds are biologically active compounds that are involved in steroid hormone biosynthesis and arachidonic acid metabolism pathways (Berthelette et al., 1997 ▸). In addition, indanone derivatives serve as scaffolds for a variety of heterocycles (Sloop et al., 2002 ▸, 2012 ▸).
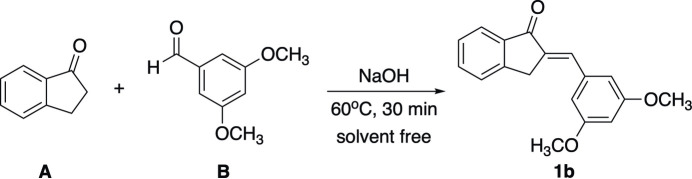
The combination of these two potential pharmacophores using greener and more efficient synthesis pathways en route to a series of highly functionalized indanone-based chalcones is now being studied by our research group. The solvent-free Claisen–Schmidt reaction undertaken in Fig. 1 ▸ minimizes reaction toxicity, limits waste production and enables easier product isolation in many cases.
Figure 1.
Green synthesis scheme for indanone-based chalcones
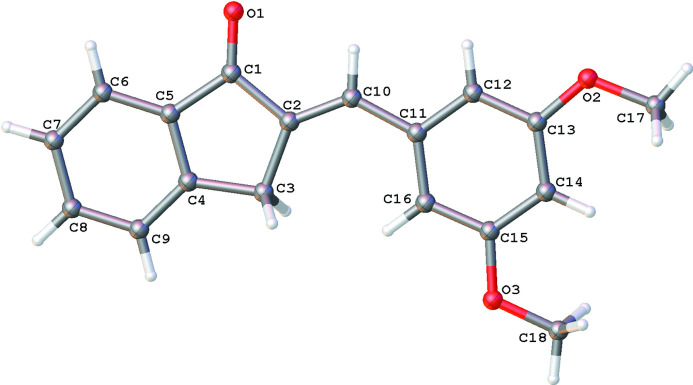
In the title molecule (Fig. 2 ▸), the dihedral angle between the indanone ring system and the benzene ring is 2.54 (4) ° and the C‘7 and C18 atoms of the methoxy groups deviate from the benzene ring by 0.087 (1) and 0.114 (1) Å, respectively. No unusual bond lengths or angles are noted after a routine Mogul geometry check (Bruno et al., 2004 ▸).
Figure 2.
Displacement ellipsoid plot of 1b. Ellipsoids are drawn at the 50% probability level.
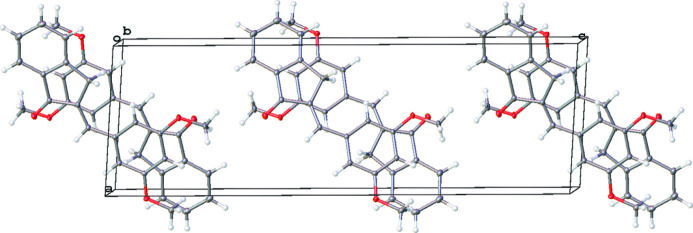
The predominant supramolecular feature of this structure (Fig. 3 ▸) are slipped stacking interactions. This consists of ring-over-atom pairings between the indanone ring and the 3-position of the dimethoxyphenyl ring of a neighboring molecule and generates a relatively close contact of 2.7 Å for the methylene H atoms of the indanone ring to the adjacent molecule.
Figure 3.
Packing diagram of 1b viewed along the b axis.
Structurally characterized 1b is consistent with known structures of similar indaneones. A search of the Cambridge Structural Database (Version 5.41, update of November 2019; Groom et al., 2016 ▸) gave 35 hits with a similar core structure. A defined three-dimensional parameter search on the distance between the carbonyl O atom and the phenyl ring gave a clear indication of the stereochemistry of the double bond. The title compound adopts the more common E isomer – along with 33 of the other structures published – indicated by an O—C distances 4.2 to 4.5 Å. Only two examples of Z isomers (O—C of 3.2 to 3.4 Å) exist [POWZUX (Zhou et al., 2009 ▸) and HAVLAR (Mori & Maeda, 1994 ▸)]. The latter has seven structure determinations as part of a light-driven solid-state isomerization study (Harada et al., 2009 ▸).
Synthesis and crystallization
A 25 mL beaker equipped with a stir bar was charged with 3,5-dimethoxybenzaldehyde (0.50 g, 3.0 mmol) and warmed to 60°C. To the liquified aldehyde was added 1-indanone (0.40 g, 3.0 mmol) and solid NaOH (0.20 g, 3.8 mmol). The reaction mixture was stirred for 30 minutes at 60°C. The resulting reaction mixture was neutralized with 4 mL of 1 M HCl, the resulting residue was washed with several 1 mL aliquots of distilled water and the crude product (0.80 g, 95% yield) isolated via vacuum filtration. Recrystallization from 95% ethanol solution via slow evaporation afforded the target chalcone, (E)-2-(3,5-dimethoxybenzylidenyl)-1-indanone (1b) as colorless needles, (0.47 g, 56% yield). Melting range: 174–175°C. IR, 1H and 13C NMR spectroscopy and single-crystal X-ray analysis (see supporting information) confirmed the product identity.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 1 ▸.
Table 1. Experimental details.
| Crystal data | |
| Chemical formula | C18H16O3 |
| M r | 280.31 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 |
| a, b, c (Å) | 7.7611 (4), 7.2894 (4), 24.0331 (13) |
| β (°) | 93.5573 (12) |
| V (Å3) | 1357.02 (13) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.39 × 0.12 × 0.05 |
| Data collection | |
| Diffractometer | Bruker-Nonius X8 Kappa APEXII |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.95, 0.99 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 30838, 5231, 4087 |
| R int | 0.040 |
| (sin θ/λ)max (Å−1) | 0.772 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.044, 0.123, 1.02 |
| No. of reflections | 5231 |
| No. of parameters | 192 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.61, −0.24 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2414314620007592/bt4094sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620007592/bt4094Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620007592/bt4094Isup3.smi
Supporting information file. DOI: 10.1107/S2414314620007592/bt4094Isup4.cml
1H NMR data. DOI: 10.1107/S2414314620007592/bt4094sup5.pdf
13C NMR data. DOI: 10.1107/S2414314620007592/bt4094sup6.pdf
CCDC reference: 1894469
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
All X-ray crystallography measurements were made in the Molecular Education, Technology, and Research Innovation Center (METRIC) at North Carolina State University.
full crystallographic data
Crystal data
| C18H16O3 | F(000) = 592 |
| Mr = 280.31 | Dx = 1.372 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.7611 (4) Å | Cell parameters from 242 reflections |
| b = 7.2894 (4) Å | θ = 3.0–33.1° |
| c = 24.0331 (13) Å | µ = 0.09 mm−1 |
| β = 93.5573 (12)° | T = 100 K |
| V = 1357.02 (13) Å3 | Needle, colourless |
| Z = 4 | 0.39 × 0.12 × 0.05 mm |
Data collection
| Bruker-Nonius X8 Kappa APEXII diffractometer | 5231 independent reflections |
| Radiation source: fine-focus sealed tube | 4087 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.040 |
| Detector resolution: 8.3333 pixels mm-1 | θmax = 33.3°, θmin = 2.6° |
| phi and ω scans | h = −11→11 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −11→11 |
| Tmin = 0.95, Tmax = 0.99 | l = −37→37 |
| 30838 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.044 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.123 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0695P)2 + 0.2851P] where P = (Fo2 + 2Fc2)/3 |
| 5231 reflections | (Δ/σ)max = 0.001 |
| 192 parameters | Δρmax = 0.61 e Å−3 |
| 0 restraints | Δρmin = −0.24 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. All hydrogen atoms were seen in the difference map of later refinements, but were placed at calculated positions and refined using a riding model, setting isotropic displacement parameters to 1.2 or 1.5 times that of the parent atom for ring H atoms and methyl groups respectively. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.47855 (9) | 0.17474 (11) | 0.33135 (3) | 0.01698 (15) | |
| O2 | 1.03362 (8) | 0.10918 (10) | 0.58143 (3) | 0.01475 (14) | |
| O3 | 0.48827 (9) | 0.32457 (10) | 0.64602 (3) | 0.01472 (14) | |
| C1 | 0.37431 (12) | 0.23058 (12) | 0.36368 (4) | 0.01126 (16) | |
| C2 | 0.40164 (11) | 0.24728 (12) | 0.42550 (4) | 0.01007 (15) | |
| C3 | 0.23699 (11) | 0.31671 (12) | 0.44870 (4) | 0.01069 (16) | |
| H3A | 0.191109 | 0.227118 | 0.474981 | 0.013* | |
| H3B | 0.256199 | 0.435489 | 0.468075 | 0.013* | |
| C4 | 0.11611 (11) | 0.33843 (12) | 0.39744 (4) | 0.01048 (16) | |
| C5 | 0.19615 (12) | 0.29325 (12) | 0.34906 (4) | 0.01129 (16) | |
| C6 | 0.11094 (12) | 0.30860 (13) | 0.29642 (4) | 0.01457 (18) | |
| H6 | 0.168019 | 0.279979 | 0.263676 | 0.017* | |
| C7 | −0.05951 (13) | 0.36685 (14) | 0.29328 (4) | 0.01710 (19) | |
| H7 | −0.120329 | 0.378944 | 0.257935 | 0.021* | |
| C8 | −0.14259 (12) | 0.40795 (13) | 0.34182 (4) | 0.01618 (18) | |
| H8 | −0.260325 | 0.444422 | 0.339062 | 0.019* | |
| C9 | −0.05538 (12) | 0.39621 (12) | 0.39403 (4) | 0.01333 (17) | |
| H9 | −0.111664 | 0.427002 | 0.426756 | 0.016* | |
| C10 | 0.55633 (12) | 0.20333 (12) | 0.44993 (4) | 0.01049 (16) | |
| H10 | 0.63798 | 0.160868 | 0.425033 | 0.013* | |
| C11 | 0.62038 (11) | 0.20978 (12) | 0.50842 (4) | 0.00944 (15) | |
| C12 | 0.79305 (11) | 0.15891 (12) | 0.52012 (4) | 0.01034 (15) | |
| H12 | 0.86179 | 0.122965 | 0.490626 | 0.012* | |
| C13 | 0.86425 (11) | 0.16081 (12) | 0.57468 (4) | 0.01032 (15) | |
| C14 | 0.76564 (11) | 0.21385 (12) | 0.61855 (4) | 0.01067 (16) | |
| H14 | 0.813948 | 0.214673 | 0.655848 | 0.013* | |
| C15 | 0.59412 (11) | 0.26573 (12) | 0.60630 (4) | 0.01004 (15) | |
| C16 | 0.52038 (11) | 0.26358 (12) | 0.55215 (4) | 0.01045 (15) | |
| H16 | 0.40318 | 0.298291 | 0.544834 | 0.013* | |
| C17 | 1.10933 (12) | 0.09692 (13) | 0.63704 (4) | 0.01411 (17) | |
| H17A | 1.229061 | 0.054895 | 0.636091 | 0.021* | |
| H17B | 1.043717 | 0.009564 | 0.658384 | 0.021* | |
| H17C | 1.107152 | 0.217881 | 0.654776 | 0.021* | |
| C18 | 0.54517 (13) | 0.30032 (15) | 0.70314 (4) | 0.01596 (18) | |
| H18A | 0.57173 | 0.170604 | 0.710108 | 0.024* | |
| H18B | 0.453887 | 0.339485 | 0.726921 | 0.024* | |
| H18C | 0.648975 | 0.374224 | 0.711613 | 0.024* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0152 (3) | 0.0258 (4) | 0.0101 (3) | 0.0022 (3) | 0.0025 (2) | −0.0029 (3) |
| O2 | 0.0093 (3) | 0.0234 (3) | 0.0114 (3) | 0.0048 (2) | −0.0004 (2) | −0.0017 (2) |
| O3 | 0.0125 (3) | 0.0249 (4) | 0.0069 (3) | 0.0053 (3) | 0.0018 (2) | −0.0017 (2) |
| C1 | 0.0120 (4) | 0.0128 (4) | 0.0089 (4) | −0.0009 (3) | 0.0004 (3) | −0.0003 (3) |
| C2 | 0.0117 (4) | 0.0111 (4) | 0.0074 (3) | −0.0006 (3) | 0.0009 (3) | −0.0002 (3) |
| C3 | 0.0112 (4) | 0.0120 (4) | 0.0090 (3) | 0.0004 (3) | 0.0015 (3) | −0.0008 (3) |
| C4 | 0.0108 (4) | 0.0096 (3) | 0.0110 (4) | −0.0010 (3) | 0.0002 (3) | 0.0002 (3) |
| C5 | 0.0119 (4) | 0.0119 (4) | 0.0099 (4) | −0.0007 (3) | −0.0007 (3) | 0.0004 (3) |
| C6 | 0.0155 (4) | 0.0170 (4) | 0.0109 (4) | −0.0010 (3) | −0.0017 (3) | 0.0006 (3) |
| C7 | 0.0164 (4) | 0.0177 (4) | 0.0165 (4) | −0.0009 (3) | −0.0053 (3) | 0.0025 (3) |
| C8 | 0.0121 (4) | 0.0142 (4) | 0.0218 (5) | 0.0007 (3) | −0.0021 (3) | 0.0023 (3) |
| C9 | 0.0116 (4) | 0.0126 (4) | 0.0159 (4) | 0.0006 (3) | 0.0012 (3) | 0.0014 (3) |
| C10 | 0.0117 (4) | 0.0116 (4) | 0.0083 (3) | 0.0002 (3) | 0.0012 (3) | −0.0007 (3) |
| C11 | 0.0100 (4) | 0.0097 (3) | 0.0086 (3) | −0.0002 (3) | 0.0006 (3) | −0.0004 (3) |
| C12 | 0.0110 (4) | 0.0116 (4) | 0.0086 (3) | 0.0012 (3) | 0.0016 (3) | −0.0005 (3) |
| C13 | 0.0089 (4) | 0.0112 (4) | 0.0109 (4) | 0.0009 (3) | 0.0007 (3) | −0.0002 (3) |
| C14 | 0.0103 (4) | 0.0125 (4) | 0.0092 (4) | 0.0011 (3) | 0.0004 (3) | −0.0005 (3) |
| C15 | 0.0101 (4) | 0.0121 (4) | 0.0080 (4) | 0.0006 (3) | 0.0017 (3) | −0.0010 (3) |
| C16 | 0.0094 (4) | 0.0127 (4) | 0.0093 (4) | 0.0011 (3) | 0.0003 (3) | −0.0004 (3) |
| C17 | 0.0123 (4) | 0.0165 (4) | 0.0132 (4) | 0.0014 (3) | −0.0025 (3) | −0.0011 (3) |
| C18 | 0.0172 (4) | 0.0238 (5) | 0.0071 (4) | 0.0020 (3) | 0.0022 (3) | −0.0007 (3) |
Geometric parameters (Å, º)
| O1—C1 | 1.2255 (11) | C8—H8 | 0.95 |
| O2—C13 | 1.3673 (11) | C9—H9 | 0.95 |
| O2—C17 | 1.4289 (11) | C10—C11 | 1.4623 (12) |
| O3—C15 | 1.3665 (11) | C10—H10 | 0.95 |
| O3—C18 | 1.4267 (11) | C11—C16 | 1.4006 (12) |
| C1—C5 | 1.4777 (13) | C11—C12 | 1.4020 (12) |
| C1—C2 | 1.4929 (12) | C12—C13 | 1.3912 (12) |
| C2—C10 | 1.3421 (12) | C12—H12 | 0.95 |
| C2—C3 | 1.5127 (13) | C13—C14 | 1.3952 (12) |
| C3—C4 | 1.5098 (13) | C14—C15 | 1.3975 (12) |
| C3—H3A | 0.99 | C14—H14 | 0.95 |
| C3—H3B | 0.99 | C15—C16 | 1.3890 (12) |
| C4—C5 | 1.3913 (12) | C16—H16 | 0.95 |
| C4—C9 | 1.3935 (12) | C17—H17A | 0.98 |
| C5—C6 | 1.3951 (12) | C17—H17B | 0.98 |
| C6—C7 | 1.3869 (14) | C17—H17C | 0.98 |
| C6—H6 | 0.95 | C18—H18A | 0.98 |
| C7—C8 | 1.3999 (15) | C18—H18B | 0.98 |
| C7—H7 | 0.95 | C18—H18C | 0.98 |
| C8—C9 | 1.3908 (13) | ||
| C13—O2—C17 | 117.69 (7) | C2—C10—H10 | 114.6 |
| C15—O3—C18 | 118.00 (7) | C11—C10—H10 | 114.6 |
| O1—C1—C5 | 126.64 (8) | C16—C11—C12 | 119.40 (8) |
| O1—C1—C2 | 126.83 (8) | C16—C11—C10 | 123.99 (8) |
| C5—C1—C2 | 106.53 (7) | C12—C11—C10 | 116.61 (8) |
| C10—C2—C1 | 118.94 (8) | C13—C12—C11 | 120.34 (8) |
| C10—C2—C3 | 132.21 (8) | C13—C12—H12 | 119.8 |
| C1—C2—C3 | 108.84 (7) | C11—C12—H12 | 119.8 |
| C4—C3—C2 | 103.34 (7) | O2—C13—C12 | 115.58 (8) |
| C4—C3—H3A | 111.1 | O2—C13—C14 | 123.71 (8) |
| C2—C3—H3A | 111.1 | C12—C13—C14 | 120.70 (8) |
| C4—C3—H3B | 111.1 | C13—C14—C15 | 118.42 (8) |
| C2—C3—H3B | 111.1 | C13—C14—H14 | 120.8 |
| H3A—C3—H3B | 109.1 | C15—C14—H14 | 120.8 |
| C5—C4—C9 | 119.79 (8) | O3—C15—C16 | 115.30 (8) |
| C5—C4—C3 | 111.71 (8) | O3—C15—C14 | 122.96 (8) |
| C9—C4—C3 | 128.50 (8) | C16—C15—C14 | 121.73 (8) |
| C4—C5—C6 | 121.84 (8) | C15—C16—C11 | 119.40 (8) |
| C4—C5—C1 | 109.53 (8) | C15—C16—H16 | 120.3 |
| C6—C5—C1 | 128.63 (8) | C11—C16—H16 | 120.3 |
| C7—C6—C5 | 118.09 (9) | O2—C17—H17A | 109.5 |
| C7—C6—H6 | 121.0 | O2—C17—H17B | 109.5 |
| C5—C6—H6 | 121.0 | H17A—C17—H17B | 109.5 |
| C6—C7—C8 | 120.48 (9) | O2—C17—H17C | 109.5 |
| C6—C7—H7 | 119.8 | H17A—C17—H17C | 109.5 |
| C8—C7—H7 | 119.8 | H17B—C17—H17C | 109.5 |
| C9—C8—C7 | 121.00 (9) | O3—C18—H18A | 109.5 |
| C9—C8—H8 | 119.5 | O3—C18—H18B | 109.5 |
| C7—C8—H8 | 119.5 | H18A—C18—H18B | 109.5 |
| C8—C9—C4 | 118.77 (9) | O3—C18—H18C | 109.5 |
| C8—C9—H9 | 120.6 | H18A—C18—H18C | 109.5 |
| C4—C9—H9 | 120.6 | H18B—C18—H18C | 109.5 |
| C2—C10—C11 | 130.87 (8) | ||
| O1—C1—C2—C10 | 2.20 (14) | C3—C4—C9—C8 | 179.35 (9) |
| C5—C1—C2—C10 | −178.24 (8) | C1—C2—C10—C11 | 178.55 (9) |
| O1—C1—C2—C3 | −178.27 (9) | C3—C2—C10—C11 | −0.85 (17) |
| C5—C1—C2—C3 | 1.28 (9) | C2—C10—C11—C16 | 1.67 (15) |
| C10—C2—C3—C4 | 179.41 (10) | C2—C10—C11—C12 | −178.08 (9) |
| C1—C2—C3—C4 | −0.03 (9) | C16—C11—C12—C13 | 0.36 (13) |
| C2—C3—C4—C5 | −1.36 (9) | C10—C11—C12—C13 | −179.87 (8) |
| C2—C3—C4—C9 | 179.02 (9) | C17—O2—C13—C12 | −175.86 (8) |
| C9—C4—C5—C6 | 1.74 (14) | C17—O2—C13—C14 | 4.54 (13) |
| C3—C4—C5—C6 | −177.92 (8) | C11—C12—C13—O2 | −179.86 (8) |
| C9—C4—C5—C1 | −178.10 (8) | C11—C12—C13—C14 | −0.25 (13) |
| C3—C4—C5—C1 | 2.24 (10) | O2—C13—C14—C15 | 179.29 (8) |
| O1—C1—C5—C4 | 177.40 (9) | C12—C13—C14—C15 | −0.29 (13) |
| C2—C1—C5—C4 | −2.16 (10) | C18—O3—C15—C16 | 169.35 (8) |
| O1—C1—C5—C6 | −2.42 (16) | C18—O3—C15—C14 | −11.69 (13) |
| C2—C1—C5—C6 | 178.02 (9) | C13—C14—C15—O3 | −178.18 (8) |
| C4—C5—C6—C7 | −1.47 (14) | C13—C14—C15—C16 | 0.72 (13) |
| C1—C5—C6—C7 | 178.33 (9) | O3—C15—C16—C11 | 178.37 (8) |
| C5—C6—C7—C8 | −0.26 (14) | C14—C15—C16—C11 | −0.61 (13) |
| C6—C7—C8—C9 | 1.73 (15) | C12—C11—C16—C15 | 0.06 (13) |
| C7—C8—C9—C4 | −1.46 (14) | C10—C11—C16—C15 | −179.69 (8) |
| C5—C4—C9—C8 | −0.24 (13) |
Funding Statement
Funding for this research was provided by: GGC STEC 4500 Research Fund.
References
- Berthelette, C., McCooye, C., Leblanc, Y., Trimble, L. A. & Tsou, N. N. (1997). J. Org. Chem. 62, 4339–4342. [DOI] [PubMed]
- Bruker (2017). Instrument Service, APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruno, I. J., Cole, J. C., Kessler, M., Luo, J., Motherwell, W. D. S., Purkis, L. H., Smith, B. R., Taylor, R., Cooper, R. I., Harris, S. E. & Orpen, A. G. (2004). J. Chem. Inf. Comput. Sci. 44, 2133–2144. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Harada, J., Harakawa, M., Sugiyama, S. & Ogawa, K. (2009). CrystEngComm, 11, 1235–1239.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Sovago, I., Cottrell, S. J., Galek, P. T. A., McCabe, P., Pidcock, E., Platings, M., Shields, G. P., Stevens, J. S., Towler, M. & Wood, P. A. (2020). J. Appl. Cryst. 53, 226–235. [DOI] [PMC free article] [PubMed]
- Mori, Y. & Maeda, K. (1994). Acta Cryst. B50, 106–112.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Singh, A., Fatima, K., Singh, A., Behl, A., Mintoo, M., Hasanain, M., Ashraf, R., Luqman, S., Shanker, K., Mondhe, D., Sarkar, J., Chanda, D. & Negi, A. S. (2015). Eur. J. Pharm. Sci. 76, 57–67. [DOI] [PubMed]
- Singh, P., Anand, A. & Kumar, V. (2014). Eur. J. Med. Chem. 85, 758–777. [DOI] [PubMed]
- Sloop, J., Boyle, P., Fountain, A. W., Gomez, C., Jackson, J., Pearman, W., Schmidt, R. & Weyand, J. (2012). Appl. Sci. 2, 61–99.
- Sloop, J., Bumgardner, C. & Loehle, W. D. (2002). J. Fluor. Chem. 118, 135–147.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
- Zhou, Y.-X., Wang, J.-Q., Du, R.-J., Tang, J.-G. & Guo, C. (2009). Acta Cryst. E65, o1936. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2414314620007592/bt4094sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620007592/bt4094Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620007592/bt4094Isup3.smi
Supporting information file. DOI: 10.1107/S2414314620007592/bt4094Isup4.cml
1H NMR data. DOI: 10.1107/S2414314620007592/bt4094sup5.pdf
13C NMR data. DOI: 10.1107/S2414314620007592/bt4094sup6.pdf
CCDC reference: 1894469
Additional supporting information: crystallographic information; 3D view; checkCIF report