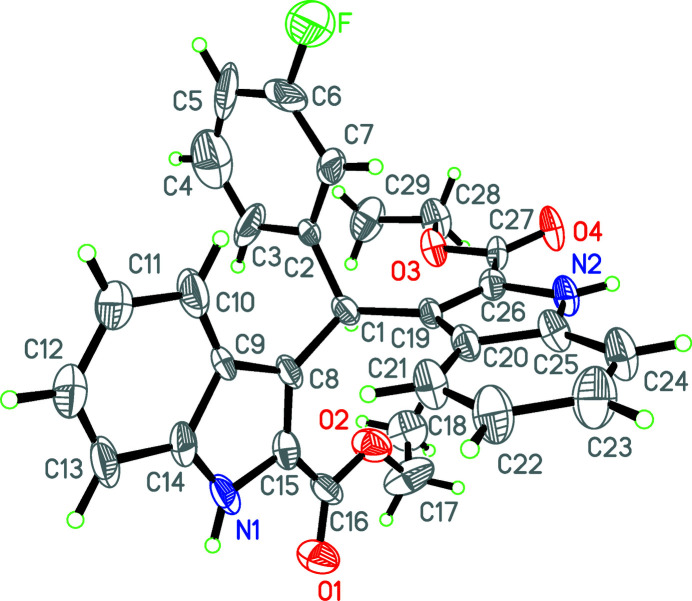
In the title compound, the indole ring systems are approximately perpendicular to one another with a dihedral angle of 88.3 (4)°.
Keywords: crystal structure, bisindole, MRI, contrast agents
Abstract
In the title compound, C29H25FN2O4, the mean planes of the indole ring systems are approximately perpendicular to one another [dihedral angle = 88.3 (4)°]. The benzene ring is twisted with respect to the indole ring systems by 49.8 (5) and 77.6 (3)°. In the crystal, pairs of N—H⋯O hydrogen bonds link the molecules into the inversion dimers which are further linked into supramolecular chains propagating along the [110] direction.
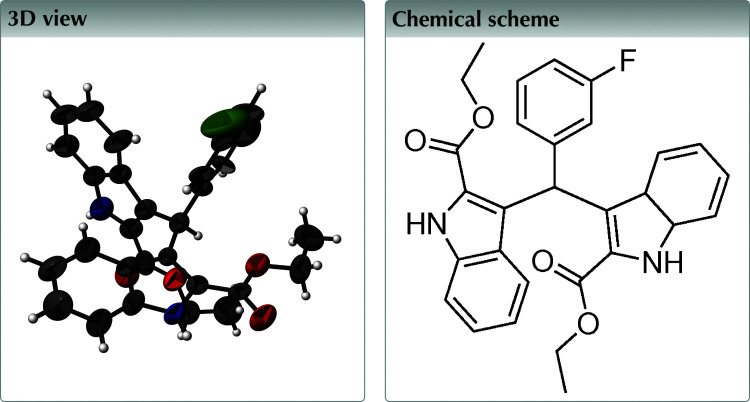
Structure description
There are abundant bis(indolyl)methane derivatives in various terrestrial and marine natural resources (Sundberg, 1996 ▸). As part of our ongoing studies of bis(indoyl)methane compounds, we now report the synthesis and crystal structure of the title compound.
The molecular structure of the title compound is shown in Fig. 1 ▸. The indole ring systems are nearly perpendicular to one another [dihedral angle = 88.3 (4)°] while the benzene ring (C2–C7) is twisted with respect to the N1/C8–C15 and N2/C19–C26 indole ring systems with dihedral angles of 49.8 (5) and 77.6 (3)°, respectively. The carboxyl groups are approximately co-planar with their attached indole ring systems, the dihedral angles between the carboxyl groups and the mean planes of the N1/C8–C15 and N2/C19–C26 indole ring systems being 6.2 (5) and 6.4 (4)°, respectively.
Figure 1.
The molecular structure of the title molecule with displacement ellipsoids drawn at the 30% probability level.
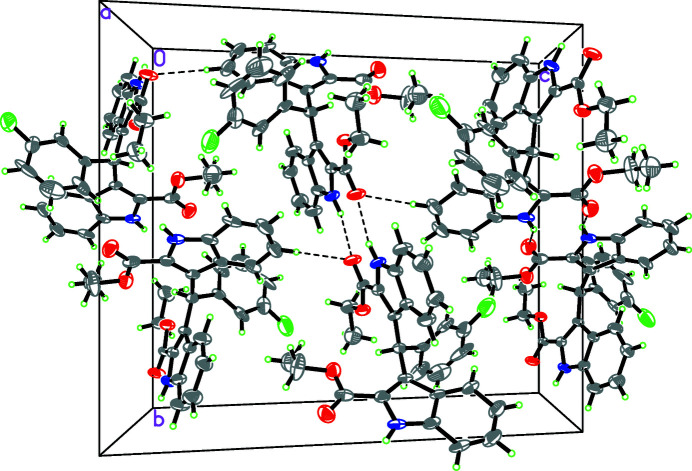
In the crystal, pairwise N1—H1A⋯O1i and N2—H2A⋯O4ii hydrogen bonds both generate
 (8) loops; together these lead to [110] chains of molecules. A weak C11—H11A⋯O4iii interaction also occurs, which links the chains into (001) sheets (Table 1 ▸ and Fig. 2 ▸).
(8) loops; together these lead to [110] chains of molecules. A weak C11—H11A⋯O4iii interaction also occurs, which links the chains into (001) sheets (Table 1 ▸ and Fig. 2 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯O1i | 0.86 | 2.16 | 2.918 (11) | 147 |
| N2—H2A⋯O4ii | 0.86 | 2.08 | 2.904 (9) | 159 |
| C11—H11A⋯O4iii | 0.93 | 2.58 | 3.498 (13) | 171 |
Symmetry codes: (i)
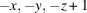 ; (ii)
; (ii)
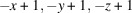 ; (iii)
; (iii)
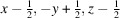 .
.
Figure 2.
A packing diagram of the title compound. Hydrogen bonds are shown as dashed lines.
Several similar structures have been reported previously, viz. diethyl 3,3′-(phenylmethylene)bis(1H-indole-2-carboxylate) (Sun et al., 2012 ▸), dimethyl 3,3′-[(3-fluorophenyl)methylene]bis(1H-indole-2-carboxylate) (Lu et al., 2014 ▸), dimethyl 3,3′-[(4-fluorophenyl)methylene]bis(1H-indole-2-carboxylate) (Sun et al., 2015 ▸) and dimethyl 3,3′-[(2-fluorophenyl)methylene]bis(1H-indole-2-carboxylate) (Lu et al., 2017 ▸). In these structures, the indole ring systems are also nearly perpendicular to one another, making dihedral angles of 82.0 (5), 87.8 (5), 84.0 (5) and 86.0 (5)°, respectively.
Synthesis and crystallization
Ethyl indole-2-carboxylate (1.88 g, 10 mmol) was dissolved in 20 ml of ethanol and 3-fluorobenzaldehyde (0.62 g, 5 mmol) and concentrated HCl (0.5 ml) was added. The mixture was heated to reflux temperature for 2 h. After cooling, the white product was filtered off and washed thoroughly with ethanol (yield = 92%). Single crystals of the title compound suitable for X-ray analysis were obtained by slow evaporation of an ethanol solution.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C29H25FN2O4 |
| M r | 484.51 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 293 |
| a, b, c (Å) | 8.9960 (18), 15.921 (3), 18.297 (4) |
| β (°) | 102.59 (3) |
| V (Å3) | 2557.6 (9) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.20 × 0.20 × 0.10 |
| Data collection | |
| Diffractometer | Enraf–Nonius CAD-4 |
| Absorption correction | ψ scan (North et al., 1968 ▸) |
| T min, T max | 0.982, 0.991 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 5000, 4685, 1424 |
| R int | 0.131 |
| (sin θ/λ)max (Å−1) | 0.603 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.143, 0.306, 1.30 |
| No. of reflections | 4685 |
| No. of parameters | 319 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.56, −0.72 |
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314620009128/hb4349sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620009128/hb4349Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620009128/hb4349Isup3.cml
CCDC reference: 2014000
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the Center of Testing and Analysis, Nanjing University, for support.
full crystallographic data
Crystal data
| C29H25FN2O4 | F(000) = 1016 |
| Mr = 484.51 | Dx = 1.258 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.9960 (18) Å | Cell parameters from 25 reflections |
| b = 15.921 (3) Å | θ = 9–12° |
| c = 18.297 (4) Å | µ = 0.09 mm−1 |
| β = 102.59 (3)° | T = 293 K |
| V = 2557.6 (9) Å3 | Block, colorless |
| Z = 4 | 0.20 × 0.20 × 0.10 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | 1424 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.131 |
| Graphite monochromator | θmax = 25.4°, θmin = 1.7° |
| ω/2θ scans | h = 0→10 |
| Absorption correction: ψ scan (North et al., 1968) | k = 0→19 |
| Tmin = 0.982, Tmax = 0.991 | l = −22→21 |
| 5000 measured reflections | 3 standard reflections every 200 reflections |
| 4685 independent reflections | intensity decay: 1% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.143 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.306 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.30 | w = 1/[σ2(Fo2) + (0.060P)2] where P = (Fo2 + 2Fc2)/3 |
| 4685 reflections | (Δ/σ)max = 0.009 |
| 319 parameters | Δρmax = 0.56 e Å−3 |
| 0 restraints | Δρmin = −0.71 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. H atoms were positioned geometrically with N—H = 0.86 Å and C—H = 0.93–0.98 Å, and constrained to ride on their parent atoms. The constraint Uiso(H) = 1.2Ueq(C,N) or 1.5Ueq(methyl C) was applied in all cases. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| F | 0.5574 (10) | 0.2828 (5) | 0.1783 (4) | 0.154 (4) | |
| O1 | 0.1733 (8) | 0.0523 (4) | 0.5601 (4) | 0.080 (2) | |
| N1 | 0.0972 (9) | 0.0284 (4) | 0.4084 (5) | 0.063 (2) | |
| H1A | 0.0468 | −0.0028 | 0.4327 | 0.076* | |
| C1 | 0.3860 (9) | 0.1906 (5) | 0.3962 (5) | 0.045 (2) | |
| H1B | 0.4561 | 0.1763 | 0.4434 | 0.054* | |
| N2 | 0.3321 (8) | 0.4124 (4) | 0.4463 (4) | 0.054 (2) | |
| H2A | 0.3634 | 0.4597 | 0.4668 | 0.064* | |
| O2 | 0.3615 (8) | 0.1390 (4) | 0.5478 (4) | 0.078 (2) | |
| C2 | 0.4782 (11) | 0.1845 (6) | 0.3395 (6) | 0.049 (3) | |
| O3 | 0.6571 (7) | 0.2781 (3) | 0.4797 (3) | 0.0609 (19) | |
| C3 | 0.5727 (12) | 0.1140 (8) | 0.3428 (7) | 0.094 (5) | |
| H3A | 0.5770 | 0.0753 | 0.3812 | 0.112* | |
| O4 | 0.6412 (7) | 0.4138 (4) | 0.5044 (4) | 0.071 (2) | |
| C4 | 0.659 (2) | 0.1009 (11) | 0.2907 (12) | 0.143 (7) | |
| H4A | 0.7197 | 0.0531 | 0.2945 | 0.172* | |
| C5 | 0.6584 (19) | 0.1558 (11) | 0.2334 (11) | 0.129 (7) | |
| H5A | 0.7151 | 0.1471 | 0.1972 | 0.155* | |
| C6 | 0.5694 (16) | 0.2231 (10) | 0.2335 (7) | 0.092 (4) | |
| C7 | 0.4733 (12) | 0.2417 (7) | 0.2840 (6) | 0.071 (3) | |
| H7A | 0.4121 | 0.2893 | 0.2792 | 0.086* | |
| C8 | 0.2671 (10) | 0.1236 (5) | 0.3855 (6) | 0.050 (3) | |
| C9 | 0.1838 (10) | 0.0882 (5) | 0.3150 (6) | 0.051 (3) | |
| C10 | 0.1875 (12) | 0.1021 (6) | 0.2409 (7) | 0.080 (4) | |
| H10A | 0.2524 | 0.1420 | 0.2276 | 0.096* | |
| C11 | 0.0911 (12) | 0.0547 (7) | 0.1867 (6) | 0.082 (4) | |
| H11A | 0.0929 | 0.0626 | 0.1366 | 0.099* | |
| C12 | −0.0061 (14) | −0.0033 (7) | 0.2053 (7) | 0.088 (4) | |
| H12A | −0.0678 | −0.0341 | 0.1673 | 0.105* | |
| C13 | −0.0162 (12) | −0.0175 (6) | 0.2762 (7) | 0.077 (3) | |
| H13A | −0.0844 | −0.0565 | 0.2878 | 0.092* | |
| C14 | 0.0816 (11) | 0.0295 (6) | 0.3329 (7) | 0.058 (3) | |
| C15 | 0.2065 (11) | 0.0855 (6) | 0.4391 (6) | 0.057 (3) | |
| C16 | 0.2487 (12) | 0.0887 (6) | 0.5223 (7) | 0.061 (3) | |
| C17 | 0.4070 (12) | 0.1489 (9) | 0.6278 (6) | 0.108 (5) | |
| H17A | 0.3662 | 0.2010 | 0.6427 | 0.129* | |
| H17B | 0.3673 | 0.1029 | 0.6525 | 0.129* | |
| C18 | 0.5630 (13) | 0.1500 (7) | 0.6483 (7) | 0.112 | |
| H18A | 0.5940 | 0.1565 | 0.7016 | 0.168* | |
| H18B | 0.6015 | 0.1960 | 0.6241 | 0.168* | |
| H18C | 0.6027 | 0.0982 | 0.6337 | 0.168* | |
| C19 | 0.3282 (9) | 0.2778 (5) | 0.4087 (5) | 0.039 (2) | |
| C20 | 0.1817 (11) | 0.3129 (5) | 0.3856 (5) | 0.049 (3) | |
| C21 | 0.0406 (12) | 0.2819 (6) | 0.3459 (6) | 0.069 (3) | |
| H21A | 0.0315 | 0.2268 | 0.3286 | 0.082* | |
| C22 | −0.0847 (13) | 0.3351 (7) | 0.3330 (6) | 0.077 (4) | |
| H22A | −0.1789 | 0.3152 | 0.3073 | 0.092* | |
| C23 | −0.0710 (13) | 0.4196 (7) | 0.3585 (6) | 0.089 (4) | |
| H23A | −0.1560 | 0.4544 | 0.3490 | 0.106* | |
| C24 | 0.0652 (12) | 0.4502 (6) | 0.3968 (6) | 0.071 (3) | |
| H24A | 0.0749 | 0.5056 | 0.4131 | 0.085* | |
| C25 | 0.1893 (11) | 0.3958 (5) | 0.4105 (5) | 0.054 (3) | |
| C26 | 0.4231 (11) | 0.3414 (5) | 0.4456 (5) | 0.044 (2) | |
| C27 | 0.5740 (11) | 0.3489 (5) | 0.4795 (5) | 0.045 (2) | |
| C28 | 0.8156 (10) | 0.2802 (6) | 0.5124 (6) | 0.075 (3) | |
| H28A | 0.8324 | 0.2942 | 0.5651 | 0.089* | |
| H28B | 0.8662 | 0.3218 | 0.4877 | 0.089* | |
| C29 | 0.8750 (12) | 0.1962 (7) | 0.5028 (6) | 0.101 (4) | |
| H29A | 0.9824 | 0.1948 | 0.5241 | 0.152* | |
| H29B | 0.8573 | 0.1830 | 0.4504 | 0.152* | |
| H29C | 0.8242 | 0.1557 | 0.5276 | 0.152* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| F | 0.256 (10) | 0.129 (7) | 0.088 (6) | −0.081 (7) | 0.063 (6) | −0.033 (5) |
| O1 | 0.085 (6) | 0.085 (5) | 0.073 (6) | −0.020 (4) | 0.022 (4) | 0.013 (4) |
| N1 | 0.076 (6) | 0.026 (4) | 0.088 (7) | −0.012 (4) | 0.016 (6) | −0.001 (5) |
| C1 | 0.050 (6) | 0.033 (5) | 0.053 (7) | −0.012 (5) | 0.011 (5) | 0.000 (5) |
| N2 | 0.058 (5) | 0.031 (4) | 0.065 (6) | −0.009 (4) | 0.001 (5) | −0.015 (4) |
| O2 | 0.081 (5) | 0.091 (6) | 0.060 (5) | −0.032 (5) | 0.014 (4) | −0.005 (4) |
| C2 | 0.057 (7) | 0.035 (6) | 0.065 (8) | −0.025 (5) | 0.033 (6) | −0.021 (5) |
| O3 | 0.068 (5) | 0.036 (4) | 0.072 (5) | 0.002 (4) | 0.001 (4) | −0.004 (3) |
| C3 | 0.068 (8) | 0.109 (11) | 0.117 (12) | −0.005 (8) | 0.049 (8) | −0.068 (9) |
| O4 | 0.079 (5) | 0.030 (4) | 0.093 (5) | −0.004 (4) | −0.008 (4) | −0.016 (4) |
| C4 | 0.132 (15) | 0.105 (14) | 0.18 (2) | −0.017 (12) | 0.016 (15) | −0.038 (13) |
| C5 | 0.132 (14) | 0.101 (13) | 0.169 (19) | −0.033 (12) | 0.065 (14) | −0.106 (13) |
| C6 | 0.113 (11) | 0.097 (11) | 0.066 (10) | −0.081 (9) | 0.022 (9) | −0.013 (9) |
| C7 | 0.079 (8) | 0.067 (8) | 0.077 (9) | −0.039 (7) | 0.036 (7) | −0.046 (7) |
| C8 | 0.062 (7) | 0.025 (5) | 0.061 (7) | −0.011 (5) | 0.013 (6) | −0.002 (5) |
| C9 | 0.063 (7) | 0.025 (5) | 0.069 (8) | −0.017 (5) | 0.023 (6) | −0.012 (6) |
| C10 | 0.104 (9) | 0.053 (7) | 0.077 (9) | −0.029 (6) | 0.009 (8) | −0.024 (7) |
| C11 | 0.097 (9) | 0.081 (8) | 0.070 (9) | −0.023 (7) | 0.021 (7) | −0.029 (7) |
| C12 | 0.105 (10) | 0.074 (9) | 0.084 (10) | −0.019 (8) | 0.023 (9) | −0.040 (8) |
| C13 | 0.084 (8) | 0.034 (6) | 0.112 (10) | −0.010 (6) | 0.019 (8) | −0.028 (7) |
| C14 | 0.063 (7) | 0.032 (6) | 0.076 (9) | 0.006 (5) | 0.006 (7) | −0.015 (6) |
| C15 | 0.069 (7) | 0.031 (6) | 0.067 (8) | 0.007 (5) | 0.010 (7) | −0.003 (6) |
| C16 | 0.053 (7) | 0.039 (6) | 0.094 (10) | −0.010 (6) | 0.022 (7) | 0.006 (6) |
| C17 | 0.062 (8) | 0.200 (14) | 0.058 (9) | −0.006 (8) | 0.008 (7) | −0.030 (9) |
| C18 | 0.112 | 0.112 | 0.112 | 0.000 | 0.024 | 0.000 |
| C19 | 0.041 (6) | 0.026 (5) | 0.049 (6) | −0.005 (4) | 0.010 (5) | −0.006 (4) |
| C20 | 0.065 (7) | 0.032 (5) | 0.047 (6) | −0.009 (5) | 0.008 (6) | −0.004 (5) |
| C21 | 0.072 (8) | 0.042 (6) | 0.085 (9) | −0.001 (6) | 0.003 (7) | −0.010 (6) |
| C22 | 0.089 (9) | 0.061 (8) | 0.070 (8) | −0.002 (7) | −0.008 (7) | −0.015 (6) |
| C23 | 0.086 (9) | 0.072 (8) | 0.098 (10) | 0.028 (7) | −0.002 (8) | −0.001 (8) |
| C24 | 0.068 (8) | 0.039 (6) | 0.097 (9) | −0.002 (6) | −0.003 (7) | −0.013 (6) |
| C25 | 0.054 (7) | 0.036 (6) | 0.063 (7) | −0.001 (5) | −0.007 (6) | 0.004 (5) |
| C26 | 0.053 (6) | 0.036 (5) | 0.044 (6) | 0.003 (5) | 0.009 (5) | −0.001 (5) |
| C27 | 0.060 (7) | 0.030 (5) | 0.042 (6) | 0.006 (5) | 0.001 (5) | −0.008 (5) |
| C28 | 0.053 (7) | 0.056 (7) | 0.100 (9) | 0.015 (6) | −0.016 (7) | 0.001 (6) |
| C29 | 0.097 (9) | 0.099 (9) | 0.104 (10) | 0.053 (8) | 0.012 (8) | −0.007 (8) |
Geometric parameters (Å, º)
| F—C6 | 1.374 (13) | C11—C12 | 1.365 (13) |
| O1—C16 | 1.216 (10) | C11—H11A | 0.9300 |
| N1—C14 | 1.356 (11) | C12—C13 | 1.339 (13) |
| N1—C15 | 1.367 (10) | C12—H12A | 0.9300 |
| N1—H1A | 0.8600 | C13—C14 | 1.418 (12) |
| C1—C2 | 1.464 (11) | C13—H13A | 0.9300 |
| C1—C8 | 1.492 (10) | C15—C16 | 1.488 (13) |
| C1—C19 | 1.517 (10) | C17—C18 | 1.371 (12) |
| C1—H1B | 0.9800 | C17—H17A | 0.9700 |
| N2—C25 | 1.335 (10) | C17—H17B | 0.9700 |
| N2—C26 | 1.398 (9) | C18—H18A | 0.9600 |
| N2—H2A | 0.8600 | C18—H18B | 0.9600 |
| O2—C16 | 1.297 (10) | C18—H18C | 0.9600 |
| O2—C17 | 1.440 (11) | C19—C26 | 1.399 (10) |
| C2—C7 | 1.359 (13) | C19—C20 | 1.409 (11) |
| C2—C3 | 1.401 (13) | C20—C25 | 1.394 (11) |
| O3—C27 | 1.351 (9) | C20—C21 | 1.407 (11) |
| O3—C28 | 1.421 (9) | C21—C22 | 1.389 (12) |
| C3—C4 | 1.370 (18) | C21—H21A | 0.9300 |
| C3—H3A | 0.9300 | C22—C23 | 1.420 (12) |
| O4—C27 | 1.234 (9) | C22—H22A | 0.9300 |
| C4—C5 | 1.363 (19) | C23—C24 | 1.361 (12) |
| C4—H4A | 0.9300 | C23—H23A | 0.9300 |
| C5—C6 | 1.339 (18) | C24—C25 | 1.392 (11) |
| C5—H5A | 0.9300 | C24—H24A | 0.9300 |
| C6—C7 | 1.426 (15) | C26—C27 | 1.370 (11) |
| C7—H7A | 0.9300 | C28—C29 | 1.465 (11) |
| C8—C15 | 1.364 (11) | C28—H28A | 0.9700 |
| C8—C9 | 1.457 (12) | C28—H28B | 0.9700 |
| C9—C10 | 1.382 (12) | C29—H29A | 0.9600 |
| C9—C14 | 1.400 (11) | C29—H29B | 0.9600 |
| C10—C11 | 1.389 (12) | C29—H29C | 0.9600 |
| C10—H10A | 0.9300 | ||
| C14—N1—C15 | 108.4 (9) | C8—C15—C16 | 131.9 (10) |
| C14—N1—H1A | 125.8 | O1—C16—O2 | 125.4 (11) |
| C15—N1—H1A | 125.8 | O1—C16—C15 | 121.1 (10) |
| C2—C1—C8 | 111.1 (7) | O2—C16—C15 | 113.2 (10) |
| C2—C1—C19 | 115.7 (7) | C18—C17—O2 | 109.1 (10) |
| C8—C1—C19 | 114.4 (7) | C18—C17—H17A | 109.9 |
| C2—C1—H1B | 104.7 | O2—C17—H17A | 109.9 |
| C8—C1—H1B | 104.7 | C18—C17—H17B | 109.9 |
| C19—C1—H1B | 104.7 | O2—C17—H17B | 109.9 |
| C25—N2—C26 | 109.7 (7) | H17A—C17—H17B | 108.3 |
| C25—N2—H2A | 125.1 | C17—C18—H18A | 109.5 |
| C26—N2—H2A | 125.1 | C17—C18—H18B | 109.5 |
| C16—O2—C17 | 117.4 (9) | H18A—C18—H18B | 109.5 |
| C7—C2—C3 | 119.2 (11) | C17—C18—H18C | 109.5 |
| C7—C2—C1 | 123.7 (10) | H18A—C18—H18C | 109.5 |
| C3—C2—C1 | 117.1 (10) | H18B—C18—H18C | 109.5 |
| C27—O3—C28 | 119.0 (7) | C26—C19—C20 | 106.9 (7) |
| C4—C3—C2 | 121.6 (15) | C26—C19—C1 | 122.8 (8) |
| C4—C3—H3A | 119.2 | C20—C19—C1 | 130.2 (7) |
| C2—C3—H3A | 119.2 | C25—C20—C21 | 118.4 (9) |
| C3—C4—C5 | 122.0 (19) | C25—C20—C19 | 107.5 (8) |
| C3—C4—H4A | 119.0 | C21—C20—C19 | 134.1 (8) |
| C5—C4—H4A | 119.0 | C22—C21—C20 | 118.8 (9) |
| C6—C5—C4 | 114.4 (18) | C22—C21—H21A | 120.6 |
| C6—C5—H5A | 122.8 | C20—C21—H21A | 120.6 |
| C4—C5—H5A | 122.8 | C21—C22—C23 | 120.8 (10) |
| C5—C6—F | 120.2 (17) | C21—C22—H22A | 119.6 |
| C5—C6—C7 | 127.9 (14) | C23—C22—H22A | 119.6 |
| F—C6—C7 | 111.8 (15) | C24—C23—C22 | 120.8 (10) |
| C2—C7—C6 | 114.8 (11) | C24—C23—H23A | 119.6 |
| C2—C7—H7A | 122.6 | C22—C23—H23A | 119.6 |
| C6—C7—H7A | 122.6 | C23—C24—C25 | 117.8 (9) |
| C15—C8—C9 | 104.8 (8) | C23—C24—H24A | 121.1 |
| C15—C8—C1 | 127.7 (9) | C25—C24—H24A | 121.1 |
| C9—C8—C1 | 127.4 (9) | N2—C25—C24 | 127.8 (9) |
| C10—C9—C14 | 119.6 (10) | N2—C25—C20 | 108.8 (8) |
| C10—C9—C8 | 133.6 (9) | C24—C25—C20 | 123.4 (9) |
| C14—C9—C8 | 106.8 (9) | C27—C26—N2 | 116.7 (8) |
| C11—C10—C9 | 117.9 (10) | C27—C26—C19 | 136.2 (8) |
| C11—C10—H10A | 121.0 | N2—C26—C19 | 107.1 (7) |
| C9—C10—H10A | 121.0 | O4—C27—O3 | 118.1 (8) |
| C12—C11—C10 | 121.6 (11) | O4—C27—C26 | 126.7 (8) |
| C12—C11—H11A | 119.2 | O3—C27—C26 | 114.9 (8) |
| C10—C11—H11A | 119.2 | O3—C28—C29 | 106.7 (8) |
| C13—C12—C11 | 122.5 (12) | O3—C28—H28A | 110.4 |
| C13—C12—H12A | 118.7 | C29—C28—H28A | 110.4 |
| C11—C12—H12A | 118.7 | O3—C28—H28B | 110.4 |
| C12—C13—C14 | 117.2 (11) | C29—C28—H28B | 110.4 |
| C12—C13—H13A | 121.4 | H28A—C28—H28B | 108.6 |
| C14—C13—H13A | 121.4 | C28—C29—H29A | 109.5 |
| N1—C14—C9 | 108.6 (9) | C28—C29—H29B | 109.5 |
| N1—C14—C13 | 130.4 (11) | H29A—C29—H29B | 109.5 |
| C9—C14—C13 | 121.0 (11) | C28—C29—H29C | 109.5 |
| N1—C15—C8 | 111.4 (9) | H29A—C29—H29C | 109.5 |
| N1—C15—C16 | 116.3 (10) | H29B—C29—H29C | 109.5 |
| C8—C1—C2—C7 | 110.8 (9) | C17—O2—C16—O1 | 3.8 (15) |
| C19—C1—C2—C7 | −21.8 (13) | C17—O2—C16—C15 | 177.6 (9) |
| C8—C1—C2—C3 | −67.4 (11) | N1—C15—C16—O1 | −11.3 (14) |
| C19—C1—C2—C3 | 159.9 (8) | C8—C15—C16—O1 | 176.3 (10) |
| C7—C2—C3—C4 | −0.8 (16) | N1—C15—C16—O2 | 174.6 (8) |
| C1—C2—C3—C4 | 177.5 (11) | C8—C15—C16—O2 | 2.1 (15) |
| C2—C3—C4—C5 | 1 (2) | C16—O2—C17—C18 | 138.6 (11) |
| C3—C4—C5—C6 | 1 (2) | C2—C1—C19—C26 | −72.7 (11) |
| C4—C5—C6—F | −179.3 (12) | C8—C1—C19—C26 | 156.2 (8) |
| C4—C5—C6—C7 | −3 (2) | C2—C1—C19—C20 | 104.3 (11) |
| C3—C2—C7—C6 | −0.4 (13) | C8—C1—C19—C20 | −26.8 (14) |
| C1—C2—C7—C6 | −178.6 (8) | C26—C19—C20—C25 | −1.7 (10) |
| C5—C6—C7—C2 | 2.2 (17) | C1—C19—C20—C25 | −179.0 (9) |
| F—C6—C7—C2 | 179.2 (8) | C26—C19—C20—C21 | 179.0 (10) |
| C2—C1—C8—C15 | 149.4 (10) | C1—C19—C20—C21 | 1.6 (18) |
| C19—C1—C8—C15 | −77.3 (12) | C25—C20—C21—C22 | 0.2 (15) |
| C2—C1—C8—C9 | −34.5 (13) | C19—C20—C21—C22 | 179.5 (10) |
| C19—C1—C8—C9 | 98.8 (11) | C20—C21—C22—C23 | 0.8 (16) |
| C15—C8—C9—C10 | 177.8 (11) | C21—C22—C23—C24 | −0.5 (18) |
| C1—C8—C9—C10 | 1.0 (17) | C22—C23—C24—C25 | −0.6 (17) |
| C15—C8—C9—C14 | −1.5 (10) | C26—N2—C25—C24 | −177.8 (10) |
| C1—C8—C9—C14 | −178.2 (8) | C26—N2—C25—C20 | 0.5 (11) |
| C14—C9—C10—C11 | −1.9 (15) | C23—C24—C25—N2 | 179.7 (10) |
| C8—C9—C10—C11 | 178.9 (10) | C23—C24—C25—C20 | 1.7 (16) |
| C9—C10—C11—C12 | 0.9 (16) | C21—C20—C25—N2 | −179.8 (8) |
| C10—C11—C12—C13 | 0.7 (19) | C19—C20—C25—N2 | 0.7 (11) |
| C11—C12—C13—C14 | −1.2 (18) | C21—C20—C25—C24 | −1.4 (15) |
| C15—N1—C14—C9 | 0.3 (10) | C19—C20—C25—C24 | 179.1 (9) |
| C15—N1—C14—C13 | −179.8 (9) | C25—N2—C26—C27 | 179.2 (8) |
| C10—C9—C14—N1 | −178.7 (9) | C25—N2—C26—C19 | −1.5 (10) |
| C8—C9—C14—N1 | 0.7 (10) | C20—C19—C26—C27 | −179.0 (11) |
| C10—C9—C14—C13 | 1.4 (14) | C1—C19—C26—C27 | −1.4 (16) |
| C8—C9—C14—C13 | −179.2 (8) | C20—C19—C26—N2 | 1.9 (10) |
| C12—C13—C14—N1 | −179.8 (10) | C1—C19—C26—N2 | 179.5 (8) |
| C12—C13—C14—C9 | 0.1 (15) | C28—O3—C27—O4 | 3.8 (13) |
| C14—N1—C15—C8 | −1.3 (11) | C28—O3—C27—C26 | 178.8 (8) |
| C14—N1—C15—C16 | −175.3 (8) | N2—C26—C27—O4 | −7.4 (14) |
| C9—C8—C15—N1 | 1.7 (10) | C19—C26—C27—O4 | 173.6 (10) |
| C1—C8—C15—N1 | 178.5 (8) | N2—C26—C27—O3 | 178.1 (7) |
| C9—C8—C15—C16 | 174.4 (9) | C19—C26—C27—O3 | −1.0 (16) |
| C1—C8—C15—C16 | −8.8 (16) | C27—O3—C28—C29 | −178.8 (8) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···O1i | 0.86 | 2.16 | 2.918 (11) | 147 |
| N2—H2A···O4ii | 0.86 | 2.08 | 2.904 (9) | 159 |
| C11—H11A···O4iii | 0.93 | 2.58 | 3.498 (13) | 171 |
Symmetry codes: (i) −x, −y, −z+1; (ii) −x+1, −y+1, −z+1; (iii) x−1/2, −y+1/2, z−1/2.
Funding Statement
Funding for this research was provided by: Natural Science Foundation of Jiangsu Province (grant No. BK20181486); Natural Science Foundation of the Jiangsu Higher Education Institutions (grant No. 17KJB320001); Training program of Students Innovation and Entrepreneurship in Jiangsu Province (grant No. 201812920002Y); Overseas Training Program for Excellent Young Teachers and Principals of Jiangsu Province; Qing Lan Project of Jiangsu Province; Natural Science Foundation of Nanjing Polytechnic Institute (grant No. NHKY-2019-07).
References
- Enraf–Nonius (1994). CAD-4 EXPRESS. Enraf–Nonius, Delft, The Netherlands.
- Harms, K. & Wocadlo, S. (1995). XCAD4. University of Marburg, Germany.
- Lu, X.-H., Sun, H.-S., Cai, Y., Chen, L.-L. & Chen, Y.-F. (2017). Acta Cryst. E73, 1790–1792. [DOI] [PMC free article] [PubMed]
- Lu, X.-H., Sun, H.-S. & Hu, J. (2014). Acta Cryst. E70, 593–595. [DOI] [PMC free article] [PubMed]
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sun, H.-S., Li, Y., Jiang, H., Xu, N. & Xu, H. (2015). Acta Cryst. E71, 1140–1142. [DOI] [PMC free article] [PubMed]
- Sun, H.-S., Li, Y.-L., Xu, N., Xu, H. & Zhang, J.-D. (2012). Acta Cryst. E68, o2764. [DOI] [PMC free article] [PubMed]
- Sundberg, R. J. (1996). The Chemistry of Indoles, p. 113. New York: Academic Press.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S2414314620009128/hb4349sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620009128/hb4349Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620009128/hb4349Isup3.cml
CCDC reference: 2014000
Additional supporting information: crystallographic information; 3D view; checkCIF report