In the title compound, the dihedral angle between the pyridine and difluorobenzene rings is 45.12 (12)°.
Keywords: crystal structure, 2-phenylpyridine, pyridine ring
Abstract
In the title compound, C27H19F2N, the five-fused-ring system is highly puckered and the dihedral angle between the central pyridine ring and pendant difluorobenzene ring is 45.12 (12)°. In the crystal, inversion dimers linked by pairwise weak C—H⋯N hydrogen bonds generate R
2
2(12) loops and the dimers are further linked by weak C—H⋯F interactions to form [
 01] chains.
01] chains.
Structure description
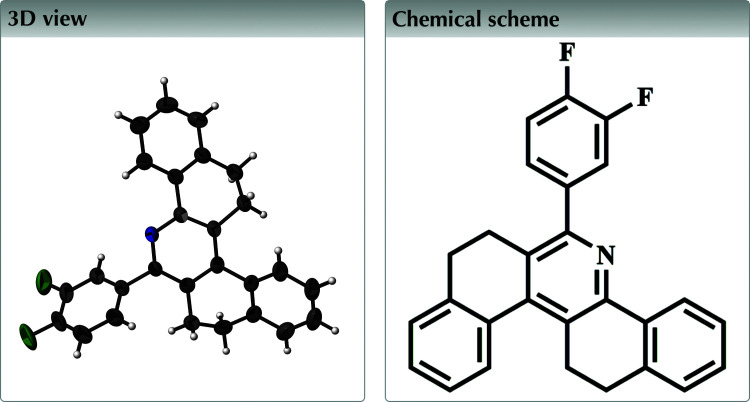
In recent years, nitrogen-containing heterocyclic molecular materials have found use as optoelectronic materials (Gu et al. 2017 ▸; Zhang et al., 2019 ▸) because of their conjugated structures and photophysical properties. In this work, we describe the synthesis and structure of the title compound (Fig. 1 ▸) in which the F atoms should increase solubility and provide strong electron-withdrawing groups.
Figure 1.
The molecular structure of the title compound showing 50% displacement ellipsoids.
The crystal structure shows that the dihedral angle between the C1–C6 difluorobenzene ring and the adjacent C7/C8/C17/C18/C27/N1 pyridine ring is 45.12 (12)°. In the crystal, pairwise weak C10—H10B⋯N1 hydrogen bonds (Table 1 ▸) link the molecules into inversion dimers, which are further linked by weak C—H⋯F interactions (Fig. 2 ▸) to form [
 01] double chains.
01] double chains.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10—H10B⋯N1i | 0.97 | 2.61 | 3.552 (4) | 165 |
| C19—H19A⋯F2ii | 0.97 | 2.56 | 3.111 (4) | 116 |
Symmetry codes: (i)
 ; (ii)
; (ii)
 .
.
Figure 2.
Partial packing diagram showing hydrogen bonds as dashed lines.
Synthesis and crystallization
Ammonium acetate (7.60 g, 0.100 mol) was dissolved in 15 ml glacial acetic acid. Then, 3,4-difluorobenzaldehyde (2.00 g, 0.014 mol) and 1-tetrahydronaphthalone (4.12 g, 0.028 mol) were added and the reaction was heated to reflux at 383 K for 5 h. Upon cooling and recrystallization from ethanol solution, 1.64 g (yield 30%) of the title compound was recovered. Crystals for X-ray analysis were obtained from the slow evaporation of an acetonitrile solution.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C27H19F2N |
| M r | 395.43 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 296 |
| a, b, c (Å) | 9.394 (5), 9.802 (5), 11.914 (6) |
| α, β, γ (°) | 88.983 (5), 73.144 (5), 69.488 (5) |
| V (Å3) | 979.0 (8) |
| Z | 2 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.03 × 0.02 × 0.01 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD area detector |
| Absorption correction | Multi-scan (SADABS; Bruker, 2013 ▸) |
| T min, T max | 0.633, 0.746 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 7892, 4051, 3053 |
| R int | 0.027 |
| (sin θ/λ)max (Å−1) | 0.651 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.063, 0.190, 1.06 |
| No. of reflections | 4051 |
| No. of parameters | 271 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.43, −0.25 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314620012419/hb4358sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620012419/hb4358Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620012419/hb4358Isup3.cml
CCDC reference: 2012681
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| C27H19F2N | Z = 2 |
| Mr = 395.43 | F(000) = 412 |
| Triclinic, P1 | Dx = 1.341 Mg m−3 |
| a = 9.394 (5) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 9.802 (5) Å | Cell parameters from 2895 reflections |
| c = 11.914 (6) Å | θ = 2.4–27.5° |
| α = 88.983 (5)° | µ = 0.09 mm−1 |
| β = 73.144 (5)° | T = 296 K |
| γ = 69.488 (5)° | Block-shaped, white |
| V = 979.0 (8) Å3 | 0.03 × 0.02 × 0.01 mm |
Data collection
| Bruker APEXII CCD area detector diffractometer | 3053 reflections with I > 2σ(I) |
| phi and ω scans | Rint = 0.027 |
| Absorption correction: multi-scan (SADABS; Bruker, 2013) | θmax = 27.6°, θmin = 1.8° |
| Tmin = 0.633, Tmax = 0.746 | h = −12→12 |
| 7892 measured reflections | k = −12→10 |
| 4051 independent reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.063 | H-atom parameters constrained |
| wR(F2) = 0.190 | w = 1/[σ2(Fo2) + (0.0837P)2 + 0.5466P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max < 0.001 |
| 4051 reflections | Δρmax = 0.43 e Å−3 |
| 271 parameters | Δρmin = −0.25 e Å−3 |
| 0 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.7467 (2) | 0.3707 (2) | 0.54946 (15) | 0.0391 (4) | |
| F1 | 1.0054 (3) | 0.3247 (2) | 0.10698 (15) | 0.0904 (6) | |
| F2 | 1.1593 (2) | 0.5132 (3) | 0.08204 (17) | 0.1047 (8) | |
| C18 | 0.5333 (2) | 0.4724 (2) | 0.73116 (18) | 0.0380 (5) | |
| C17 | 0.5140 (2) | 0.6141 (2) | 0.69776 (18) | 0.0381 (5) | |
| C8 | 0.6055 (3) | 0.6315 (2) | 0.58465 (19) | 0.0391 (5) | |
| C7 | 0.7235 (2) | 0.5064 (2) | 0.51581 (18) | 0.0363 (5) | |
| C5 | 0.8364 (2) | 0.5126 (2) | 0.39951 (18) | 0.0386 (5) | |
| C27 | 0.6517 (2) | 0.3545 (2) | 0.65376 (18) | 0.0374 (5) | |
| C16 | 0.3991 (3) | 0.7498 (2) | 0.7746 (2) | 0.0417 (5) | |
| C26 | 0.6825 (3) | 0.2019 (2) | 0.68702 (19) | 0.0410 (5) | |
| C21 | 0.5609 (3) | 0.1703 (3) | 0.7718 (2) | 0.0439 (5) | |
| C6 | 0.8684 (3) | 0.4139 (3) | 0.30477 (19) | 0.0443 (5) | |
| H6 | 0.817554 | 0.346510 | 0.313138 | 0.053* | |
| C11 | 0.3292 (3) | 0.8721 (3) | 0.7200 (2) | 0.0456 (6) | |
| C22 | 0.5900 (3) | 0.0269 (3) | 0.8019 (2) | 0.0534 (6) | |
| H22 | 0.510292 | 0.005181 | 0.857526 | 0.064* | |
| C4 | 0.9152 (3) | 0.6117 (3) | 0.3848 (2) | 0.0510 (6) | |
| H4 | 0.894687 | 0.678252 | 0.447433 | 0.061* | |
| C19 | 0.4315 (3) | 0.4353 (3) | 0.84374 (19) | 0.0462 (6) | |
| H19A | 0.328771 | 0.514474 | 0.870742 | 0.055* | |
| H19B | 0.483188 | 0.426509 | 0.904573 | 0.055* | |
| C20 | 0.4060 (3) | 0.2928 (3) | 0.8250 (2) | 0.0508 (6) | |
| H20A | 0.350530 | 0.267914 | 0.899957 | 0.061* | |
| H20B | 0.339713 | 0.306392 | 0.773529 | 0.061* | |
| C15 | 0.3651 (3) | 0.7608 (3) | 0.8972 (2) | 0.0517 (6) | |
| H15 | 0.416490 | 0.682039 | 0.933526 | 0.062* | |
| C1 | 0.9756 (3) | 0.4166 (3) | 0.1989 (2) | 0.0549 (7) | |
| C25 | 0.8280 (3) | 0.0895 (3) | 0.6371 (2) | 0.0508 (6) | |
| H25 | 0.908948 | 0.109709 | 0.581439 | 0.061* | |
| C9 | 0.5619 (3) | 0.7816 (3) | 0.5415 (2) | 0.0480 (6) | |
| H9A | 0.595581 | 0.772922 | 0.456085 | 0.058* | |
| H9B | 0.616434 | 0.836198 | 0.568047 | 0.058* | |
| C10 | 0.3802 (3) | 0.8638 (3) | 0.5889 (2) | 0.0510 (6) | |
| H10A | 0.352889 | 0.961687 | 0.563338 | 0.061* | |
| H10B | 0.325601 | 0.812838 | 0.558290 | 0.061* | |
| C2 | 1.0529 (3) | 0.5146 (3) | 0.1853 (2) | 0.0615 (8) | |
| C12 | 0.2169 (3) | 0.9997 (3) | 0.7903 (3) | 0.0588 (7) | |
| H12 | 0.166393 | 1.080199 | 0.755108 | 0.071* | |
| C3 | 1.0245 (3) | 0.6118 (3) | 0.2769 (3) | 0.0617 (7) | |
| H3 | 1.077498 | 0.677447 | 0.267400 | 0.074* | |
| C24 | 0.8549 (3) | −0.0521 (3) | 0.6687 (3) | 0.0618 (7) | |
| H24 | 0.953022 | −0.126144 | 0.634986 | 0.074* | |
| C23 | 0.7334 (4) | −0.0818 (3) | 0.7512 (3) | 0.0617 (7) | |
| H23 | 0.749976 | −0.176760 | 0.772106 | 0.074* | |
| C13 | 0.1814 (3) | 1.0058 (3) | 0.9111 (3) | 0.0670 (8) | |
| H13 | 0.106587 | 1.090484 | 0.956823 | 0.080* | |
| C14 | 0.2552 (3) | 0.8882 (3) | 0.9651 (2) | 0.0616 (8) | |
| H14 | 0.231630 | 0.894100 | 1.046662 | 0.074* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0375 (9) | 0.0410 (10) | 0.0340 (9) | −0.0146 (8) | −0.0032 (7) | 0.0015 (7) |
| F1 | 0.0965 (14) | 0.0996 (14) | 0.0468 (9) | −0.0197 (11) | 0.0020 (9) | −0.0195 (9) |
| F2 | 0.0783 (13) | 0.1363 (19) | 0.0616 (11) | −0.0315 (13) | 0.0245 (10) | 0.0223 (11) |
| C18 | 0.0354 (11) | 0.0462 (12) | 0.0301 (10) | −0.0133 (9) | −0.0084 (8) | 0.0024 (9) |
| C17 | 0.0362 (11) | 0.0438 (12) | 0.0323 (10) | −0.0126 (9) | −0.0093 (9) | −0.0029 (9) |
| C8 | 0.0441 (12) | 0.0381 (12) | 0.0367 (11) | −0.0150 (9) | −0.0142 (9) | 0.0026 (9) |
| C7 | 0.0363 (11) | 0.0404 (12) | 0.0321 (10) | −0.0161 (9) | −0.0073 (9) | 0.0031 (8) |
| C5 | 0.0335 (10) | 0.0440 (12) | 0.0359 (11) | −0.0130 (9) | −0.0086 (9) | 0.0076 (9) |
| C27 | 0.0356 (11) | 0.0430 (12) | 0.0322 (10) | −0.0146 (9) | −0.0080 (9) | 0.0039 (9) |
| C16 | 0.0388 (11) | 0.0412 (12) | 0.0435 (12) | −0.0159 (10) | −0.0079 (10) | −0.0038 (9) |
| C26 | 0.0436 (12) | 0.0450 (13) | 0.0365 (11) | −0.0182 (10) | −0.0126 (9) | 0.0065 (9) |
| C21 | 0.0465 (13) | 0.0485 (13) | 0.0405 (12) | −0.0211 (11) | −0.0141 (10) | 0.0079 (10) |
| C6 | 0.0407 (12) | 0.0491 (13) | 0.0385 (12) | −0.0128 (10) | −0.0096 (10) | 0.0056 (10) |
| C11 | 0.0409 (12) | 0.0415 (12) | 0.0525 (13) | −0.0166 (10) | −0.0086 (10) | −0.0058 (10) |
| C22 | 0.0581 (15) | 0.0546 (15) | 0.0588 (15) | −0.0317 (13) | −0.0212 (13) | 0.0177 (12) |
| C4 | 0.0481 (14) | 0.0571 (15) | 0.0507 (14) | −0.0268 (12) | −0.0098 (11) | 0.0074 (11) |
| C19 | 0.0460 (13) | 0.0494 (14) | 0.0342 (11) | −0.0139 (11) | −0.0030 (10) | 0.0051 (10) |
| C20 | 0.0458 (13) | 0.0538 (15) | 0.0490 (14) | −0.0199 (11) | −0.0068 (11) | 0.0125 (11) |
| C15 | 0.0518 (14) | 0.0596 (16) | 0.0416 (12) | −0.0247 (12) | −0.0048 (11) | −0.0093 (11) |
| C1 | 0.0470 (14) | 0.0653 (16) | 0.0347 (12) | −0.0045 (12) | −0.0055 (10) | 0.0015 (11) |
| C25 | 0.0453 (13) | 0.0473 (14) | 0.0509 (14) | −0.0126 (11) | −0.0068 (11) | 0.0055 (11) |
| C9 | 0.0611 (15) | 0.0432 (13) | 0.0378 (12) | −0.0214 (11) | −0.0088 (11) | 0.0036 (10) |
| C10 | 0.0601 (15) | 0.0402 (13) | 0.0547 (14) | −0.0169 (11) | −0.0221 (12) | 0.0105 (11) |
| C2 | 0.0416 (13) | 0.0790 (19) | 0.0458 (14) | −0.0149 (13) | 0.0038 (11) | 0.0188 (13) |
| C12 | 0.0491 (14) | 0.0445 (14) | 0.0769 (19) | −0.0148 (12) | −0.0126 (13) | −0.0036 (13) |
| C3 | 0.0443 (14) | 0.0709 (18) | 0.0691 (18) | −0.0283 (13) | −0.0078 (13) | 0.0229 (15) |
| C24 | 0.0564 (16) | 0.0481 (15) | 0.0728 (18) | −0.0126 (12) | −0.0157 (14) | 0.0090 (13) |
| C23 | 0.0649 (17) | 0.0479 (15) | 0.0777 (19) | −0.0218 (13) | −0.0285 (15) | 0.0190 (13) |
| C13 | 0.0520 (15) | 0.0613 (18) | 0.0717 (19) | −0.0205 (14) | 0.0065 (14) | −0.0248 (15) |
| C14 | 0.0593 (16) | 0.0686 (18) | 0.0491 (14) | −0.0277 (14) | 0.0024 (12) | −0.0188 (13) |
Geometric parameters (Å, º)
| N1—C7 | 1.344 (3) | C4—H4 | 0.9300 |
| N1—C27 | 1.348 (3) | C4—C3 | 1.394 (3) |
| F1—C1 | 1.330 (3) | C19—H19A | 0.9700 |
| F2—C2 | 1.338 (3) | C19—H19B | 0.9700 |
| C18—C17 | 1.401 (3) | C19—C20 | 1.528 (4) |
| C18—C27 | 1.402 (3) | C20—H20A | 0.9700 |
| C18—C19 | 1.526 (3) | C20—H20B | 0.9700 |
| C17—C8 | 1.417 (3) | C15—H15 | 0.9300 |
| C17—C16 | 1.495 (3) | C15—C14 | 1.388 (4) |
| C8—C7 | 1.401 (3) | C1—C2 | 1.376 (4) |
| C8—C9 | 1.506 (3) | C25—H25 | 0.9300 |
| C7—C5 | 1.496 (3) | C25—C24 | 1.386 (4) |
| C5—C6 | 1.393 (3) | C9—H9A | 0.9700 |
| C5—C4 | 1.395 (3) | C9—H9B | 0.9700 |
| C27—C26 | 1.490 (3) | C9—C10 | 1.541 (4) |
| C16—C11 | 1.399 (4) | C10—H10A | 0.9700 |
| C16—C15 | 1.400 (3) | C10—H10B | 0.9700 |
| C26—C21 | 1.410 (3) | C2—C3 | 1.363 (4) |
| C26—C25 | 1.390 (3) | C12—H12 | 0.9300 |
| C21—C22 | 1.396 (3) | C12—C13 | 1.377 (4) |
| C21—C20 | 1.493 (3) | C3—H3 | 0.9300 |
| C6—H6 | 0.9300 | C24—H24 | 0.9300 |
| C6—C1 | 1.374 (3) | C24—C23 | 1.388 (4) |
| C11—C10 | 1.490 (4) | C23—H23 | 0.9300 |
| C11—C12 | 1.407 (3) | C13—H13 | 0.9300 |
| C22—H22 | 0.9300 | C13—C14 | 1.377 (4) |
| C22—C23 | 1.364 (4) | C14—H14 | 0.9300 |
| C7—N1—C27 | 118.46 (18) | C21—C20—H20A | 109.4 |
| C17—C18—C27 | 117.92 (19) | C21—C20—H20B | 109.4 |
| C17—C18—C19 | 125.17 (19) | C19—C20—H20A | 109.4 |
| C27—C18—C19 | 116.9 (2) | C19—C20—H20B | 109.4 |
| C18—C17—C8 | 118.99 (19) | H20A—C20—H20B | 108.0 |
| C18—C17—C16 | 123.57 (19) | C16—C15—H15 | 119.7 |
| C8—C17—C16 | 117.4 (2) | C14—C15—C16 | 120.6 (3) |
| C17—C8—C9 | 118.13 (19) | C14—C15—H15 | 119.7 |
| C7—C8—C17 | 118.1 (2) | F1—C1—C6 | 120.3 (3) |
| C7—C8—C9 | 123.6 (2) | F1—C1—C2 | 118.7 (2) |
| N1—C7—C8 | 122.89 (19) | C6—C1—C2 | 121.0 (2) |
| N1—C7—C5 | 114.26 (18) | C26—C25—H25 | 119.4 |
| C8—C7—C5 | 122.84 (19) | C24—C25—C26 | 121.3 (2) |
| C6—C5—C7 | 119.4 (2) | C24—C25—H25 | 119.4 |
| C6—C5—C4 | 119.0 (2) | C8—C9—H9A | 109.7 |
| C4—C5—C7 | 121.5 (2) | C8—C9—H9B | 109.7 |
| N1—C27—C18 | 123.3 (2) | C8—C9—C10 | 110.01 (19) |
| N1—C27—C26 | 116.34 (19) | H9A—C9—H9B | 108.2 |
| C18—C27—C26 | 120.27 (19) | C10—C9—H9A | 109.7 |
| C11—C16—C17 | 118.0 (2) | C10—C9—H9B | 109.7 |
| C11—C16—C15 | 119.3 (2) | C11—C10—C9 | 109.1 (2) |
| C15—C16—C17 | 122.7 (2) | C11—C10—H10A | 109.9 |
| C21—C26—C27 | 119.1 (2) | C11—C10—H10B | 109.9 |
| C25—C26—C27 | 121.9 (2) | C9—C10—H10A | 109.9 |
| C25—C26—C21 | 119.0 (2) | C9—C10—H10B | 109.9 |
| C26—C21—C20 | 118.1 (2) | H10A—C10—H10B | 108.3 |
| C22—C21—C26 | 118.9 (2) | F2—C2—C1 | 119.9 (3) |
| C22—C21—C20 | 123.0 (2) | F2—C2—C3 | 119.5 (3) |
| C5—C6—H6 | 120.2 | C3—C2—C1 | 120.6 (2) |
| C1—C6—C5 | 119.6 (2) | C11—C12—H12 | 119.9 |
| C1—C6—H6 | 120.2 | C13—C12—C11 | 120.2 (3) |
| C16—C11—C10 | 118.6 (2) | C13—C12—H12 | 119.9 |
| C16—C11—C12 | 119.2 (2) | C4—C3—H3 | 120.3 |
| C12—C11—C10 | 122.2 (2) | C2—C3—C4 | 119.4 (3) |
| C21—C22—H22 | 119.4 | C2—C3—H3 | 120.3 |
| C23—C22—C21 | 121.2 (2) | C25—C24—H24 | 120.4 |
| C23—C22—H22 | 119.4 | C25—C24—C23 | 119.2 (3) |
| C5—C4—H4 | 119.8 | C23—C24—H24 | 120.4 |
| C3—C4—C5 | 120.4 (3) | C22—C23—C24 | 120.6 (3) |
| C3—C4—H4 | 119.8 | C22—C23—H23 | 119.7 |
| C18—C19—H19A | 109.2 | C24—C23—H23 | 119.7 |
| C18—C19—H19B | 109.2 | C12—C13—H13 | 119.6 |
| C18—C19—C20 | 111.98 (19) | C14—C13—C12 | 120.9 (3) |
| H19A—C19—H19B | 107.9 | C14—C13—H13 | 119.6 |
| C20—C19—H19A | 109.2 | C15—C14—H14 | 120.2 |
| C20—C19—H19B | 109.2 | C13—C14—C15 | 119.7 (3) |
| C21—C20—C19 | 111.3 (2) | C13—C14—H14 | 120.2 |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10—H10B···N1i | 0.97 | 2.61 | 3.552 (4) | 165 |
| C19—H19A···F2ii | 0.97 | 2.56 | 3.111 (4) | 116 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) x−1, y, z+1.
Funding Statement
Funding for this research was provided by: National Natural Science Foundation of China (award Nos. 21871003 and 51672002).
References
- Bruker (2013). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Gu, B., Wu, W. B., Xu, G. X., Feng, G. X., Yin, F., Chong, P. H. J., Qu, J. L., Yong, K. T. & Liu, B. (2017). Adv. Mater. 29, 1701076. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Zhang, C., Zhao, Y., Li, D., Liu, J., Han, H., He, D., Tian, X., Li, S., Wu, J. & Tian, Y. (2019). Chem. Commun. 55, 1450–1453. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314620012419/hb4358sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620012419/hb4358Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620012419/hb4358Isup3.cml
CCDC reference: 2012681
Additional supporting information: crystallographic information; 3D view; checkCIF report




