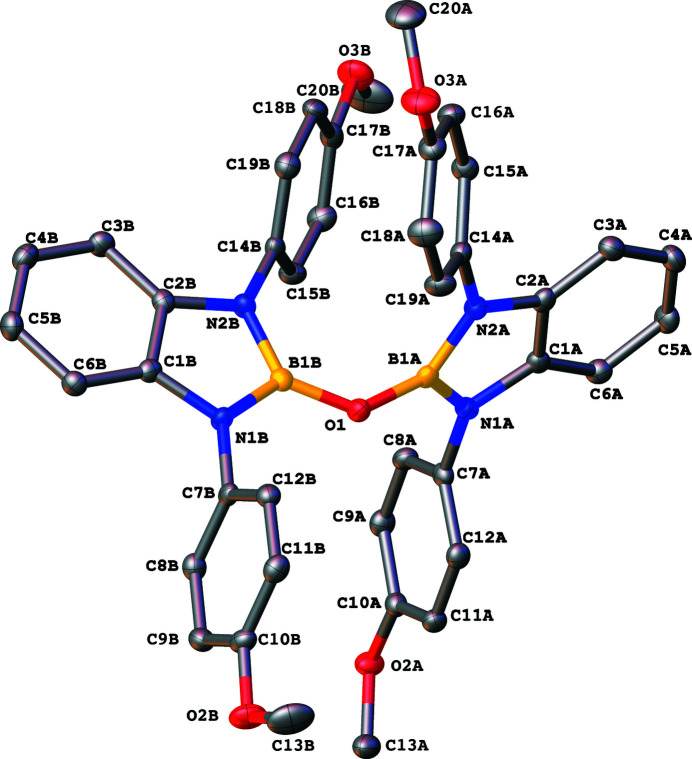
The title compound features a B—O—B bond angle of 132.75 (13)°.
Keywords: crystal structure, bridging μ-oxo, boron
Abstract
In the title compound, C40H36B2N4O5, the B—O—B bond angle is 132.75 (13) and the dihedral angle between the benzodiazborole rings is 73.02 (5)°. In the crystal, weak C—H⋯O interactions link the molecules.
Structure description
The field of cooperative catalysis has given scientists the ability to access more complex molecular transformations using cheaper, readily available metals (Allen et al., 2012 ▸; Lohr & Marks, 2015 ▸). The title compound, C40H36B2N4O5, was synthesized using elements from the main group of the periodic table, which are cheaper and more accessible than the traditionally used transition metals (Karunananda et al., 2017 ▸; Power, 2010 ▸).
The title compound has a pincer-like orientation formed by an oxygen single-atom bridge connected to two Lewis-acidic boron centers (Fig. 1 ▸). The diamine moieties bound to the boron atoms provide redox-active sites, which give the structure the electron equivalents that boron lacks while also modulating the steric environment (Prier et al., 2013 ▸; Pye et al., 2017 ▸; Bellemin-Laponnaz et al., 2014 ▸). The pincer shape might allow the compound to use the boron atoms and the redox-active ligands to create a binding pocket for coordination and bridging of a small molecule substrate.
Figure 1.
The molecular structure of the title compound. Hydrogen atoms have been omitted for clarity. Ellipsoids are at 50% probability.
The B1A—O1—B1B bond angle is 132.75 (13)°, which is reasonable given the steric bulk that is present in the diazaborole moiety. Additionally, it is likely that a p-type electronic interaction exists between O1 and the adjacent boron atoms (B1A and B1B) that would serve to open up the bond angle substantially beyond the textbook angle of 109.5° for an O atom bearing two lone pairs of electrons. As a result of steric encumbrance, the B1A and B1B benzodiazaborole rings are angled away from one another to a near perpendicular orientation, with a plane-to-plane tilt of 73.02 (5)°. The dihedral angles between the B1A benzodiazaborole ring system and its pendant p-methoxybenzene rings are 80.49 (6) and 49.84 (7)° for the C7A and C14A rings, respectively. Comparable data for the B1B ring system and its pendant C7B and C14B rings are 78.32 (6) and 65.96 (7)°, respectively. The C atoms of the methoxy groups are all close to their respective ring planes: C13A [deviation = 0.333 (2) Å]; C20A [0.254 (2) Å]; C13B [−0.040 (2 Å)]; C20B [0.193 (2) Å].
In the crystal, weak C—H⋯O interactions (Table 1 ▸) link the molecules.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C8B—H8B⋯O2A i | 0.95 | 2.40 | 3.233 (2) | 147 |
| C13B—H13E⋯O3B ii | 0.98 | 2.46 | 3.374 (3) | 155 |
Symmetry codes: (i)
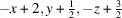 ; (ii)
; (ii)
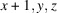 .
.
Synthesis and crystallization
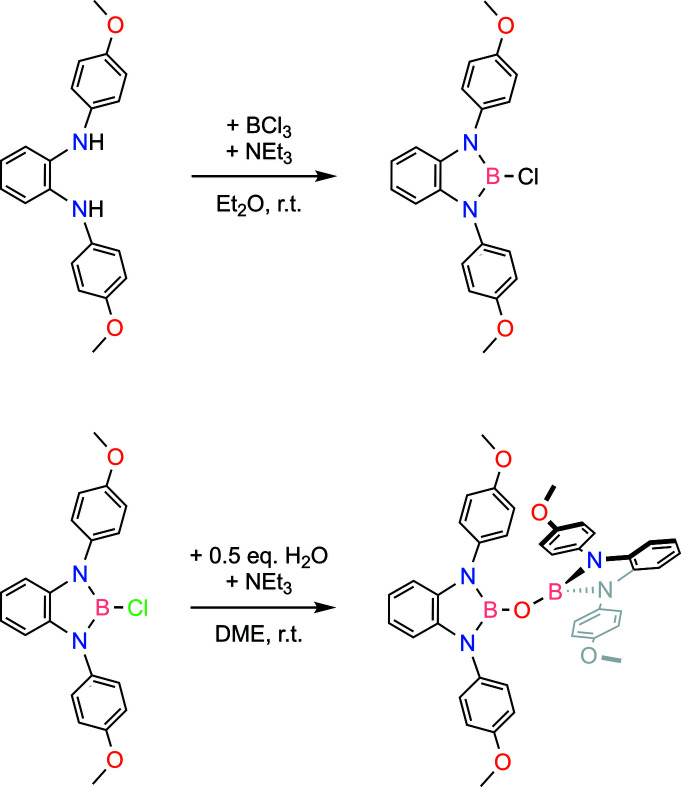
The title compound was synthesized in two steps (Fig. 2 ▸) from the previously reported precursor, N 1,N 2-bis(4-methoxyphenyl)benzene-1,2-diamine (Xiong et al., 2018 ▸; Wang et al., 2018 ▸).
Figure 2.
Chemical scheme for the synthesis of the title compound.
Under an anhydrous nitrogen atmosphere, 12 mmol of the diamine precursor was dissolved in 400 ml of diethyl ether. An excess of triethylamine, four equivalents, was then added. A stoichiometric amount of boron trichloride was added to this stirred solution whereupon a white precipitate composed of a mixture of triethylammonium chloride and the monomeric diazaborole chloride was formed. The volatiles were removed under reduced pressure to give a white solid. The solid was extracted in a fritted glass filter with a minimum volume of benzene, and the filtrate was evaporated under reduced pressure to give the crude diazaborole chloride. This crude solid was recrystallized from a toluene/hexanes mixture. The diazaborole chloride, (II), was obtained in 87% yield. The single-crystal X-ray structure of the diazaborole chloride has been deposited with the Cambridge Structural Database (Mallard et al., 2020 ▸).
Under an anhydrous nitrogen atmosphere, a solution was prepared that contained 3.0 mmol of (II), four equivalents of triethylamine, and ∼200 ml of 1,2-dimethoxyethane. This solution was then treated with half an equivalent of water (used as a 1 M solution in 1,2-dimethoxyethane). After stirring overnight, a white precipitate of the triethylammonium chloride formed that was then filtered and discarded. The filtrate was dried under reduced pressure to give the crude product. The solid was extracted in a fritted glass filter with a minimum volume of benzene, and the filtrate was evaporated under reduced pressure to give the title compound in 85% yield.
Single crystals suitable for X-ray analysis were obtained from a saturated solution of hexanes. The solution was allowed to stand overnight whereupon small colorless crystals formed.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. A small number of intense low-angle reflections are missing from this data set due to the arrangement of the instrument with a conservatively sized beam stop. The large number of reflections in the data set ensures that no particular bias has been introduced.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C40H36B2N4O5 |
| M r | 674.35 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 100 |
| a, b, c (Å) | 16.7584 (15), 13.6696 (14), 16.0291 (17) |
| β (°) | 111.125 (5) |
| V (Å3) | 3425.2 (6) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.09 |
| Crystal size (mm) | 0.17 × 0.07 × 0.05 |
| Data collection | |
| Diffractometer | Bruker D8QUEST |
| Absorption correction | Multi-scan (SADABS; Bruker, 2016 ▸) |
| T min, T max | 0.696, 0.745 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 46461, 6305, 4773 |
| R int | 0.063 |
| (sin θ/λ)max (Å−1) | 0.604 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.039, 0.093, 1.02 |
| No. of reflections | 6305 |
| No. of parameters | 464 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.19, −0.23 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2414314620012481/hb4361sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620012481/hb4361Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620012481/hb4361Isup4.cml
CCDC reference: 2031384
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
The authors thank the University of Pennsylvania for data-collection services and both Professor Louise Dawe (Wilfrid Laurier University) and Dr Amy Sarjeant (Bristol Myers Squibb) for their patient teaching on our journey into crystallography.
full crystallographic data
Crystal data
| C40H36B2N4O5 | F(000) = 1416 |
| Mr = 674.35 | Dx = 1.308 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 16.7584 (15) Å | Cell parameters from 9875 reflections |
| b = 13.6696 (14) Å | θ = 2.6–25.3° |
| c = 16.0291 (17) Å | µ = 0.09 mm−1 |
| β = 111.125 (5)° | T = 100 K |
| V = 3425.2 (6) Å3 | Plank, clear colourless |
| Z = 4 | 0.17 × 0.07 × 0.05 mm |
Data collection
| Bruker D8QUEST diffractometer | 4773 reflections with I > 2σ(I) |
| ω and φ scans | Rint = 0.063 |
| Absorption correction: multi-scan (SADABS; Bruker, 2016) | θmax = 25.4°, θmin = 2.0° |
| Tmin = 0.696, Tmax = 0.745 | h = −20→19 |
| 46461 measured reflections | k = −16→16 |
| 6305 independent reflections | l = −19→19 |
Refinement
| Refinement on F2 | Primary atom site location: iterative |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.039 | H-atom parameters constrained |
| wR(F2) = 0.093 | w = 1/[σ2(Fo2) + (0.0417P)2 + 0.8558P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max = 0.001 |
| 6305 reflections | Δρmax = 0.19 e Å−3 |
| 464 parameters | Δρmin = −0.23 e Å−3 |
| 0 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. The hydrogen atoms were treated in calculated positions and refined in the riding model approximation with distances of C—H = 0.95 and 0.98 Å for the aryl and methyl groups, respectively. Methyl group H atoms were allowed to rotate, but not to tip, in order to find the best rotameric conformation. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.85920 (6) | 0.72089 (8) | 0.47878 (7) | 0.0182 (3) | |
| O2A | 0.94929 (7) | 0.28970 (8) | 0.67336 (7) | 0.0215 (3) | |
| O2B | 1.26822 (7) | 0.80020 (9) | 0.56662 (8) | 0.0271 (3) | |
| O3B | 0.46275 (7) | 0.72061 (9) | 0.49287 (9) | 0.0293 (3) | |
| N2A | 0.72444 (7) | 0.71200 (9) | 0.34165 (9) | 0.0164 (3) | |
| N1B | 0.94279 (7) | 0.84989 (9) | 0.58254 (9) | 0.0153 (3) | |
| N2B | 0.80077 (7) | 0.83556 (9) | 0.56577 (9) | 0.0165 (3) | |
| N1A | 0.77512 (8) | 0.57002 (9) | 0.42085 (9) | 0.0165 (3) | |
| O3A | 0.67160 (7) | 1.09930 (8) | 0.22224 (9) | 0.0306 (3) | |
| C8A | 0.78840 (9) | 0.46361 (11) | 0.54824 (11) | 0.0179 (3) | |
| H8A | 0.736024 | 0.488985 | 0.549372 | 0.022* | |
| C1B | 0.92486 (9) | 0.91823 (11) | 0.63878 (10) | 0.0155 (3) | |
| C4A | 0.56664 (10) | 0.53896 (12) | 0.18616 (11) | 0.0220 (4) | |
| H4A | 0.519745 | 0.535105 | 0.130943 | 0.026* | |
| C14B | 0.71442 (9) | 0.80361 (11) | 0.54539 (10) | 0.0156 (3) | |
| C19B | 0.64607 (10) | 0.86514 (12) | 0.50179 (11) | 0.0196 (4) | |
| H19B | 0.656451 | 0.928549 | 0.483657 | 0.024* | |
| C11A | 0.94122 (9) | 0.38913 (12) | 0.54478 (11) | 0.0181 (4) | |
| H11A | 0.993502 | 0.363627 | 0.543511 | 0.022* | |
| C7B | 1.02652 (9) | 0.83921 (11) | 0.57765 (10) | 0.0153 (3) | |
| C12B | 1.04462 (9) | 0.88045 (11) | 0.50785 (11) | 0.0173 (3) | |
| H12B | 1.001788 | 0.917154 | 0.463646 | 0.021* | |
| C9B | 1.16985 (10) | 0.77675 (12) | 0.63780 (11) | 0.0196 (4) | |
| H9B | 1.213171 | 0.741701 | 0.683007 | 0.024* | |
| C2A | 0.67165 (9) | 0.63331 (11) | 0.29705 (10) | 0.0163 (3) | |
| C8B | 1.09010 (9) | 0.78759 (11) | 0.64347 (11) | 0.0181 (3) | |
| H8B | 1.078610 | 0.759811 | 0.692302 | 0.022* | |
| C7A | 0.82051 (9) | 0.49728 (11) | 0.48451 (10) | 0.0160 (3) | |
| C12A | 0.89620 (9) | 0.45872 (11) | 0.48261 (11) | 0.0178 (3) | |
| H12A | 0.917565 | 0.480128 | 0.438260 | 0.021* | |
| C11B | 1.12469 (10) | 0.86905 (12) | 0.50128 (11) | 0.0196 (4) | |
| H11B | 1.136209 | 0.896959 | 0.452520 | 0.024* | |
| C10A | 0.90946 (9) | 0.35708 (11) | 0.60870 (10) | 0.0169 (3) | |
| C9A | 0.83218 (10) | 0.39372 (11) | 0.60967 (11) | 0.0191 (4) | |
| H9A | 0.809716 | 0.370545 | 0.652606 | 0.023* | |
| C10B | 1.18735 (9) | 0.81671 (12) | 0.56643 (11) | 0.0190 (4) | |
| C2B | 0.83793 (9) | 0.91005 (11) | 0.62841 (10) | 0.0155 (3) | |
| C5A | 0.59719 (10) | 0.45422 (12) | 0.23465 (11) | 0.0218 (4) | |
| H5A | 0.570709 | 0.393380 | 0.212281 | 0.026* | |
| C15B | 0.69809 (9) | 0.71079 (12) | 0.56962 (11) | 0.0191 (4) | |
| H15B | 0.744615 | 0.668406 | 0.599301 | 0.023* | |
| C4B | 0.85715 (10) | 1.03368 (12) | 0.73819 (11) | 0.0216 (4) | |
| H4B | 0.834773 | 1.074256 | 0.772644 | 0.026* | |
| C6A | 0.66609 (9) | 0.45685 (12) | 0.31563 (11) | 0.0184 (4) | |
| H6A | 0.687046 | 0.398918 | 0.349096 | 0.022* | |
| C18B | 0.56315 (10) | 0.83459 (12) | 0.48467 (11) | 0.0202 (4) | |
| H18B | 0.516732 | 0.877299 | 0.455501 | 0.024* | |
| C15A | 0.62849 (10) | 0.85263 (12) | 0.28888 (11) | 0.0195 (4) | |
| H15A | 0.582685 | 0.814596 | 0.293193 | 0.023* | |
| C13A | 1.03594 (10) | 0.26680 (13) | 0.68586 (11) | 0.0235 (4) | |
| H13A | 1.037828 | 0.231596 | 0.633340 | 0.035* | |
| H13B | 1.060410 | 0.225671 | 0.739098 | 0.035* | |
| H13C | 1.069072 | 0.327420 | 0.693708 | 0.035* | |
| C16B | 0.61476 (10) | 0.67826 (12) | 0.55134 (11) | 0.0217 (4) | |
| H16B | 0.604205 | 0.613775 | 0.566929 | 0.026* | |
| C17B | 0.54747 (9) | 0.74158 (12) | 0.51001 (11) | 0.0197 (4) | |
| C17A | 0.68022 (10) | 1.00504 (12) | 0.25366 (11) | 0.0218 (4) | |
| C5B | 0.94306 (10) | 1.04153 (12) | 0.74906 (11) | 0.0206 (4) | |
| H5B | 0.978511 | 1.086867 | 0.791098 | 0.025* | |
| C19A | 0.77567 (10) | 0.86889 (12) | 0.30786 (11) | 0.0223 (4) | |
| H19A | 0.831507 | 0.841940 | 0.324531 | 0.027* | |
| C3A | 0.60342 (9) | 0.62980 (12) | 0.21689 (11) | 0.0195 (4) | |
| H3A | 0.582056 | 0.687674 | 0.183498 | 0.023* | |
| C14A | 0.70906 (9) | 0.81108 (11) | 0.31211 (10) | 0.0169 (3) | |
| C3B | 0.80324 (10) | 0.96752 (12) | 0.67782 (11) | 0.0189 (4) | |
| H3B | 0.744661 | 0.962050 | 0.670814 | 0.023* | |
| C6B | 0.97784 (9) | 0.98366 (11) | 0.69901 (11) | 0.0172 (3) | |
| H6B | 1.036467 | 0.989175 | 0.706222 | 0.021* | |
| C1A | 0.70288 (9) | 0.54691 (11) | 0.34553 (10) | 0.0165 (3) | |
| B1B | 0.86581 (11) | 0.79655 (13) | 0.53674 (12) | 0.0156 (4) | |
| C16A | 0.61347 (10) | 0.94878 (12) | 0.25942 (11) | 0.0213 (4) | |
| H16A | 0.557743 | 0.975921 | 0.243290 | 0.026* | |
| C18A | 0.76183 (10) | 0.96511 (12) | 0.27972 (12) | 0.0263 (4) | |
| H18A | 0.808247 | 1.004032 | 0.278184 | 0.032* | |
| B1A | 0.79043 (11) | 0.67265 (13) | 0.41912 (12) | 0.0164 (4) | |
| C13B | 1.28701 (12) | 0.84058 (15) | 0.49346 (15) | 0.0377 (5) | |
| H13D | 1.248532 | 0.812024 | 0.437283 | 0.057* | |
| H13E | 1.346415 | 0.825729 | 0.500977 | 0.057* | |
| H13F | 1.278949 | 0.911650 | 0.491983 | 0.057* | |
| C20A | 0.59608 (11) | 1.15048 (14) | 0.21765 (14) | 0.0363 (5) | |
| H20A | 0.545972 | 1.117823 | 0.174811 | 0.055* | |
| H20B | 0.598966 | 1.217911 | 0.198158 | 0.055* | |
| H20C | 0.591353 | 1.150928 | 0.276835 | 0.055* | |
| C20B | 0.44238 (12) | 0.62958 (15) | 0.52332 (17) | 0.0465 (6) | |
| H20D | 0.457484 | 0.576027 | 0.491127 | 0.070* | |
| H20E | 0.380958 | 0.627181 | 0.512244 | 0.070* | |
| H20F | 0.474669 | 0.622809 | 0.587577 | 0.070* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0145 (5) | 0.0188 (6) | 0.0201 (6) | −0.0002 (4) | 0.0048 (5) | −0.0043 (5) |
| O2A | 0.0220 (6) | 0.0240 (6) | 0.0192 (6) | 0.0064 (5) | 0.0082 (5) | 0.0051 (5) |
| O2B | 0.0190 (6) | 0.0296 (7) | 0.0381 (8) | 0.0035 (5) | 0.0169 (5) | 0.0044 (6) |
| O3B | 0.0151 (6) | 0.0332 (7) | 0.0390 (8) | −0.0048 (5) | 0.0092 (5) | −0.0001 (6) |
| N2A | 0.0159 (6) | 0.0148 (7) | 0.0180 (7) | −0.0012 (5) | 0.0056 (5) | −0.0014 (6) |
| N1B | 0.0144 (6) | 0.0164 (7) | 0.0169 (7) | −0.0012 (5) | 0.0078 (5) | −0.0029 (6) |
| N2B | 0.0136 (6) | 0.0184 (7) | 0.0174 (7) | −0.0029 (5) | 0.0054 (5) | −0.0025 (6) |
| N1A | 0.0144 (6) | 0.0170 (7) | 0.0166 (7) | 0.0009 (5) | 0.0037 (5) | −0.0004 (6) |
| O3A | 0.0296 (7) | 0.0184 (6) | 0.0426 (8) | 0.0034 (5) | 0.0115 (6) | 0.0083 (6) |
| C8A | 0.0146 (8) | 0.0184 (9) | 0.0215 (9) | 0.0006 (6) | 0.0074 (7) | −0.0020 (7) |
| C1B | 0.0175 (8) | 0.0163 (8) | 0.0143 (8) | 0.0010 (6) | 0.0075 (6) | 0.0024 (6) |
| C4A | 0.0181 (8) | 0.0260 (9) | 0.0185 (9) | −0.0018 (7) | 0.0026 (7) | −0.0026 (7) |
| C14B | 0.0143 (7) | 0.0201 (9) | 0.0129 (8) | −0.0014 (6) | 0.0056 (6) | −0.0031 (6) |
| C19B | 0.0219 (8) | 0.0185 (9) | 0.0194 (9) | −0.0011 (7) | 0.0085 (7) | 0.0003 (7) |
| C11A | 0.0158 (8) | 0.0206 (9) | 0.0184 (9) | 0.0014 (6) | 0.0067 (7) | −0.0016 (7) |
| C7B | 0.0140 (7) | 0.0143 (8) | 0.0173 (8) | −0.0023 (6) | 0.0055 (6) | −0.0055 (6) |
| C12B | 0.0179 (8) | 0.0156 (8) | 0.0176 (9) | 0.0014 (6) | 0.0054 (7) | 0.0004 (7) |
| C9B | 0.0169 (8) | 0.0198 (9) | 0.0196 (9) | 0.0018 (6) | 0.0035 (7) | −0.0004 (7) |
| C2A | 0.0159 (8) | 0.0167 (8) | 0.0190 (9) | −0.0021 (6) | 0.0095 (7) | −0.0026 (7) |
| C8B | 0.0194 (8) | 0.0187 (8) | 0.0167 (9) | −0.0031 (7) | 0.0072 (7) | −0.0009 (7) |
| C7A | 0.0158 (8) | 0.0135 (8) | 0.0163 (8) | −0.0023 (6) | 0.0027 (6) | −0.0024 (7) |
| C12A | 0.0186 (8) | 0.0204 (9) | 0.0161 (8) | −0.0013 (7) | 0.0082 (7) | −0.0009 (7) |
| C11B | 0.0233 (8) | 0.0182 (9) | 0.0207 (9) | −0.0022 (7) | 0.0121 (7) | −0.0006 (7) |
| C10A | 0.0186 (8) | 0.0154 (8) | 0.0150 (8) | −0.0001 (6) | 0.0041 (7) | −0.0013 (7) |
| C9A | 0.0206 (8) | 0.0210 (9) | 0.0184 (9) | −0.0018 (7) | 0.0101 (7) | −0.0004 (7) |
| C10B | 0.0157 (8) | 0.0171 (8) | 0.0257 (9) | −0.0008 (6) | 0.0092 (7) | −0.0032 (7) |
| C2B | 0.0169 (8) | 0.0141 (8) | 0.0143 (8) | −0.0015 (6) | 0.0043 (6) | 0.0018 (6) |
| C5A | 0.0189 (8) | 0.0205 (9) | 0.0246 (9) | −0.0062 (7) | 0.0063 (7) | −0.0063 (7) |
| C15B | 0.0162 (8) | 0.0204 (9) | 0.0207 (9) | 0.0034 (6) | 0.0067 (7) | 0.0020 (7) |
| C4B | 0.0257 (9) | 0.0208 (9) | 0.0211 (9) | 0.0021 (7) | 0.0116 (7) | −0.0016 (7) |
| C6A | 0.0172 (8) | 0.0171 (8) | 0.0219 (9) | −0.0009 (6) | 0.0083 (7) | 0.0004 (7) |
| C18B | 0.0163 (8) | 0.0264 (9) | 0.0161 (9) | 0.0054 (7) | 0.0036 (7) | 0.0005 (7) |
| C15A | 0.0164 (8) | 0.0207 (9) | 0.0220 (9) | −0.0020 (6) | 0.0077 (7) | −0.0003 (7) |
| C13A | 0.0222 (9) | 0.0271 (10) | 0.0202 (9) | 0.0070 (7) | 0.0064 (7) | 0.0008 (7) |
| C16B | 0.0216 (8) | 0.0177 (9) | 0.0275 (10) | −0.0019 (7) | 0.0108 (7) | −0.0002 (7) |
| C17B | 0.0148 (8) | 0.0268 (9) | 0.0181 (9) | −0.0042 (7) | 0.0068 (7) | −0.0051 (7) |
| C17A | 0.0247 (9) | 0.0160 (8) | 0.0236 (9) | 0.0009 (7) | 0.0075 (7) | 0.0005 (7) |
| C5B | 0.0225 (8) | 0.0198 (9) | 0.0184 (9) | −0.0041 (7) | 0.0063 (7) | −0.0036 (7) |
| C19A | 0.0162 (8) | 0.0212 (9) | 0.0305 (10) | 0.0014 (7) | 0.0097 (7) | 0.0009 (8) |
| C3A | 0.0173 (8) | 0.0217 (9) | 0.0190 (9) | 0.0010 (7) | 0.0060 (7) | 0.0014 (7) |
| C14A | 0.0191 (8) | 0.0159 (8) | 0.0153 (8) | 0.0000 (6) | 0.0060 (7) | −0.0025 (7) |
| C3B | 0.0169 (8) | 0.0223 (9) | 0.0194 (9) | −0.0008 (7) | 0.0088 (7) | 0.0006 (7) |
| C6B | 0.0153 (8) | 0.0178 (9) | 0.0188 (9) | −0.0025 (6) | 0.0065 (7) | 0.0004 (7) |
| C1A | 0.0136 (7) | 0.0199 (9) | 0.0172 (9) | −0.0006 (6) | 0.0067 (7) | −0.0018 (7) |
| B1B | 0.0160 (9) | 0.0152 (9) | 0.0146 (9) | 0.0000 (7) | 0.0045 (7) | 0.0011 (7) |
| C16A | 0.0175 (8) | 0.0217 (9) | 0.0239 (9) | 0.0034 (7) | 0.0066 (7) | −0.0018 (7) |
| C18A | 0.0214 (8) | 0.0222 (9) | 0.0372 (11) | −0.0038 (7) | 0.0130 (8) | 0.0027 (8) |
| B1A | 0.0137 (8) | 0.0197 (10) | 0.0180 (10) | −0.0005 (7) | 0.0081 (7) | −0.0041 (8) |
| C13B | 0.0304 (10) | 0.0370 (12) | 0.0588 (14) | 0.0033 (8) | 0.0320 (10) | 0.0105 (10) |
| C20A | 0.0372 (11) | 0.0230 (10) | 0.0457 (13) | 0.0108 (8) | 0.0112 (9) | 0.0073 (9) |
| C20B | 0.0231 (10) | 0.0426 (13) | 0.0723 (17) | −0.0100 (9) | 0.0155 (10) | 0.0125 (12) |
Geometric parameters (Å, º)
| O1—B1B | 1.368 (2) | C7A—C12A | 1.384 (2) |
| O1—B1A | 1.372 (2) | C12A—H12A | 0.9500 |
| O2A—C10A | 1.3673 (18) | C11B—H11B | 0.9500 |
| O2A—C13A | 1.4272 (18) | C11B—C10B | 1.384 (2) |
| O2B—C10B | 1.3730 (18) | C10A—C9A | 1.394 (2) |
| O2B—C13B | 1.430 (2) | C9A—H9A | 0.9500 |
| O3B—C17B | 1.3757 (18) | C2B—C3B | 1.384 (2) |
| O3B—C20B | 1.422 (2) | C5A—H5A | 0.9500 |
| N2A—C2A | 1.4124 (19) | C5A—C6A | 1.392 (2) |
| N2A—C14A | 1.427 (2) | C15B—H15B | 0.9500 |
| N2A—B1A | 1.437 (2) | C15B—C16B | 1.392 (2) |
| N1B—C1B | 1.4040 (19) | C4B—H4B | 0.9500 |
| N1B—C7B | 1.4407 (19) | C4B—C5B | 1.391 (2) |
| N1B—B1B | 1.432 (2) | C4B—C3B | 1.393 (2) |
| N2B—C14B | 1.4322 (19) | C6A—H6A | 0.9500 |
| N2B—C2B | 1.4079 (19) | C6A—C1A | 1.383 (2) |
| N2B—B1B | 1.433 (2) | C18B—H18B | 0.9500 |
| N1A—C7A | 1.431 (2) | C18B—C17B | 1.388 (2) |
| N1A—C1A | 1.4034 (19) | C15A—H15A | 0.9500 |
| N1A—B1A | 1.428 (2) | C15A—C14A | 1.386 (2) |
| O3A—C17A | 1.3720 (19) | C15A—C16A | 1.389 (2) |
| O3A—C20A | 1.425 (2) | C13A—H13A | 0.9800 |
| C8A—H8A | 0.9500 | C13A—H13B | 0.9800 |
| C8A—C7A | 1.393 (2) | C13A—H13C | 0.9800 |
| C8A—C9A | 1.378 (2) | C16B—H16B | 0.9500 |
| C1B—C2B | 1.410 (2) | C16B—C17B | 1.386 (2) |
| C1B—C6B | 1.379 (2) | C17A—C16A | 1.388 (2) |
| C4A—H4A | 0.9500 | C17A—C18A | 1.390 (2) |
| C4A—C5A | 1.386 (2) | C5B—H5B | 0.9500 |
| C4A—C3A | 1.395 (2) | C5B—C6B | 1.395 (2) |
| C14B—C19B | 1.390 (2) | C19A—H19A | 0.9500 |
| C14B—C15B | 1.383 (2) | C19A—C14A | 1.389 (2) |
| C19B—H19B | 0.9500 | C19A—C18A | 1.383 (2) |
| C19B—C18B | 1.380 (2) | C3A—H3A | 0.9500 |
| C11A—H11A | 0.9500 | C3B—H3B | 0.9500 |
| C11A—C12A | 1.388 (2) | C6B—H6B | 0.9500 |
| C11A—C10A | 1.385 (2) | C16A—H16A | 0.9500 |
| C7B—C12B | 1.381 (2) | C18A—H18A | 0.9500 |
| C7B—C8B | 1.391 (2) | C13B—H13D | 0.9800 |
| C12B—H12B | 0.9500 | C13B—H13E | 0.9800 |
| C12B—C11B | 1.392 (2) | C13B—H13F | 0.9800 |
| C9B—H9B | 0.9500 | C20A—H20A | 0.9800 |
| C9B—C8B | 1.380 (2) | C20A—H20B | 0.9800 |
| C9B—C10B | 1.391 (2) | C20A—H20C | 0.9800 |
| C2A—C3A | 1.379 (2) | C20B—H20D | 0.9800 |
| C2A—C1A | 1.407 (2) | C20B—H20E | 0.9800 |
| C8B—H8B | 0.9500 | C20B—H20F | 0.9800 |
| B1B—O1—B1A | 132.75 (13) | C5B—C4B—C3B | 121.34 (15) |
| C10A—O2A—C13A | 116.62 (12) | C3B—C4B—H4B | 119.3 |
| C10B—O2B—C13B | 116.33 (13) | C5A—C6A—H6A | 121.2 |
| C17B—O3B—C20B | 118.15 (13) | C1A—C6A—C5A | 117.54 (15) |
| C2A—N2A—C14A | 123.34 (13) | C1A—C6A—H6A | 121.2 |
| C2A—N2A—B1A | 107.38 (13) | C19B—C18B—H18B | 119.9 |
| C14A—N2A—B1A | 129.26 (13) | C19B—C18B—C17B | 120.13 (15) |
| C1B—N1B—C7B | 122.71 (12) | C17B—C18B—H18B | 119.9 |
| C1B—N1B—B1B | 107.83 (12) | C14A—C15A—H15A | 119.4 |
| B1B—N1B—C7B | 129.45 (13) | C14A—C15A—C16A | 121.25 (15) |
| C14B—N2B—B1B | 129.55 (13) | C16A—C15A—H15A | 119.4 |
| C2B—N2B—C14B | 122.27 (12) | O2A—C13A—H13A | 109.5 |
| C2B—N2B—B1B | 107.94 (12) | O2A—C13A—H13B | 109.5 |
| C1A—N1A—C7A | 121.93 (13) | O2A—C13A—H13C | 109.5 |
| C1A—N1A—B1A | 108.04 (13) | H13A—C13A—H13B | 109.5 |
| B1A—N1A—C7A | 130.03 (13) | H13A—C13A—H13C | 109.5 |
| C17A—O3A—C20A | 117.01 (13) | H13B—C13A—H13C | 109.5 |
| C7A—C8A—H8A | 119.8 | C15B—C16B—H16B | 120.6 |
| C9A—C8A—H8A | 119.8 | C17B—C16B—C15B | 118.85 (15) |
| C9A—C8A—C7A | 120.39 (14) | C17B—C16B—H16B | 120.6 |
| N1B—C1B—C2B | 108.82 (13) | O3B—C17B—C18B | 115.02 (14) |
| C6B—C1B—N1B | 130.41 (14) | O3B—C17B—C16B | 124.66 (15) |
| C6B—C1B—C2B | 120.74 (14) | C16B—C17B—C18B | 120.30 (14) |
| C5A—C4A—H4A | 119.4 | O3A—C17A—C16A | 124.35 (14) |
| C5A—C4A—C3A | 121.27 (15) | O3A—C17A—C18A | 116.17 (14) |
| C3A—C4A—H4A | 119.4 | C16A—C17A—C18A | 119.47 (15) |
| C19B—C14B—N2B | 120.81 (14) | C4B—C5B—H5B | 119.6 |
| C15B—C14B—N2B | 120.11 (13) | C4B—C5B—C6B | 120.83 (15) |
| C15B—C14B—C19B | 119.08 (14) | C6B—C5B—H5B | 119.6 |
| C14B—C19B—H19B | 119.8 | C14A—C19A—H19A | 119.5 |
| C18B—C19B—C14B | 120.34 (15) | C18A—C19A—H19A | 119.5 |
| C18B—C19B—H19B | 119.8 | C18A—C19A—C14A | 120.92 (15) |
| C12A—C11A—H11A | 120.2 | C4A—C3A—H3A | 120.9 |
| C10A—C11A—H11A | 120.2 | C2A—C3A—C4A | 118.20 (15) |
| C10A—C11A—C12A | 119.59 (14) | C2A—C3A—H3A | 120.9 |
| C12B—C7B—N1B | 120.36 (14) | C15A—C14A—N2A | 121.32 (14) |
| C12B—C7B—C8B | 119.32 (14) | C15A—C14A—C19A | 118.41 (15) |
| C8B—C7B—N1B | 120.32 (14) | C19A—C14A—N2A | 120.25 (13) |
| C7B—C12B—H12B | 119.5 | C2B—C3B—C4B | 117.73 (14) |
| C7B—C12B—C11B | 120.99 (15) | C2B—C3B—H3B | 121.1 |
| C11B—C12B—H12B | 119.5 | C4B—C3B—H3B | 121.1 |
| C8B—C9B—H9B | 119.7 | C1B—C6B—C5B | 118.25 (14) |
| C8B—C9B—C10B | 120.55 (15) | C1B—C6B—H6B | 120.9 |
| C10B—C9B—H9B | 119.7 | C5B—C6B—H6B | 120.9 |
| C3A—C2A—N2A | 130.98 (15) | N1A—C1A—C2A | 108.66 (13) |
| C3A—C2A—C1A | 120.25 (14) | C6A—C1A—N1A | 129.58 (14) |
| C1A—C2A—N2A | 108.63 (13) | C6A—C1A—C2A | 121.68 (14) |
| C7B—C8B—H8B | 120.0 | O1—B1B—N1B | 124.79 (14) |
| C9B—C8B—C7B | 119.98 (15) | O1—B1B—N2B | 128.08 (14) |
| C9B—C8B—H8B | 120.0 | N1B—B1B—N2B | 107.10 (14) |
| C8A—C7A—N1A | 120.37 (13) | C15A—C16A—H16A | 120.1 |
| C12A—C7A—N1A | 120.31 (14) | C17A—C16A—C15A | 119.70 (14) |
| C12A—C7A—C8A | 119.31 (14) | C17A—C16A—H16A | 120.1 |
| C11A—C12A—H12A | 119.6 | C17A—C18A—H18A | 119.9 |
| C7A—C12A—C11A | 120.70 (15) | C19A—C18A—C17A | 120.19 (15) |
| C7A—C12A—H12A | 119.6 | C19A—C18A—H18A | 119.9 |
| C12B—C11B—H11B | 120.3 | O1—B1A—N2A | 127.87 (15) |
| C10B—C11B—C12B | 119.39 (15) | O1—B1A—N1A | 124.76 (15) |
| C10B—C11B—H11B | 120.3 | N1A—B1A—N2A | 107.27 (13) |
| O2A—C10A—C11A | 124.14 (14) | O2B—C13B—H13D | 109.5 |
| O2A—C10A—C9A | 115.80 (14) | O2B—C13B—H13E | 109.5 |
| C11A—C10A—C9A | 120.06 (15) | O2B—C13B—H13F | 109.5 |
| C8A—C9A—C10A | 119.91 (15) | H13D—C13B—H13E | 109.5 |
| C8A—C9A—H9A | 120.0 | H13D—C13B—H13F | 109.5 |
| C10A—C9A—H9A | 120.0 | H13E—C13B—H13F | 109.5 |
| O2B—C10B—C9B | 115.73 (14) | O3A—C20A—H20A | 109.5 |
| O2B—C10B—C11B | 124.52 (15) | O3A—C20A—H20B | 109.5 |
| C11B—C10B—C9B | 119.75 (14) | O3A—C20A—H20C | 109.5 |
| N2B—C2B—C1B | 108.31 (13) | H20A—C20A—H20B | 109.5 |
| C3B—C2B—N2B | 130.53 (14) | H20A—C20A—H20C | 109.5 |
| C3B—C2B—C1B | 121.12 (14) | H20B—C20A—H20C | 109.5 |
| C4A—C5A—H5A | 119.5 | O3B—C20B—H20D | 109.5 |
| C4A—C5A—C6A | 121.04 (15) | O3B—C20B—H20E | 109.5 |
| C6A—C5A—H5A | 119.5 | O3B—C20B—H20F | 109.5 |
| C14B—C15B—H15B | 119.4 | H20D—C20B—H20E | 109.5 |
| C14B—C15B—C16B | 121.26 (14) | H20D—C20B—H20F | 109.5 |
| C16B—C15B—H15B | 119.4 | H20E—C20B—H20F | 109.5 |
| C5B—C4B—H4B | 119.3 | ||
| O2A—C10A—C9A—C8A | 178.55 (14) | C2B—N2B—B1B—N1B | −0.61 (17) |
| N2A—C2A—C3A—C4A | 175.54 (15) | C2B—C1B—C6B—C5B | −0.2 (2) |
| N2A—C2A—C1A—N1A | 0.14 (17) | C5A—C4A—C3A—C2A | 0.3 (2) |
| N2A—C2A—C1A—C6A | −177.10 (14) | C5A—C6A—C1A—N1A | −175.77 (15) |
| N1B—C1B—C2B—N2B | 0.47 (17) | C5A—C6A—C1A—C2A | 0.8 (2) |
| N1B—C1B—C2B—C3B | 178.43 (14) | C15B—C14B—C19B—C18B | 1.4 (2) |
| N1B—C1B—C6B—C5B | −177.82 (15) | C15B—C16B—C17B—O3B | −176.14 (15) |
| N1B—C7B—C12B—C11B | −178.68 (14) | C15B—C16B—C17B—C18B | 2.2 (2) |
| N1B—C7B—C8B—C9B | 179.28 (14) | C4B—C5B—C6B—C1B | −0.3 (2) |
| N2B—C14B—C19B—C18B | −178.20 (14) | C13A—O2A—C10A—C11A | 13.0 (2) |
| N2B—C14B—C15B—C16B | 179.40 (14) | C13A—O2A—C10A—C9A | −167.12 (14) |
| N2B—C2B—C3B—C4B | 177.41 (15) | C5B—C4B—C3B—C2B | −0.4 (2) |
| N1A—C7A—C12A—C11A | 179.14 (14) | C3A—C4A—C5A—C6A | −0.3 (2) |
| O3A—C17A—C16A—C15A | 177.38 (16) | C3A—C2A—C1A—N1A | 176.32 (14) |
| O3A—C17A—C18A—C19A | −176.64 (16) | C3A—C2A—C1A—C6A | −0.9 (2) |
| C8A—C7A—C12A—C11A | −1.7 (2) | C14A—N2A—C2A—C3A | 6.7 (3) |
| C1B—N1B—C7B—C12B | −100.79 (18) | C14A—N2A—C2A—C1A | −177.71 (13) |
| C1B—N1B—C7B—C8B | 78.92 (19) | C14A—N2A—B1A—O1 | −6.6 (3) |
| C1B—N1B—B1B—O1 | −177.24 (15) | C14A—N2A—B1A—N1A | 177.07 (14) |
| C1B—N1B—B1B—N2B | 0.90 (17) | C14A—C15A—C16A—C17A | −0.5 (3) |
| C1B—C2B—C3B—C4B | 0.0 (2) | C14A—C19A—C18A—C17A | −1.1 (3) |
| C4A—C5A—C6A—C1A | −0.2 (2) | C3B—C4B—C5B—C6B | 0.6 (3) |
| C14B—N2B—C2B—C1B | 174.97 (13) | C6B—C1B—C2B—N2B | −177.64 (14) |
| C14B—N2B—C2B—C3B | −2.7 (3) | C6B—C1B—C2B—C3B | 0.3 (2) |
| C14B—N2B—B1B—O1 | 3.1 (3) | C1A—N1A—C7A—C8A | −78.00 (19) |
| C14B—N2B—B1B—N1B | −174.99 (14) | C1A—N1A—C7A—C12A | 101.15 (17) |
| C14B—C19B—C18B—C17B | −0.8 (2) | C1A—N1A—B1A—O1 | −175.29 (15) |
| C14B—C15B—C16B—C17B | −1.6 (2) | C1A—N1A—B1A—N2A | 1.21 (17) |
| C19B—C14B—C15B—C16B | −0.2 (2) | C1A—C2A—C3A—C4A | 0.3 (2) |
| C19B—C18B—C17B—O3B | 177.45 (14) | B1B—O1—B1A—N2A | 62.9 (3) |
| C19B—C18B—C17B—C16B | −1.0 (2) | B1B—O1—B1A—N1A | −121.33 (19) |
| C11A—C10A—C9A—C8A | −1.5 (2) | B1B—N1B—C1B—C2B | −0.85 (17) |
| C7B—N1B—C1B—C2B | −179.69 (13) | B1B—N1B—C1B—C6B | 177.02 (16) |
| C7B—N1B—C1B—C6B | −1.8 (2) | B1B—N1B—C7B—C12B | 80.6 (2) |
| C7B—N1B—B1B—O1 | 1.5 (3) | B1B—N1B—C7B—C8B | −99.6 (2) |
| C7B—N1B—B1B—N2B | 179.63 (14) | B1B—N2B—C14B—C19B | −119.01 (18) |
| C7B—C12B—C11B—C10B | −0.9 (2) | B1B—N2B—C14B—C15B | 61.4 (2) |
| C12B—C7B—C8B—C9B | −1.0 (2) | B1B—N2B—C2B—C1B | 0.10 (17) |
| C12B—C11B—C10B—O2B | 179.54 (14) | B1B—N2B—C2B—C3B | −177.61 (17) |
| C12B—C11B—C10B—C9B | −0.4 (2) | C16A—C15A—C14A—N2A | −179.44 (15) |
| C2A—N2A—C14A—C15A | 47.6 (2) | C16A—C15A—C14A—C19A | 1.9 (2) |
| C2A—N2A—C14A—C19A | −133.79 (16) | C16A—C17A—C18A—C19A | 2.5 (3) |
| C2A—N2A—B1A—O1 | 175.24 (15) | C18A—C17A—C16A—C15A | −1.7 (3) |
| C2A—N2A—B1A—N1A | −1.12 (17) | C18A—C19A—C14A—N2A | −179.75 (16) |
| C8B—C7B—C12B—C11B | 1.6 (2) | C18A—C19A—C14A—C15A | −1.1 (3) |
| C8B—C9B—C10B—O2B | −178.96 (14) | B1A—O1—B1B—N1B | −167.79 (16) |
| C8B—C9B—C10B—C11B | 1.0 (2) | B1A—O1—B1B—N2B | 14.5 (3) |
| C7A—N1A—C1A—C2A | 178.84 (13) | B1A—N2A—C2A—C3A | −175.02 (16) |
| C7A—N1A—C1A—C6A | −4.2 (2) | B1A—N2A—C2A—C1A | 0.61 (17) |
| C7A—N1A—B1A—O1 | 5.1 (3) | B1A—N2A—C14A—C15A | −130.34 (17) |
| C7A—N1A—B1A—N2A | −178.45 (14) | B1A—N2A—C14A—C19A | 48.3 (2) |
| C7A—C8A—C9A—C10A | 0.6 (2) | B1A—N1A—C7A—C8A | 101.61 (19) |
| C12A—C11A—C10A—O2A | −179.27 (14) | B1A—N1A—C7A—C12A | −79.2 (2) |
| C12A—C11A—C10A—C9A | 0.8 (2) | B1A—N1A—C1A—C2A | −0.85 (17) |
| C10A—C11A—C12A—C7A | 0.8 (2) | B1A—N1A—C1A—C6A | 176.11 (16) |
| C9A—C8A—C7A—N1A | −179.86 (14) | C13B—O2B—C10B—C9B | 179.79 (15) |
| C9A—C8A—C7A—C12A | 1.0 (2) | C13B—O2B—C10B—C11B | −0.2 (2) |
| C10B—C9B—C8B—C7B | −0.3 (2) | C20A—O3A—C17A—C16A | 17.1 (2) |
| C2B—N2B—C14B—C19B | 67.3 (2) | C20A—O3A—C17A—C18A | −163.81 (16) |
| C2B—N2B—C14B—C15B | −112.25 (17) | C20B—O3B—C17B—C18B | −175.17 (17) |
| C2B—N2B—B1B—O1 | 177.44 (16) | C20B—O3B—C17B—C16B | 3.2 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C8B—H8B···O2Ai | 0.95 | 2.40 | 3.233 (2) | 147 |
| C13B—H13E···O3Bii | 0.98 | 2.46 | 3.374 (3) | 155 |
Symmetry codes: (i) −x+2, y+1/2, −z+3/2; (ii) x+1, y, z.
Funding Statement
Funding for this research was provided by program manager Dr Imre Gyuk through the US Department of Energy, Office of Electricity. Sandia National Laboratories is a multi-mission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC., a wholly owned subsidiary of Honeywell International, Inc., for the US Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. The views expressed in this article do not necessarily represent the views of the US Department of Energy or the United States Government. Davidson College and the Davidson Research Institute are acknowledged for scholarships to HHM and NAR.
References
- Allen, A. E. & MacMillan, D. W. C. (2012). Chem. Sci. 3, 633–658. [DOI] [PMC free article] [PubMed]
- Bellemin-Laponnaz, S. & Dagorne, S. (2014). Chem. Rev. 114, 8747–8774. [DOI] [PubMed]
- Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2015). Acta Cryst. A71, 59–75. [DOI] [PMC free article] [PubMed]
- Bruker (2016). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Karunananda, M. K. & Mankad, N. P. (2017). ACS Catal. 7, 6110–6119.
- Lohr, T. L. & Marks, T. J. (2015). Nat. Chem. 7, 477–482. [DOI] [PubMed]
- Mallard, H. H., Anstey, M. R., Kennedy, N. D., Rudman, N. A., Greenwood, A. M., Angle, C. E., Nicoleau, J., Torquato, N. A., Gau, M. R. & Carroll, P. J. (2020). CSD Communication (refcode CCDC 2015021). CCDC, Cambridge, England.
- Power, P. P. (2010). Nature, 463, 171–177. [DOI] [PubMed]
- Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. (2013). Chem. Rev. 113, 5322–5363. [DOI] [PMC free article] [PubMed]
- Pye, D. R. & Mankad, N. P. (2017). Chem. Sci. 8, 1705–1718. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Wang, Z., Chen, X., Xie, H., Wang, D., Huang, H. & Deng, G.-J. (2018). Org. Lett. 20, 5470–5473. [DOI] [PubMed]
- Xiong, M., Gao, Z., Liang, X., Cai, P., Zhu, H. & Pan, Y. (2018). Chem. Commun. 54, 9679–9682. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2414314620012481/hb4361sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314620012481/hb4361Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314620012481/hb4361Isup4.cml
CCDC reference: 2031384
Additional supporting information: crystallographic information; 3D view; checkCIF report