The crystal structure of cis-[MoO2(acac)2] has been redetermined at 100 K, providing a more precise description of the structure including hydrogen atoms and intermolecular contacts.
Keywords: crystal structure, molybdenum, oxo, acetylacetonate, acac
Abstract
The title compound, [Mo(C5H7O2)2O2] or cis-[MoO2(acac)2] (acac is acetylacetonate), contains a molybdenum(VI) atom coordinated by two acetylacetonate ligands and two doubly bonded oxido ligands in a distorted octahedral shape. The molecule is chiral and the asymmetric unit contains two independent molecules (one Δ, one Λ). Extensive C—H⋯O contacts are present throughout the structure. Data were collected at 100 K, providing higher precision of unit-cell parameters and atomic positions than previous determinations [Kamenar et al. (1973 ▸). Cryst. Struct. Commun.
2, 41–44.; Krasochka et al. (1975). Zh. Strukt. Khim.
16, 696–698].

Structure description
The title compound is a versatile starting material for the preparation of cis-dioxidomolybdenum complexes, including complexes containing organodinitrogen ligands (Bustos et al., 1994 ▸) and molybdenyl adducts of platinum μ-S dimers (Henderson et al., 2011 ▸). MoO2(acac)2 has also been used to prepare dioxidomolybdenum(VI) complexes with O,N,N′ chelating ligands (Ceylan et al., 2015 ▸) and an amine bis(phenolate) ligand (Bowen & Wile, 2021 ▸). Many of these complexes have been prepared and studied for their catalytic activities, including complexes with acylpyrazolonate ligands that catalyze the deoxygenation of epoxides (Hills et al., 2013 ▸; Begines et al., 2018 ▸) and dioxidomolybdenum(VI) complexes with salicylamide ligands for the epoxidation of olefins (Annese et al., 2019 ▸). Molybdenum(VI) dioxido complexes with acetylacetonato ligands have also been investigated for their catalytic properties in the dehydrogenation of alcohols (Korstanje et al., 2013 ▸). These complexes are of particular interest due to their close structural similarities to the active sites of several molybdoenyzmes such as sulfite oxidase, xanthine oxidase, and DMSO reductase (Sousa & Fernandes, 2015 ▸).
Two previous structural determinations of cis-dioxidobis(acetylacetonato)molybdenum(VI) were published in the mid-1970s (Kamenar et al., 1973 ▸; Krasochka et al., 1975) based on photographic methods and room-temperature data collections. Additionally, Craven et al. (1971 ▸) cite an unpublished diffraction study that also confirms the cis coordination and includes additional structural information consistent with the current study. None of the previously published structure solutions attempted to locate the positions of any of the hydrogen atoms. Several closely related structures have been determined, including cis-dioxido-molybdenum complexes with 1,3-diphenylpropanedianoto ligands (Kojić-Prodić et al., 1974 ▸; Korstanje et al., 2013 ▸) and tert-butylacetylacetonato ligands (Nass et al., 2001 ▸). The structure of the product from the reaction of cis-[MoO2(acac)2] with the strong Lewis acid B(C6F5)3 (Galsworthy et al., 1997 ▸) displays a nearly linear Mo=O—B arrangement [171.2 (1)°] and lengthening of the donating Mo=O bond by about 0.1 Å.
The asymmetric unit of the title compound contains two crystallographically independent cis-[MoO2(acac)2] molecules, one each of the Δ and Λ forms (Fig. 1 ▸). The molecular structure adopts a distorted octahedral arrangement around the MoVI atoms, with oxido ligands in a cis arrangement and oxido-molybdenum-oxido angles of 105.40 (4) and 105.59 (5)°. As observed previously (Krasochka, 1973 ▸; Kojić-Prodić et al., 1974 ▸), the Mo—O bond distances trans to the molybdenum-oxygen double bonds are significantly lengthened [avg = 2.185 (5) Å] relative to the other molybdenum–oxygen distances [avg = 1.999 (11) Å] (see Table 1 ▸ for selected bond distances and angles). The four molybdenum oxygen distances for the doubly-bonded oxido ligands average 1.7012 (16) Å, in agreement with the average distance found for over 140 similar cis-dioxido molybdenum complexes in the Cambridge Structural Database (Groom et al., 2016 ▸). These metrics are also in agreement with relatively narrow distribution of molybdenum–oxygen distances observed by Mayer (1988 ▸) for cis-dioxido complexes.
Figure 1.
Displacement ellipsoid (50% probability) diagram of the two independent molecules with the numbering scheme for the non-hydrogen atoms.
Table 1. Selected geometric parameters (Å, °).
| Mo1—O1 | 1.7029 (9) | Mo2—O7 | 1.6996 (9) |
| Mo1—O2 | 1.7001 (9) | Mo2—O8 | 1.7021 (9) |
| Mo1—O3 | 2.1825 (8) | Mo2—O9 | 2.1808 (8) |
| Mo1—O4 | 2.1921 (8) | Mo2—O10 | 2.1848 (9) |
| Mo1—O5 | 2.0060 (8) | Mo2—O11 | 1.9898 (8) |
| Mo1—O6 | 1.9897 (8) | Mo2—O12 | 2.0106 (8) |
| O2—Mo1—O1 | 105.40 (4) | O7—Mo2—O8 | 105.59 (5) |
All of the hydrogen-bonding contacts are weak C—H⋯O interactions with D⋯A distances between 3.3 and 3.5 Å (see Table 2 ▸ and Fig. 2 ▸). There are contacts between C—H atoms and all four of the oxido ligands, including two contacts to O1 and three contacts to O8. Additional C—H contacts are made to most of the acetylacetonate oxygen atoms as well.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H1A⋯O8i | 0.93 (1) | 2.62 (2) | 3.4834 (16) | 155 (2) |
| C5—H5B⋯O10ii | 0.98 (1) | 2.50 (1) | 3.4652 (15) | 170 (2) |
| C6—H6A⋯O8 | 0.97 (1) | 2.51 (2) | 3.3894 (15) | 151 (2) |
| C8—H8⋯O7iii | 0.91 (1) | 2.79 (2) | 3.4071 (14) | 126 (1) |
| C10—H10A⋯O6iv | 0.95 (1) | 2.68 (2) | 3.3334 (15) | 127 (1) |
| C10—H10C⋯O1v | 0.97 (1) | 2.50 (2) | 3.3126 (15) | 141 (2) |
| C11—H11B⋯O3i | 0.99 (1) | 2.48 (1) | 3.4415 (14) | 163 (2) |
| C15—H15A⋯O1ii | 0.93 (1) | 2.55 (2) | 3.4592 (15) | 167 (2) |
| C15—H15C⋯O4 | 0.96 (1) | 2.53 (2) | 3.4018 (15) | 152 (2) |
| C16—H16A⋯O11vi | 0.94 (1) | 2.66 (2) | 3.3127 (15) | 128 (2) |
| C16—H16B⋯O8vii | 0.96 (2) | 2.52 (2) | 3.3971 (17) | 153 (2) |
| C18—H18⋯O2viii | 0.92 (1) | 2.82 (2) | 3.4646 (15) | 128 (1) |
Symmetry codes: (i)
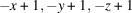 ; (ii)
; (ii)
 ; (iii)
; (iii)
 ; (iv)
; (iv)
 ; (v)
; (v)
 ; (vi)
; (vi)
 ; (vii)
; (vii)
 ; (viii)
; (viii)
 .
.
Figure 2.
Packing diagram (viewed along a), showing extensive weak C—H⋯O contacts (red dotted lines) throughout the crystal structure.
Synthesis and crystallization
The title compound was prepared using the Inorganic Syntheses procedure (Chakravorti & Bandyopadhyay, 1992 ▸) with some modifications adapted from Arnáiz (1995 ▸). A sample of 3.0 grams of ammonium para-molybdate was dissolved in 6.0 ml of 24%wt aqueous ammonia. A syringe was used to add 7.0 ml of 2,4-pentanedione with stirring. Concentrated nitric acid (5.0 ml) was added and the solution was stirred for 30 min. The product precipitated as a pale-yellow solid and was isolated by filtration and washed with deionized water (2 × 10 ml), followed by ethanol (1 × 10 ml), and diethyl ether (1 × 10 ml). Over multiple preparations the yield averaged around 90%. Characterization by 1H NMR and FTIR agrees with previously reported values (Chakravorti & Bandyopadhyay, 1992 ▸; Arnáiz, 1995 ▸).
Three different crystallization methods were utilized: slow evaporation from a concentrated solution in 2,4-pentanedione, vapor diffusion (dichloromethane/diethyl ether), and layering (dichloromethane/diethyl ether) in a standard 5 mm NMR tube. All three methods produced crystals, but the highest quality crystals and those used in this study were produced from solvent layering.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 3 ▸.
Table 3. Experimental details.
| Crystal data | |
| Chemical formula | [Mo(C5H7O2)2O2] |
| M r | 326.15 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 100 |
| a, b, c (Å) | 8.0111 (3), 12.4143 (4), 12.6847 (4) |
| α, β, γ (°) | 75.649 (1), 89.272 (1), 87.072 (1) |
| V (Å3) | 1220.56 (7) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 1.09 |
| Crystal size (mm) | 0.28 × 0.22 × 0.14 |
| Data collection | |
| Diffractometer | Bruker APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.676, 0.747 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 82074, 11855, 10556 |
| R int | 0.035 |
| (sin θ/λ)max (Å−1) | 0.835 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.023, 0.061, 1.04 |
| No. of reflections | 11855 |
| No. of parameters | 391 |
| No. of restraints | 28 |
| H-atom treatment | Only H-atom coordinates refined |
| Δρmax, Δρmin (e Å−3) | 1.19, −1.11 |
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2414314621007781/wm4150sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314621007781/wm4150Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314621007781/wm4150Isup3.mol
CCDC reference: 2100177
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| [Mo(C5H7O2)2O2] | Z = 4 |
| Mr = 326.15 | F(000) = 656 |
| Triclinic, P1 | Dx = 1.775 Mg m−3 |
| a = 8.0111 (3) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 12.4143 (4) Å | Cell parameters from 9760 reflections |
| c = 12.6847 (4) Å | θ = 3.0–36.3° |
| α = 75.649 (1)° | µ = 1.09 mm−1 |
| β = 89.272 (1)° | T = 100 K |
| γ = 87.072 (1)° | Block, yellow |
| V = 1220.56 (7) Å3 | 0.28 × 0.22 × 0.14 mm |
Data collection
| Bruker APEXII CCD diffractometer | 10556 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.035 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | θmax = 36.4°, θmin = 1.7° |
| Tmin = 0.676, Tmax = 0.747 | h = −13→13 |
| 82074 measured reflections | k = −20→20 |
| 11855 independent reflections | l = −21→21 |
Refinement
| Refinement on F2 | 28 restraints |
| Least-squares matrix: full | 0 constraints |
| R[F2 > 2σ(F2)] = 0.023 | Only H-atom coordinates refined |
| wR(F2) = 0.061 | w = 1/[σ2(Fo2) + (0.0307P)2 + 0.524P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.003 |
| 11855 reflections | Δρmax = 1.19 e Å−3 |
| 391 parameters | Δρmin = −1.11 e Å−3 |
Special details
| Refinement. All H atoms were located in a difference-Fourier map. Hydrogen atom positions were refined with C—H distances restrained to 0.98 (2) Å (CH3) or 0.95 (2) Å (ring C) and with Uiso(H) = 1.5Ueq(C) (methyl) or Uiso(H) = 1.2Ueq(C) (ring). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Mo1 | 0.19729 (2) | 0.76693 (2) | 0.56793 (2) | 0.01071 (2) | |
| O1 | 0.01992 (11) | 0.69604 (7) | 0.57195 (8) | 0.01646 (15) | |
| O2 | 0.14825 (12) | 0.86914 (7) | 0.63247 (7) | 0.01718 (15) | |
| O3 | 0.45296 (11) | 0.82176 (7) | 0.55088 (7) | 0.01484 (14) | |
| O4 | 0.32120 (11) | 0.65709 (7) | 0.47653 (7) | 0.01407 (14) | |
| O5 | 0.30012 (10) | 0.65609 (7) | 0.69609 (7) | 0.01323 (13) | |
| O6 | 0.17058 (11) | 0.86448 (7) | 0.41831 (7) | 0.01522 (14) | |
| C1 | 0.37036 (18) | 0.55651 (10) | 0.34261 (11) | 0.0194 (2) | |
| H1A | 0.408 (2) | 0.5708 (16) | 0.2707 (13) | 0.029* | |
| H1B | 0.457 (2) | 0.5162 (15) | 0.3884 (16) | 0.029* | |
| H1C | 0.286 (2) | 0.5032 (14) | 0.3486 (16) | 0.029* | |
| C2 | 0.30151 (14) | 0.65672 (9) | 0.37795 (9) | 0.01315 (17) | |
| C3 | 0.21648 (16) | 0.74411 (10) | 0.30091 (9) | 0.01650 (19) | |
| H3 | 0.205 (2) | 0.7355 (14) | 0.2306 (12) | 0.020* | |
| C4 | 0.16005 (14) | 0.84288 (9) | 0.32317 (9) | 0.01329 (17) | |
| C5 | 0.08566 (17) | 0.93714 (10) | 0.23647 (10) | 0.0183 (2) | |
| H5A | 0.170 (2) | 0.9885 (14) | 0.2000 (15) | 0.028* | |
| H5B | 0.033 (2) | 0.9095 (15) | 0.1802 (14) | 0.028* | |
| H5C | 0.002 (2) | 0.9801 (15) | 0.2663 (16) | 0.028* | |
| C6 | 0.42700 (16) | 0.58188 (10) | 0.86696 (9) | 0.0170 (2) | |
| H6A | 0.416 (2) | 0.5069 (12) | 0.8582 (16) | 0.026* | |
| H6B | 0.333 (2) | 0.5953 (15) | 0.9090 (15) | 0.026* | |
| H6C | 0.5274 (19) | 0.5743 (15) | 0.9049 (15) | 0.026* | |
| C7 | 0.42379 (14) | 0.66499 (9) | 0.75910 (8) | 0.01228 (16) | |
| C8 | 0.54477 (15) | 0.74177 (10) | 0.73039 (9) | 0.01541 (18) | |
| H8 | 0.6317 (19) | 0.7423 (14) | 0.7754 (13) | 0.018* | |
| C9 | 0.56069 (13) | 0.81282 (9) | 0.62473 (9) | 0.01234 (17) | |
| C10 | 0.71393 (15) | 0.87800 (10) | 0.59652 (11) | 0.0180 (2) | |
| H10A | 0.689 (2) | 0.9523 (12) | 0.5569 (15) | 0.027* | |
| H10B | 0.776 (2) | 0.8840 (15) | 0.6561 (13) | 0.027* | |
| H10C | 0.788 (2) | 0.8426 (15) | 0.5532 (15) | 0.027* | |
| Mo2 | 0.31234 (2) | 0.23314 (2) | 0.94971 (2) | 0.01062 (2) | |
| O7 | 0.35933 (12) | 0.13590 (7) | 1.06697 (7) | 0.01702 (15) | |
| O8 | 0.49058 (11) | 0.30206 (7) | 0.91316 (8) | 0.01682 (15) | |
| O9 | 0.18784 (11) | 0.33673 (7) | 0.80520 (7) | 0.01435 (14) | |
| O10 | 0.05669 (11) | 0.17773 (7) | 0.96628 (7) | 0.01557 (15) | |
| O11 | 0.33915 (11) | 0.12896 (7) | 0.85341 (7) | 0.01430 (14) | |
| O12 | 0.20855 (11) | 0.34966 (7) | 1.01892 (7) | 0.01405 (14) | |
| C11 | 0.42605 (16) | 0.05038 (9) | 0.70948 (10) | 0.01590 (19) | |
| H11A | 0.506 (2) | 0.0052 (14) | 0.7626 (15) | 0.024* | |
| H11B | 0.481 (2) | 0.0759 (15) | 0.6381 (12) | 0.024* | |
| H11C | 0.338 (2) | 0.0040 (14) | 0.6990 (15) | 0.024* | |
| C12 | 0.35213 (13) | 0.14698 (9) | 0.74799 (9) | 0.01179 (16) | |
| C13 | 0.29618 (16) | 0.24372 (9) | 0.67571 (9) | 0.01583 (19) | |
| H13 | 0.312 (2) | 0.2494 (14) | 0.6000 (11) | 0.019* | |
| C14 | 0.20743 (13) | 0.33250 (9) | 0.70739 (9) | 0.01210 (16) | |
| C15 | 0.13314 (16) | 0.42696 (10) | 0.62128 (10) | 0.01670 (19) | |
| H15A | 0.095 (2) | 0.4053 (15) | 0.5610 (13) | 0.025* | |
| H15B | 0.043 (2) | 0.4672 (15) | 0.6472 (16) | 0.025* | |
| H15C | 0.219 (2) | 0.4773 (14) | 0.5942 (15) | 0.025* | |
| C16 | −0.20236 (16) | 0.12360 (11) | 1.04748 (12) | 0.0219 (2) | |
| H16A | −0.178 (2) | 0.0492 (13) | 1.0454 (17) | 0.033* | |
| H16B | −0.280 (2) | 0.1597 (16) | 0.9911 (15) | 0.033* | |
| H16C | −0.265 (2) | 0.1222 (16) | 1.1124 (14) | 0.033* | |
| C17 | −0.04876 (14) | 0.18876 (9) | 1.03822 (9) | 0.01463 (18) | |
| C18 | −0.03130 (16) | 0.26312 (11) | 1.10536 (10) | 0.0182 (2) | |
| H18 | −0.116 (2) | 0.2651 (15) | 1.1543 (14) | 0.022* | |
| C19 | 0.08525 (14) | 0.34274 (9) | 1.08878 (9) | 0.01445 (18) | |
| C20 | 0.07649 (18) | 0.43275 (12) | 1.14881 (11) | 0.0220 (2) | |
| H20A | 0.087 (3) | 0.5039 (13) | 1.1019 (16) | 0.033* | |
| H20B | −0.027 (2) | 0.4408 (16) | 1.1843 (16) | 0.033* | |
| H20C | 0.163 (2) | 0.4253 (17) | 1.1994 (15) | 0.033* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mo1 | 0.01212 (4) | 0.01239 (4) | 0.00833 (4) | −0.00019 (3) | −0.00151 (3) | −0.00391 (3) |
| O1 | 0.0145 (3) | 0.0165 (4) | 0.0190 (4) | −0.0011 (3) | −0.0029 (3) | −0.0055 (3) |
| O2 | 0.0220 (4) | 0.0167 (4) | 0.0150 (4) | −0.0011 (3) | 0.0008 (3) | −0.0078 (3) |
| O3 | 0.0153 (3) | 0.0185 (4) | 0.0102 (3) | −0.0043 (3) | −0.0011 (3) | −0.0016 (3) |
| O4 | 0.0174 (4) | 0.0143 (3) | 0.0106 (3) | 0.0010 (3) | −0.0004 (3) | −0.0037 (3) |
| O5 | 0.0147 (3) | 0.0152 (3) | 0.0094 (3) | −0.0023 (3) | −0.0017 (3) | −0.0018 (3) |
| O6 | 0.0229 (4) | 0.0129 (3) | 0.0099 (3) | 0.0008 (3) | −0.0041 (3) | −0.0031 (3) |
| C1 | 0.0265 (6) | 0.0172 (5) | 0.0161 (5) | 0.0006 (4) | 0.0036 (4) | −0.0079 (4) |
| C2 | 0.0152 (4) | 0.0140 (4) | 0.0111 (4) | −0.0026 (3) | 0.0016 (3) | −0.0043 (3) |
| C3 | 0.0244 (5) | 0.0163 (5) | 0.0096 (4) | −0.0001 (4) | −0.0026 (4) | −0.0048 (3) |
| C4 | 0.0157 (4) | 0.0138 (4) | 0.0102 (4) | −0.0028 (3) | −0.0027 (3) | −0.0020 (3) |
| C5 | 0.0240 (5) | 0.0165 (5) | 0.0127 (4) | −0.0004 (4) | −0.0066 (4) | 0.0000 (4) |
| C6 | 0.0228 (5) | 0.0171 (5) | 0.0096 (4) | 0.0007 (4) | −0.0027 (4) | −0.0004 (3) |
| C7 | 0.0156 (4) | 0.0126 (4) | 0.0090 (4) | 0.0017 (3) | −0.0015 (3) | −0.0037 (3) |
| C8 | 0.0176 (5) | 0.0159 (4) | 0.0126 (4) | −0.0016 (4) | −0.0050 (4) | −0.0029 (3) |
| C9 | 0.0133 (4) | 0.0114 (4) | 0.0134 (4) | 0.0000 (3) | −0.0004 (3) | −0.0051 (3) |
| C10 | 0.0146 (5) | 0.0172 (5) | 0.0234 (6) | −0.0040 (4) | 0.0003 (4) | −0.0070 (4) |
| Mo2 | 0.01235 (4) | 0.01240 (4) | 0.00711 (4) | 0.00003 (3) | 0.00073 (3) | −0.00262 (3) |
| O7 | 0.0226 (4) | 0.0168 (4) | 0.0105 (3) | −0.0003 (3) | −0.0010 (3) | −0.0013 (3) |
| O8 | 0.0153 (4) | 0.0165 (4) | 0.0181 (4) | −0.0016 (3) | 0.0027 (3) | −0.0033 (3) |
| O9 | 0.0188 (4) | 0.0146 (3) | 0.0095 (3) | 0.0024 (3) | −0.0003 (3) | −0.0034 (3) |
| O10 | 0.0151 (3) | 0.0185 (4) | 0.0147 (4) | −0.0040 (3) | 0.0028 (3) | −0.0066 (3) |
| O11 | 0.0214 (4) | 0.0131 (3) | 0.0083 (3) | 0.0013 (3) | 0.0016 (3) | −0.0029 (2) |
| O12 | 0.0161 (3) | 0.0159 (3) | 0.0115 (3) | −0.0007 (3) | 0.0016 (3) | −0.0062 (3) |
| C11 | 0.0213 (5) | 0.0137 (4) | 0.0135 (4) | 0.0010 (4) | 0.0031 (4) | −0.0052 (3) |
| C12 | 0.0132 (4) | 0.0124 (4) | 0.0102 (4) | −0.0018 (3) | 0.0025 (3) | −0.0037 (3) |
| C13 | 0.0244 (5) | 0.0145 (4) | 0.0085 (4) | 0.0012 (4) | 0.0011 (4) | −0.0031 (3) |
| C14 | 0.0146 (4) | 0.0116 (4) | 0.0100 (4) | −0.0021 (3) | −0.0006 (3) | −0.0020 (3) |
| C15 | 0.0233 (5) | 0.0129 (4) | 0.0127 (4) | −0.0002 (4) | −0.0046 (4) | −0.0009 (3) |
| C16 | 0.0159 (5) | 0.0220 (5) | 0.0248 (6) | −0.0044 (4) | 0.0025 (4) | 0.0002 (4) |
| C17 | 0.0139 (4) | 0.0151 (4) | 0.0127 (4) | 0.0001 (3) | 0.0004 (3) | 0.0007 (3) |
| C18 | 0.0185 (5) | 0.0222 (5) | 0.0141 (5) | 0.0002 (4) | 0.0065 (4) | −0.0054 (4) |
| C19 | 0.0173 (4) | 0.0173 (4) | 0.0091 (4) | 0.0039 (4) | −0.0006 (3) | −0.0050 (3) |
| C20 | 0.0263 (6) | 0.0251 (6) | 0.0181 (5) | 0.0048 (5) | −0.0002 (4) | −0.0135 (5) |
Geometric parameters (Å, º)
| Mo1—O1 | 1.7029 (9) | Mo2—O7 | 1.6996 (9) |
| Mo1—O2 | 1.7001 (9) | Mo2—O8 | 1.7021 (9) |
| Mo1—O3 | 2.1825 (8) | Mo2—O9 | 2.1808 (8) |
| Mo1—O4 | 2.1921 (8) | Mo2—O10 | 2.1848 (9) |
| Mo1—O5 | 2.0060 (8) | Mo2—O11 | 1.9898 (8) |
| Mo1—O6 | 1.9897 (8) | Mo2—O12 | 2.0106 (8) |
| O3—C9 | 1.2624 (13) | O9—C14 | 1.2623 (13) |
| O4—C2 | 1.2635 (13) | O10—C17 | 1.2632 (14) |
| O5—C7 | 1.3067 (13) | O11—C12 | 1.3036 (13) |
| O6—C4 | 1.3041 (13) | O12—C19 | 1.3104 (14) |
| C1—H1A | 0.933 (14) | C11—H11A | 0.984 (14) |
| C1—H1B | 0.947 (15) | C11—H11B | 0.988 (14) |
| C1—H1C | 0.957 (14) | C11—H11C | 0.962 (14) |
| C1—C2 | 1.5010 (16) | C11—C12 | 1.4961 (15) |
| C2—C3 | 1.4183 (16) | C12—C13 | 1.3761 (16) |
| C3—H3 | 0.931 (14) | C13—H13 | 0.953 (14) |
| C3—C4 | 1.3782 (16) | C13—C14 | 1.4191 (16) |
| C4—C5 | 1.4971 (16) | C14—C15 | 1.4942 (16) |
| C5—H5A | 0.983 (14) | C15—H15A | 0.932 (14) |
| C5—H5B | 0.975 (14) | C15—H15B | 0.961 (14) |
| C5—H5C | 0.968 (15) | C15—H15C | 0.957 (14) |
| C6—H6A | 0.973 (14) | C16—H16A | 0.941 (14) |
| C6—H6B | 0.946 (14) | C16—H16B | 0.961 (15) |
| C6—H6C | 0.931 (14) | C16—H16C | 0.955 (15) |
| C6—C7 | 1.4952 (16) | C16—C17 | 1.4944 (17) |
| C7—C8 | 1.3762 (16) | C17—C18 | 1.4152 (17) |
| C8—H8 | 0.907 (14) | C18—H18 | 0.917 (14) |
| C8—C9 | 1.4184 (16) | C18—C19 | 1.3709 (17) |
| C9—C10 | 1.4955 (16) | C19—C20 | 1.4986 (17) |
| C10—H10A | 0.948 (14) | C20—H20A | 0.942 (15) |
| C10—H10B | 0.931 (14) | C20—H20B | 0.950 (15) |
| C10—H10C | 0.965 (14) | C20—H20C | 0.935 (15) |
| O1—Mo1—O3 | 165.66 (4) | O7—Mo2—O8 | 105.59 (5) |
| O1—Mo1—O4 | 89.35 (4) | O7—Mo2—O9 | 164.58 (4) |
| O1—Mo1—O5 | 93.60 (4) | O7—Mo2—O10 | 87.98 (4) |
| O1—Mo1—O6 | 98.03 (4) | O7—Mo2—O11 | 95.53 (4) |
| O2—Mo1—O1 | 105.40 (4) | O7—Mo2—O12 | 96.98 (4) |
| O2—Mo1—O3 | 88.56 (4) | O8—Mo2—O9 | 89.81 (4) |
| O2—Mo1—O4 | 165.18 (4) | O8—Mo2—O10 | 166.15 (4) |
| O2—Mo1—O5 | 97.16 (4) | O8—Mo2—O11 | 97.67 (4) |
| O2—Mo1—O6 | 95.35 (4) | O8—Mo2—O12 | 94.14 (4) |
| O3—Mo1—O4 | 76.80 (3) | O9—Mo2—O10 | 76.68 (3) |
| O5—Mo1—O3 | 81.17 (3) | O11—Mo2—O9 | 81.38 (3) |
| O5—Mo1—O4 | 82.99 (3) | O11—Mo2—O10 | 83.47 (3) |
| O6—Mo1—O3 | 83.63 (3) | O11—Mo2—O12 | 159.82 (4) |
| O6—Mo1—O4 | 80.95 (3) | O12—Mo2—O9 | 82.36 (3) |
| O6—Mo1—O5 | 160.01 (4) | O12—Mo2—O10 | 81.22 (3) |
| C9—O3—Mo1 | 127.94 (7) | C14—O9—Mo2 | 128.23 (7) |
| C2—O4—Mo1 | 128.41 (7) | C17—O10—Mo2 | 127.20 (8) |
| C7—O5—Mo1 | 130.40 (7) | C12—O11—Mo2 | 131.47 (7) |
| C4—O6—Mo1 | 132.43 (7) | C19—O12—Mo2 | 129.74 (7) |
| H1A—C1—H1B | 108.5 (17) | H11A—C11—H11B | 109.9 (16) |
| H1A—C1—H1C | 105.8 (17) | H11A—C11—H11C | 108.6 (15) |
| H1B—C1—H1C | 103.1 (16) | H11B—C11—H11C | 106.5 (15) |
| C2—C1—H1A | 115.0 (12) | C12—C11—H11A | 111.4 (11) |
| C2—C1—H1B | 113.3 (13) | C12—C11—H11B | 110.9 (10) |
| C2—C1—H1C | 110.2 (12) | C12—C11—H11C | 109.4 (11) |
| O4—C2—C1 | 117.22 (10) | O11—C12—C11 | 114.43 (9) |
| O4—C2—C3 | 123.67 (10) | O11—C12—C13 | 124.15 (10) |
| C3—C2—C1 | 119.10 (10) | C13—C12—C11 | 121.35 (10) |
| C2—C3—H3 | 117.7 (11) | C12—C13—H13 | 118.0 (10) |
| C4—C3—C2 | 123.42 (10) | C12—C13—C14 | 123.58 (10) |
| C4—C3—H3 | 118.8 (11) | C14—C13—H13 | 118.3 (10) |
| O6—C4—C3 | 124.17 (10) | O9—C14—C13 | 123.53 (10) |
| O6—C4—C5 | 114.20 (10) | O9—C14—C15 | 117.53 (10) |
| C3—C4—C5 | 121.60 (10) | C13—C14—C15 | 118.93 (10) |
| C4—C5—H5A | 112.3 (11) | C14—C15—H15A | 113.4 (12) |
| C4—C5—H5B | 110.8 (11) | C14—C15—H15B | 113.3 (12) |
| C4—C5—H5C | 111.2 (12) | C14—C15—H15C | 108.1 (11) |
| H5A—C5—H5B | 106.9 (16) | H15A—C15—H15B | 107.6 (16) |
| H5A—C5—H5C | 107.7 (16) | H15A—C15—H15C | 105.6 (16) |
| H5B—C5—H5C | 107.8 (16) | H15B—C15—H15C | 108.5 (15) |
| H6A—C6—H6B | 105.2 (16) | H16A—C16—H16B | 110.9 (18) |
| H6A—C6—H6C | 101.7 (16) | H16A—C16—H16C | 107.2 (17) |
| H6B—C6—H6C | 112.9 (17) | H16B—C16—H16C | 102.8 (17) |
| C7—C6—H6A | 110.9 (12) | C17—C16—H16A | 112.3 (12) |
| C7—C6—H6B | 110.0 (12) | C17—C16—H16B | 109.7 (12) |
| C7—C6—H6C | 115.2 (12) | C17—C16—H16C | 113.5 (12) |
| O5—C7—C6 | 114.28 (10) | O10—C17—C16 | 117.36 (11) |
| O5—C7—C8 | 124.26 (10) | O10—C17—C18 | 123.12 (11) |
| C8—C7—C6 | 121.45 (10) | C18—C17—C16 | 119.44 (11) |
| C7—C8—H8 | 121.1 (11) | C17—C18—H18 | 115.8 (12) |
| C7—C8—C9 | 123.91 (10) | C19—C18—C17 | 124.01 (10) |
| C9—C8—H8 | 114.2 (11) | C19—C18—H18 | 119.2 (11) |
| O3—C9—C8 | 123.05 (10) | O12—C19—C18 | 124.32 (10) |
| O3—C9—C10 | 117.50 (10) | O12—C19—C20 | 114.39 (11) |
| C8—C9—C10 | 119.42 (10) | C18—C19—C20 | 121.27 (11) |
| C9—C10—H10A | 112.2 (12) | C19—C20—H20A | 112.1 (13) |
| C9—C10—H10B | 114.7 (12) | C19—C20—H20B | 114.9 (12) |
| C9—C10—H10C | 109.6 (11) | C19—C20—H20C | 112.8 (13) |
| H10A—C10—H10B | 105.2 (16) | H20A—C20—H20B | 102.8 (17) |
| H10A—C10—H10C | 108.5 (16) | H20A—C20—H20C | 104.7 (18) |
| H10B—C10—H10C | 106.2 (16) | H20B—C20—H20C | 108.6 (18) |
| Mo1—O3—C9—C8 | 14.91 (16) | Mo2—O9—C14—C13 | 11.74 (16) |
| Mo1—O3—C9—C10 | −167.23 (8) | Mo2—O9—C14—C15 | −167.95 (8) |
| Mo1—O4—C2—C1 | −165.78 (8) | Mo2—O10—C17—C16 | −167.33 (8) |
| Mo1—O4—C2—C3 | 13.14 (16) | Mo2—O10—C17—C18 | 15.86 (16) |
| Mo1—O5—C7—C6 | 159.72 (8) | Mo2—O11—C12—C11 | 160.69 (8) |
| Mo1—O5—C7—C8 | −21.55 (16) | Mo2—O11—C12—C13 | −22.43 (17) |
| Mo1—O6—C4—C3 | −21.18 (18) | Mo2—O12—C19—C18 | −20.31 (17) |
| Mo1—O6—C4—C5 | 160.91 (8) | Mo2—O12—C19—C20 | 161.61 (8) |
| O4—C2—C3—C4 | 6.18 (19) | O10—C17—C18—C19 | 10.74 (19) |
| O5—C7—C8—C9 | −7.08 (18) | O11—C12—C13—C14 | −4.41 (19) |
| C1—C2—C3—C4 | −174.93 (11) | C11—C12—C13—C14 | 172.26 (11) |
| C2—C3—C4—O6 | −3.75 (19) | C12—C13—C14—O9 | 8.31 (19) |
| C2—C3—C4—C5 | 174.01 (11) | C12—C13—C14—C15 | −172.00 (11) |
| C6—C7—C8—C9 | 171.56 (11) | C16—C17—C18—C19 | −166.01 (12) |
| C7—C8—C9—O3 | 9.00 (18) | C17—C18—C19—O12 | −10.0 (2) |
| C7—C8—C9—C10 | −168.82 (11) | C17—C18—C19—C20 | 167.99 (12) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H1A···O8i | 0.93 (1) | 2.62 (2) | 3.4834 (16) | 155 (2) |
| C5—H5B···O10ii | 0.98 (1) | 2.50 (1) | 3.4652 (15) | 170 (2) |
| C6—H6A···O8 | 0.97 (1) | 2.51 (2) | 3.3894 (15) | 151 (2) |
| C8—H8···O7iii | 0.91 (1) | 2.79 (2) | 3.4071 (14) | 126 (1) |
| C10—H10A···O6iv | 0.95 (1) | 2.68 (2) | 3.3334 (15) | 127 (1) |
| C10—H10C···O1v | 0.97 (1) | 2.50 (2) | 3.3126 (15) | 141 (2) |
| C11—H11B···O3i | 0.99 (1) | 2.48 (1) | 3.4415 (14) | 163 (2) |
| C15—H15A···O1ii | 0.93 (1) | 2.55 (2) | 3.4592 (15) | 167 (2) |
| C15—H15C···O4 | 0.96 (1) | 2.53 (2) | 3.4018 (15) | 152 (2) |
| C16—H16A···O11vi | 0.94 (1) | 2.66 (2) | 3.3127 (15) | 128 (2) |
| C16—H16B···O8vii | 0.96 (2) | 2.52 (2) | 3.3971 (17) | 153 (2) |
| C18—H18···O2viii | 0.92 (1) | 2.82 (2) | 3.4646 (15) | 128 (1) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x, −y+1, −z+1; (iii) −x+1, −y+1, −z+2; (iv) −x+1, −y+2, −z+1; (v) x+1, y, z; (vi) −x, −y, −z+2; (vii) x−1, y, z; (viii) −x, −y+1, −z+2.
Funding Statement
Funding for this research was provided by: National Science Foundation, Directorate for Education and Human Resources (grant No. 0942850 to Dean Johnston); Otterbein University Student Research Fund (grant to Calvin King, Aileen Seitz, Mia Sethi).
References
- Annese, C., Caputo, D., D’Accolti, L., Fusco, C., Nacci, A., Rossin, A., Tuci, G. & Giambastiani, G. (2019). Eur. J. Inorg. Chem. pp. 221–229.
- Arnáiz, F. J. (1995). J. Chem. Educ. 72, A7.
- Begines, E., Carrasco, C. J., Montilla, F., Álvarez, E., Marchetti, F., Pettinari, R., Pettinari, C. & Galindo, A. (2018). Dalton Trans. 47, 197–208. [DOI] [PubMed]
- Bowen, C. L. & Wile, B. M. (2021). IUCrData, 6, x210516. [DOI] [PMC free article] [PubMed]
- Bruker (2020). APEX3 and SAINT. Bruker AXS, Madison, Wisconsin, USA.
- Bustos, C., Manzur, C., Carrillo, D., Robert, F. & Gouzerh, P. (1994). Inorg. Chem. 33, 1427–1433.
- Ceylan, B. İ., Deniz, N. G., Kahraman, S. & Ulkuseven, B. (2015). Spectrochim. Acta A, 141, 272–277. [DOI] [PubMed]
- Chakravorti, M. C. & Bandyopadhyay, D. (1992). Inorganic Syntheses, Vol. 29, edited by R. N. Grimes, pp. 129–131. New York: John Wiley & Sons.
- Craven, B. M., Ramey, K. C. & Wise, W. B. (1971). Inorg. Chem. 10, 2626–2628.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Galsworthy, J. R., Green, M. L. H., Müller, M. & Prout, K. (1997). J. Chem. Soc. Dalton Trans. pp. 1309–1313.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Henderson, W., Nicholson, B. K., Bridson, J. H., Kueh, J. T. & Andy Hor, T. S. (2011). Inorg. Chim. Acta, 375, 142–149.
- Hills, L., Moyano, R., Montilla, F., Pastor, A., Galindo, A., Álvarez, E., Marchetti, F. & Pettinari, C. (2013). Eur. J. Inorg. Chem. pp. 3352–3361.
- Kamenar, B., Penavic, M. & Prout, C. K. (1973). Cryst. Struct. Commun. 2, 41–44.
- Kojić-Prodić, B., Rużić-Toroš, Ž., Grdenić, D. & Golič, L. (1974). Acta Cryst. B30, 300–305.
- Korstanje, T. J., Folkertsma, E., Lutz, M., Jastrzebski, J. T. B. H. & Klein Gebbink, R. J. M. (2013). Eur. J. Inorg. Chem. pp. 2195–2204.
- Krasochka, O. N., Sokolova, Yu. A. & Atovmyan, L. O. (1973). Zh. Strukt. Khim. 16, 696–698.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Mayer, J. M. (1988). Inorg. Chem. 27, 3899–3903.
- Nass, M., Schürmann, M., Preut, H. & Krause, N. (2001). Z. Krist. New Cryst. Struct. 216, 461–464.
- Palmer, D. C. (2019). CrystalMaker. CrystalMaker Software Ltd, Begbroke, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Sousa, S. C. A. & Fernandes, A. C. (2015). Coord. Chem. Rev. 284, 67–92.
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S2414314621007781/wm4150sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314621007781/wm4150Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314621007781/wm4150Isup3.mol
CCDC reference: 2100177
Additional supporting information: crystallographic information; 3D view; checkCIF report




