Abstract
Background
Isolated distal deep vein thrombosis (IDDVT) is a common subtype of deep vein thrombosis (DVT). Consensus guidelines provide conflicting recommendations for IDDVT management; some recommend anticoagulant treatment, while others suggest serial compression ultrasonography (CUS) monitoring for patients not at “high risk” of proximal extension. The purpose of this study was to describe outcomes of serial CUS-monitored IDDVT and identify risk factors for proximal thrombus extension or anticoagulant treatment initiation.
Methods
A retrospective descriptive study was conducted using electronic data from University of Utah Health. Adult subjects with objectively confirmed, serial CUS-monitored IDDVT were included. Subjects were followed for 30 days for occurrence of a composite outcome of proximal thrombus extension or anticoagulant treatment initiation. Descriptive statistics were used to summarize characteristics of the study population. Characteristics were compared across outcome groups using inferential statistics.
Results
A total of 182 subjects were included, with 53 subjects (29.1%) experiencing the composite outcome. Of these, 12 (22.6%) experienced proximal thrombus extension and 41 (77.4%) initiated anticoagulant treatment. A prior history of venous thromboembolism (VTE) was significantly higher in those who experienced the composite outcome than in those who did not.
Conclusions
Our results suggest that 70% of patients with serial CUS-monitored IDDVT did not experience thrombus extension or require anticoagulant treatment within 30 days of diagnosis, regardless of risk factors for proximal extension. Serial CUS monitoring may be a useful management strategy for IDDVT. A history of VTE may identify patients more likely to experience proximal thrombus extension or require anticoagulation.
Keywords: Venous thrombosis, Isolated distal deep vein thrombosis, Retrospective studies, Ultrasonography, Anticoagulants
Introduction
Isolated distal deep vein thrombosis (IDDVT) is a common subtype of deep vein thrombosis (DVT). IDDVT is defined as a thrombotic process involving only veins of the lower leg distal to, but not including, the popliteal vein [1]. These distal veins include the anterior and posterior tibial veins, the peroneal veins, and the veins of the soleus and gastrocnemius muscles [2]. IDDVT makes up about one-half of all DVT cases diagnosed with whole-leg compression ultrasonography (CUS) [2–5]. This translates to roughly 150,000 – 300,000 IDDVT cases diagnosed every year in the United States [5–6].Despite the high incidence of IDDVT, high-certainty evidence pertaining to optimal IDDVT management strategies is lacking. This was demonstrated by a recent Cochrane systematic review that specifically called for additional research on IDDVT treatment strategies [7].Thus, studies that can provide clearer evidence for optimal IDDVT management are needed.
There are eight published randomized controlled trials (RCTs) specifically studying patients with IDDVT [8–14]. Results from the two most recent RCTs illustrate the unclear benefit of IDDVT treatment using anticoagulants. The Anticoagulant Therapy for Symptomatic Calf Deep Vein Thrombosis (CACTUS) trial found no significant difference in a composite outcome of thrombus extension, contralateral proximal DVT, and symptomatic pulmonary embolism (PE) between patients with IDDVT randomized to treatment with nadroparin or placebo [12]. The Anticoagulation of Calf Thrombosis (ACT) pilot study found a nonsignificant difference (p=0.11) in a composite outcome of proximal thrombus progression, PE, VTE death, and major bleeding between those randomized to anticoagulation treatment and no anticoagulation treatment [6]. The results of these trials do not provide compelling evidence for treating IDDVT with anticoagulants. Furthermore, the unclear benefit of anticoagulant treatment must be weighed against the known bleeding risk with these agents. For some patients, watchful waiting strategies such as surveillance with serial CUS may be viable alternatives for IDDVT management.
International evidence-based consensus guidelines provide differing recommendations for IDDVT management, likely due to the aforementioned lack of high-certainty evidence. The American College of Chest Physicians (ACCP) 2016 VTE Guidelines suggest anticoagulation in patients with severe IDDVT symptoms or “risk factors” for proximal extension (defined as positive D-dimer, extensive thrombus, thrombus in close proximity to proximal veins, absence of a reversible provoking factor, active cancer, history of VTE, and inpatient status) [4].For other patients, monitoring with repeated (serial) CUS is suggested [4]. These recommendations are graded as 2C, meaning that they are based on low- or very low-certainty evidence and that additional evidence may lead to alternative recommendations [4]. The 2013 International Consensus Statement on Prevention and Treatment of Venous Thromboembolism, however, recommends that all patients with symptomatic IDDVT be treated with anticoagulation [15]. The 2020 American Society of Hematology recommendations make no recommendations regarding IDDVT treatment [16]. These conflicting recommendations leave practitioners and patients to make treatment decisions based on low-certainty evidence or expert opinion.
Describing the outcomes of untreated, serial CUS-monitored IDDVT can aid practitioners in identifying patients who might benefit from initial anticoagulation treatment, and those in whom the risks of anticoagulation likely outweigh the benefits. Without clear guidelines, practitioners can use this knowledge to facilitate robust shared decision-making conversations with patients. Therefore, the objective of our study was to describe outcomes of untreated, CUS-monitored IDDVT and to identify any significant differences between those who experience thrombus extension or initiate anticoagulant treatment and those who do not.
Methods
Study Setting
A retrospective descriptive study was conducted using electronic data from the University of Utah Health system between June 1, 2014 and January 1, 2020. The University of Utah Health system includes four hospitals and twelve community clinics, which serve patients from Utah and its neighboring states. This study was performed under IRB exemption IRB_00085773.
Study Population
Medical records of subjects with objectively confirmed IDDVT were identified using natural language processing (NLP), which is a validated method shown to have a sensitivity of 94% for identifying DVT [17]. The specific NLP methodology used in this study has been internally validated with a sensitivity of 96.0% and a specificity of 97.7% for detecting venous thromboembolism. For the purposes of this study, objectively confirmed IDDVT was defined as thrombus in the lower leg distal to, and not including, the popliteal vein detected by CUS. Records were manually reviewed to identify subjects who met the following inclusion criteria: age of 18 years or older with IDDVT who were managed using serial CUS monitoring surveillance. Serial CUS monitoring was defined as at least one follow-up CUS study within 14 days of the CUS used to establish the IDDVT diagnosis. This definition is consistent with ACCP recommendation for serial CUS monitoring for a duration of two weeks in patients with IDDVT [4]. Subjects were excluded if anticoagulant treatment was initiated within 48 hours of the IDDVT diagnosis. Anticoagulant treatment was defined as any new prescription for a direct oral anticoagulant (DOAC), warfarin, low molecular weight heparin (LMWH), unfractionated heparin infusion, or switching existing prophylactic anticoagulant treatment to therapeutic dosing.
Data Collection and Storage
Data was collected in a standardized collection form and stored for subsequent analysis using REDCap, a secure web application for managing study databases. Chart review and data collection was completed manually by a trained chart abstractor (A.C.B.), and uncertainties in eligibility criteria and study outcomes were reviewed by a second trained abstractor (A.E.J.). Any disagreements between chart abstractors were adjudicated by a third reviewer (D.M.W.).
Study Variables
Baseline patient characteristics included age, sex, race, ethnicity, smoking status, body mass index (BMI), prior history of VTE, D-dimer level prior to IDDVT diagnosis (if available), use of medications associated with increased thrombotic risk, use of medications for thromboprophylaxis, medical comorbidities, history of orthopedic surgery within 90 days, the presence and character of symptoms at the time of IDDVT diagnosis, weight-bearing status at the time of IDDVT diagnosis, and inpatient status at the time of IDDVT diagnosis. Subjects were followed for 30 days after IDDVT diagnosis to determine if they experienced a composite outcome consisting of proximal thrombus extension or anticoagulant treatment initiation, which were study endpoints. Proximal thrombus extension was defined as any thrombus that extended into the popliteal, femoral, or iliac veins, or PE. Anticoagulation treatment initiation was defined as described previously that occurred at least 48 hours after IDDVT diagnosis. Outcome data sources consisted of CUS reports, computed tomography pulmonary angiogram (CTPA) reports, electronic prescription records, and electronic provider notes.
Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics of the overall study population and categorized by those who did and did not experience the composite outcome. Categorical variables were summarized using frequency and percentages. Continuous variables were summarized using mean and standard deviation or median and interquartile range. Characteristics of subjects who experienced and did not experience the composite outcome were compared using student t-tests or Mann Whitney-U for continuous variables and Chi-squared or Fisher’s exact tests for categorical variables, as appropriate. P-values <0.05 were considered statistically significant and all tests were two-tailed. Subgroup analyses were performed by comparing characteristics of subjects experiencing the separate components of the composite outcome to those without the outcome using one-way ANOVA and Chi-squared or Fisher’s exact tests. The p-values for pairwise group comparisons were adjusted for multiplicity using Hommel’s multiple comparison procedure [18]. Risk ratios and associated 95% confidence intervals were calculated for some variables that differed significantly between groups.
Results
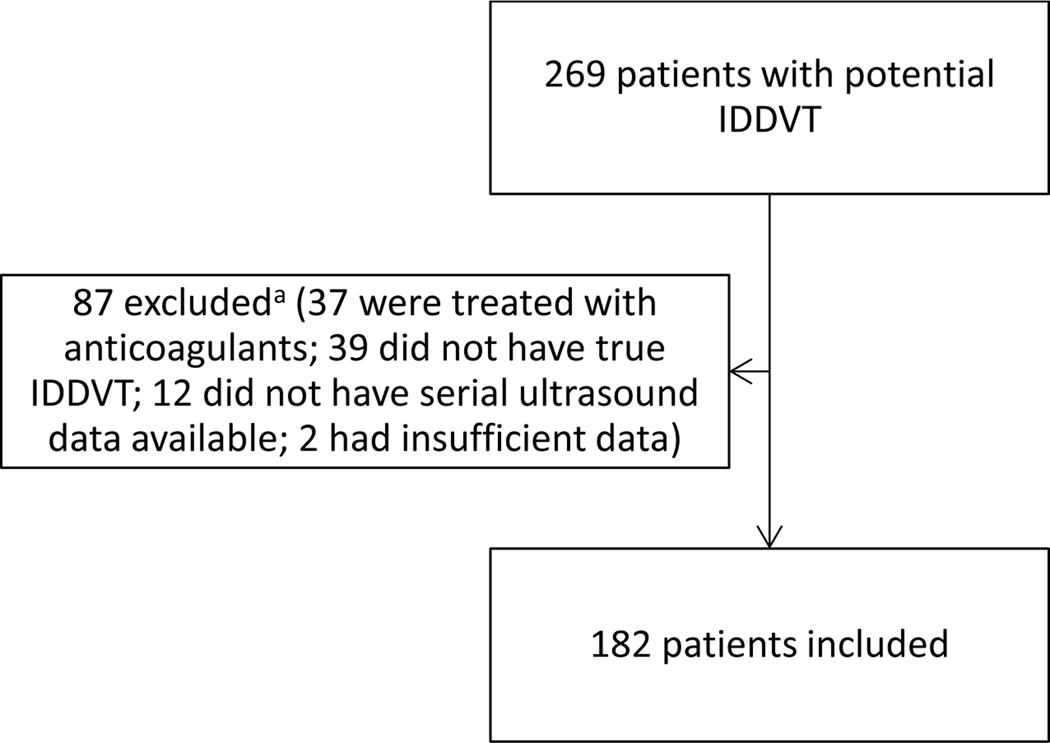
We identified 269 patients with potential IDDVT and 87 were subsequently excluded (Figure 1). Of the 87 excluded patients, 37 were excluded due to starting anticoagulant treatment within 48 hours of the index IDDVT diagnosis (Figure 1). A total of 182 patients were included in the final analysis. Baseline characteristics of included patients are summarized in Table 1. Patients were on average 57.3 years old, predominantly of Caucasian race (85.7%), and 44.5% female. Fifty-three (29.1%) subjects experienced the composite outcome of proximal thrombus extension or anticoagulant treatment initiation. Of these, 12 (22.6%) experienced proximal thrombus extension and 41 (77.4%) initiated anticoagulant treatment. Among subjects who experienced proximal thrombus extension, nine experienced extension to the popliteal vein, six experienced extension to the femoral vein, two experienced extension to the iliac vein, and four experienced PE. The overall incidence of PE was 2.2%. Prior history of VTE was more common in patients who experienced the composite outcome (52.8% vs. 17.1%, p < 0.001) (Table 1). Patients with a history of VTE were nearly 3 times as likely to experience the composite outcome than patients with no history of VTE (RR 2.96 [95% CI: 1.92 – 4.55]). This significant finding persisted in the subgroup analysis of those who experienced the separate components of the composite outcome (Table 2). Of the 12 patients who experienced proximal extension, 11 (91.7%) had a prior history of VTE (Table 2). Patients with a history of VTE were 36 times as likely to experience proximal extension (RR 36.0 [95% CI: 4.83 – 268.60]) and more than twice as likely to initiate anticoagulant treatment (RR 2.38 [95% CI: 1.43 – 3.95]) than patients without a history of VTE. D-dimer was numerically higher among patients who experienced proximal thrombus extension than in those who did not (6.95 mcg/mL vs. 1.87 mcg/mL, respectively, p = 0.18) (Table 2), but this difference was not statistically significant.
Figure 1.
Patient Selection Flow Diagram
aPatients may have been excluded for multiple reasons
Table 1.
Baseline characteristics at time of isolated distal deep vein thrombosis (IDDVT) diagnosis of University of Utah patients monitored with serial compression ultrasound categorized by composite outcome status.
| All patients (N = 182) | Experienced composite outcome (N = 53) | Did not experience composite outcome (N 129) | P-Value | |
|---|---|---|---|---|
| Age (years) (SD) | 57.3 (15.9) | 58.0 (14.1) | 57.0 (16.7) | 0.68 |
| Female sex (%) | 81 (44.5) | 25 (47.2) | 56 (43.4) | 0.64 |
| Race/ethnicity (%) | ||||
| Caucasian | 156 (85.7) | 47 (88.7) | 109 (84.5) | 0.46 |
| African | 2(1.1) | 0(0) | 2 (1.6) | 1.0 |
| American | ||||
| American Indian or Alaska Native | 2(1.1) | 0(0) | 2 (1.6) | 1.0 |
| Hawaiian/Pacific Islander | 2(1.1) | 0(0) | 2 (1.6) | 1.0 |
| Not specified | 2(1.1) | 1 (1.9) | 1 (0.8) | 0.50 |
| Other | 18 (9.9) | 5 (9.4) | 13 (10.1) | 0.89 |
| Hispanic ethnicity | 20 (11.0) | 7 (13.2) | 13 (10.1) | 0.54 |
| Smoking status (%) | ||||
| Current | 13 (7.1) | 3 (5.7) | 10 (7.8) | 0.76 |
| Former | 45 (24.7) | 14 (26.4) | 31 (24.0) | 0.73 |
| Never | 124 (68.1) | 36 (67.9) | 88 (68.2) | 0.97 |
| BMI (kg/m2) (SD) | 31.1 (10.1) | 31.9 (14.2) | 30.8 (7.9) | 0.51 |
| Median D-dimer prior to IDDVT diagnosisa (μg/ mL) (IQR) | 2.35 (1.41–3.90) | 3.05 (1.44–3.60) | 1.87 (1.32–4.58) | 0.84 |
| History of VTE (%) | 50 (27.5) | 28 (52.8) | 22 (17.1) | <0.001 |
| Use of thrombus-provoking medicationb (%) | 14 (7.7) | 4 (7.5) | 10 (7.8) | 1.0 |
| Prophylactic anticoagulant use at time of IDDVT diagnosisc, d (%) | ||||
| Aspirin | 64(35.2) | 18 (34.0) | 46 (35.7) | 0.83 |
| Warfarin | 2(1.1) | 1 (1.9) | 1 (0.8) | 0.50 |
| Enoxaparin | 24 (13.2) | 9 (17.0) | 15(11.6) | 0.33 |
| Other | 18 (9.9) | 4 (7.5) | 14 (10.9) | 0.59 |
| Comorbiditiese (%) | ||||
| Coronary artery disease | 19 (10.4) | 6 (11.3) | 13 (10.1) | 0.80 |
| Stroke | 23 (12.6) | 6 (11.3) | 17 (13.2) | 0.73 |
| Diabetes mellitus | 35 (19.2) | 11 (20.8) | 24 (18.6) | 0.74 |
| Hypertension | 75 (41.2) | 22 (41.5) | 53 (41.1) | 0.96 |
| Vascular disease | 25 (13.7) | 6 (11.3) | 19 (14.7) | 0.54 |
| Heart failure | 13 (7.1) | 4 (7.5) | 9 (7.0) | 1.0 |
| Cancer | 24 (13.2) | 7 (13.2) | 17 (13.2) | 1.0 |
| Renal disease | 16 (8.8) | 7 (13.2) | 9 (7.0) | 0.18 |
| Orthopedic surgery within 90 daysf (%) | ||||
| Total hip replacement | 1 (0.5) | 1 (1.9) | 0(0) | 0.29 |
| Hip fracture | 6 (3.3) | 1 (1.9) | 5 (3.9) | 0.67 |
| Total knee replacement | 7 (3.8) | 1 (1.9) | 6 (4.7) | 0.68 |
| Ankle fracture | 8 (4.4) | 3 (5.7) | 5 (3.9) | 0.69 |
| Achilles tendon repair | 5 (2.7) | 1 (1.9) | 4 (3.1) | 1.0 |
| Spinal surgery | 10 (5.5) | 2 (3.8) | 8 (6.2) | 0.73 |
| ACL repair | 4(2.2) | 0(0) | 4 (3.1) | 0.32 |
| Foot/heel/toe surgery | 12(6.6) | 2 (3.8) | 10 (7.8) | 0.51 |
| Other orthopedic procedures | 14 (7.7) | 3 (5.7) | 11 (8.5) | 0.76 |
| Presence of symptoms at IDDVTg (%) | 143 (78.6) | 39 (73.6) | 104 (80.6) | 0.29 |
| Weight-bearing at time of IDDVT (%) | 75 (41.2) | 24 (45.3) | 51 (39.5) | 0.47 |
| Inpatient Status at time of IDDVT (%) | 63 (34.6) | 21 (39.6) | 42 (32.6) | 0.36 |
SD-standard deviation; BMI-body mass index; IDDVT-isolated distal deep vein thrombosis; VTE-venous thromboembolism; ACL-anterior cruciate ligament.
D-dimer values were not available for all patients (N = 22).
Defined as estrogen, tamoxifen, raloxifene, testosterone, erythropoietin, and ponatinib.
Patients may not have been on prophylaxis at the time of diagnosis.
Patients may have used multiple forms of prophylaxis.
Patients may have had multiple comorbidities or no comorbidities.
Not all included patients had an orthopedic procedure within 90 days (N = 67).
Defined as swelling, redness, pain, or warmth.
Table 2.
Subgroup analysis – Characteristics of patients who experienced proximal extension or initiation of anticoagulant treatment
| Initiated anticoagulant treatment (N = 41) | Experienced Proximal extension (N = 12) | Did not experience composite outcome (N = 129) | P-Value | |
|---|---|---|---|---|
| Age (years) (SD) | 57.9 (15.4) | 58.5 (8.5) | 57.0 (16.7) | 0.91 |
|
| ||||
| Female sex (%) | 19 (46.3) | 6 (50.0) | 56 (43.4) | 0.88 |
|
| ||||
| Caucasian race | 36 (87.8) | 11 (91.7) | 109 (84.5) | 0.72 |
|
| ||||
| BMI (kg/m2) (SD) | 32.5 (15.6) | 29.8 (7.5) | 30.8 (7.9) | 0.58 |
|
| ||||
| Median D-dimer Prior to IDDVT Diagnosisa (mcg/mL) (IQR) | 2.18 (1.41–3.35) | 6.95 (5.08–8.83) | 1.87 (1.32–4.58) | 0.18 |
|
| ||||
| History of VTE (%) | 17 (41.5) | 11 (91.7) | 22 (17.1) | <0.001b |
|
| ||||
| Use of thrombus-provoking medicationsc (%) | 3 (7.3) | 1 (8.3) | 10 (7.8) | 1.0 |
|
| ||||
| Prophylactic anticoagulant use at time of IDDVT diagnosisd, e (%) | ||||
|
| ||||
| Aspirin | 15 (36.6) | (25.0) | 46 (35.7) | 0.81 |
| Warfarin | 1 (2.4) | 0 (0) | 1 (0.8) | 0.50 |
| Enoxaparin | 5 (12.2) | 4 (33.3) | 15 (11.6) | 0.13 |
| Other | 2 (4.9) | 2 (16.7) | 14 (10.9) | 0.31 |
|
| ||||
| Comorbiditiesf (%) | ||||
|
| ||||
| Coronary artery disease | 6 (14.6) | 0 (0) | 13 (10.1) | 0.40 |
| Stroke | 4 (9.8) | 2 (16.7) | 17 (13.2) | 0.74 |
| Diabetes | 8 (19.5) | 3 (25.0) | 24 (18.6) | 0.85 |
| Hypertension | 16 (39.0) | 6 (50.0) | 53 (41.1) | 0.79 |
| Vascular Disease | 6 (14.6) | 0 (0) | 19 (14.7) | 0.50 |
| Heart Failure | 4 (15.4) | 0 (0) | 9 (7.0) | 0.61 |
| Cancer | 6 (14.6) | 1 (8.3) | 17 (13.2) | 0.93 |
| Renal Disease | 7 (17.1) | 0 (0) | 9 (7.0) | 0.11 |
|
| ||||
| Presence of symptoms at IDDVTg (%) | 32 (78.0) | 7 (58.3) | 104 (80.6) | 0.20 |
|
| ||||
| Number of symptoms at IDDVT (SD) | 1.3 (1.0) | 0.75 (0.75) | 1.18 (0.8) | 0.15 |
|
| ||||
| Weight-bearing at IDDVT (%) | 20 (48.8) | 4 (33.3) | 51 (39.5) | 0.50 |
|
| ||||
| Inpatient Status at time of IDDVT (%) | 15 (36.6) | 6 (50.0) | 42 (32.6) | 0.46 |
D-dimer values were not available for all patients (N = 22)
p = 0.002 for Initiation of anticoagulation vs Proximal extension, p < 0.001 for Proximal extension vs No composite outcome, p = 0.002 for No composite outcome vs Initiation of anticoagulation
Defined as estrogen, tamoxifen, raloxifene, testosterone, erythropoietin, and ponatinib
Patients may not have been on prophylaxis at the time of diagnosis
Patients may have used multiple forms of prophylaxis
Patients may have had multiple comorbidities or no comorbidities
Defined as swelling, redness, pain, or warmth; SD-standard deviation; BMI-body mass index; IDDVT-isolated distal deep vein thrombosis; VTE-venous thromboembolism; ACL-anterior cruciate ligament
Discussion
In this study we aimed to describe outcomes of patients with IDDVT managed using serial CUS and identify any differences between those who did and did not experience proximal thrombus extension or initiation of anticoagulation therapy. Our results indicate that approximately 7 out of 10 patients with untreated IDDVT did not experience the primary outcome of proximal thrombus extension or anticoagulant treatment initiation within 30 days of the index IDDVT diagnosis. This adds to the current body of evidence demonstrating that there are patients diagnosed with IDDVT who can be successfully managed with serial CUS [6,12]. The rate of proximal extension in our study (6.7%) aligns closely with the outcome rate (5.0%) in the placebo arm of the CACTUS trial, which looked at a composite outcome of proximal extension, contralateral proximal DVT, or symptomatic pulmonary embolism [12]. Our rate of proximal extension is also similar to those in two other studies of untreated, ultrasound monitored IDDVT. In the Evolution of Untreated Calf Deep-vein Thrombosis in High Risk Symptomatic Outpatients (CALTHRO) study, the rate of proximal extension (proximal DVT and PE) was 6.3%; in the Calf Vein Thrombosis Outcomes Comparing Anticoagulation and Serial Ultrasound Imaging Management Strategies study, the rate of proximal extension in the surveillance group was 9.5% [19–20]. The observation that our outcome rate is similar to those in other studies of untreated patients provides confidence that our study reasonably captured the natural history of IDDVT. Among those who experienced the composite outcome, the majority (77.4%) initiated anticoagulant treatment in the absence of proximal thrombus extension. Prior history of VTE was present in over half of patients who experienced the composite outcome and in 9 of 10 patients with proximal thrombus extension. This observation indicates that patients with prior VTE who present with IDDVT may not be optimal candidates for monitoring with serial CUS and may be candidates for initiation of anticoagulation, an observation that deserves further study. This supports the ACCP definition of “risk factors” for proximal extension that might warrant anticoagulant treatment of IDDVT [4]. Higher D-dimer levels prior to IDDVT diagnosis approached significance in the subgroup analysis of subjects with the separate components of the composite outcome compared to those with no outcome, which was likely driven by those who experienced proximal extension. D-dimer level is another “risk factor,” suggested by the ACCP guidelines to identify patients who might benefit from initiation of anticoagulation therapy. However, only 22 of the 182 patients included in our study had a pre-IDDVT diagnosis D-dimer value available, which brings into question the real-world practicality of using D-dimer as a surrogate marker for potentially unfavorable outcomes in patients with IDDVT. If the clinical probability of a thrombus is moderate or high, many providers may forego a D-dimer and proceed with CUS evaluation, and if a thrombus is subsequently identified, a D-dimer is no longer indicated.
Our findings contrast recommendations from the 2016 ACCP VTE Guidelines and the International Consensus Statement on VTE Treatment, which suggest initiating anticoagulation therapy in patients with “severe symptoms” or any symptoms, respectively [4,15].We found no significant differences in IDDVT symptoms between those who experienced the outcome (and its components) and those who did not. In fact, the presence of symptoms was numerically higher in those who did not experience the composite outcome than in those who did. This brings into question the utility of using symptoms as a means of identifying a subset of patients at higher risk for unfavorable IDDVT outcomes. The presence of IDDVT symptoms is partly subjective. Furthermore, the term “severe symptoms” leaves substantial room for interpretation.
One of the strengths of our study is that it adds to the body of research surrounding IDDVT management, which is unfortunately associated with a lack of high-certainty evidence to guide management decisions [7]. To our knowledge, there are no randomized controlled trials to date directly comparing serial CUS monitoring to other management strategies for IDDVT [7]. Our study results indicate that serial CUS monitoring may be an appropriate management strategy for many patients with IDDVT and thus calls for additional research exploring this strategy. However, it is important to note that our study did not compare serial CUS monitoring to anticoagulant treatment for IDDVT management, which may be a useful comparison in future research. Another strength of our study lies in the subgroup analysis, which brought to light findings that challenge the utility of some of the current evidence-based recommendations for IDDVT management. This illustrates a need for clarified or revised guidelines, which may need to be preceded by additional high-quality research in this area.
Our study is not without limitations. A major limitation is the relatively small sample size of 178 patients. It is possible that a larger sample may have revealed more significant differences in clinical characteristics between those with and without proximal extension or initiation of anticoagulation therapy. Our study follow-up was also limited to 30-days, and longer-term outcome data may have contributed greater perspective on the potential consequences of untreated, ultrasound-monitored IDDVT. Our follow-up period was ultimately chosen based on ACCP guideline recommendations of serial imaging for two weeks [4]. Another limitation of our study is that it was conducted within a single healthcare system, the University of Utah, which may limit the generalizability of our results. Furthermore, some event and characteristic data may have been missed if patients sought care at a different healthcare system. Health data from some surrounding healthcare systems are integrated into our database through agreements between sites. Lastly, our study was retrospective and observational in nature, which can potentially introduce information bias and confounding bias. Chart abstractors were trained, and multiple abstractors were used to settle uncertainties surrounding eligibility criteria and study outcomes. Data surrounding potential confounding factors and covariates (i.e., symptoms at the time of diagnosis, smoking status, recent orthopedic surgery) was collected and summarized within groups for completeness. However, some data was not available for all of the subjects in our study, including the reasons for anticoagulant initiation for some subjects who did not experience proximal extension. This data would have been useful to further describe patients with this outcome. It is also possible that potentially relevant variables were missing due to the retrospective design of the present study.
Conclusion
In summary, our study results showed that 70% of patients with serial CUS-monitored IDDVT did not require anticoagulation treatment within 30 days of IDDVT diagnosis. A history of VTE was more common among those who experienced the composite outcome or its components and those who did not. Our results indicate that, in lieu of evidence suggesting a clear benefit of treating IDDVT with anticoagulants, serial CUS monitoring may be an appropriate management strategy for many patients with IDDVT. Additional high-quality research is warranted, specifically research that compares serial CUS monitoring to other IDDVT management strategies.
Highlights.
Optimal management of isolated distal deep vein thrombosis (IDDVT) is unclear
Some guidelines suggest treating IDDVT with anticoagulants
Some guidelines suggest monitoring IDDVT with serial ultrasounds
70% of subjects with ultrasound-monitored IDDVT did not require anticoagulation
Patients with a history of VTE were more likely to experience thrombus extension
Acknowledgments
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002538. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interests
Dr. Witt has received research funding from Roche Diagnostics and is supported by funding from the Agency for Healthcare Research and Quality (Grant #R18 HS027960-01)
Abbreviations
- IDDVT
Isolated distal deep vein thrombosis
- DVT
Deep vein thrombosis
- CUS
Compression ultrasonography
- VTE
Venous thromboembolism
- RCT
Randomized controlled trial
- PE
Pulmonary embolism
- ACCP
The American College of Chest Physicians
- NLP
Natural language processing
- DOAC
Direct oral anticoagulant
- LMWH
Low molecular weight heparin
- BMI
Body mass index
- CTPA
Computed tomography pulmonary angiogram
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Righini M, Paris S, Le Gal G, Laroche J, Perrier A, & Bounameaux H. (2006). Clinical relevance of distal deep vein thrombosis. Review of literature data. Thrombosis and Haemostasis, 95(1), 56–64. doi: 10.1160/TH05-08-0588. [DOI] [PubMed] [Google Scholar]
- [2].Palareti G, & Sartori M. (2016). Treatment of Isolated Below the Knee Deep Vein Thrombosis. Current Atherosclerosis Reports, 18(7). doi: 10.1007/s11883-016-0594-1. [DOI] [PubMed] [Google Scholar]
- [3].Porfidia A, Carnicelli A, Bonadia N, Pola R, & Landolfi R. (2016). Controversies in venous thromboembolism: the unique case of isolated distal deep vein thrombosis. Internal and Emergency Medicine, 11(6), 775–779. doi: 10.1007/s11739-016-1453-3. [DOI] [PubMed] [Google Scholar]
- [4].Kearon C, Akl E, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, … Moores L. (2016). Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest, 149(2), 315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- [5].Utter G, Dhillon T, Salcedo E, Shouldice D, Reynolds C, Humphries M, White R. (2016). Therapeutic anticoagulation for isolated calf deep vein thrombosis. JAMA Surgery, 151(9). doi: 10.1001/jamasurg.2016.1770. [DOI] [PubMed] [Google Scholar]
- [6].Horner D, Hogg K, Body R, Nash M, Baglin T, Mackway-Jones K. (2014). The Anticoagulation of Calf Thrombosis (ACT) Project. Chest, 146(6), 1468–77. doi: 10.1378/chest.14-0235. [DOI] [PubMed] [Google Scholar]
- [7].Kirkilesis G, Kakkos SK., Bicknell C, Salim S, Kakavia K. (2020). Treatment of distal deep vein thrombosis. Cochrane Database of Systematic Reviews, issue 4. doi: 10.1002/14651858.CD013422.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ferrara F, Meli F, Amato C, Cospite V, Raimondi F, Novo G. (2006). Optimal duration of treatment in surgical patients with calf venous thrombosis involving one or more veins. Angiology, 57, 418–23. [DOI] [PubMed] [Google Scholar]
- [9].Lagerstedt C, Olsson CG., Fagher BO, Oqvist BW, Albrechtsson U. (1985). Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet, 2(8454), 515–8. [DOI] [PubMed] [Google Scholar]
- [10].Nielsen HK., Husted SE, Krusell LR., Fasting H, Charles P, Hansen HH, et al. (1994). Anticoagulant therapy in deep venous thrombosis. A randomized controlled study. Thrombosis Research, 73, 215–26. [DOI] [PubMed] [Google Scholar]
- [11].Pinede L, Ninet J, Duhaut P, Chabaud S, Demolombe-Rague S, Durieu I, et al. (2001). Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation, 103(20), 2453–60. doi: 10.1161/01.CIR.103.20.2453. [DOI] [PubMed] [Google Scholar]
- [12].Righini M, Galanaud J-P, Guenneguez H, Brisot D, Diard A, Faisse P, … Quere I. (2016). Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. The Lancet Haematology, 3(12), e556–e562. doi: 10.1016/s2352-3026(16)30131-4. [DOI] [PubMed] [Google Scholar]
- [13].Schulman S, Rhedin A, Lindmarker P, Carlsson A, Lafrars G, Nicol P, et al. (1995). A comparison of six week with six months of oral anticoagulation therapy after a first episode of venous thromboembolism. New England Journal of Medicine, 332(25), 1661–5. [DOI] [PubMed] [Google Scholar]
- [14].Schwarz T, Buschmann L, Beyer J, Halbritter K, Rastan A, Schellong S. (2010). Therapy of isolated calf muscle vein thrombosis: a randomized, controlled study. Journal of Vascular Surgery, 52(5), 1246–50. doi: 10.1016/j.jvs.2010.05.094. [DOI] [PubMed] [Google Scholar]
- [15].Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism– international consensus statement. (2013). International Angiology, 32(2), 111–260. [PubMed] [Google Scholar]
- [16].Ortel TL., Neumann G, Ageno W, Beyth R, Clark NP, Cuker A, et al. (2020). American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Advances, 4(19), 4693–4738. doi: 10.1182/bloodadvances.2020001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tian Z, Sun S, Eguale T, Rochefort C. (2017). Automated Extraction of VTE Events from Narrative Radiology Reports in Electronic Health Records. A Validation Study. Medical Care, 55(10), e73–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wright SP. (1992). Adjusted P-Values for Simultaneous Inference. Biometrics. 48:1005–1013. [Google Scholar]
- [19].Palareti G, Cosmi B, Lessiani G, Rodorigo G, Guazzaloca G, Brusi C, et al. (2010). Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: The blind, prospective CALTHRO study. Thromb Haemost, 104, 1063–1070. doi: 10.1160/TH10-06-0351. [DOI] [PubMed] [Google Scholar]
- [20].Kuczmik W, Wysokinski WE., Macedo T, Froehling D, Daniels P, Casanegra A, et al. Calf Vein Thrombosis Outcomes Comparing Anticoagulation and Serial Ultrasound Imaging Management Strategies. (2021). Mayo Clin Proc, 96(5), 1184–1192. doi: 10.1016/j.mayocp.2021.01.024. [DOI] [PubMed] [Google Scholar]