PURPOSE
Patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) have poor prognosis. For these patients, treatment options are limited after first-line systemic therapy.
PATIENTS AND METHODS
In this open-label phase III clinical study, patients with advanced or metastatic ESCC, whose tumor progressed after first-line systemic treatment, were randomly assigned (1:1) to receive intravenous tislelizumab, an anti–programmed cell death protein 1 antibody, 200 mg every 3 weeks or chemotherapy (investigator's choice of paclitaxel, docetaxel, or irinotecan). The primary end point was overall survival (OS) in all patients. The key secondary end point was OS in patients with programmed death-ligand 1 tumor area positivity (TAP) score ≥ 10%.
RESULTS
In total, 512 patients across 11 countries/regions were randomly assigned. At final analysis, conducted after 410 death events occurred, OS was significantly longer with tislelizumab versus chemotherapy in all patients (median, 8.6 v 6.3 months; hazard ratio [HR], 0.70 [95% CI, 0.57 to 0.85]; one-sided P = .0001), and in patients with TAP ≥ 10% (median, 10.3 months v 6.8 months; HR, 0.54 [95% CI, 0.36 to 0.79]; one-sided P = .0006). Survival benefit was consistently observed across all predefined subgroups, including those defined by baseline TAP score, region, and race. Treatment with tislelizumab was associated with higher objective response rate (20.3% v 9.8%) and a more durable antitumor response (median, 7.1 months v 4.0 months) versus chemotherapy in all patients. Fewer patients experienced ≥ grade 3 treatment-related adverse events (18.8% v 55.8%) with tislelizumab versus chemotherapy.
CONCLUSION
Tislelizumab significantly improved OS compared with chemotherapy as second-line therapy in patients with advanced or metastatic ESCC, with a tolerable safety profile. Patients with programmed death-ligand 1 TAP ≥ 10% also demonstrated statistically significant survival benefit with tislelizumab versus chemotherapy.
INTRODUCTION
In 2020, esophageal cancer (EC) was ranked the seventh most common cancer worldwide and sixth most common cause of cancer-related deaths.1 Esophageal squamous cell carcinoma (ESCC) is the most common histologic subtype, accounting for more than 85% of ECs worldwide.2,3 Reports from the SEER Program show that between 2011 and 2017, the prognosis of metastatic ESCC was poor, with a 5-year survival rate of 5.2%.4
CONTEXT
Key Objective
Esophageal squamous cell carcinoma (ESCC) is an aggressive cancer associated with a 5-year survival rate of 5%. The phase III RATIONALE-302 study that enrolled a global population of 512 patients with advanced or metastatic ESCC evaluated whether tislelizumab monotherapy improved overall survival versus chemotherapy when used as second-line treatment for patients with advanced or metastatic ESCC.
Knowledge Generated
Tislelizumab demonstrated statistically significant and clinically meaningful improvement in overall survival versus chemotherapy, with a tolerable safety profile, in patients with advanced or metastatic ESCC in a global population in second-line treatment. In patients with programmed death-ligand 1 tumor area positivity ≥ 10%, tislelizumab also demonstrated statistically significant survival benefit. Survival benefit of tislelizumab over chemotherapy was observed across subgroups of region, race, and programmed death-ligand 1 expression level.
Relevance
The results of RATIONALE-302 suggest that tislelizumab is an appropriate treatment option for patients with advanced or metastatic ESCC in second-line treatment setting.
First-line systemic therapy for advanced or metastatic ESCC typically consists of a fluoropyrimidine- and platinum-based regimen.5-7 In the second-line setting, single-agent taxane or irinotecan is typically used; however, these are associated with significant toxicities and marginal antitumor activity with poor long-term survival.8-12 Recently, trials studying the programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway have demonstrated prolonged survival and safety benefits with anti–PD-1 antibodies versus chemotherapy in patients with advanced or metastatic ESCC whose disease progressed after first-line systemic therapy.10-13 These studies demonstrated survival benefit specifically in patients with high PD-L1 expression, or in Asian or Chinese patients irrespective of PD-L1 expression level.10-12
Tislelizumab is an investigational humanized immunoglobulin G4 monoclonal antibody with high affinity for PD-1, designed to minimize binding to FcγR on macrophages to limit antibody-dependent phagocytosis, a potential mechanism of resistance to anti–PD-1 therapy.14 In early-phase clinical studies, tislelizumab monotherapy or in combination with chemotherapy demonstrated antitumor activity in patients with solid tumors, including ECs, and showed a safety profile similar to other anti–PD-1 antibodies.15-17 Here, we report the efficacy and safety results from the global, randomized phase III RATIONALE-302 study (ClinicalTrials.gov identifier: NCT03430843) of tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic ESCC.
PATIENTS AND METHODS
Patients
Eligible patients were adults (age ≥ 18 years) with histologically confirmed ESCC who had advanced or metastatic disease that progressed after first-line systemic treatment. Patients who had tumor progression within 6 months after definitive chemoradiotherapy, neoadjuvant, or adjuvant therapy were also eligible. Patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, at least one measurable/evaluable lesion by RECIST v1.1, and adequate hematologic, hepatic, renal, and coagulation function. Exclusion criteria included patients who had received prior therapies targeting PD-1 or PD-L1, active brain or leptomeningeal metastasis, active autoimmune disease, or other prior malignancies active within 2 years before random assignment. Full eligibility criteria are provided in the Data Supplement (online only).
The Protocol (online only) was approved by the relevant Institutional Review Board/Independent Ethics Committee for each study site. The full Protocol is available with the Data Supplement. The study was carried out in accordance with the International Conference on Harmonisation Good Clinical Practice Guideline, the principles of the Declaration of Helsinki, and local laws and regulations. All patients provided written informed consent before participation.
Trial Design and Treatment
This open-label, randomized, active-controlled, multicenter, phase III clinical study recruited patients across 11 countries/regions (Belgium, mainland China, France, Germany, Italy, Japan, Republic of Korea, Spain, Taiwan, the United Kingdom, and the United States). Eligible patients were randomly assigned (1:1) to receive tislelizumab or investigator's choice of the following single-agent chemotherapies: paclitaxel, docetaxel, or irinotecan. Tislelizumab was administered intravenously (IV) 200 mg once every three weeks. Paclitaxel was administered as 135-175 mg/m2 IV once every 3 weeks, or in doses of 80-100 mg/m2 once weekly as per regional guidelines. In Japan, paclitaxel was administered as 100 mg/m2 IV in cycles consisting of once weekly dosing for 6 weeks, followed by one week of rest. Docetaxel was administered as 75 mg/m2 IV once every 3 weeks (70 mg/m2 IV once every 3 weeks in Japan). Irinotecan 125 mg/m2 IV was administered on days 1 and 8, every 21 days. Stratified randomization was used and was stratified by region (Asia [excluding Japan] v Japan v Europe/North America), ECOG PS (0 v 1), and investigator-chosen chemotherapy (paclitaxel v docetaxel v irinotecan). Patients were treated until disease progression, unacceptable toxicity, or withdrawal for other reasons. At the discretion of the investigator, patients receiving tislelizumab could continue to receive treatment after progression if the patient was likely to benefit from continued treatment, provided that the patient provided written informed consent. Details of random assignment and tumor response assessment methods are described in the Data Supplement.
Assessments
PD-L1 expression was centrally assessed using the analytically validated VENTANA PD-L1 (SP263) assay with tumor area positivity (TAP) score, which is defined as the total percentage of the tumor area covered by tumor cells with any membrane staining above background and tumor-associated immune cells with any staining above background. Patients with PD-L1–positive expression were defined as having a TAP score of ≥ 10%. Tumor responses were assessed using computed tomography or magnetic resonance imaging, every 6 weeks for 6 months, and then every 9 weeks, by the investigator per RECIST v1.1. Adverse events (AEs) were assessed throughout the study and up to 30 days after the last dose of study drug or initiation of a new anticancer therapy, whichever occurred first, according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.03. Immune-related AEs were recorded up to 90 days after the last dose of study drug, regardless of whether the patient started a new anticancer therapy. All suspected study-drug–related serious AEs continued to be recorded by the investigators after treatment discontinuation.
End Points
The primary end point of this study was overall survival (OS) in all randomly assigned patients (the intent-to-treat [ITT] population). The key secondary end point was OS in patients with PD-L1 TAP ≥ 10%. Other secondary end points included progression-free survival (PFS), objective response rate (ORR), and duration of response (DoR), assessed by the investigator per RECIST v1.1 in the ITT population and in patients with PD-L1 TAP ≥ 10%, and safety and tolerability. A complete list of study end points is provided in the Data Supplement.
Statistical Analyses
The OS hazard ratio (HR) of interest for tislelizumab versus chemotherapy was assumed to be 0.75 with a median OS of 8 versus 6 months, respectively. Approximately 400 death events were required to provide a power of 82% at a one-sided significance level of 0.025 to detect superiority of tislelizumab over chemotherapy. Assuming a 26-month period to observe the target number of death events and a dropout rate of 5% per year, approximately 500 patients were to be enrolled. The target number of death events was estimated to occur approximately 30.2 months after the first patient enrolled.
For OS analysis, P values for the comparison between treatment arms were estimated from a one-sided log-rank test stratified by ECOG PS and investigator-chosen chemotherapy. HRs and associated two-sided 95% CIs were estimated from a stratified Cox regression model including treatment arm as a covariate and with chemotherapy option and ECOG PS as strata. Median OS and 95% CI were calculated using a generalized Brookmeyer and Crowley method, and the cumulative probability of OS at 6 and 12 months was calculated (with two-sided 95% CI) using Greenwood's formula. Kaplan-Meier survival curves were also presented for each arm. When superiority in OS in the ITT population was determined, a hierarchical hypothesis testing approach for the key secondary end point of OS in patients with PD-L1 TAP ≥ 10% was used to preserve a study-wise type I error rate at 5%. The subgroup analysis of OS in the ITT population was prespecified in the Statistical Analysis Plan provided in the Data Supplement.
Median PFS and median DoR with 95% CI were calculated using a generalized Brookmeyer and Crowley method. For ORR, a stratified Cochran-Mantel-Haenszel test was used to calculate common odds ratios and associated two-sided 95% CIs. ORR, difference in ORR, and Clopper-Pearson 95% CI were also calculated.
Safety was evaluated in all randomly assigned patients who received at least one dose of study drug and analyzed using descriptive statistics. All calculations and analyses were conducted using SAS version 9.4 or higher. Full statistical methods are provided in the Statistical Analysis Plan provided in the Data Supplement.
RESULTS
Patients and Treatment
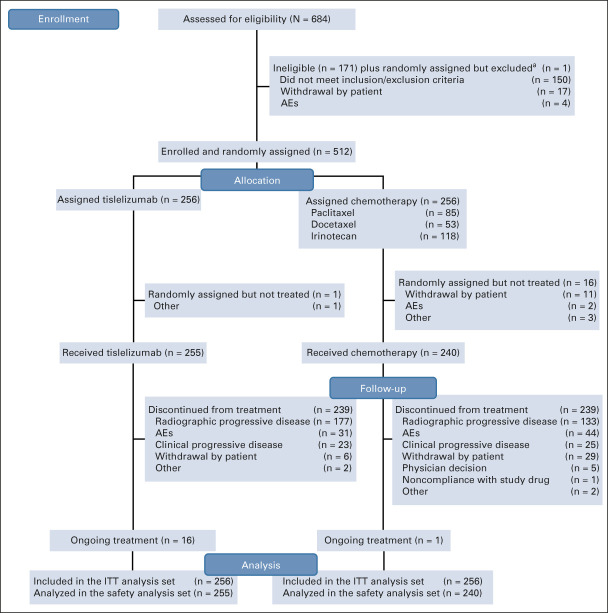
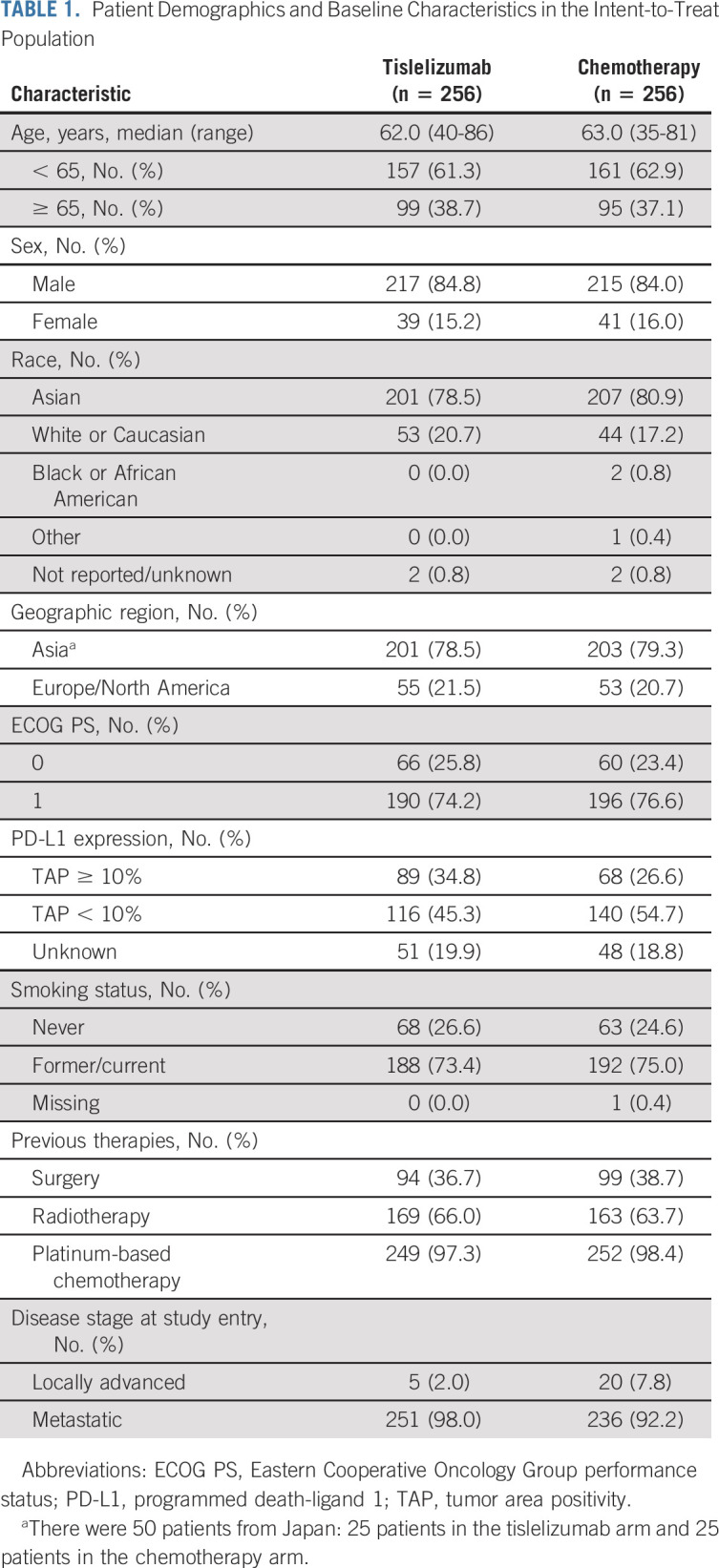
Between January 2018 and March 2020, 512 patients in Asia (404 patients [78.9%]) and Europe and North America (108 patients [21.1%]) were randomly assigned to receive tislelizumab (n = 256) or chemotherapy (n = 256) and were included in the ITT population (Fig 1). Of these, 255 patients in the tislelizumab arm and 240 patients in the chemotherapy arm received at least one dose of assigned treatment (Fig 1). Patient characteristics and demographics were generally balanced between treatment arms (Table 1). The median age of patients was 62 years, 79.7% of patients were Asian, and 84.4% of patients were male. A total of 487 (95.1%) patients had metastatic disease at study entry, and 157 (30.7%) patients had PD-L1 TAP ≥ 10% tumors. More patients in the tislelizumab arm had PD-L1 TAP ≥ 10% versus patients in the chemotherapy arm (34.8% v 26.6%).
FIG 1.
CONSORT diagram. aOne patient died before random assignment and was inadvertently randomly assigned into the study. Data cutoff: December 1, 2020. AE, adverse event; ITT, intent-to-treat.
TABLE 1.
Patient Demographics and Baseline Characteristics in the Intent-to-Treat Population
At the time of data cutoff (December 1, 2020), median follow-up from random assignment to data cutoff or death, whichever came first, was 8.5 months (0.2 to 31.7 months) for tislelizumab and 5.8 months (0.0 to 30.8 months) for chemotherapy. The median duration of exposure was 84.0 days (7-862 days) to tislelizumab and 45.5 days (7-584 days) to chemotherapy. More patients in the tislelizumab arm received ≥ 6 months of study treatment versus patients in the chemotherapy arm (25.5% v 8.7%).
Efficacy
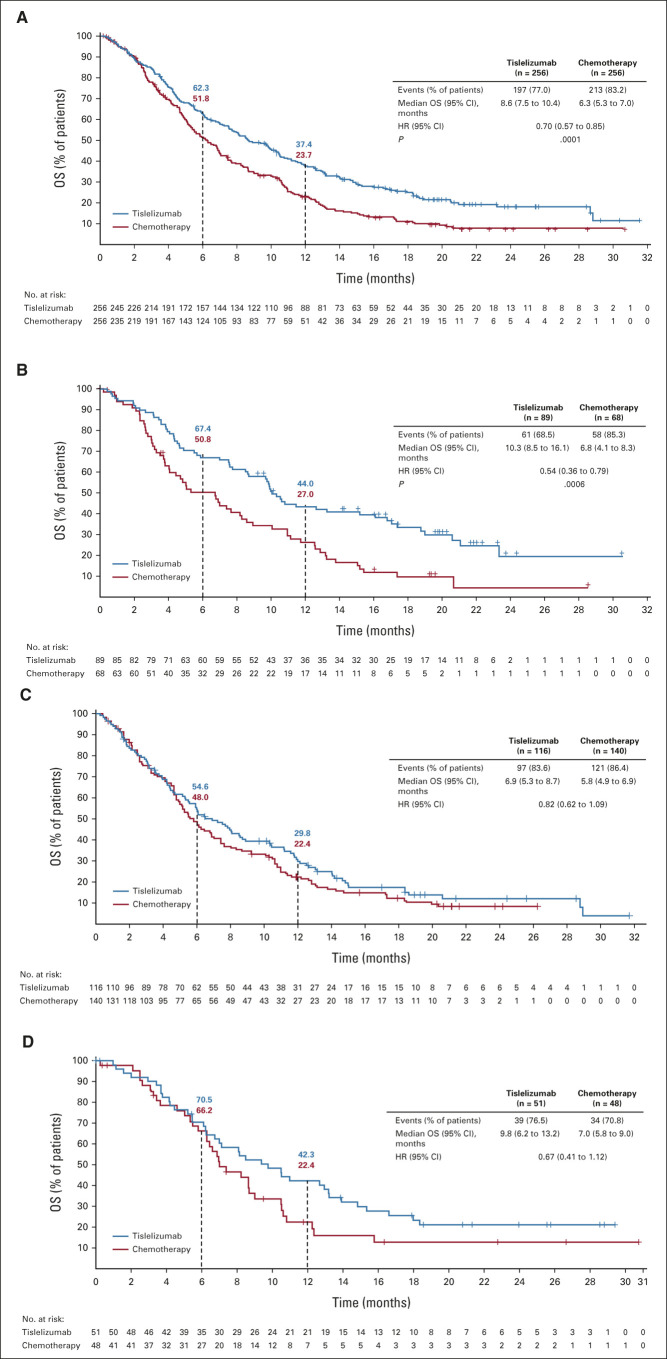
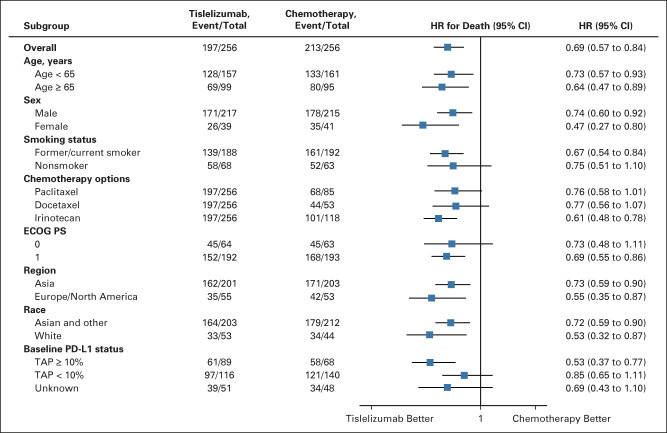
At final analysis, a total of 197 (77.0%) versus 213 (83.2%) deaths occurred in the tislelizumab and chemotherapy arms, respectively. OS was significantly improved in the tislelizumab arm versus the chemotherapy arm (median 8.6 months [95% CI, 7.5 to 10.4] v 6.3 months [95% CI, 5.3 to 7.0]; HR for death 0.70, 95% CI, 0.57 to 0.85; one-sided P = .0001; Fig 2). The 12-month OS rate was 37.4% (95% CI, 31.4 to 43.4) versus 23.7% (95% CI, 18.5 to 29.3) in the tislelizumab and chemotherapy arms, respectively. In total, 11 patients (4.3%) versus 55 patients (21.5%) in the tislelizumab and chemotherapy arms, respectively, had received anti–PD-1 or anti–PD-L1 therapy after discontinuation of study treatment (Data Supplement). Post hoc adjustment analyses for baseline PD-L1 expression status confirmed that the imbalance between treatment arms in baseline PD-L1 expression status had little impact on the estimate of the treatment effect on OS results (Data Supplement). Survival benefit of tislelizumab versus chemotherapy was observed in all predefined subgroups, including those defined by baseline PD-L1 expression status, region, and race (Fig 3; Data Supplement).
FIG 2.
Kaplan-Meier plot of OS in the (A) intent-to-treat population, (B) PD-L1 TAP ≥ 10%, (C) PD-L1 TAP < 10%, and (D) TAP unknown populations. One-sided P value was estimated from a log-rank test stratified by ECOG PS and chemotherapy option. HR was based on a Cox regression model including treatment as a covariate and ECOG PS and chemotherapy option as strata. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1; TAP, tumor area positivity.
FIG 3.
Subgroup analysis of overall survival in the intent-to-treat population. HR was based on an unstratified Cox regression model including treatment as a covariate. The race subcategory other includes Black or African American, not reported, unknown, and other. ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; PD-L1, programmed death-ligand 1; TAP, tumor area positivity.
In patients with TAP ≥ 10%, tislelizumab significantly improved OS versus chemotherapy (median 10.3 months; 95% CI, 8.5 to 16.1 v 6.8 months; 95% CI, 4.1 to 8.3; HR 0.54; 95% CI, 0.36 to 0.79; one-sided P = .0006; Fig 2, Data Supplement). Survival benefits with tislelizumab versus chemotherapy were also observed in patients with TAP < 10% (HR, 0.82; 95% CI, 0.62 to 1.09) and TAP unknown (HR, 0.67; 95% CI, 0.41 to 1.12; Fig 2, Data Supplement). The post hoc interaction analysis between treatment group and baseline PD-L1 expression status showed the P value was .21, indicating no significant interaction of treatment effect by PD-L1 status (P value ≥ .15).
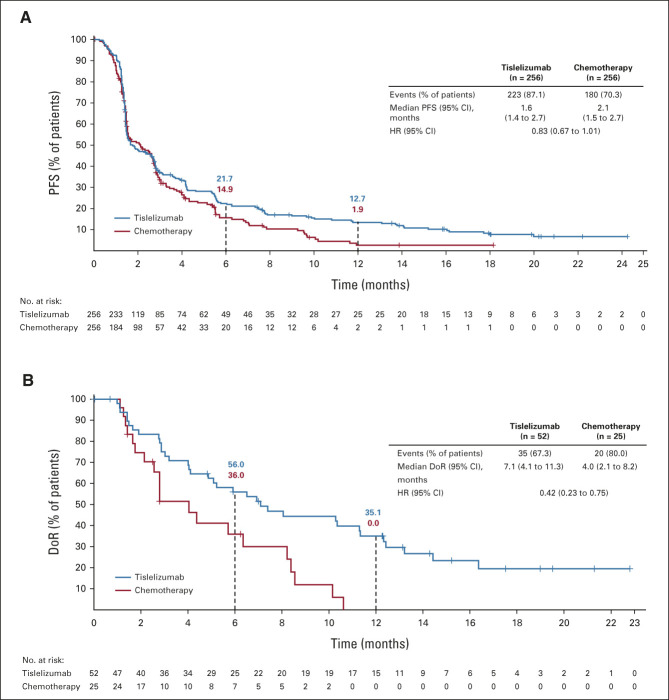
A total of 223 (87.1%) versus 180 (70.3%) patients had disease progression or died at data cutoff in the tislelizumab and chemotherapy arms, respectively. Median PFS was 1.6 months (95% CI, 1.4 to 2.7) versus 2.1 months (95% CI, 1.5 to 2.7) in the tislelizumab and chemotherapy arms, respectively (HR, 0.83; 95% CI, 0.67 to 1.01; Fig 4). The PFS Kaplan-Meier curves began to separate at approximately 3 months in favor of tislelizumab versus chemotherapy. The estimated PFS rates in the tislelizumab versus chemotherapy arms were 21.7% versus 14.9% at 6 months and 12.7% versus 1.9% at 12 months (Fig 4). PFS results in patients with TAP ≥ 10% are shown in the Data Supplement.
FIG 4.
Kaplan-Meier plot of (A) PFS and (B) DoR in the intent-to-treat population. HR was based on a Cox regression model including treatment as a covariate and Eastern Cooperative Oncology Group performance status and chemotherapy option as strata. DoR, duration of response; HR, hazard ratio; PFS, progression-free survival.
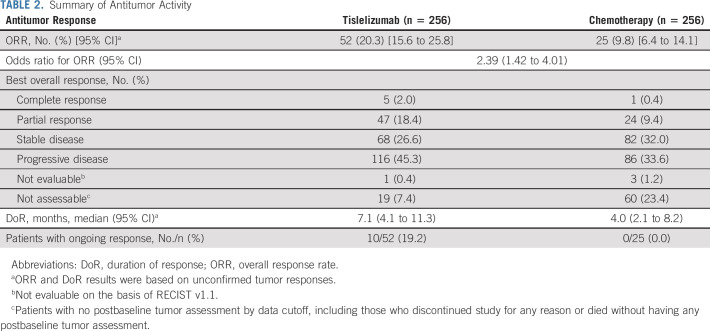
A total of 52 patients (20.3% [95% CI, 15.6 to 25.8]) in the tislelizumab arm versus 25 patients (9.8% [95% CI, 6.4 to 14.1]) in the chemotherapy arm achieved an objective response (Table 2). Moreover, 5 (2.0%) patients versus 1 (0.4%) patient had a complete response in the tislelizumab and chemotherapy arms, respectively. Median DoR was 7.1 months (95% CI, 4.1 to 11.3) in the tislelizumab arm versus 4.0 months (95% CI, 2.1 to 8.2) in the chemotherapy arm (Table 2, Fig 4). ORR also favored tislelizumab versus chemotherapy in patients with TAP ≥ 10% (Data Supplement).
TABLE 2.
Summary of Antitumor Activity
Safety and Tolerability
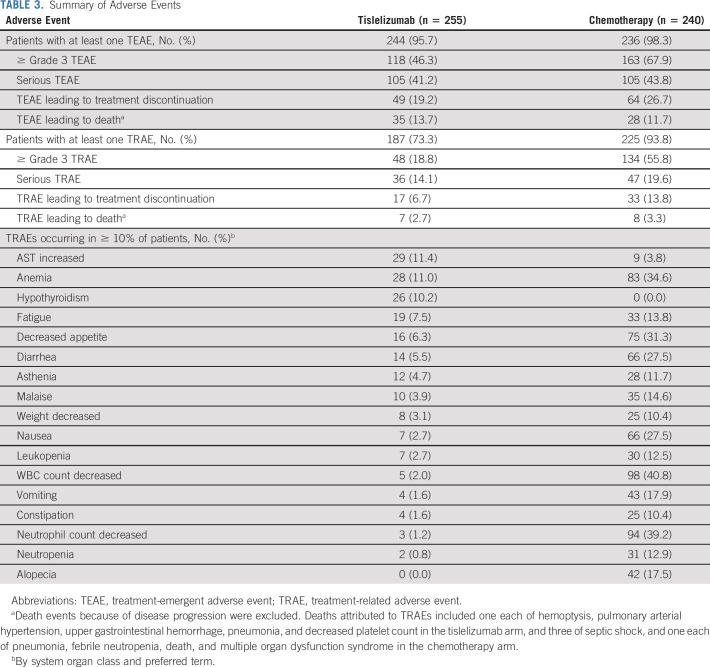
Fewer patients experienced treatment-related AEs (TRAEs) with tislelizumab versus chemotherapy (73.3% v 93.8%; Table 3). The most common TRAEs with tislelizumab were increased aspartate aminotransferase (11.4%), anemia (11.0%), and hypothyroidism (10.2%). The most common TRAEs with chemotherapy were decreased white blood cell count (40.8%), decreased neutrophil count (39.2%), and anemia (34.6%). Fewer patients had ≥ grade 3 TRAEs with tislelizumab versus chemotherapy (18.8% v 55.8%). The incidence of serious TRAEs was 14.1% versus 19.6% with tislelizumab versus chemotherapy, respectively. Fewer patients discontinued tislelizumab versus chemotherapy (6.7% v 13.8%) because of a TRAE (Table 3).
TABLE 3.
Summary of Adverse Events
In both treatment arms, the primary cause of death was disease progression, which occurred at a lower frequency with tislelizumab versus chemotherapy (59.8% v 66.0%). Deaths attributed to TRAEs were reported for 5 (2.0%) patients in the tislelizumab arm versus 7 (2.9%) patients in the chemotherapy arm.
DISCUSSION
The RATIONALE-302 phase III study was designed to detect the superiority of tislelizumab versus chemotherapy in improving survival in all randomly assigned patients and met this primary end point at final analysis. Treatment with tislelizumab showed a statistically significant and clinically meaningful improvement in OS versus chemotherapy in patients with advanced or metastatic ESCC whose disease progressed during or after first-line therapy. Survival benefit was observed across all prespecified subgroups, including region, race, and PD-L1 expression level.
RATIONALE-302 was, to our knowledge, the first study that demonstrated significant survival benefit with an anti–PD-1 antibody in a global ESCC population from Asia and Europe/North America. The OS improvement (30% reduction in the risk of death and 2.3-month extension in median OS) was similar to other studies investigating anti–PD-1 antibodies as second-line treatment for ESCC.10,12 In ATTRACTION-3, which mainly enrolled Asian patients (96%), OS was significantly improved with nivolumab versus chemotherapy (paclitaxel or docetaxel): 10.9 months versus 8.4 months, HR = 0.77, P = .019.10 In ESCORT, a study conducted in solely in China, significant improvement in OS was seen with camrelizumab versus chemotherapy (docetaxel or irinotecan): 8.3 months versus 6.2 months, HR = 0.71, P = .001.12 KEYNOTE-181 was a global study that enrolled patients with ESCC and esophageal adenocarcinoma. However, its favorable improvement in OS with pembrolizumab versus chemotherapy (paclitaxel, docetaxel, or irinotecan) in patients with ESCC (8.2 months v 7.1 months, HR = 0.78, P = .0095) failed to meet the prespecified boundary for statistical significance.11 Notably, the magnitude of OS benefit with tislelizumab in the RATIONALE-302 study was observed in the context of a much higher rate of subsequent anti–PD-1/PD-L1 treatment after discontinuing from study treatment in the chemotherapy arm (21.5%) versus the tislelizumab arm (4.3%), whereas in other studies, 6%-9% of patients in the chemotherapy arm received subsequent anti–PD-1/PD-L1 antibodies.10,12
RATIONALE-302 enrolled 79% of patients from Asia and 21% of patients from Europe/North America, which reflected the global distribution of patients with ESCC. The survival benefit of tislelizumab versus chemotherapy was observed in both Asian (HR, 0.73) and non-Asian patients (HR, 0.55). Although Asian patients with ESCC in KEYNOTE-181 (58% from Asia) appeared to have enhanced benefit with pembrolizumab versus chemotherapy, this was not observed with other anti–PD-1 antibodies, including tislelizumab in RATIONALE-302 and nivolumab in two other large global phase III studies investigating nivolumab versus placebo in the adjuvant treatment of EC (Checkmate-577) and nivolumab plus chemotherapy versus chemotherapy in first-line treatment of EC (Checkmate-648).18,19
RATIONALE-302 enrolled patients regardless of PD-L1 expression status. The TAP score (SP263) used to assess PD-L1 expression demonstrated comparable efficacy association in gastric cancer to CPS (22C3).20 According to the hierarchical testing, OS was significantly improved with tislelizumab over chemotherapy in patients with PD-L1 TAP ≥ 10% (HR, 0.54), and in the prespecified exploratory analysis, tislelizumab also showed a favorable trend of improvement in OS versus chemotherapy in patients with PD-L1 TAP < 10% (HR, 0.82). Although the OS benefit appeared to be enriched in patients with PD-L1 TAP ≥ 10% in RATIONALE-302, post hoc analysis of OS with the adjustment for PD-L1 expression status confirmed the OS benefit in all randomly assigned patients (HR, 0.70; 95% CI, 0.57 to 0.87), and post hoc interaction analysis between treatment and baseline PD-L1 expression status showed that OS benefit was observed regardless of PD-L1 expression level. Similarly, consistent survival benefit was also observed across patients with different PD-L1 expression levels with nivolumab and camrelizumab over chemotherapy in ATTRACTION-3 and ESCORT studies, which measured PD-L1 expression status using alternative assays.10,12
Consistent with OS findings, tislelizumab showed a greater and more durable antitumor response than chemotherapy. ORR was twice as high with tislelizumab versus chemotherapy. Median DoR was 3.1 months longer with tislelizumab versus chemotherapy, with more responders exhibiting ongoing responses at data cutoff. Although median PFS was shorter with tislelizumab versus chemotherapy, the numerically favorable HR of PFS and increasing separation of the Kaplan-Meier curves after 3 months suggested a potential benefit in PFS. Similar separation of Kaplan-Meier curves of PFS in favor of anti–PD-1 antibodies was also observed in other studies conducted in this setting.11,12 One hypothesis postulated for this observation is the longer time to the onset of antitumor effect seen with immunotherapies versus cytotoxic drugs.21
Despite longer drug exposure with tislelizumab, fewer TRAEs were observed versus chemotherapy. The incidence of ≥ grade 3 TRAEs, serious TRAEs, and TRAEs leading to treatment discontinuation was also lower in the tislelizumab arm versus the chemotherapy arm. Overall, the safety profile of tislelizumab was favorable over chemotherapy.
Limitations of this study include the open-label study design, which may have affected compliance, and a lack of blinded review of response data by an independent committee, which may have affected response data (ORR, PFS, and DoR). In addition, future studies are needed to explore the analytical concordance of the assays and the predictiveness of PD-L1 TAP expression in ESCC.
In conclusion, tislelizumab provided a statistically significant and clinically meaningful improvement in OS versus chemotherapy in patients with advanced or metastatic ESCC who had disease progression after first-line systemic therapy, with a tolerable safety profile. Patients with PD-L1 TAP ≥ 10% also demonstrated statistically significant survival benefit with tislelizumab versus chemotherapy.
ACKNOWLEDGMENT
The authors would like to thank the participants of the study and all the study staff for their contributions to the study. A full list of investigators is provided in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
List of RATIONALE-302 Investigators
Lin Shen
Consulting or Advisory Role: MSD, Bristol-Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji Biopharmaceutical, Harbour BioMed, Merck
Speakers' Bureau: Hutchison Whampoa, MSD
Research Funding: Nanjing Yaojieankang Biotechnology (Inst), Baiji Shenzhou (Beijing) Biotechnology Co Ltd (Inst), Beijing Xiantong Biomedical Technology Co Ltd (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst)
Ken Kato
Honoraria: Lilly, BMS, Ono Pharmaceutical
Consulting or Advisory Role: Ono Pharmaceutical, BeiGene, MSD, Oncolys BioPharma, Bayer
Speakers' Bureau: Ono Pharmaceutical, Bristol Myers Squibb Japan, MSD
Research Funding: Ono Pharmaceutical (Inst), Shionogi (Inst), MSD Oncology (Inst), Beigene (Inst), Chugai Pharma (Inst), Bayer (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst)
Sung-Bae Kim
Stock and Other Ownership Interests: Neogene TC Corp, Genopeak
Honoraria: DAEHWA Pharmaceutical, ISU Abxis
Consulting or Advisory Role: Lilly (Inst), AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis, BeiGene, Daiichi Sankyo/AstraZeneca
Research Funding: Novartis (Inst), Dongkook Pharma (Inst), Genzyme (Inst)
Jaffer A. Ajani
Honoraria: Lilly, Bristol Myers Squibb, Merck, Aduro Biotech, DAVA Pharmaceuticals, AstraZeneca, Acrotech Biopharma, Zymeworks, Astellas Pharma, Amgen, OncoTherics, Daiichi Sankyo, Novartis, Servier, Gilead Sciences, BeiGene, Fresenius Kabi, Boehringer Ingelheim, GRAIL
Consulting or Advisory Role: American Cancer Society, BeiGene, Vaccinogen, Insys Therapeutics, Merck, Bristol Myers Squibb
Research Funding: Novartis, Bristol Myers Squibb, Taiho Pharmaceutical, Roche/Genentech, Amgen, Lilly/ImClone, Merck, Delta-Fly Pharma, Gilead Sciences, Takeda, ProLynx, Zymeworks, Daiichi Sankyo, Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I have research funding from Genentech, Roche, BMS, Taiho, MedImmune, Merck, Amgen, and Lilly
Xinmin Yu
Research Funding: BeiGene, Innovent Biologics, BMS, MSD, Hansoh
Jianhua Chen
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma
Hiroki Hara
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Merck Serono, Yakult Honsha, Lilly, Ono Pharmaceutical, Takeda, Bristol Myers Squibb, Sanofi, MSD, Daiichi Sankyo, Kyowa Hakko Kirin, Bayer, Asahi Kasei
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Boehringer Ingelheim, Dainippon Sumitomo, Bristol Myers Squibb Japan, Daiichi Sankyo/UCB Japan
Research Funding: AstraZeneca (Inst), Merck Serono (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Dainippon Sumitomo Pharma (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Incyte (Inst), BeiGene (Inst), Astellas Pharma (Inst), Bayer (Inst), Amgen (Inst), Chugai Pharma (Inst), Janssen Oncology (Inst)
Taroh Satoh
Honoraria: Chugai Pharma, Merck Serono, Bristol Myers Squibb, Takeda, Yakult Honsha, Lilly, Bayer Yakuhin, Ono Pharmaceutical, Merck, Astellas Pharma, Taiho Pharmaceutical, Nihonkayaku, Daiihi-Sankyo
Consulting or Advisory Role: Bayer Yakuhin, Lilly, Ono Pharmaceutical, Takara Bio, Merck Serono, Nihonkayaku
Research Funding: Yakult Honsha (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Sanofi (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Merck (Inst), Merck Serono (Inst), Gilead Sciences (Inst), Dainippon Sumitomo Pharma (Inst), IQVIA (Inst)
Roberto Pazo-Cid
Consulting or Advisory Role: Baxalta/Shire, Celgene, Lilly, Roche, Bristol Myers Squibb/Celgene, Servier
Travel, Accommodations, Expenses: Celgene, Lilly
Hendrik-Tobias Arkenau
Employment: Hospital Corporation of America
Honoraria: Roche, Guardant Health, Bicycle Therapeutics, Servier, Merck KGaA, BeiGene, Bayer
Consulting or Advisory Role: iOnctura, Engitix
Research Funding: Sarah Cannon Research Institute
Christophe Borg
Consulting or Advisory Role: Roche/Genentech, MSD Oncology, Bayer, Pierre Fabre
Research Funding: Roche/Genentech (Inst)
Florian Lordick
Honoraria: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Elsevier, BioNTech, Servier, Merck KGaA, Roche, Medscape, Incyte, Art Tempi, Medupdate, Streamedup!
Consulting or Advisory Role: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, Astellas Pharma, Servier, Zymeworks, Amgen, Daichi Sankyo, Novartis, Beigene
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly
Liyun Li
Employment: BeiGene, Johnson & Johnson/Janssen
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: BeiGene, Johnson & Johnson/Janssen
Ningning Ding
Employment: BeiGene
Jingwen Shi
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Roche, Servier, Bristol Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Halozyme, Array BioPharma, Biocartis, GlaxoSmithKline, Daiichi Sankyo, Pierre Fabre, Sirtex Medical, Taiho Pharmaceutical, Incyte, Astellas Pharma
Research Funding: Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), Bristol Myers Squibb (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the ASCO annual meeting, virtual, June 4-8, 2021; the ESMO World Gastrointestinal Congress, virtual, June 30-July 3, 2021; and in part at the Chinese Society of Clinical Oncology, Beijing, China, September 15-19, 2021.
SUPPORT
Sponsored by BeiGene, Ltd. Medical writing support, under the direction of the authors, was provided by Yasmin Issop, PhD, of Ashfield MedComms, an Ashfield Health company, and was funded by BeiGene, Ltd.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
On request, and subject to certain criteria, conditions, and exceptions, BeiGene, Ltd, will provide access to individual deidentified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. BeiGene will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data requests may be submitted to DataDisclosure@beigene.com.
AUTHOR CONTRIBUTIONS
Conception and design: Lin Shen, Ken Kato, Sung-Bae Kim, Jaffer A. Ajani, Kuaile Zhao, Zhiyong He, Qi Luo, Jufeng Wang, Zhendong Chen, Zuoxing Niu, Longzhen Zhang, Tienan Yi, Jianhua Chen, Guohua Yu, Chen-Yuan Lin, Qing Bi, Hendrick-Tobias Arkenau, Liyun Li, Ningning Ding, Aiyang Tao, Eric Van Cutsem
Administrative support: Yongqian Shu, Roberto Pazo-Cid
Provision of study materials or patients: Yongqian Shu, Taroh Satoh, Roberto Pazo-Cid, Christophe Borg, Florian Lordick, Eric Van Cutsem
Collection and assembly of data: Lin Shen, Ken Kato, Sung-Bae Kim, Kuaile Zhao, Zhiyong He, Xinmin Yu, Yongqian Shu, Jufeng Wang, Zhendong Chen, Zuoxing Niu, Longzhen Zhang, Tienan Yi, Jong-Mu Sun, Jianhua Chen, Guohua Yu, Chen-Yuan Lin, Hiroki Hara, Qing Bi, Taroh Satoh, Roberto Pazo-Cid, Hendrick-Tobias Arkenau, Christophe Borg, Florian Lordick, Liyun Li, Ningning Ding, Aiyang Tao, Eric Van Cutsem
Data analysis and interpretation: Lin Shen, Ken Kato, Sung-Bae Kim, Jaffer A. Ajani, Chen-Yuan Lin, Taroh Satoh, Roberto Pazo-Cid, Hendrick-Tobias Arkenau, Florian Lordick, Liyun Li, Ningning Ding, Aiyang Tao, Jingwen Shi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tislelizumab Versus Chemotherapy as Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma (RATIONALE-302): A Randomized Phase III Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lin Shen
Consulting or Advisory Role: MSD, Bristol-Myers Squib, AstraZeneca, Daiichi Sankyo, Roche, Mingji Biopharmaceutical, Harbour BioMed, Merck
Speakers' Bureau: Hutchison Whampoa, MSD
Research Funding: Nanjing Yaojieankang Biotechnology (Inst), Baiji Shenzhou (Beijing) Biotechnology Co Ltd (Inst), Beijing Xiantong Biomedical Technology Co Ltd (Inst), QiLu Pharmaceutical (Inst), Zaiding Pharmaceutical (Inst)
Ken Kato
Honoraria: Lilly, BMS, Ono Pharmaceutical
Consulting or Advisory Role: Ono Pharmaceutical, BeiGene, MSD, Oncolys BioPharma, Bayer
Speakers' Bureau: Ono Pharmaceutical, Bristol Myers Squibb Japan, MSD
Research Funding: Ono Pharmaceutical (Inst), Shionogi (Inst), MSD Oncology (Inst), Beigene (Inst), Chugai Pharma (Inst), Bayer (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst)
Sung-Bae Kim
Stock and Other Ownership Interests: Neogene TC Corp, Genopeak
Honoraria: DAEHWA Pharmaceutical, ISU Abxis
Consulting or Advisory Role: Lilly (Inst), AstraZeneca, DAEHWA Pharmaceutical, ISU Abxis, BeiGene, Daiichi Sankyo/AstraZeneca
Research Funding: Novartis (Inst), Dongkook Pharma (Inst), Genzyme (Inst)
Jaffer A. Ajani
Honoraria: Lilly, Bristol Myers Squibb, Merck, Aduro Biotech, DAVA Pharmaceuticals, AstraZeneca, Acrotech Biopharma, Zymeworks, Astellas Pharma, Amgen, OncoTherics, Daiichi Sankyo, Novartis, Servier, Gilead Sciences, BeiGene, Fresenius Kabi, Boehringer Ingelheim, GRAIL
Consulting or Advisory Role: American Cancer Society, BeiGene, Vaccinogen, Insys Therapeutics, Merck, Bristol Myers Squibb
Research Funding: Novartis, Bristol Myers Squibb, Taiho Pharmaceutical, Roche/Genentech, Amgen, Lilly/ImClone, Merck, Delta-Fly Pharma, Gilead Sciences, Takeda, ProLynx, Zymeworks, Daiichi Sankyo, Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I have research funding from Genentech, Roche, BMS, Taiho, MedImmune, Merck, Amgen, and Lilly
Xinmin Yu
Research Funding: BeiGene, Innovent Biologics, BMS, MSD, Hansoh
Jianhua Chen
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma
Hiroki Hara
Honoraria: Chugai Pharma, Taiho Pharmaceutical, Merck Serono, Yakult Honsha, Lilly, Ono Pharmaceutical, Takeda, Bristol Myers Squibb, Sanofi, MSD, Daiichi Sankyo, Kyowa Hakko Kirin, Bayer, Asahi Kasei
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Boehringer Ingelheim, Dainippon Sumitomo, Bristol Myers Squibb Japan, Daiichi Sankyo/UCB Japan
Research Funding: AstraZeneca (Inst), Merck Serono (Inst), MSD (Inst), Ono Pharmaceutical (Inst), Taiho Pharmaceutical (Inst), Boehringer Ingelheim (Inst), Dainippon Sumitomo Pharma (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Incyte (Inst), BeiGene (Inst), Astellas Pharma (Inst), Bayer (Inst), Amgen (Inst), Chugai Pharma (Inst), Janssen Oncology (Inst)
Taroh Satoh
Honoraria: Chugai Pharma, Merck Serono, Bristol Myers Squibb, Takeda, Yakult Honsha, Lilly, Bayer Yakuhin, Ono Pharmaceutical, Merck, Astellas Pharma, Taiho Pharmaceutical, Nihonkayaku, Daiihi-Sankyo
Consulting or Advisory Role: Bayer Yakuhin, Lilly, Ono Pharmaceutical, Takara Bio, Merck Serono, Nihonkayaku
Research Funding: Yakult Honsha (Inst), Chugai Pharma (Inst), Ono Pharmaceutical (Inst), Sanofi (Inst), Lilly (Inst), Daiichi Sankyo (Inst), Merck (Inst), Merck Serono (Inst), Gilead Sciences (Inst), Dainippon Sumitomo Pharma (Inst), IQVIA (Inst)
Roberto Pazo-Cid
Consulting or Advisory Role: Baxalta/Shire, Celgene, Lilly, Roche, Bristol Myers Squibb/Celgene, Servier
Travel, Accommodations, Expenses: Celgene, Lilly
Hendrik-Tobias Arkenau
Employment: Hospital Corporation of America
Honoraria: Roche, Guardant Health, Bicycle Therapeutics, Servier, Merck KGaA, BeiGene, Bayer
Consulting or Advisory Role: iOnctura, Engitix
Research Funding: Sarah Cannon Research Institute
Christophe Borg
Consulting or Advisory Role: Roche/Genentech, MSD Oncology, Bayer, Pierre Fabre
Research Funding: Roche/Genentech (Inst)
Florian Lordick
Honoraria: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Elsevier, BioNTech, Servier, Merck KGaA, Roche, Medscape, Incyte, Art Tempi, Medupdate, Streamedup!
Consulting or Advisory Role: Lilly, Merck Sharp & Dohme, Bristol Myers Squibb, Astellas Pharma, Servier, Zymeworks, Amgen, Daichi Sankyo, Novartis, Beigene
Research Funding: Bristol Myers Squibb (Inst), MSD (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Lilly
Liyun Li
Employment: BeiGene, Johnson & Johnson/Janssen
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: BeiGene, Johnson & Johnson/Janssen
Ningning Ding
Employment: BeiGene
Jingwen Shi
Employment: BeiGene
Stock and Other Ownership Interests: BeiGene
Eric Van Cutsem
Consulting or Advisory Role: Bayer, Lilly, Roche, Servier, Bristol Myers Squibb, Celgene, Merck Sharp & Dohme, Merck KGaA, Novartis, AstraZeneca, Halozyme, Array BioPharma, Biocartis, GlaxoSmithKline, Daiichi Sankyo, Pierre Fabre, Sirtex Medical, Taiho Pharmaceutical, Incyte, Astellas Pharma
Research Funding: Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Ipsen (Inst), Merck (Inst), Merck KGaA (Inst), Servier (Inst), Bristol Myers Squibb (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Wang QL, Xie SH, Wahlin K, et al. : Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol 10:717-728, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang FL, Yu SJ: Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 41:210-215, 2018 [DOI] [PubMed] [Google Scholar]
- 4.SEER: Cancer Stat Facts: Esophageal Cancer . 2021. https://seer.cancer.gov/statfacts/html/esoph.html [Google Scholar]
- 5.Shah MA, Kennedy EB, Catenacci DV, et al. : Treatment of locally advanced esophageal carcinoma: ASCO Guideline. J Clin Oncol 38:2677-2694, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Lordick F, Mariette C, Haustermans K, et al. : Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27:v50-v57, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Muro K, Lordick F, Tsushima T, et al. : Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic oesophageal cancer: A JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol 30:34-43, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Assersohn L, Brown G, Cunningham D, et al. : Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol 15:64-69, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Ford HE, Marshall A, Bridgewater JA, et al. : Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): An open-label, phase 3 randomised controlled trial. Lancet Oncol 15:78-86, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Kato K, Cho BC, Takahashi M, et al. : Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:1506-1517, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Kojima T, Shah MA, Muro K, et al. : Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol 38:4138-4148, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Xu J, Chen Y, et al. : Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol 21:832-842, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Lu Z, Peng Z, Liu C, et al. : Current status and future perspective of immunotherapy in gastrointestinal cancers. Innovation (N Y) 1:100041, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T, Song X, Xu L, et al. : The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother 67:1079-1090, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai J, Deva S, Lee JS, et al. : Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors. J Immunother Cancer 8:e000453, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Bai Y, Xu N, et al. : Tislelizumab plus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma and gastric/gastroesophageal junction adenocarcinoma. Clin Cancer Res 26:4542-4550, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Guo J, Zhang Q, et al. : Tislelizumab in Chinese patients with advanced solid tumors: An open-label, non-comparative, phase 1/2 study. J Immunother Cancer 8:e000437, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chau I, Doki Y, Ajani JA, et al. : Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol 39, 2021. (abstr LBA4001) [Google Scholar]
- 19.Kelly RJ, Ajani JA, Kuzdzal J, et al. : Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 384:1191-1203, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Chao Y, Yang S, Zhang Y, et al. : 154P Investigation of PD-L1 expression and tislelizumab efficacy in gastroesophageal adenocarcinoma using a novel tumor and immune cell score with VENTANA PD-L1 (SP263) assay and Combined Positive Score (CPS). Ann Oncol 31:S300, 2020 [Google Scholar]
- 21.Boland P, Pavlick AC, Weber J, et al. : Immunotherapy to treat malignancy in patients with pre-existing autoimmunity. J Immunother Cancer 8:e000356, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]