PURPOSE
Cisplatin is the main systemic treatment modality for male type II germ cell tumors (GCTs). Although generally very effective, 5%-10% of patients suffer from cisplatin-resistant disease. Identification of the driving mechanisms of resistance will enable improved risk stratification and development of alternative treatments.
METHODS
We developed and characterized cisplatin-resistant GCT cell line models and compared their molecular characteristics with patient samples with cisplatin resistance and/or a poor clinical outcome. Subsequently, the association between the overlapping genetic features and clinical data was assessed. Finally, we used Cox regression to determine the prognostic relevance of these features within the currently used risk classification.
RESULTS
Gain of chromosome 3p25.3 was detected in all cisplatin-resistant cell lines, and copy number of this region correlated with the level of resistance (R = 0.96, P = 1.5e-04). Gain of this region was detected at low frequencies in primary tumors and at higher frequencies in relapsed and/or cisplatin-resistant tumors. Chromosome 3p25.3 gain was associated with shorter progression-free survival and overall survival, with the strongest association observed in nonseminomas excluding pure teratomas. 3p25.3 gain was more frequently observed in tumors with yolk sac tumor histology and predicted adverse outcome independent of the International Germ Cell Cancer Collaborative Group risk classification and the presence of TP53/MDM2 alterations.
CONCLUSION
On the basis of both in vitro analyses and clinical data, we found 3p25.3 to be strongly associated with cisplatin resistance and poor clinical outcome in male type II GCTs. Using genomic profiling, 3p25.3 status could help to improve risk stratification in male patients with type II GCT. Further characterization of this locus and underlying mechanisms of resistance is warranted to guide development of novel treatment approaches for cisplatin-resistant disease.
INTRODUCTION
Germ cell tumors (GCTs) comprise a heterogeneous group of neoplasms derived from the germ cell lineage, with multiple subtypes mirroring different cells of origin. The most common subtype are the malignant GCTs of the adult testis (type II), which are the most frequent solid malignancy in men until the age of 34 years.1 Despite an increasing incidence across the globe, mortality rates have decreased remarkably since the introduction of cisplatin-based combination chemotherapies,2 leading to current 5-year survival rates exceeding 90%.3 Despite its association with significant long-term side effects, cisplatin yet remains the most effective cytotoxic drug in GCTs and is therefore considered the cornerstone of standard chemotherapy regimens used in the clinic.4,5 However, resistance to cisplatin emerges in a small but clinically meaningful number of patients and, apart from high-dose chemotherapy, no alternative treatment options are available.6
CONTEXT
Key Objective
Cisplatin is highly effective in treatment of type II male germ cell tumors (GCTs); however, resistance occurs in 5%-10% of patients. Understanding of this clinically relevant observation has been hampered by scarcity of suitable material and tools for evaluation. We aim to identify biomarkers of cisplatin resistance through a unique integrated analysis of experimental data and publicly available GCT patient data sets.
Knowledge Generated
Copy number gain of chromosome 3 cytoband p25.3 was identified in laboratory cell line models as a possible driver of cisplatin resistance. In multiple GCT cohorts, it was associated with cisplatin resistance, yolk sac tumor histology, and significantly poorer progression-free survival and overall survival. Finally, 3p25.3 gain was demonstrated to be a predictor of poor outcome, independent of the International Germ Cell Cancer Collaborative Group model currently used for GCT risk classification.
Relevance
Incorporating chromosome 3p25.3 copy number status in GCT risk classification can help to identify patients who will respond poorly to cisplatin-based therapy.
Cisplatin resistance is known to be associated with histological composition in male type II GCTs. Tumors can consist of seminoma and nonseminoma histologies, with nonseminomas being further subdivided into embryonal carcinoma, yolk sac tumor (YST), choriocarcinoma, and teratoma.7,8 Seminomas are less likely to develop cisplatin resistance than nonseminomas,9 whereas teratomas are inherently cisplatin-resistant because of their benign nature.10 Another determinant of cisplatin resistance is anatomic localization of the tumor, with mediastinal tumors showing resistance more frequently.
Interestingly, these tumors show frequent TP53 mutations, implicating this pathway in cisplatin resistance in GCTs.11 It is currently impossible to reliably predict which tumors will respond poorly to cisplatin. The International Germ Cell Cancer Collaborative Group (IGCCCG) classification is a risk staging system that takes into account levels of marker proteins, histology, and location, and classifies patients in good, intermediate, and poor prognosis.12 Although this classification is the reference for assessing expected outcome, there is still considerable heterogeneity in response to treatment, even within the patients belonging to the poor prognosis subgroup. Consequently, a deeper understanding of cisplatin-resistance mechanisms of male type II GCTs may affect up-front patient stratification and would also help development of alternative targeted therapies in this challenging clinical setting.
METHODS
Patient Inclusion
Use of patient samples was based on written informed consent and approved for research by the Medical Ethical Committee of the Erasmus Medical Center (the Netherlands), Permit No. 02.981. Samples were used according to the Code for Proper Secondary Use of Human Tissue in the Netherlands developed by the Dutch Federation of Medical Scientific Societies (FMWV, version, 2002; update 2011).
Methylation and Copy Number Alteration Profiling
Analyses were performed as previously described.13,14 In brief, copy number analyses were performed using the conumee package (Hovestadt V, Zapatka M. conumee: Enhanced copy number variation analysis using Illumina DNA methylation arrays. R package version 1.9.0).15 Data were generated for bins with at least 25 probes. Other settings were default. The reference set was composed of 64 normal male samples from an in-house set (n = 14) and from the German Cancer Research Center (n = 50, DKFZ, Heidelberg, obtained from Dr Martin Sill).
Public Data Sets
Processed data from the TGCA, MSKCC-2016, and MSKCC-2017 cohorts11,16,17 were downloaded from cBioportal (February 2021). Copy number data from the MSKCC-2008 cohort18 were downloaded from NCBI GEO (GSE8614) and subjected to segmentation using the DNA copy R-package (version 1.64.0) using standard settings. MSKCC-2008 contains more than one tumor per patient for some patients. If either of these contains a 3p25.3 gain, the respective patient was classified as positive.
Data Analysis
Tumors were scored as positive for 3p25.3 gain if any segment that falls within this region (chr3:870000-11800000, HG19) had a higher log2ratio CN than 0.1 (0.2 for TCGA). Using these cutoffs, approximately 90% of tumors showed gain of chromosome 12p in every data set, in line with previously reported frequencies of isochromosome 12 presence in type II GCTs.19,20 Relationship between categorical variables was determined using Fisher's exact test or chi-square test while the relationship between numerical variables was determined using the Pearson correlation coefficient. Cox regression was used to determine multivariable relationships with progression-free survival (PFS) and overall survival (OS). Analysis and visualization were performed in R (v4.0.2), and the R2 bioinformatics analysis platform (R2.amc.nl) was used for visualization.
RESULTS
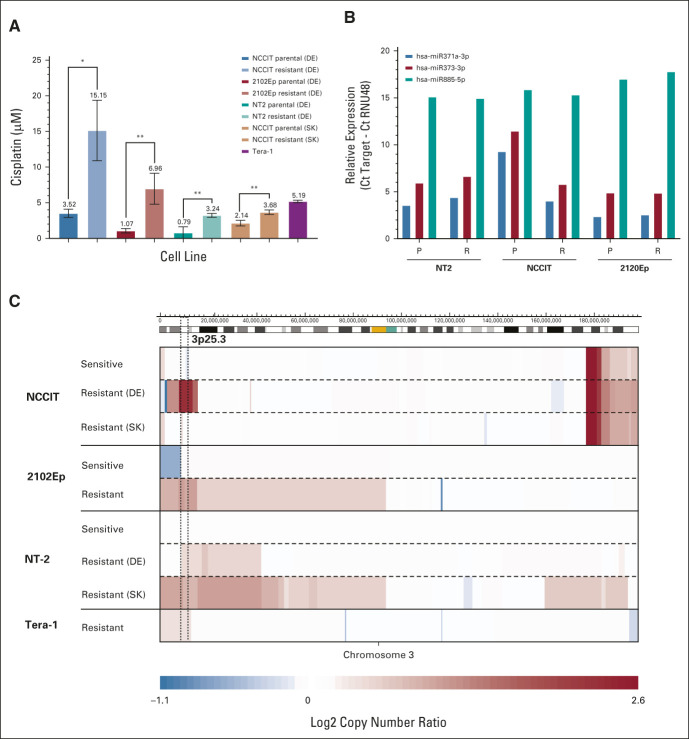
This study includes a unique set of matched sensitive (ie, parental) and cisplatin-resistant male type II GCT nonseminoma cell lines generated in two laboratories independently (Data Supplement, online only). Cisplatin concentrations at which cell growth was inhibited by 50% (IC50s) were significantly higher in the resistant subclones of NCCIT, 2102Ep, and NT2 than in their parental counterparts (Fig 1A). No chemotherapy-naive subclone was available for Tera-1; however, its IC50 is higher than all sensitive subclones and in the same order of magnitude as found in the resistant cell lines.
FIG 1.
Characterization of cisplatin-resistant cell lines indicates 3p25.3 gain as a potential driver of cisplatin resistance in GCTs. (A) IC50s of cisplatin-sensitive lines and resistant counterparts. Bars represent the means and standard deviations of triplicate experiments. *P ≤ .05, **P ≤ .01. (B) Expression of selected microRNAs in cell lines as determined by using RT-qPCR. Bars represent expression relative to the RNU48 snoRNA. (C) Copy number plots of selected cell lines on chromosome 3. Copy numbers were determined using WGS. Blue indicates loss and red indicates gain of the respective region, with color intensity reflecting the extent which is indicated in the scale bar below. The 3p25.3 region is indicated with dashed lines. DE, Germany; GCT, germ cell tumor; IC50, half maximal inhibitory concentration; P, parental; R, resistant; RT-qPCR, quantitative reverse transcription PCR; SK, Slovakia; WGS, whole-genome sequencing.
To determine the mechanism behind the observed resistance, parental and resistant cell lines were subjected to complete molecular characterization. Cisplatin resistance has been shown to be associated with differentiation in GCTs21; however, no changes were identified in microRNAs 371-3p, 373-3p, and 885-5p, which are associated with the differentiation status in GCTs22 (Fig 1B). Concordantly, no major consistent changes in RNA expression and methylation status were identified in the resistant lines (Data Supplement), which would be expected if resistant lines underwent differentiation. Overall, this demonstrated that the observed acquired resistance was not driven by differentiation, which has been identified as a major mechanism of intrinsic treatment resistance in teratomas. Moreover, no clearly enriched processes were identified in the differentially expressed/methylated genes (data not shown).
Analysis of DNA copy number variation by whole-genome sequencing identified a recurrent copy number gain involving chromosome 3p, cytoband 25.3 in all the resistant lines compared with their parental counterparts (Fig 1C), suggesting that this aberration could be associated with cisplatin resistance in male type II GCTs. Copy number of this region showed a strong correlation with the IC50 in the corresponding cell lines, suggesting that there is a dose dependent effect (Data Supplement).
The two resistant clones for NCCIT and NT2 that were independently generated showed different breakpoints for the 3p25.3 region, precluding the selection of an existing subclone, therefore indicating a de novo event likely promoted through repeated cisplatin exposure (Data Supplement).
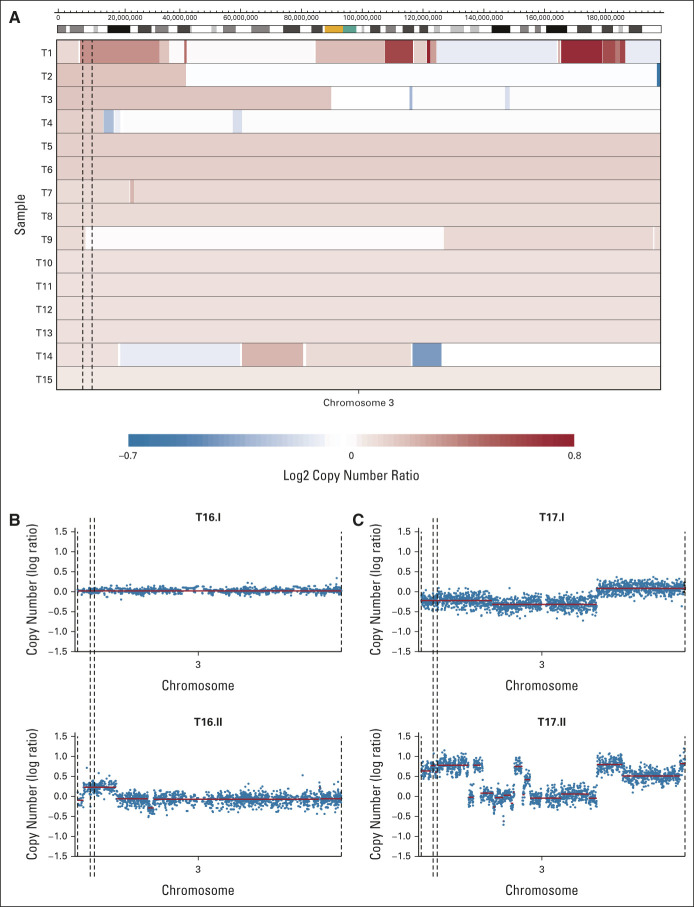
To assess whether copy number gain of chromosome 3p25.3 also occurred in GCT tissue samples, unselected primary type II male GCTs were screened for the presence of this aberration. Copy number gain on chromosome 3p25.3 (defined as a log2 copy number ratio > 0.1) was identified in 15 of 221 tumors (6.8%), indicating that it is a rare event in primary GCTs. Both segmental gains and whole-chromosome gains were observed (Fig 2A). In metastasized and/or cisplatin-resistant GCTs, the frequency of 3p25.3 gain was higher (29%, 2 of 7), although the number of cases was limited. In addition, 3p25.3 gain was present in several relapses/metastases, although it was not detectable or present at lower copy number in the matched primary tumor (Figs 2B and 2C). This suggests that 3p25.3 gain either might occur as a de novo event, as we observed in our resistant cell lines, or could already be present at diagnosis, possibly at lower frequencies, providing a selective advantage during treatment and progression. To determine whether the observed frequencies are representative, several publicly available male type II GCT cohorts were analyzed (Data Supplement). The cohort from the TCGA contains untreated primary male type II GCTs and yields a somewhat higher frequency of 3p25.3 gain than our cohort (13 of 133, 9.7%). The tumors in the MSKCC-2016 cohort are classified as cisplatin-sensitive or cisplatin-resistant on the basis of their treatment response. A frequency of 17.1% (13 of 76) was found in the chemotherapy-sensitive tumors and 33.7% (35 of 104) in the chemotherapy-resistant tumors (Fig 3A, P = .02 as determined by using Fisher's exact test). In the MSKCC-2008 cohort, which contains primary GCTs, treated samples, and metastases, 3p25.3 gain was identified in 12.2% of tumors (9 of 74). In summary, 3p25.3 gain is relatively rare in primary, untreated GCTs while this frequency increases in metastasized and/or cisplatin-resistant tumors.
FIG 2.
3p25.3 gain is rare in primary tumors and more frequent in resistant and/or metastasized tumors. (A) Copy number plots of all tumors in our cohort that show 3p25.3 gain. Copy numbers were determined using methylation profiling. Blue indicates loss and red indicates gain of the respective region, with color intensity reflecting the extent which is indicated in the scale bar below. The 3p25.3 region is indicated with dashed lines. (B) Copy number plots of a primary metastasis pair that has a 3p25.3 gain in the metastasis (lower) and not in the primary tumor (upper). Blue dots represent bins and red lines represent copy number segments. The 3p25.3 region is indicated with dashed lines. (C) Copy number plots of a pre-post treatment tumor pair that has a 3p25.3 gain in the pretreatment tumor (upper), which shows an increase in copy number in the post-treatment tumor (lower). Blue dots represent bins and red lines represent copy number segments. The 3p25.3 region is indicated with dashed lines.
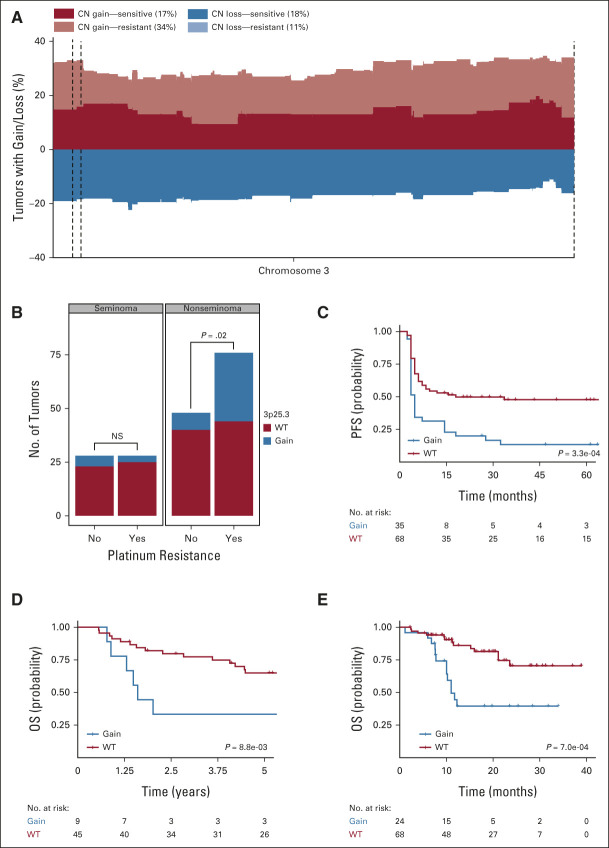
FIG 3.
3p25.3 gain is more frequent in cisplatin-resistant tumors and is associated with poor prognosis, especially in nonseminoma tumors. (A) Frequency plot of gain and loss on chromosome 3 in the MSKCC-2016 cohort. Gain/loss in the cisplatin-sensitive tumors is plotted in deep red/blue while gain/loss in the cisplatin-resistant tumors is superimposed in a lighter color. The 3p25.3 region is indicated by dashed lines. Frequencies at which 3p25.3 gain/loss is identified in the sensitive/resistant tumors are shown in the legend within parentheses. Note that the light blue bars are not visible in the figure since the cisplatin-resistant tumors consistently show lower frequencies of chromosome 3 loss than sensitive tumors. (B) Bar graph showing the number of tumors with 3p25.3 gain in cisplatin-sensitive and cisplatin-resistant tumors in seminomas and nonseminomas, respectively. Lines show the significance of the ratio 3p gained versus nongained in the sensitive and resistant tumors in each subtype as determined by using Fisher's exact test. (C) Kaplan-Meier plot of PFS in nonseminoma tumors in the MSKCC-2016 cohort on the basis of the presence of 3p25.3 gain. The P value was generated using the log-rank test. (D) Kaplan-Meier plot of OS in nonseminomas in the MSKCC-2008 cohort on the basis of the presence of 3p25.3 gain. The P value was generated using the log-rank test. (E) Kaplan-Meier plot of OS in nonseminomas in the MSKCC-2017 cohort on the basis of the presence of 3p25.3 gain. The P value was generated using the log-rank test. CN, copy number; OS, overall survival; PFS, progression-free survival; WT, wild-type.
In line with a proposed role for 3p25.3 gain in cisplatin resistance, patients with a 3p copy number gain have a significantly poorer prognosis than patients without this aberration, as determined in the MSKCC-2016 patient series (Data Supplement). Seminomas generally respond better to chemotherapy and are less likely to develop resistance than nonseminomas9 and, therefore, the occurrence of 3p25.3 gain was analyzed separately in both histologic subgroups. Gain of 3p25.3 was less frequent in seminomas, where it was observed in similar frequencies in resistant and nonresistant tumors, whereas in nonseminomas, it was significantly more frequent in resistant tumors (Fig 3B, P = .003, Fisher's exact test). This suggests that 3p25.3 gain contributes to cisplatin resistance only in nonseminomas. Concordantly, in nonseminomas, there was a highly significant correlation between gain of 3p25.3 and shorter PFS (Fig 3C) while no such association was found in seminomas (Data Supplement). In the MSKCC-2008 cohort, which consists of only nonseminomas, there is a strong association between 3p25.3 gain and shorter OS (Fig 3D). Teratomas are known to be intrinsically cisplatin-resistant, and that is why they were excluded from the MSKCC-2016 cohort. If excluded from the MSKCC-2008 cohort, the relationship between 3p25.3 gain and shorter OS became even more apparent (Data Supplement). Finally, a GCT data set from the pan-cancer MSK-impact series17 was assembled, focusing only on male tumors. This set has a large overlap with the MSKCC-2016 data set; however, for this set, the OS instead of PFS was reported for 124 tumors. In this set (MSKCC-2017), there was also a significant association between 3p25.3 gain and shorter OS, specifically in nonseminomas (Fig 3E, Data Supplement). The GCTs that were previously reported in the MSKCC-2016 showed a similar effect on OS as the other tumors from the GCT MSKCC-2017 cohort, excluding the possibility that the observed OS effect is solely driven by the observed PFS effect shown in Figure 3C (Data Supplement). This reinforces the presumed role of 3p25.3 gain in cisplatin resistance and the poor prognosis that is associated with it, especially in nonseminomas.
Correlating clinical data with gain of 3p25.3 in nonseminomas revealed a strong association with YST histology (Fig 4A, P value = 7.5e-0.3, Fisher's exact test). The same association was observed in the other cohorts (Data Supplement). Hypothetically, YST histology is the main determinant of poor survival, which may determine the association between 3p25.3 and poorer survival outcomes. However, in the MSKCC-impact data set, there was no clear association between histology and PFS in general, whereas within the YSTs, 3p25.3 gain was borderline associated with a poor prognosis. Moreover, within the nonseminomas, 3p25.3 gain was still strongly associated with poor prognosis if YSTs were excluded from the analysis (Data Supplement). This suggests that 3p25.3 gain occurs preferentially but not exclusively in YSTs and may serve as a biomarker of treatment resistance and poor prognosis for all nonteratomatous nonseminomas.
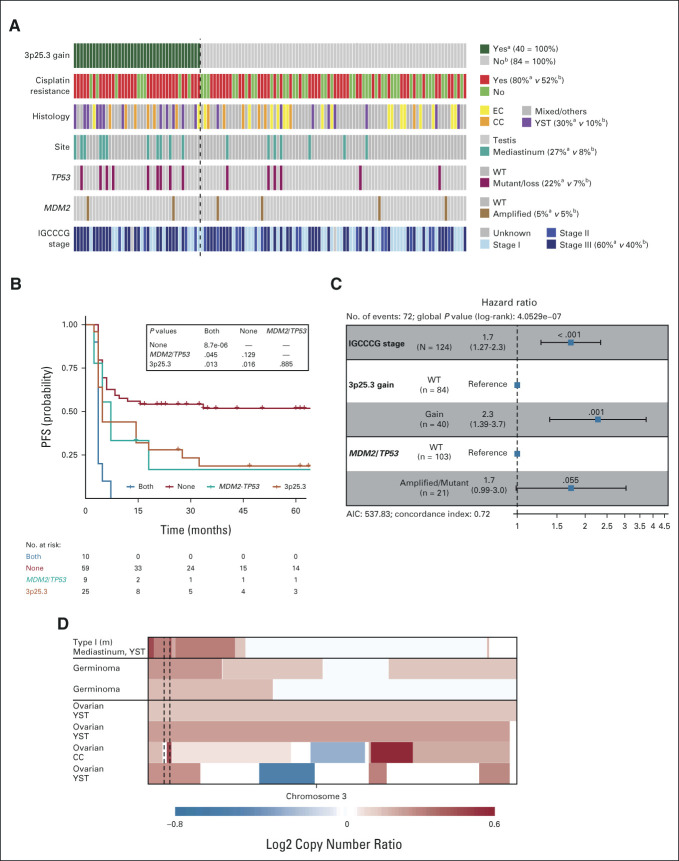
FIG 4.
3p25.3 gain is an independent predictor for poor prognosis in male type II nonseminomas. (A) Oncoprint of 3p25.3 gain and various other genetic and clinical parameters of the 124 nonseminomas in the MSKCC-2016 cohort. Columns represent tumors with colors in rows representing the various characteristics per tumor. The dashed line separates the tumors with 3p gain from the tumors without. Numbers to the right of the legend represent the percentage of tumors with the indicated characteristic in the a3p25.3 gained and bWT tumors, respectively. (B) Kaplan-Meier analysis of the MSKCC-2016 nonseminoma cohort separated by TP53/MDM2 and 3p25.3 gain status. The table shows P values for between-group comparisons which were generated using the log-rank test with Benjamini-Hochberg multiple testing correction. (C) Forest plot of the hazard ratios calculated by Cox regression survival analysis on the MSKCC-2016 nonseminoma cohort. (D) Copy number plots of selected GCTs on chromosome 3. Copy numbers were generated using methylation profiling/next-generation sequencing. Blue indicates loss and red indicates gain of the respective region, with color intensity reflecting the extent which is indicated in the scale bar below. The 3p25.3 region is indicated with dashed lines. AIC, Akaike's information criterion; CC, choriocarcinoma; EC, embryonal carcinoma; GCT, germ cell tumor; PFS, progression-free survival; TE, teratoma; WT, wild-type; YST, yolk sac tumor.
Genetic aberrations in TP53 and MDM2 have previously been suggested to be involved in cisplatin resistance in male type II GCTs.11 To determine how the presence of these aberrations relates to 3p25.3 gain and survival, TP53/MDM2 and 3p25.3 status were analyzed together. Aberrations in TP53 and gain of 3p25.3 co-occured more frequently than expected by chance alone (Fig 4A, P = .02, Fisher's exact test) while there was no association with an MDM2 amplification (P = 1, Fisher's exact test). In line with the observation that TP53 mutations are frequently seen in mediastinal tumors, there was also an enrichment of 3p25.3 gain in mediastinal tumors (P = .01, Fisher's exact test). GCTs that had both 3p25.3 gain and aberrations in TP53 or MDM2 had a poorer prognosis than tumors with a single aberration while there was no difference in survival between patients who harbored only 3p25.3 gain and no TP53/MDM2 aberrations and patients who showed the inverse pattern (Fig 4B). This suggests that P53 pathway inactivation and 3p25.3 gain are two separate mechanisms leading to cisplatin resistance in GCTs. If they co-occur in the same tumor, the prognosis seems to be even worse. The main predictor for the prognosis of primarily advanced GCTs is currently the IGCCCG risk classification12; however, there was no significant association between IGCCCG poor-risk stage and 3p25.3 gain (P = .167, chi-square test). To determine how IGCCCG stage and 3p25.3 status relate to prognosis and whether 3p25.3 status could add to GCT risk classification, a multivariable Cox regression analysis was performed. This showed that 3p25.3 gain was a strong predictor of poor PFS, independent of IGCCCG risk category, in this cohort of nonseminomas (Data Supplement). Moreover, if TP53/MDM2 status was added to the model, 3p25.3 gain remained a strong independent predictor of PFS while TP53/MDM2 status was not significant anymore (Fig 4C). Within the MSKCC-2008 set, 3p25.3 gain showed a similar effect as an independent predictor of OS when analyzed together with the IGCCCG risk classification, although it does not reach significance, possibly because of the low number of cases (Data Supplement). Overall, this indicates that 3p25.3 status may aid in risk classification of male type II GCTs. Although the postpubertal type II male GCTs are the most frequent manifestation of GCTs, they can also occur in pediatric patients (type I) and in postpubertal girls (type II ovarian), and in the brain (i.e. germinoma or dysgerminoma).7 Our data and the MSKCC-impact data were screened, and gain of 3p25.3 was identified in one male pediatric type I tumor, two germinomas, and four ovarian type II GCTs (Fig 4D). Interestingly, four of these seven tumors contained a YST histology, suggesting that 3p25.3 could be associated with YST histology also in other GCTs.
DISCUSSION
Despite the excellent overall 5-year survival rates of male type II GCTs since the introduction of cisplatin-based combination chemotherapy, intrinsic or acquired cisplatin resistance remains a major clinical challenge with unfavorable prognostic impact. Understanding the biology of this phenomenon is paramount to identify (1) biomarkers to predict treatment resistance, (2) ways to prevent it from developing, and (3) novel effective targeted therapies for relapsed and refractory disease. Research on this topic is hampered by the overall low availability of metastatic cisplatin-resistant tumors, especially with histologically proven, viable nonteratomatous disease.
Therefore, we initially used several independent sets of male type II GCT-derived cell lines to screen for general mechanisms of cisplatin resistance. The cisplatin-resistant cell line subclones showed different magnitudes of cisplatin resistance and were generated using different methodologies; however, they all showed de novo gain of chromosome 3p25.3. No other recurrent molecular changes were identified, although the lines were extensively investigated. Moreover, copy number on chromosome 3p25.3 showed a strong dose-dependent relationship with cisplatin sensitivity in our cell line models.
The subsequent analysis of patient samples demonstrated that the observed 3p25.3 gain can be found in patient samples and is not an in vitro artifact. It was identified at low frequencies in untreated primary GCTs and at significantly higher frequencies in metastasized, pretreated, and/or cisplatin-resistant tumors, which highlights a possible role in cisplatin resistance. There was considerable heterogeneity in the frequencies at which 3p25.3 gain was identified in the different data sets; however, we propose that this is at least partially driven by heterogeneity in the patient composition of the individual data sets investigated. Especially the various MSKCC data sets are heavily enriched for metastasized and pretreated tumors and are not a representative sampling of all male type II GCTs.11,18 Gain of 3p25.3, however, remains strongly associated with both PFS and OS independently of data set composition, indicating that this gain in chromosomal material may possibly serve as a stable predictive and prognostic biomarker of cisplatin-resistant disease. The fact that 3p25.3 gain is associated with worse PFS and OS suggests that cisplatin resistance through 3p25.3 gain is not readily overcome by high-dose chemotherapy.
The preferential presence of the 3p25.3 amplification in YSTs was identified before18 and is in keeping with identification of 3p25.3 gain in a cisplatin-resistant ovarian YST cell line.23 In addition, about 20% of ovarian YSTs showed increased copy numbers of 3p25.3.24 Although this predominance of 3p25.3 amplification in YSTs remains to be explained, it might be related to a higher vulnerability of this histological element to develop copy number aberrations because of less stringent DNA maintenance mechanisms because of the limited life span of this tissue type under physiological circumstances.8 Interestingly, the presence of 3p25.3 gain remains associated with poor prognosis, even within the YSTs, indicating that it is not a bystander but a driving event in cisplatin resistance.
Gain of 3p25.3 was observed across all histological GCT subtypes; however, it is not associated with a worse prognosis in seminomas. Furthermore, if teratomas are excluded, the relationship with poor outcome becomes stronger. Consequently, we propose that 3p35.3 gain should only be considered as a marker for poor prognosis in nonseminomas excluding pure teratomas. Most observed male type II GCTs contain mixed histologies at diagnosis.25 Our data do indicate that 3p25.3 gain promises to be a useful prognostic marker for this group as well. However, more research is needed to identify whether the histological composition is associated with the prognostic power of 3p25.3 gain.
The fact that 3p25.3 gain was found in both pediatric (type I) and adult (type II) GCTs, as well as in testicular, mediastinal, and ovarian primaries, suggests that it could represent a more general, possibly universal type of mechanism of cisplatin resistance in GCTs. Whether there is an association with survival, and whether this is specific to certain histological subtypes, remains to be determined. Interestingly, loss of chromosome 3p is generally much more common than gain of this region, especially in squamous cell tumors.26 Further research should indicate whether 3p25.3 gain as a proposed mechanism of cisplatin resistance is specific to GCTs or is a more general mechanism that has not been identified so far in other tumor types.
We detect a positive correlation between the presence of 3p25.3 gain and TP53 mutations, but the background and functional impact of this remains to be determined. Hypothetically, this could be caused by a higher tolerance/propensity for acquiring genetic aberrations in specific tumors or that the presence of one aberration increases the chances of acquiring the other. The survival analyses showed that tumors with both 3p25.3 and TP53/MDM2 aberrations had an even poorer prognosis than tumors harboring only one alteration. This suggests that 3p25.3 gain and aberrations in the TP53/MDM2 axis are independent mechanisms of cisplatin resistance, in line with a recently described independent functional role of P53 in GCT treatment resistance.27 The IGCCCG risk staging is an established tool for risk classification and treatment decision making in male type II GCTs. Our analyses suggest that 3p25.3 gain could be a strong independent predictor of poor prognosis, even when accounting for the IGCCCG risk categories. It has recently been described that the presence of TP53/MDM2 mutations could also add to patient stratification in male type II GCTs,11 but even when this information was added to the regression model, gain of 3p25.3 remains a strong independent risk factor for poor outcome. Although this finding awaits validation using more clinical data sets, we believe that gain of 3p25.3 could be a valuable diagnostic tool and prognostic biomarker for the identification of cisplatin-resistant tumors. Identification of a genomically defined high-risk group of patients which is already a clinical routine in many other malignancies, eg, leukemias or myeloma,28,29 could improve risk stratification and potentially guide treatment decisions in patients with GCT, too. Finally, further research on the mechanism(s) through which 3p25.3 gain drives cisplatin resistance could open new therapeutic avenues to treat refractory patients for whom currently little curative treatment options are available.
Harmen J.G. van de Werken
Stock and Other Ownership Interests: Cergentis
Honoraria: Bayer
Christoph Oing
Honoraria: Ipsen, AstraZeneca, Roche, Ipsen
Consulting or Advisory Role: Novartis
Research Funding: PharmaMar
Travel, Accommodations, Expenses: Ipsen
Friedemann Honecker
Honoraria: Janssen Oncology
Leendert H.J. Looijenga
Patents, Royalties, Other Intellectual Property: Patent related to 3p amplification and treatment resistance of germ cell tumors (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Princess Máxima Center for Pediatric Oncology and the Bergh in het Zadel Foundation.
D.M.T. and T.F.E. equally contributed to this work.
DATA SHARING STATEMENT
WGS Sequencing data ENA PRJEB38262. Methylation data, Array Express, accession number E-MTAB-9114 and GSA data, Array Express, accession number E- MTAB-9266. The primary data sets are available via the various repositories.
AUTHOR CONTRIBUTIONS
Conception and design: Dennis M. Timmerman, Thomas F. Eleveld, Leendert H.J. Looijenga
Financial support: Leendert H.J. Looijenga
Administrative support: Leendert H.J. Looijenga
Provision of study materials or patients: Silvia Schmidtova, Katarina Kalavska, Christoph Oing, Friedemann Honecker, Michal Mego, Leendert H.J. Looijenga
Collection and assembly of data: Dennis M. Timmerman, Thomas F. Eleveld, Lambert C.J. Dorssers, Ad J.M. Gillis, Silvia Schmidtova, Katarina Kalavska, Michal Mego, Leendert H.J. Looijenga
Data analysis and interpretation: Dennis M. Timmerman, Thomas F. Eleveld, Sruthi Sriram, Lambert C.J. Dorssers, Harmen J.G. van de Werken, Christoph Oing, Michal Mego, Leendert H.J. Looijenga
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chromosome 3p25.3 Gain Is Associated With Cisplatin Resistance and Is an Independent Predictor of Poor Outcome in Male Malignant Germ Cell Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Harmen J.G. van de Werken
Stock and Other Ownership Interests: Cergentis
Honoraria: Bayer
Christoph Oing
Honoraria: Ipsen, AstraZeneca, Roche, Ipsen
Consulting or Advisory Role: Novartis
Research Funding: PharmaMar
Travel, Accommodations, Expenses: Ipsen
Friedemann Honecker
Honoraria: Janssen Oncology
Leendert H.J. Looijenga
Patents, Royalties, Other Intellectual Property: Patent related to 3p amplification and treatment resistance of germ cell tumors (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Richiardi L, Ekbom A, et al. : Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. Int J Cancer 118:3099-3111, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Einhorn LH, Donohue J: Cis-diamminedichloroplatinum, vinblastine, and 384 bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med 87:293-298, 1977 [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2021. CA Cancer J Clin 71:7-33, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Chovanec M, Abu Zaid M, Hanna N, et al. : Long-term toxicity of cisplatin in germ-cell tumor survivors. Ann Oncol 28:2670-2679, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curreri SA, Fung C, Beard CJ: Secondary malignant neoplasms in testicular cancer survivors. Urol Oncol 33:392-398, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen C, Honecker F: Cisplatin resistance in germ cell tumours: Models and mechanisms. Andrology 3:111-121, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Oosterhuis JW, Looijenga LH: Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 5:210-222, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Oosterhuis JW, Looijenga LHJ: Germ cell tumors from a developmental perspective. Nat Rev Cancer 19:522-537, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Adra N, Einhorn LH: Testicular cancer update. Clin Adv Hematol Oncol 15:386-396, 2017 [PubMed] [Google Scholar]
- 10.Korkola JE, Houldsworth J, Bosl GJ, et al. : Molecular events in germ cell tumours: Linking chromosome-12 gain, acquisition of pluripotency and response to cisplatin. BJU Int 104:1334-1338, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Bagrodia A, Lee BH, Lee W, et al. : Genetic determinants of cisplatin resistance in patients with advanced germ cell tumors. J Clin Oncol 34:4000-4007, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillessen S, Sauvé N, Collette L, et al. : Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): Results from the IGCCCG update consortium. J Clin Oncol 39:1563-1574, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorssers LCJ, Gillis AJM, Stoop H, et al. : Molecular heterogeneity and early metastatic clone selection in testicular germ cell cancer development. Br J Cancer 120:444-452, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rijlaarsdam MA, Tax DM, Gillis AJ, et al. : Genome wide DNA methylationprofiles provide clues to the origin and pathogenesis of germ cell tumors. PLoS One 10:e0122146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovestadt V, Zapatka M: conumee: Enhanced copy-number variation analysis using illumina DNA methylation arrays. R package version 1.9.0. http://bioconductor.org/packages/conumee [DOI] [PMC free article] [PubMed]
- 16.Shen H, Shih J, Hollern DP, et al. : Integrated molecular characterization of testicular germ cell tumors. Cell Rep 23:3392-3406, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korkola JE, Heck S, Olshen AB, et al. : In vivo differentiation and genomic evolution in adult male germ cell tumors. Genes Chromosomes Cancer 47:43-55, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kraggerud SM, Skotheim RI, Szymanska J, et al. : Genome profiles of familial/bilateral and sporadic testicular germ cell tumors. Genes Chromosomes Cancer 34:168-174, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Mostert MM, van de Pol M, Olde Weghuis D, et al. : Comparative genomic hybridization of germ cell tumors of the adult testis: Confirmation of karyotypic findings and identification of a 12p-amplicon. Cancer Genet Cytogenet 89:146-152, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Abada PB, Howell SB: Cisplatin induces resistance by triggering differentiation of testicular embryonal carcinoma cells. PLoS One 9:e87444, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo J, Gillis AJM, van den Berg A, et al. : Identification and validation model for informative liquid biopsy-based microRNA biomarkers: Insights from germ cell tumor in vitro, in vivo and patient-derived data. Cells 8:1637, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidtova S, Dorssers LCJ, Kalavska K, et al. : Napabucasin overcomes cisplatin resistance in ovarian germ cell tumor-derived cell line by inhibiting cancer stemness. Cancer Cel Int 20:364, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, et al. : Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: Implications for pathogenesis. Endocr Rev 34:339-376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostofi FK, Sesterhenn IA: Pathology of germ cell tumors of testes. Prog Clin Biol Res 203:1-34, 1985 [PubMed] [Google Scholar]
- 26.Taylor AM, Shih J, Ha G, et al. : Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell 33:676-689.e3, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmerman DM, Eleveld TF, Gillis AJM, et al. : The role of TP53 in cisplatin resistance in mediastinal and testicular germ cell tumors. Int J Mol Sci 22:11774, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrot A, Corre J, Avet-Loiseau H: Risk stratification and targets in multiple myeloma: From genomics to the bedside. Am Soc Clin Oncol Ed Book 38:675-680, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Papaemmanuil E, Gerstung M, Bullinger L, et al. : Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374:2209-2221, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]