PURPOSE
Vaccine-induced neutralizing antibodies (nAbs) play a critical role in protection from SARS CoV-2. Patients with B-cell malignancies including myeloma are at increased risk of COVID-19–related mortality and exhibit variable serologic response to the vaccine. The capacity of vaccine-induced antibodies in these patients to neutralize SARS CoV-2 or its variants is not known.
METHODS
Sera from 238 patients with multiple myeloma (MM) undergoing SARS CoV-2 vaccination were analyzed. Antibodies against the SARS CoV-2 spike receptor-binding domain (RBD) and viral nucleocapsid were measured to detect serologic response to vaccine and environmental exposure to the virus. The capacity of antibodies to neutralize virus was quantified using pseudovirus neutralization assay and live virus neutralization against the initial SARS CoV-2 strain and the B1.617.2 (Delta) variant.
RESULTS
Vaccine-induced nAbs are detectable at much lower rates (54%) than estimated in previous seroconversion studies in MM, which did not monitor viral neutralization. In 33% of patients, vaccine-induced antispike RBD antibodies lack detectable neutralizing capacity, including against the B1.617.2 variant. Induction of nAbs is affected by race, disease, and treatment-related factors. Patients receiving mRNA1273 vaccine (Moderna) achieved significantly greater induction of nAbs compared with those receiving BNT162b2 (Pfizer; 67% v 48%, P = .006).
CONCLUSION
These data show that vaccine-induced antibodies in several patients with MM lack detectable virus-neutralizing activity. Vaccine-mediated induction of nAbs is affected by race, disease, vaccine, and treatment characteristics. These data have several implications for the emerging application of booster vaccines in immunocompromised hosts.
INTRODUCTION
Patients with hematologic malignancies are at an increased risk of infection with SARS CoV-2 and COVID-19–related mortality.1 Multiple myeloma (MM) is a common hematologic malignancy characterized by growth of malignant plasma cells. Initial studies in patients with MM suggested variable serologic response to SARS CoV-2 vaccines with > 80% seroconversion rates.2-6 However, these studies were limited to the detection of antibodies against CoV-2 spike proteins/receptor-binding domain (RBD)2,4,5 or surrogate assays for competition with binding between RBD and its cellular receptor angiotensin converting enzyme-2.3,6 Neutralizing antibodies (nAbs) are highly predictive of immune protection from symptomatic SARS CoV-2 infection and are the desired targets of vaccination.7 Vaccine-induced nAbs may also provide cross-reactive protection against emergence of variants.8 However, data regarding the induction of SARS CoV-2 nAbs in vaccinated patients with MM and clinical features that affect the induction of these antibodies in these patients are lacking. Understanding the determinants of vaccine-mediated induction of nAbs in patients with MM is critical to developing strategies to protect these patients from SARS CoV-2. In addition, patients with B-cell malignancies such as MM may serve as reservoirs for the generation of viral variants.9 Optimizing protection of these patients may also therefore be critical for control of the pandemic.
CONTEXT
Key Objective
What is the efficacy of SARS CoV-2 vaccines in inducing antibodies with virus-neutralizing capacity in patients with myeloma?
Knowledge Generated
Neutralizing antibodies (nAbs) are generated in only 54% of myeloma patients with current RNA vaccines. Induction of nAbs is affected by race (higher in Black patients), vaccine (higher with Moderna versus Pfizer), and myeloma therapy (lower with CD38 antibodies).
Relevance (J.W. Friedberg)
-
mRNA1273 (Moderna) leads to higher virus-neutralizing responses against SARS CoV-2 in these patients.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
METHODS
To evaluate the immune response to SARS CoV-2 vaccination, we obtained blood specimens from 238 patients with MM (patient characteristics are given in the Data Supplement [online only]) receiving SARS CoV-2 vaccines after informed consent approved by the Emory Institutional Review Board. Serologic response to the vaccine was measured with an ELISA to detect antibodies against SARS CoV-2 spike RBD.10,11 Antibodies against nucleocapsid (NC) were monitored to evaluate environmental exposure to the virus. In parallel, a pseudovirus neutralization assay was used to detect nAbs.11 Further validation of the assays was performed using a Mesoscale Discovery (MSD) assay for detection of antispike antibodies against SARS CoV-2 and other coronaviruses. In addition, antibodies were also tested for live virus neutralization against the initial SARS CoV-2 strain and the current dominant B1.617.2 (Delta) variant.12 See the Data Supplement (online only) for details of methods.
RESULTS
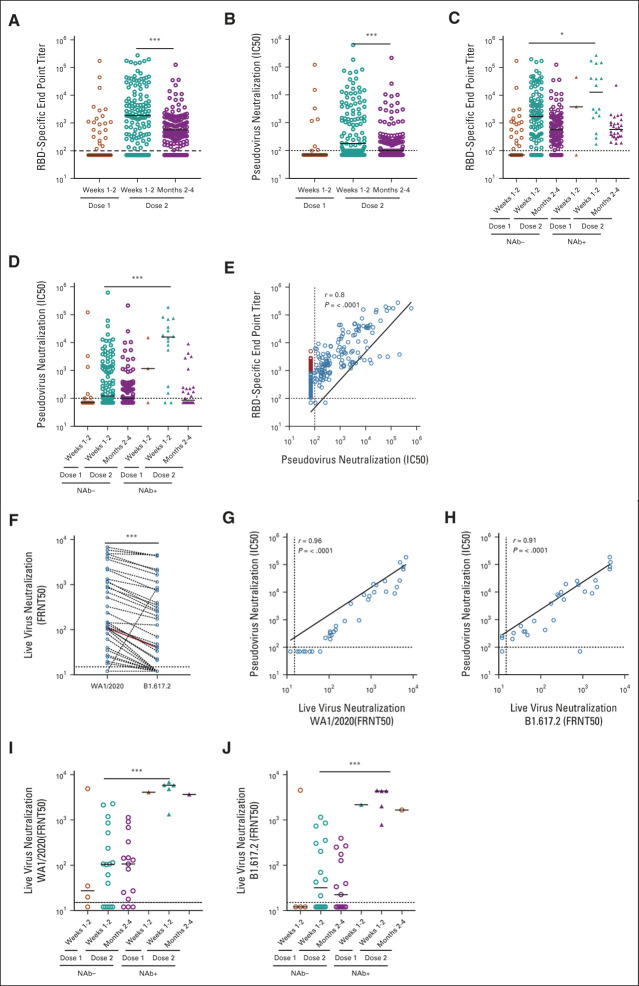
Overall, RBD-specific antibodies were detected after the second vaccine in 208 of 238 (87%) patients tested, consistent with previous studies2,4 (Fig 1A and Data Supplement). However, only 128 patients (54%) had SARS CoV-2 nAbs detected by a pseudovirus neutralization assay (Fig 1B and Data Supplement). Both anti-RBD and nAb titers peaked at 1-2 weeks after second vaccine and declined by 3 months (Data Supplement). NC antibodies indicative of prior SARS CoV-2 exposure were detected in 33 of 225 patients tested (14.6%), most of whom had no documented COVID-19 in the medical record (Data Supplement). Both anti-RBD and nAbs after vaccination were higher in patients with prior SARS CoV-2 exposure (Figs 1C and 1D). Overall, there was a strong correlation between anti-RBD antibodies and nAbs (r = 0.8; Fig 1E). However, a subset of patients had no detectable nAbs, despite clearly detectable anti-RBD antibodies at titers exceeding 10e3 AU/ml (Fig 1E). To validate the detection of RBD-specific antibodies with an independent assay, we used a MSD platform for the detection of these antibodies in a subset of patients. Data from the two assays were highly correlated (Data Supplement) and again identified a subset of patients with anti-RBD antibodies but lacking detectable neutralizing activity (Data Supplement). Vaccine-induced antispike RBD antibodies against SARS CoV-2 detected by this assay also correlated with antispike antibodies against related alpha coronaviruses such as SARS CoV-1, but not those against betacoronaviruses, consistent with known sequence homology in the spike proteins (Data Supplement).
FIG 1.
Serologic response to SARS CoV-2 vaccination in patients with myeloma. (A) RBD-specific end point IgG titer measured at weeks 1-2 (n = 51) after dose 1 and at weeks 1-2 (n = 126) and months 2-4 (n = 149) after dose 2. (B) Pseudovirus neutralization titer measured at weeks 1-2 (n = 51) after dose 1 and at weeks 1-2 (n = 126) and months 2-4 (n = 149) after dose 2. (C) and (D) Difference between NC antibody–negative (dose 1 weeks 1-2, n = 48; dose 2 weeks 1-2, n = 110; months 2-4, n = 112) and NC antibody–positive (dose 1 weeks 1-2, n = 3; dose 2 weeks 1-2, n = 16; months 2-4, n = 29) myeloma patient’s RBD-specific end point IgG titer and pseudovirus neutralization titer, respectively. Each circle represents a sample. The black line indicates the median. The horizontal dotted line indicates the detection limit of the assay. The statistical difference was measured using the Mann-Whitney test. (E) Correlation between RBD-specific end point IgG titer and pseudovirus neutralization titer in dose 1 (weeks 1-2, n = 51) and dose 2 (week 1-2, n = 126 and month 2-4, n = 149)–vaccinated patients with myeloma. Correlation analysis was performed by simple linear regression analysis, and P values were obtained from the Pearson r correlation method. (F) Pairwise comparison of neutralization activity of plasma samples against wild-type and delta SARS-CoV-2 (n = 44). The FRNT50 titers were determined by a FRNT assay using an immunostain to detect infected foci. (G) Comparison between pseudo and wild-type live virus neutralization FRNT50 titers (dose 1 weeks 1-2, n = 5; dose 2 weeks 1-2, n = 23; month 2-4, n = 16). Correlation analysis was performed by simple linear regression analysis, and P values were obtained from the Pearson r correlation method. (H) Comparison between pseudo and delta (B1.617.2) live virus neutralization FRNT50 titers (dose 1 weeks 1-2, n = 5; dose 2 weeks 1-2, n = 23; months 2-4, n = 16). Correlation analysis was performed by simple linear regression analysis, and P values were obtained from the Pearson r correlation method. (I) and (J) Difference between NC antibody–negative (dose 1 weeks 1-2, n = 4; dose 2 weeks 1-2, n = 18; months 2-4, n = 15) and NC antibody–positive (dose 1 weeks 1-2, n = 1; dose 2 weeks 1-2, n = 5; months 2-4, n = 1) myeloma patient's neutralization FRNT50 titers against wild-type and delta SARS CoV-2, respectively. *P < .05, ***P < .001. FRNT, focus reduction neutralization test; IC, inhibitory concentration; NAb, neutralizing antibody; RBD, receptor-binding domain.
Neutralization of live virus serves as the gold standard for detecting the neutralizing capacity of antiviral antibodies and has been correlated with protection from symptomatic infection.7 Therefore, we analyzed the induction of vaccine-induced nAbs against parent Wuhan strain WA1 and B1.617.2 delta variant (the current dominant variant in the United States) in a subset of these patients.13 Overall, nAb titers against B1.617.2 were 2.1-fold lower than those against WA1, consistent with our previous studies in vaccinated healthy individuals.12 The focus reduction neutralization test (FRNT)-50 for live virus correlated with IC50 for pseudovirus neutralization, for both WA1 and B1.617.2 (Figs 1G and 1H). These assays also correlated with RBD antibody assays with both platforms (Data Supplement). Notably, vaccinated patients with prior SARS CoV-2 exposure had higher FRNT50 against both WA1 and B1.617.2 variants than those without prior virus exposure (Figs 1I and 1J). Taken together, these data show that the current vaccines do not elicit detectable nAbs in a high proportion of patients with MM.
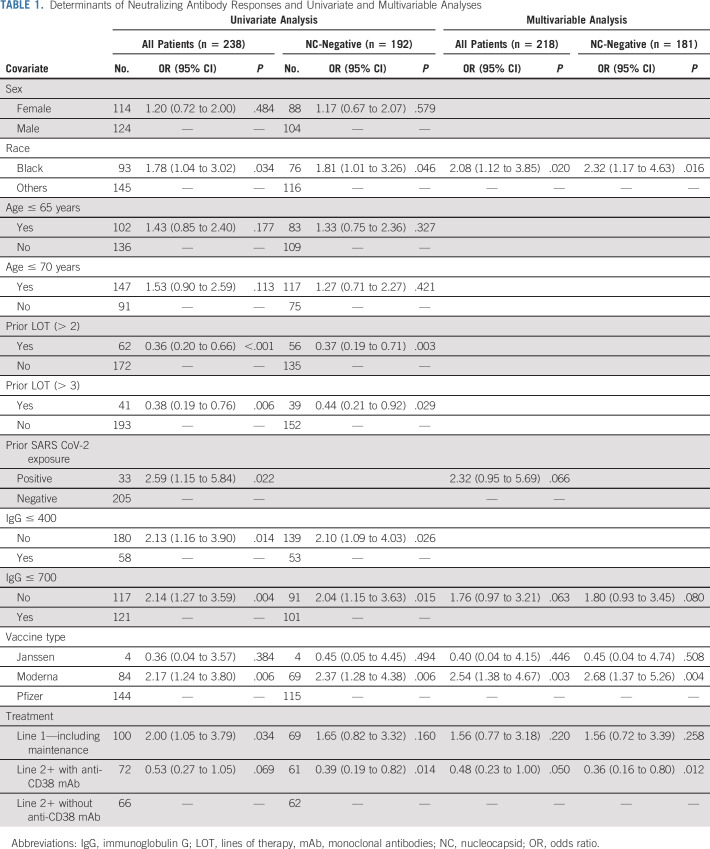
To better understand the clinical determinants of immunogenicity of the vaccine, we carried out a detailed analysis of host, disease, and treatment-related features and correlated them with the induction of SARS CoV-2 antibodies. Induction of anti-RBD antibodies was negatively correlated with the presence of hypogammaglobulinemia and prior lines of therapy (LOT), consistent with previous studies2 (Data Supplement). These parameters also correlated with the induction of nAbs. Despite similar seroconversion rates, induction of nAbs was higher in Black patients relative to their White counterparts (Data Supplement). Although patients who received mRNA1273 (Moderna) and BNT162b2 (Pfizer) vaccines had similar seroconversion rates, the induction of nAbs was significantly higher after mRNA1273 than after BNT162b2 vaccines (67% v 48%, P = .006; Data Supplement). Induction of SARS CoV-2–specific antibodies was also affected by specific MM therapies. The most pronounced effect was with the use of anti-CD38 antibodies. Interestingly, although the impact of anti-CD38 antibody therapy on the induction of anti-RBD antibodies was modest (81% v 90%, P = .05), patients receiving these therapies were less likely to mount detectable nAbs (36.5% v 61.6%, P < .0001). Therapies targeting B-cell maturation antigen (BCMA) such as belantamab mafodotin and BCL2 (Venetoclax) were associated with lower induction of vaccine-induced RBD antibodies. Conversely, patients on maintenance therapy had superior induction of anti-RBD and nAbs (Data Supplement). These differences were also evident if the analysis was restricted to patients lacking NC reactivity (Data Supplement). Taken together, these data show that several host, disease, and treatment-related features correlate with the probability of vaccine-mediated induction of nAbs in MM (Table 1). Although the negative impact of autologous stem cell transplantation or chimeric antigen-receptor T-cell therapy before vaccination is not evident in the current study, this could be attributed to the sample size and the fact that most patients in our cohort had received these therapies > 1 year before vaccination. The impact of timing from autologous stem cell transplantation or chimeric antigen-receptor therapy on vaccine responses has also been shown in other studies.14
TABLE 1.
Determinants of Neutralizing Antibody Responses and Univariate and Multivariable Analyses
Upon univariate analysis, for patients who are not newly diagnosed and those with increased prior LOT (more than two LOT), low immunoglobulin G (IgG) values (< 700 mg/dl) were associated with reduced ability to mount vaccine-induced nAbs. Those patients receiving two or more LOT with anti-CD38 monoclonal antibody (mAb) or anti-CD38 combinations who had not been exposed to SARS CoV-2 (NC-negative patients) had significantly reduced vaccine-induced nAbs. On the other hand, Black race, those receiving mRNA1273 (Moderna), and those receiving frontline therapy (including maintenance) had better nAb responses (Table 1). On multivariable analysis, Black race and receiving mRNA1273 vaccine (Moderna) remained as independent predictors of higher nAb responses at low IgG values (< 700 mg/dl); those patients receiving two or more LOT with anti-CD38 mAbs or their combinations remained as independent predictors for lower nAb responses (Table 1). Of the variables tested, only receiving one line of therapy including maintenance remained as a significant predictor of higher antispike RBD-binding antibodies in multivariable analysis (Data Supplement).
DISCUSSION
Here, we show that although > 80% of patients with MM mount serologic response to current SARS Co-V2 vaccines, many of these patients lack detectable nAbs, which are accepted to be critical for protective immunity. Susceptibility to SARS CoV-2 in our cohort is also supported by high rates of viral exposure, detected in nearly 15% of patients. Our data also illustrate the importance of monitoring nAbs and SARS CoV-2 exposure when evaluating the immunogenicity of vaccines in these patients. Such differences between anti-RBD antibodies and nAbs were not seen with these assays in vaccinated healthy donors.15 Reduced capacity to induce nAbs may be a reflection of underlying depletion of naïve B cells or defects in B-cell maturation as a manifestation of the underlying B-cell/plasma cell malignancy,16 further compounded by B-cell/plasma cell–targeted therapies. Ongoing studies characterizing the nature of antigen-specific B cells and T-cell responses should shed further light on the breadth of antiviral immunity.
Although both mRNA1273 (Moderna) and BNT162b2 (Pfizer) vaccines have yielded broadly comparable immunogenicity in healthy adults,17 mRNA1273 led to significantly higher rates of nAbs in this cohort. These data therefore support the choice of mRNA1273 as the preferred initial vaccine in this patient population. The reason behind these differences is not known but may relate in part to higher antigen dose in the Moderna vaccine or differences in the vaccine schedule. Previous studies have indeed demonstrated enhanced immunogenicity of higher-dose vaccines (such as influenza) in this patient population.18 It is notable that despite underlying immune paresis, patients with prior SARS CoV-2 exposure who were able to produce anti-NC antibodies also achieved high levels of nAbs (including against the B1.617.2 variant) after vaccination. These data therefore support the current recommendation to pursue vaccination even in patients with prior SARS CoV-2 exposure and testing further booster vaccines including heterologous high-dose boosters in this patient population.19
Outcomes in MM have improved in recent years with the introduction of several therapies, including those targeting CD38 and BCMA. Although both CD38- and BCMA-targeted therapies were associated with lower seroconversion rates, consistent with recent studies,2,3 anti-CD38 antibodies, in particular, had a profound impact on the induction of nAbs. As anti-CD38 antibodies also target normal plasmablasts, this finding is consistent with the emerging appreciation that germinal center reaction and induction of plasmablasts may be important for the induction of nAbs.20 Among other therapies, other novel insights from this analysis include the favorable impact of IMiD maintenance and adverse impact of BCL2 inhibitors. These data may therefore affect risk/benefit considerations during patient management.
Strengths of this analysis include serial specimens from a racially diverse cohort including Black patients with MM under-represented in most previous studies and analysis of nAbs with both pseudovirus and live virus neutralization assays, including against variants. Measurement for viral neutralization remains the gold standard for testing nAbs in viral immunology. Previous studies claiming evaluation of nAbs in MM relied entirely on surrogate assays without actually testing viral neutralization. These data therefore provide several novel insights and address several limitations of existing studies, which did not measure viral neutralization, including against the B1.617.2 (delta) variant, the current dominant circulating variant in the United States (Data Supplement). Further studies are needed to test whether the disproportionately low induction of nAbs as opposed to binding antibodies in MM represents a dysfunctional B-cell response. The finding that Black patients with MM achieved higher nAb response to the vaccine may help address current vaccine hesitancy in this population.21 Weakness includes lack of data on antigen-specific B- and T-cell responses, which will further enrich understanding of immunogenicity of vaccines in these cohorts. Although this analysis did not include concurrent healthy control cohort, the observed immunogenicity of the vaccine in MM is much lower than that in previous studies from our group using these assays in vaccinated healthy individuals.12,15 Overall, these data show that a large number of vaccinated patients with MM lack detectable nAbs to SARS CoV-2, which is affected by the nature of MM therapies. The susceptibility of these patients to SARS CoV-2 infection is also supported by the high rates of viral exposure in this cohort.
These data have several implications for the management of patients during the pandemic. Among the currently approved vaccines, we suggest mRNA1273 as the preferred vaccine for this population. However, a large proportion of vaccinated patients with MM may remain susceptible to SARS CoV-2 infection. Therefore, strategies to reduce exposure of these patients, including vaccination of household contacts and caretakers, should be pursued. Infected patients should be considered for early administration of passive immune therapies such as mAbs or antivirals. Patients on certain therapies such as anti-CD38 antibodies seem to be at highest risk and may require increased surveillance. By contrast, maintenance therapies do not adversely affect response to vaccines. These findings also emphasize the urgent need to pursue additional strategies to protect these patients, with higher-dose booster vaccines, or prophylactic administration of mAbs and surveillance for emergence of variants in this population.
ACKNOWLEDGMENT
The authors acknowledge the support of Cancer Tissue and Pathology, Immune monitoring, Data and Technology Applications, and Biostatistics shared resource of the Winship Cancer Institute of Emory University and NIH/NCI under award No. P30CA138292. The authors acknowledge the support of M. Johns and H. Von Hollen and their team for help with specimen collection.
Ajay K. Nooka
Consulting or Advisory Role: Amgen, Janssen Oncology, Bristol Myers Squibb, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Research Funding: Amgen (Inst), Janssen Oncology (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Hans Verkerke
Stock and Other Ownership Interests: Moderna Therapeutics
Patents, Royalties, Other Intellectual Property: Applying for a patent for a novel neutralizing antibody detection platform through Emory's IP pipeline. Not relevant to the work described here
Jonathan L. Kaufman
Consulting or Advisory Role: Celgene, TG Therapeutics, Genentech, Bristol Myers Squibb/Celgene, AbbVie, Incyte
Research Funding: Merck (Inst), Celgene (Inst), Janssen (Inst), Sutro Biopharma (Inst), Fortis (Inst), Amgen (Inst), AbbVie/Genentech (Inst), BMS (Inst)
Craig C. Hofmeister
Honoraria: Amgen, Bluebird Bio
Consulting or Advisory Role: Sanofi Pasteur, Bristol Myers Squibb, GlaxoSmithKline, Oncopeptides, Genzyme, Celgene, Janssen medical Affairs
Research Funding: Celgene (Inst), Bristol Myers Squibb (Inst), Oncopeptides (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Patent related to REC-2282 (formerly AR-42, NSC D736012)
Sagar Lonial
Stock and Other Ownership Interests: TG Therapeutics
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, AbbVie, Takeda, Merck, Sanofi
Research Funding: Celgene, Bristol Myers Squibb, Takeda
Other Relationship: TG Therapeutics
John D. Roback
Stock and Other Ownership Interests: Cambium Medical Technologies, Secure Transfusion Services
Honoraria: Secure Transfusion Services
Patents, Royalties, Other Intellectual Property: Patents held by Cambium Medical Technologies, I am listed as a coinventory on an Emory University–held patent for SARS-CoV-2 serology assays
Rafi Ahmed
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Patents on PD-1–directed immunotherapy, I am listed as a coinventor on an Emory University–held patent for SARS-CoV-2 serology assays
Mehul S. Suthar
Consulting or Advisory Role: Moderna Therapeutics, Ocugen
Research Funding: Moderna Therapeutics (Inst), Ocugen (Inst)
Madhav V. Dhodapkar
Consulting or Advisory Role: Roche/Genentech, Amgen, Kite, a Gilead company, Lava therapeutics, Janssen Oncology, Celgene
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by NCI U54CA260563. M.V.D. was also supported by funds from the NCI R35CA197603 and SCOR award from LLS. K.M.D. was supported in part by funds from NIH (CA238471 and AR077926). M.S.S. was supported in part by grants (P51 OD011132, HHSN272201400004C, and U19AI090023) from National Institutes of Health (NIH), by the Emory Executive Vice President for Health Affairs Synergy Fund award, the Pediatric Research Alliance Center for Childhood Infections and Vaccines and Children's Healthcare of Atlanta, COVID-Catalyst-I3 Funds from the Woodruff Health Sciences Center and Emory School of Medicine, Woodruff Health Sciences Center 2020 COVID-19 CURE Award, and the Emory-UGA Center of Excellence for Influenza Research and Surveillance.
A.K.N. and U.S. share authorship.
AUTHOR CONTRIBUTIONS
Conception and design: Ajay K. Nooka, Sagar Lonial, Andres Chang, John D. Roback, Rafi Ahmed, Mehul S. Suthar, Andrew S. Neish, Madhav V. Dhodapkar
Financial support: Mehul S. Suthar, Madhav V. Dhodapkar
Provision of study material or patients: Jonathan L. Kaufman, Sagar Lonial, John D. Roback, Rafi Ahmed, Madhav V. Dhodapkar
Collection and assembly of data: Ajay K. Nooka, Uma Shanmugasundaram, Hans Verkerke, Venkata V. Edara, Rajesh Valanparambil, Jonathan L. Kaufman, Nisha S. Joseph, Sagar Lonial, Maryam Azeem, Julia Manalo, Kavita M. Dhodapkar, Mehul S. Suthar, Andrew S. Neish, Madhav V. Dhodapkar
Data analysis and interpretation: Ajay K. Nooka, Uma Shanmugasundaram, Hans Verkerke, Craig C. Hofmeister, Sagar Lonial, Jeffrey M. Switchenko, Andres Chang, Susanne L. Linderman, John D. Roback, Kavita M. Dhodapkar, Mehul S. Suthar, Andrew S. Neish, Madhav V. Dhodapkar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Determinants of Neutralizing Antibody Response After SARS CoV-2 Vaccination in Patients With Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ajay K. Nooka
Consulting or Advisory Role: Amgen, Janssen Oncology, Bristol Myers Squibb, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Research Funding: Amgen (Inst), Janssen Oncology (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Hans Verkerke
Stock and Other Ownership Interests: Moderna Therapeutics
Patents, Royalties, Other Intellectual Property: Applying for a patent for a novel neutralizing antibody detection platform through Emory's IP pipeline. Not relevant to the work described here
Jonathan L. Kaufman
Consulting or Advisory Role: Celgene, TG Therapeutics, Genentech, Bristol Myers Squibb/Celgene, AbbVie, Incyte
Research Funding: Merck (Inst), Celgene (Inst), Janssen (Inst), Sutro Biopharma (Inst), Fortis (Inst), Amgen (Inst), AbbVie/Genentech (Inst), BMS (Inst)
Craig C. Hofmeister
Honoraria: Amgen, Bluebird Bio
Consulting or Advisory Role: Sanofi Pasteur, Bristol Myers Squibb, GlaxoSmithKline, Oncopeptides, Genzyme, Celgene, Janssen medical Affairs
Research Funding: Celgene (Inst), Bristol Myers Squibb (Inst), Oncopeptides (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Patent related to REC-2282 (formerly AR-42, NSC D736012)
Sagar Lonial
Stock and Other Ownership Interests: TG Therapeutics
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, AbbVie, Takeda, Merck, Sanofi
Research Funding: Celgene, Bristol Myers Squibb, Takeda
Other Relationship: TG Therapeutics
John D. Roback
Stock and Other Ownership Interests: Cambium Medical Technologies, Secure Transfusion Services
Honoraria: Secure Transfusion Services
Patents, Royalties, Other Intellectual Property: Patents held by Cambium Medical Technologies, I am listed as a coinventory on an Emory University–held patent for SARS-CoV-2 serology assays
Rafi Ahmed
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Patents on PD-1–directed immunotherapy, I am listed as a coinventor on an Emory University–held patent for SARS-CoV-2 serology assays
Mehul S. Suthar
Consulting or Advisory Role: Moderna Therapeutics, Ocugen
Research Funding: Moderna Therapeutics (Inst), Ocugen (Inst)
Madhav V. Dhodapkar
Consulting or Advisory Role: Roche/Genentech, Amgen, Kite, a Gilead company, Lava therapeutics, Janssen Oncology, Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dhodapkar M, Dhodapkar K, Ahmed R: Viral immunity and vaccines in hematologic malignancies: Implications for COVID-19. Blood Cancer Discov 2:9-12, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Oekelen O, Gleason CR, Agte S, et al. : Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell 39:1028-1030, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. : The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J 11:138, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberger LM, Saltzman LA, Senefeld JW, et al. : Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 39:10311033, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stampfer SD, Goldwater MS, Jew S, et al. : Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 35:3534-3541, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung DJ, Shah GL, Devlin SM, et al. : Disease and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov 2:568-576, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury DS, Cromer D, Reynaldi A, et al. : Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27:1205-1211, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Stamatatos L, Czartoski J, Wan Y-H, et al. : mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 372:1413-1418, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moelling K: Within-host and between-host evolution in SARS-CoV-2-new variant's source. Viruses 13:751, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suthar MS, Zimmerman MG, Kauffman RC, et al. : Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med 1:100040, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verkerke H, Horwath M, Saeedi B, et al. : Comparison of antibody ClassSpecific SARS-CoV-2 serologies for the diagnosis of acute COVID-19. J Clin Microbiol 59:e02026-20, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edara VV, Pinsky BA, Suthar MS, et al. : Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med 385:664-666, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edara VV, Hudson WH, Xie X, et al. : Neutralizing antibodies against SARS-CoV-2 variants after infection and vaccination. JAMA 325:1896-1898, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhakal B, Abedin S, Fenske T, et al. : Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR T-cell therapy. Blood 138:1278-1281, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson EJ, Rouphael NG, Widge AT, et al. : Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383:2427-2438, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailur JK, McCachren SS, Doxie DB, et al. : Early alterations in stemlike/resident T cells, innate and myeloid cells in the bone marrow in preneoplastic gammopathy. JCI Insight 5:e127807, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creech CB, Walker SC, Samuels RJ: SARS-CoV-2 vaccines. JAMA 325:1318-1320, 2021 [DOI] [PubMed] [Google Scholar]
- 18.Branagan AR, Duffy E, Gan G, et al. : Tandem high-dose influenza vaccination is associated with more durable serologic immunity in patients with plasma cell dyscrasias. Blood Adv 5:1535-1539, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebinger JE, Fert-Bober J, Printsev I, et al. : Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 27:981-984, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner JS, O'Halloran JA, Kalaidina E, et al. : SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 596:109-113, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momplaisir FM, Kuter BJ, Ghadimi F, et al. : Racial/ethnic differences in COVID-19 vaccine hesitancy among health care workers in 2 large academic hospitals. JAMA Netw Open 4:e2121931, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]