Abstract
Syphilis is a sexually transmitted infectious disease caused by Treponema pallidum. Here, we report a rare case of secondary pulmonary syphilis, which was diagnosed by metagenomic next-generation sequencing (mNGS) in bronchoalveolar lavage fluid (BALF). Direct pulmonary involvement by T. pallidum was suggested by a positive mNGS result in BALF. One month after treatment with benzathine penicillin G (2.4 million units, three doses), a repeated CT scan showed the radiological resolution. To our knowledge, this is the first reported case of secondary pulmonary syphilis diagnosed by mNGS in BALF.
Keywords: pulmonary, secondary syphilis, Treponema pallidum, mNGS
Introduction
Syphilis is a sexually transmitted infectious disease caused by Treponema pallidum. In recent years, the prevalence of syphilis has substantially increased worldwide.1 Syphilis is classified into primary, secondary, latent, and tertiary stages. Secondary syphilis with pulmonary involvement is rare. Coleman first proposed the criteria for the clinical diagnosis of secondary pulmonary syphilis in 1983.2 Despite this, pulmonary syphilis is still challenging to diagnose due to asymptomatic presentation and ability to mimic other diseases. An ideal method to diagnose secondary pulmonary syphilis is still not available. Here, we report a rare case of secondary pulmonary syphilis, which was diagnosed by metagenomic next-generation sequencing (mNGS) in bronchoalveolar lavage fluid (BALF).
Case Report
A 32-year-old heterosexual male was admitted to the Respiratory and Critical Care Department for a three-day history of left pleuritic chest pain and mild cough. He denied fever, weight loss, dyspnea, or nervous system symptoms. He had a medical history of 10-year chronic hepatitis B.
Physical examination revealed normal body temperature, normal breath sound, lack of mucosal or genitourinary lesion, and presence of erythematous papular rash over the palms and soles. The laboratory tests showed a white blood cell count of 8370/mL. The procalcitonin level of 0.336 ng/mL (normal range, <0.5) was normal. The erythrocyte sedimentation rate was increased to 69 mm/h. The serological tests of hepatitis C antibody and HIV antibody were negative, while the hepatitis B surface antigen was positive. The TP passive particle agglutination assay (TPPA) was positive, and the toluidine red unheated serum test (TRUST) revealed titer of 1:64. The TPPA kit was purchased from FUJIREBIO INC. (Tokyo, Japan), and the TRUST kit was obtained from Shanghai Rongsheng‐Biotech (Shanghai, China). Based on the history of sex with partners, erythematous papular rash over the palms and soles, and positive serological tests, secondary syphilis was considered in this patient after consultation with a dermatologist.
Chest computed tomography (CT) showed left lower lobe pneumonia, mild left pleural effusion, and mediastinal adenopathy (Figures 1 and 2). Bronchoalveolar lavage fluid (BALF) samples were collected from the patient and sent to our microbiological laboratory for smear and culture and to Guangzhou Sagene Biotech Co., Ltd. (Guangzhou, China) for mNGS. The smear and culture were negative. The mNGS analysis of the BALF identified 56 sequence reads uniquely corresponding to the T. pallidum genome, with 0.37% coverage (Figure 3). The details of mNGS sequencing and analysis in the Guangzhou Sagene Biotech Co., Ltd were provided in Supplementary Materials. Combining with clinical manifestations and laboratory data, the diagnosis of secondary syphilis with pulmonary involvement was supported. Benzathine penicillin G (2.4 million units 3 doses, 1 dose per week × 3 weeks) was administered intramuscularly. After one month, the CT scan showed that pleural effusion disappeared and pneumonia was resolved (Figure 4). The TRUST titer decreased to 1:16.
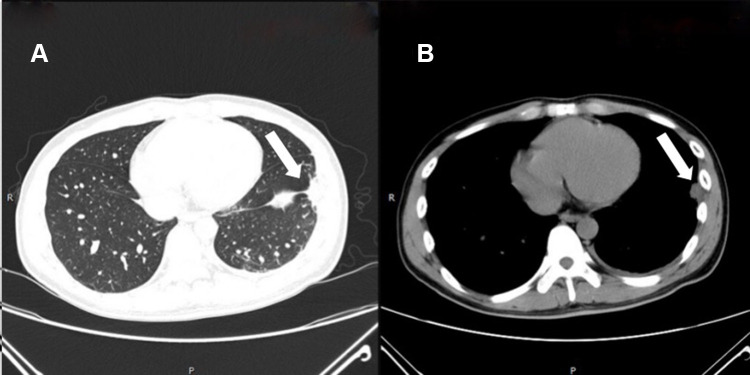
Figure 1.
Chest computed tomography on the first visit to our hospital: pneumonia can be seen in the left lower lobe on lung window (A) and mediastinal window (B) (arrows).
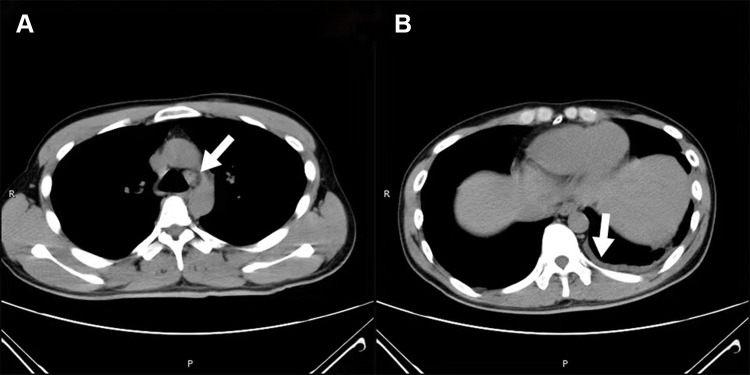
Figure 2.
(A) Arrow shows mediastinal adenopathy, and (B) arrow shows mild left pleural effusion.
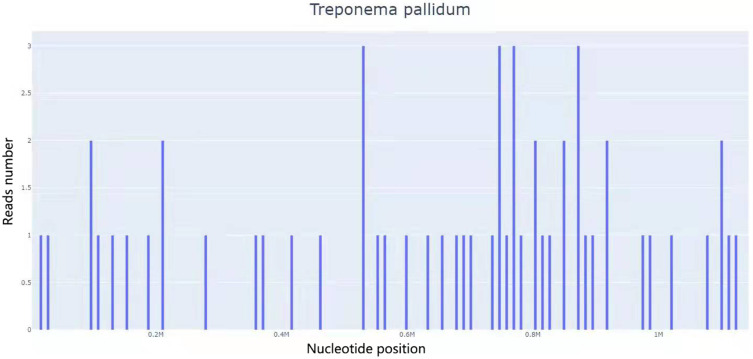
Figure 3.
A total of 56 DNA readings of Treponema pallidum in BALF, with coverage of 0.37%.
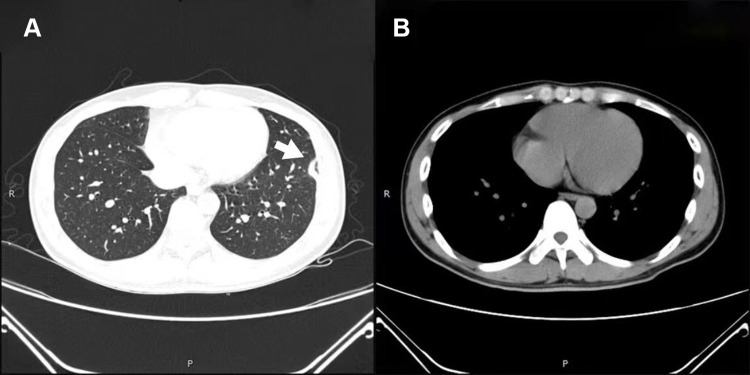
Figure 4.
Chest CT after 1 month of treatment. (A) Arrow shows that pneumonia was resolved, and (B) arrow shows that pleural effusion disappeared.
Discussion
Secondary pulmonary syphilis is extremely rare. However, with the increasing number of patients with syphilis worldwide, dozens of reports revealed pulmonary involvement in patients with secondary syphilis.2–11 The PubMed was searched with the keywords “syphilis”, “pulmonary”, and “lung” to identify case reports published in the English language up to December 31, 2021. We identified 25 cases of secondary pulmonary syphilis in the literature. The characteristics of the cases are provided in Table S1.
Among the 25 patients, the age at diagnosis ranged from 31 to 69 years old, and the number of males was 24. The respiratory symptoms of secondary pulmonary syphilis involved chest pain, cough, dyspnea, and epigastric pain, and the most common respiratory symptom was chest pain (11/24). The radiological presentation of secondary pulmonary syphilis appeared as nodule, infiltration, consolidation with pleural effusion, and adenopathy. The most common chest imaging manifestation was nodule.
For the diagnosis of secondary pulmonary syphilis, obtaining an ideal sample has not been standardized. Coleman reported that the criteria to diagnose secondary pulmonary syphilis include history and clinical findings, positive serologic test results, radiographic pulmonary abnormalities excluding other pulmonary diseases, and therapeutic response to antisyphilis treatment.2 However, the criterion to diagnose secondary pulmonary syphilis is exclusive. Differential diagnosis, such as primary or metastatic cancer, mycotic or bacterial abscess formation, sarcoidosis, and tuberculosis, should be considered.12,24 A direct evidence of secondary pulmonary syphilis is especially important. Some studies reported that polymerase chain reaction (PCR) of BALF samples, bronchial aspirate, transbronchial biopsy (TBB), or computed tomography-guided percutaneous needle aspiration (CTNA) are useful for the diagnosis of secondary pulmonary syphilis.3,13,16,26 A study showed that immunohistochemistry (IHC) allows the visualization of treponemes in BALF in one patient.5 In one case, a small number of spirochetes were found in the pleural sample.23 With the development of molecular biology, an increasing number of researchers studied the performance of mNGS in microbial detection. A study demonstrated that mNGS can detect pathogens, which are difficult to culture, especially for viruses and atypical pathogens, thereby solving the shortcomings of traditional culture methods.27 In our case, the results of mNGS in BALF samples showed the presence of T. pallidum. Thus, the diagnosis of secondary syphilis with pulmonary involvement was confirmed.
Penicillin is the treatment of choice for secondary pulmonary syphilis, and the WHO STI guideline recommends benzathine penicillin G. Other alternatives for patients include oral doxycycline, ceftriaxone, oral amoxicillin plus probenecid, and azithromycin.1 In our case, we treated the patient with benzathine penicillin G (2.4 million units 3 doses, 1 dose per week × 3 weeks) intramuscularly. After one month, a repeated CT scan showed radiological resolution.
To our knowledge, this is the first case of secondary pulmonary syphilis diagnosed by mNGS in BALF. This case showed that mNGS could be a useful method for the diagnosis of pulmonary involvement of secondary syphilis. Physicians should be aware of secondary syphilis with pulmonary involvement in the event of respiratory symptoms or lung radiological changes. The diagnosis should be confirmed by applying mNGS, PCR, or IHC in BALF, pleural fluid, or lung tissue.
Acknowledgments
The authors are very grateful to the patient in this study.
Funding Statement
Authors state no funding involved.
Abbreviations
mNGS, metagenomic next-generation sequencing; BALF, bronchoalveolar lavage fluid; PCR, polymerase chain reaction; TBB, transbronchial biopsy; CTNA, computed tomography-guided percutaneous needle aspiration; IHC, immunohistochemistry.
Ethics Approval and Consent to Participate
Institutional approval was acquired to publish the case details.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report.
Disclosure
The authors declare no competing interests.
References
- 1.World Health Organization. WHO Guidelines for the Treatment of Treponema Pallidum (Syphilis). WHO Guidelines Approved by the Guidelines Review Committee. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 2.Coleman DL, McPhee SJ, Ross TF, et al. Secondary syphilis with pulmonary involvement. West J Med. 1983;138(6):875–878. [PMC free article] [PubMed] [Google Scholar]
- 3.Bell N, Bracchi M, Pozniak A. A case of pulmonary nodules in a patient living with HIV diagnosed with secondary syphilis. BMJ Case Rep. 2021;14(8):e243765. doi: 10.1136/bcr-2021-243765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassem YH, Blind A, Chouta Ngaha F, et al. Secondary pulmonary syphilis: case report and review of literature. Ann Dermatol Venereol. 2018;145(4):278–287. doi: 10.1016/j.annder.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 5.Jeny F, Fargelat A, Laurent-Roussel S, et al. Pulmonary consolidations due to secondary syphilis with positive bronchial washing immunohistochemistry. Am J Respir Crit Care Med. 2016;193(9):1061–1062. doi: 10.1164/rccm.201511-2296IM [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Lee J-H, Lee E-S, et al. A case of secondary syphilis presenting as multiple pulmonary nodules. Korean J Intern Med. 2013;28(2):231–235. doi: 10.3904/kjim.2013.28.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson AL, Gutman JA, Welsh CH. A 50-year-old man with skin lesions and multiple pulmonary nodules. Chest. 2004;125(6):2322–2327. doi: 10.1378/chest.125.6.2322 [DOI] [PubMed] [Google Scholar]
- 8.Dooley DP, Tomski S. Syphilitic pneumonitis in an HIV-infected patient. Chest. 1994;105(2):629–631. doi: 10.1378/chest.105.2.629 [DOI] [PubMed] [Google Scholar]
- 9.Schibli H, Harms M. Tumour-like pulmonary lesion in secondary syphilis. A case report. Br J Vener Dis. 1981;57(6):367–371. doi: 10.1136/sti.57.6.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurumaji Y, Katoh T, Ohtaki N, et al. A case of secondary syphilis with a solitary pulmonary lesion. Dermatologica. 1987;174(1):23–27. doi: 10.1159/000248975 [DOI] [PubMed] [Google Scholar]
- 11.Biro L, Hill AC, Kuflik EG. Secondary syphilis with unusual clinical and laboratory findings. JAMA. 1968;206(4):889–891. doi: 10.1001/jama.1968.03150040101027 [DOI] [PubMed] [Google Scholar]
- 12.Benainous R, Alunji M, Brillet P-Y, et al. Pulmonary involvement in secondary syphilis. Eur J Case Rep Intern Med. 2021;8(7):002487. doi: 10.12890/2021_002487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Futami S, Takimoto T, Nakagami F, et al. A lung abscess caused by secondary syphilis - the utility of polymerase chain reaction techniques in transbronchial biopsy: a case report. BMC Infect Dis. 2019;19(1):598. doi: 10.1186/s12879-019-4236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komeno Y, Ota Y, Koibuchi T, et al. Secondary syphilis with tonsillar and cervical lymphadenopathy and a pulmonary lesion mimicking malignant lymphoma. Am J Case Rep. 2018;19:238–243. doi: 10.12659/AJCR.907127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta A, Furusyo N, Kishihara Y, et al. Secondary syphilis with pulmonary involvement. Intern Med. 2018;57(1):121–126. doi: 10.2169/internalmedicine.8439-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visuttichaikit S, Suwantarat N, Apisarnthanarak A, et al. A case of secondary syphilis with pulmonary involvement and review of the literature. Int J STD AIDS. 2018;29(10):1027–1032. doi: 10.1177/0956462418765834 [DOI] [PubMed] [Google Scholar]
- 17.Riganti J, Martin M, Torre AC, et al. Secondary syphilis with pulmonary involvement. J Eur Acad Dermatol Venereol. 2016;30(12):e177–e179. doi: 10.1111/jdv.13483 [DOI] [PubMed] [Google Scholar]
- 18.Soares SAJ, Soares Souza A, Zanetti G, et al. A skin rash with multiple pulmonary nodules. Eur Respir Rev. 2015;24(138):682–683. doi: 10.1183/16000617.00001815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Z, Zhang J, Li Q, et al. A case of secondary syphilis involving tonsil, pulmonary, and multiple lymph nodes: 18F-FDG PET/CT findings. Clin Nucl Med. 2015;40(4):335–337. doi: 10.1097/RLU.0000000000000662 [DOI] [PubMed] [Google Scholar]
- 20.Elzouki AN, Al-Kawaaz M, Tafesh Z. Secondary syphilis with pleural effusion: case report and literature review. Case Rep Infect Dis. 2012;2012:409896. doi: 10.1155/2012/409896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCready JB, Skrastins R, Downey JF, et al. Necrotic pulmonary nodules in secondary syphilis. CMAJ. 2011;183(3):E163–6. doi: 10.1503/cmaj.091479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David G, Perpoint T, Boibieux A, et al. Secondary pulmonary syphilis: report of a likely case and literature review. Clin Infect Dis. 2006;42(3):e11–5. doi: 10.1086/499104 [DOI] [PubMed] [Google Scholar]
- 23.Zaharopoulos P, Wong J. Cytologic diagnosis of syphilitic pleuritis: a case report. Diagn Cytopathol. 1997;16(1):35–38. doi: [DOI] [PubMed] [Google Scholar]
- 24.Cholankeril JV, Greenberg A-L, Matari HM, et al. Solitary pulmonary nodule in secondary syphilis. Clin Imaging. 1992;16(2):125–128. doi: 10.1016/0899-7071(92)90126-T [DOI] [PubMed] [Google Scholar]
- 25.Geer LL, Warshauer DM, Delany DJ. Pulmonary nodule in secondary syphilis. Australas Radiol. 1985;29(3):240–242. doi: 10.1111/j.1440-1673.1985.tb01702.x [DOI] [PubMed] [Google Scholar]
- 26.Ogawa Y, Imai Y, Yoshihara S, et al. Pulmonary involvement of secondary syphilis. Int J STD AIDS. 2018;29(1):89–91. doi: 10.1177/0956462417717653 [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Yin Y, Gao H, et al. Clinical utility of in-house metagenomic next-generation sequencing for the diagnosis of lower respiratory tract infections and analysis of the host immune response. Clin Infect Dis. 2020;71(Suppl 4):S416–S426. doi: 10.1093/cid/ciaa1516 [DOI] [PubMed] [Google Scholar]