Abstract
The brain is supplied by an elaborate vascular network that originates extracranially and reaches deep into the brain. The concept of the neurovascular unit provides a useful framework to investigate how neuronal signals regulate nearby microvessels to support the metabolic needs of the brain, but it does not consider the role of larger cerebral arteries and systemic vasoactive signals. Furthermore, the recently emerged molecular heterogeneity of cerebrovascular cells indicates that there is no prototypical neurovascular unit replicated at all levels of the vascular network. Here, we examine the cellular and molecular diversity of the cerebrovascular tree and the relative contribution of systemic and brain-intrinsic factors to neurovascular function. Evidence supports the concept of a ‘neurovascular complex’ composed of segmentally diverse functional modules that implement coordinated vascular responses to central and peripheral signals to maintain homeostasis of the brain. This concept has major implications for neurovascular regulation in health and disease and for brain imaging.
As one of most complex and metabolically active organs of the body, the brain is equipped with a sophisticated vascular system that enters intimate contact with its cellular constituents to supply energy substrates and nutrients, remove unwanted proteins and metabolites, enable neuroimmune trafficking and maintain homeostatic balance of the brain1-6. Formally introduced in 2001, the concept of the neurovascular unit (NVU) emphasizes the close developmental, structural and functional association between brain cells and the microvasculature and their coordinated reaction to injury1. The NVU concept was enthusiastically embraced by the scientific community and attracted much interest for its implications for normal brain function7, the interpretation of functional MRI (fMRI) signals8 and for brain diseases, including neurodegenerative diseases9. Consequently, much emphasis has been placed on neurovascular signaling at the level of the microvasculature, which is composed of endothelial cells, mural cells (comprising vascular smooth muscle cells (SMCs) and pericytes) and astrocytic end-feet7,10,11. However, the contribution of upstream and downstream vascular segments, as well as the profound influence of systemic factors on neurovascular function, have received less attention. Furthermore, single-cell RNA sequencing (RNA-seq) studies have revealed a remarkable segmental diversity of vascular cell transcriptomes. Here, we review the heterogeneity of the cerebrovascular tree and neurovascular associations in light of single-cell transcriptomics and functional data. We then examine how the cerebrovascular network integrates systemic vasoactive signals with those arising from the brain in the moment-to-moment regulation of cerebral blood flow (CBF). Finally, we consider the implications of such segmental diversity and integrative responses for neurovascular research in health and disease.
Cerebrovascular tree heterogeneity
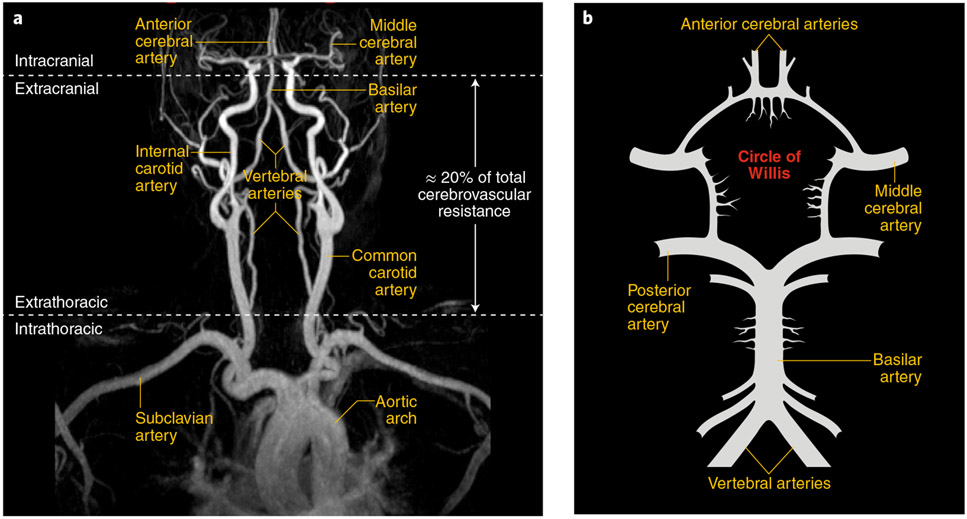
The brain is supplied by four major arteries arising from the aortic arch or its major branches. These vessels and their major branches are described in Fig. 1a,b.
Fig. 1 ∣. Anatomy of the large vessels supplying the brain.
a, Common carotid arteries arise from large intrathoracic arteries and give rise to the internal carotid arteries that enter the skull and merge into the circle of Willis. Vertebral arteries run along the cervical vertebrae and enter the skull and join to form the basilar artery, which merges into the circle of Willis. On the basis of AP gradient measurements, extracranial arteries are responsible for ~20% of the total cerebrovascular resistance34, which suggests that they contribute to the regulation of cerebral perfusion. b, Schematic representation of the circle of Willis and its major branches. Image provided by A. Gupta.
Heterogeneity of the vessel wall.
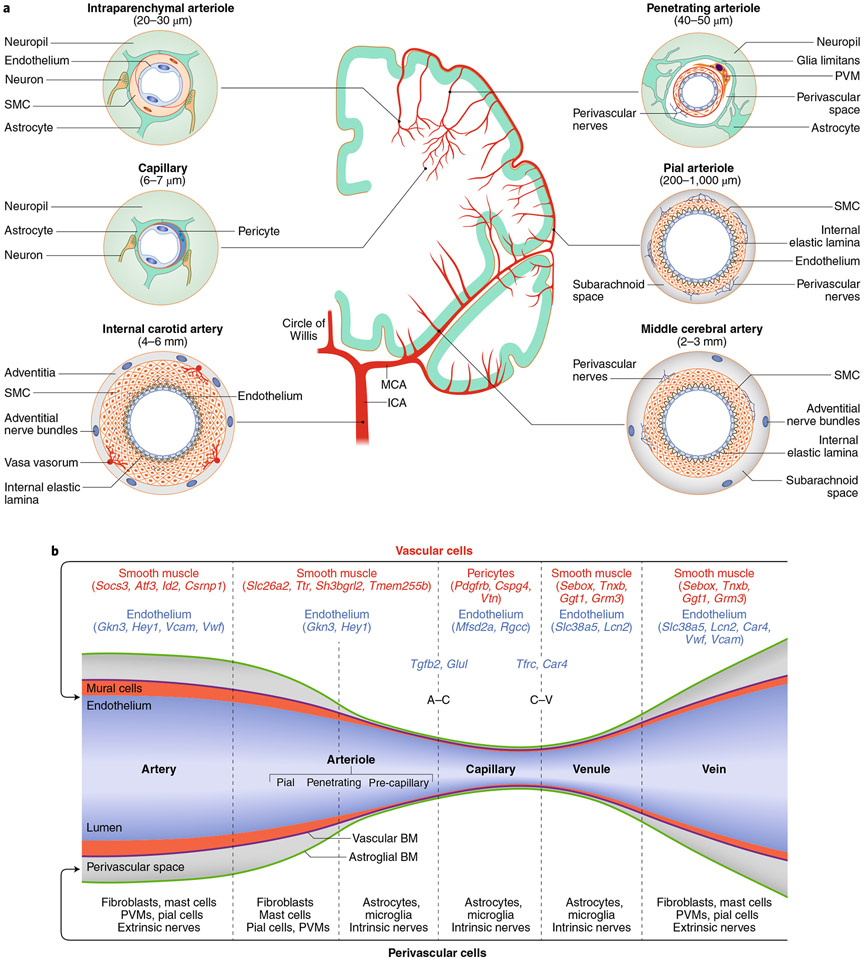
Large extracranial and intracranial cerebral arteries have multiple layers of contractile SMCs, which are ring-shaped cells encircling the endothelial basement membrane (Fig. 2a). As the arteries become smaller, the number of SMCs decreases, down to a single layer in arterioles. At this level, the endothelial and SMC basement membrane join together and are separated from the astrocytic basement membrane by the perivascular space12 (Fig. 2a,b). In smaller arterioles, the vascular basement membrane joins the basement membrane enveloping astrocytic end-feet and vessel-associated microglia, and the perivascular space disappears3,12 (Fig. 2a,b). In capillaries, SMCs are replaced by pericytes nestled into the endothelial basement membrane (Fig. 2a,b). On the venous side, SMCs reappear surrounded by the vascular basement membranes and the perivascular space (Fig. 2b). Compared to arteries, veins are endowed with fewer SMCs, which differ from arterial SMCs with respect to their flattened shape, reduced contractility and distinct transcriptomics profile13,14.
Fig. 2 ∣. Segmental heterogeneity of cerebral arteries and diversity of vascular and perivascular cells.
a, The internal carotid artery has a thick layer of SMCs surrounded by nerves arising from cranial autonomic ganglia (extrinsic innervation) embedded in perivascular connective tissue (adventitia). The internal elastic lamina separates SMCs from the endothelial cell monolayer. In the middle cerebral artery (MCA) and pial arteriolar branches, the SMC layer becomes progressively thinner, and a perivascular nerve plexus surrounds the vascular wall. Penetrating arterioles dive into the substance of the brain surrounded by a perivascular space where perivascular macrophages (PVMs) and other cells reside. As the vessel becomes smaller (intraparenchymal arterioles), the vascular basement membrane fuses with the glial basement membrane and perivascular nerves are replaced by nerve terminals from interneurons or subcortical pathways (intrinsic innervation). In capillaries, SMCs are replaced by pericytes. Vascular diameters indicated under the vascular segments refer to the human cerebral circulation. Venous SMCs are morphologically, functionally and molecularly distinct from arterial SMCs. b, Each segment of the cerebrovascular tree is characterized by diverse vascular and perivascular cells. The vascular and astroglial membranes delimit the perivascular space, which disappears when these membranes fuse together. Pial arterioles give rise to penetrating arterioles, the first-order branch of which is defined as precapillary arterioles14. For mural and endothelial cells, genes enriched in each vascular segment are also indicated. For SMCs, we used the database from ref. 13, in which segmental assignment was validated by in situ hybridization. For endothelial cells, we used ref. 17, in which the segmental assignment was predicted in silico. A–C represents marker endothelial genes at the arteriolar–capillary transition and C–V at the capillary–venular transition. BM, basement membrane; ICA, internal carotid artery.
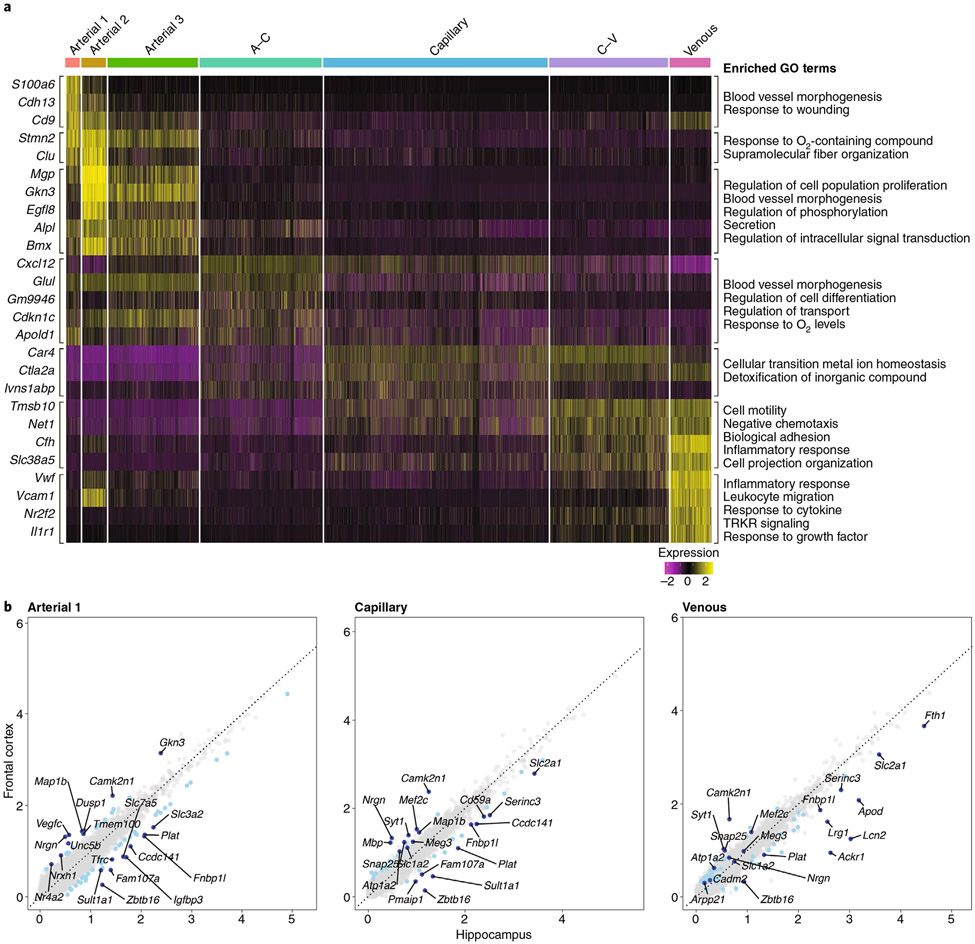
Recent single-cell RNA-seq studies have revealed several clusters of endothelial cells and mural cells assigned to different vascular segments and brain regions13,15,16. Despite their morphological homogeneity, endothelial cells are more heterogeneous at the transcriptomics level than mural cells13,17, and their molecular signature is markedly distinct from that of endothelial cells of other organs17. We mined the single-cell RNA-seq dataset from Saunders et al.15 and found seven distinct clusters of endothelial cells assigned to specific vascular segments based on previously established markers13,17 (Fig. 3a). Gene ontology (GO) analysis revealed substantial differences among endothelial cell clusters, such as enrichment of remodeling and structural-organization-related genes in arteries (S100a6, Cdh13, Clu, Alpl and Glul), in transport, in metabolism and in O2-response genes in capillaries (Slc16a1, Car4, Fmo2 and Ivns1abp) and in inflammation-response genes in veins (Cfh, Il1r1, Vwf and Vcam1) (Fig. 3a), which is in line with the wall remodeling property of arteries, the transport function of capillaries and the sensitivity of veins to inflammatory signaling11,18,19. Substantial diversity was also observed when endothelial cells were stratified by brain region, such as the frontal cortex and the hippocampus (Fig. 3b). For example, consistent with the susceptibility of the hippocampus to vascular inflammation and injury20-22, inflammatory response and cell death genes (Serinc3, Lcn2, Pmai1, Ackr1 and Plat) were relatively more enriched in the hippocampus than the cortex (Fig. 3b and Supplementary Table 1). Furthermore, consistent with the involvement of the endothelium in hippocampal neuroplasticity23,24, enrichment of GO terms reflecting blood vessel morphogenesis (Lrg1), cell adhesion (Fam107a, Zbtb16 and Apod), proliferation and plasticity (CD59a, Fth1 and Apod) was observed (Fig. 3b and Supplementary Table 1). How such molecular heterogeneity leads to functional diversity in health and disease remains to be established (see the section “Conclusions and future directions”).
Fig. 3 ∣. Endothelial expression heatmap and scatter plot of differentially expressed genes in the neocortex and the hippocampus.
a, Analysis of single-cell RNA-seq data from the Saunders database of whole-brain endothelial cells15. The heatmap shows scaled, log-normalized expression of the top discriminative genes per endothelial cell cluster (left) identified using the SEURAT toolkit. The color bar (top) denotes assignment for endothelial cells ordered by position in the vascular tree according to validated markers13,17. On the right, the GO terms that define the biological process in which the differentially expressed genes may be involved are presented. GO terms were derived from the biological process subset of MSigDB’s v7.1 GO gene sets (C5) for Mus musculus. All significant differentially expressed genes were used for analysis, and resulting pathways with a false discovery rate q-value of <0.05 are presented (see Supplementary Methods for details). b, Scatter plot of differentially expressed genes in neocortical and hippocampal endothelial cells. Comparative analysis of endothelial genes (gray dots) scaled, log-normalized expression in the frontal cortex and the hippocampus mined from the Saunders database15. Differentially expressed genes between the frontal cortex and the hippocampus are indicated in light blue, with the top ten most regulated genes indicated in dark blue. The GO terms referring to these differentially expressed genes are presented in Supplementary Table 1 (see Supplementary Methods for details).
Heterogeneity of perivascular cell types.
The cerebrovascular tree is surrounded by a wide variety of cells closely associated with the outer vessel wall. The internal carotid artery is surrounded by fibroblast-like cells embedded into the connective tissue of the adventitia (Fig. 2a,b). As they enter the subarachnoid space, arteries and veins become enveloped by an intricate network of fibrous septations of the arachnoid membrane together with fibroblasts, leptomeningeal cells lining the pia and arachnoid, mast cells and meningeal macrophages12,25-27 (Fig. 2a,b). Penetrating arterioles and ascending venules are surrounded by perivascular macrophages and by leptomeningeal cells from the pia, as well other less well-defined cell types12,15,16,26-28. Unbiased RNA-seq has unveiled several clusters of perivascular fibroblasts enriched with matrix-related genes that may contribute to the formation of basement membranes15,16 and, in neuroinflammation, to the fibrotic scar28.
Heterogeneity of neurovascular associations.
In humans as in animals, neural elements and vessels are in close contact throughout the neurovascular network. Nerve bundles originating from cranial autonomic ganglia encircle extracranial and intracranial cerebral arteries to form a dense plexus29 (Fig. 2a). As penetrating arterioles dive into the brain, the perivascular nerve plexus eventually disappears. At this level, vessels are in close contact with dendrites and terminals originating from local interneurons and subcortical nuclei, mainly the locus coeruleus, the ventral tegmental area, the raphe nucleus and the basal forebrain10,29-31. These subcortical projections often terminate on interneurons32.
Role of systemic versus brain factors
The introduction of brain imaging methods to monitor regional CBF changes in the behaving human brain1 emphasized the role of intrinsic neuronal mechanisms regulating cerebral perfusion, such as neurovascular coupling and the blood–brain barrier (BBB)1,7,11,33. While this shift in interest has led to a better understanding of neurovascular interactions and blood–brain exchange, it has de-emphasized the profound impact of systemic factors, such as blood pressure and blood gases, on CBF and the role of extracerebral arteries in regulating cerebrovascular resistance19,34 (Fig. 1a). As discussed in the next section, the moment-to-moment control of cerebrovascular function depends on the delicate balance between systemic and brain-intrinsic regulatory mechanisms.
Systemic factors.
Factors extrinsic to the brain exert powerful and widespread effects on CBF that engage the cerebrovascular tree at all levels and act with segmental specificity. Many systemic factors can influence CBF, such as posture, circulating glucose, hormones and peptides, hematocrit, blood viscosity and cold stress, among others35,36. Here, we focus on selected mechanisms that contribute to the integrated control of the cerebral vasculature: arterial pressure (AP) and blood gases.
Arterial pressure.
AP is the driving force propelling blood through cerebral blood vessels. Consequently, adequate cerebral perfusion is highly dependent on sufficient perfusion pressure. When AP falls by more than 30–40%, CBF falls, brain function ceases and loss of consciousness ensues within seconds37. Owing to the sensitivity of CBF to variations in AP, brain vessels are equipped with a buffering system that attempts to stabilize CBF during AP changes (cerebrovascular autoregulation). However, two features limit the ability of autoregulation to counteract AP changes: (1) it is effective within a limited range of APs (between +20 and −20 mmHg of baseline AP) and (2) it is engaged with a latency of several seconds35. Therefore, sudden AP changes or changes that exceed the regulated range in either direction induce passive changes in CBF. Considering that AP in humans fluctuates rapidly and widely during activities of daily living38, AP emerges as a major determinant of regional cerebral perfusion. Cerebrovascular autoregulation depends on the intrinsic property of SMCs to induce vasoconstriction when intravascular pressure increases, to impede flow, and vasodilatation when pressure decreases, to facilitate flow. This property, termed the myogenic response39, results from the interplay between stretch-activated ion channels, G-coupled receptors and modulation of Ca2+ sensitivity of the contractile apparatus. Ultimately, increases in AP lead to Ca2+ rises in SMCs that activate myosin light-chain kinase and trigger actin–myosin filament crosslinking, which in turn induces SMC contraction and vasoconstriction. These vascular adjustments involve extracranial, intracranial and intracerebral arteries and arterioles19,39.
Blood gases.
Elevations in the arterial partial pressure of CO2 (pCO2; hypercapnia) or reductions in the arterial partial pressure of O2 (pO2; hypoxia) induce global increases in CBF40. Reductions in pCO2 (hypocapnia) reduce CBF40. Hypercapnia and hypoxia induce dilatation of all segments of the cerebral circulation40-42, and are often associated with increases in AP that amplify their impact on CBF40. Since cerebral blood vessels are most sensitive to pCO2 changes in the physiological range (in humans, pCO2 of 30–50 mmHg)40, pCO2 is another powerful determinant of the moment-to-moment regulation of CBF. The mechanisms of hypercapnic vasodilation include not only SMC relaxation produced by the direct effect of CO2 and pH but also neuronal nitric oxide (NO) and acid-sensing channels43,44. Hypoxia increases CBF only when pO2 is below 50 mmHg (80% O2 saturation)41. Therefore, systemic arterial pO2 is not a major regulation of CBF in the normal state, but its effects are critical for preserving cerebral oxygenation in systemic hypoxia (see the section “Integrated vascular responses”). In brain tissue, O2 changes influence local CBF and may regulate capillary flow45. Mechanisms implicated in hypoxic cerebral vasodilatation include direct effects of hypoxemia on the blood vessel wall, vasoactivity of mediators released from the hypoxic brain, such as lactate and adenosine1, as well as activation of brainstem O2-sensing nuclei46.
Intrinsic factors.
Unlike systemic factors, intrinsic signals regulating CBF arise within the substance of the brain and spread retrogradely through the cerebrovascular tree. The resulting changes in CBF can be highly localized or diffuse. Here, we focus on neurovascular coupling (NVC) and on the increase in CBF induced by the activation of subcortical neural pathways.
Neurovascular coupling.
The close relationship between neuronal and vascular function is one of the most distinctive properties of the cerebral circulation and underlies functional imaging signals8. The profound impact that neurons exert on the brain vasculature is exemplified by the observation that activation of the visual cortex by transitioning from 7 days of dark housing to light induces more transcriptomics changes in vascular cells than in neurons47. While delivery of O2 and glucose through blood flow is one of its preeminent roles, NVC is also involved in proteostasis, neuroimmune trafficking, waste clearance, brain temperature regulation and experience-dependent hippocampal neurogenesis23,48-50.
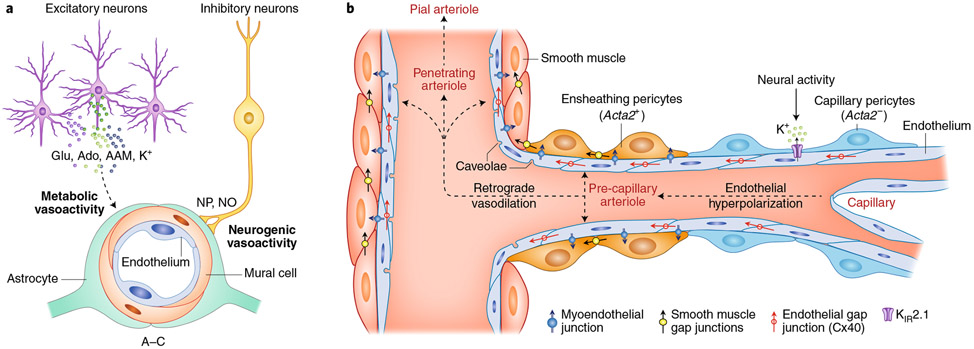
NVC results from a sequence of highly coordinated multicellular events: a local response involving microvessels near the active site and a remote response transmitted upstream to larger arteries9. The local response is thought to result from the release from brain cells of a multitude of vasoactive mediators, including prostanoids, neurotransmitters, neuropeptides, ions, NO and local hypoxia, which leads to adenosine production (see ref. 1 for more details) (Fig. 4a). These mediators may target specific vascular segments. For example, neuronal NO, which is also implicated in NVC in humans1,51, may specifically target intracerebral arterioles52. Activation of either excitatory or inhibitory neurons modulates CBF through NO, prostanoids, neurotransmitters and neuropeptides53-57. However, the vascular response driven by excitatory neurons leads to large increases in neural activity and O2 consumption, while interneurons induce powerful vascular responses with minimal or no increases in neural activity or energy expenditure54,58-60. These observations, in concert with the well known microvascular proximity of interneurons and their processes29,31,32, suggest that there are distinct mechanisms through which neural activity increases CBF. That is, a metabolically driven increase mediated by vasoactive mediators released by excitatory neurons and a neurogenically driven increase resulting from interneurons (Fig. 4a). The relative contribution of these mechanisms to NVC in vivo remains unclear. Supporting the involvement of interneurons, depletion of cerebellar interneurons (stellate cells) suppresses the increase in CBF induced by sensory stimulation without affecting local neural activity61.
Fig. 4 ∣. Local and remote vascular components of NVC.
a, Excitatory neurons have a powerful effect on local neural activity and energy metabolism, and may drive local vascular changes through neurotransmitters, vasoactive ions, as well as by-products of neural activity such as adenosine (Ado) and arachidonic acid metabolites (AAMs). Astrocytes may also participate in this process. Interneurons, which have little impact on local neural activity, may signal blood vessels through direct neurovascular connections releasing neuropeptides (NPs) and NO. Glu, glutamate. b, The local vascular response elicited by overall neural activity leads to hyperpolarization of endothelial cells through KIR2.1 channels. Whether mesh and thin-strand (capillary) pericytes (ACTA2-negative)71 participate in this process remains unclear. Hyperpolarization is propagated retrogradely through inter-endothelial gap junctions and is transmitted to contractile mural cells, ACTA2-containing ensheathing pericytes71 and SMCs, likely via myoendothelial junctions and KIR channels. In turn, mural cell hyperpolarization suppresses voltage-gated Ca2+ channel activity, resulting in intracellular Ca2+ depletion, relaxation of the contractile apparatus and vasodilatation. The relaxation is then transmitted to adjacent mural cells through intercellular gap junctions, leading to retrograde vasodilation, which eventually reaches pial arterioles at the brain surface.
There has been a growing interest in the role of capillaries in NVC62,63. The involvement of capillaries was suggested by descriptions of activity-induced capillary dilatations due to relaxation of pericytes62 and by vascular modeling studies pointing to the capillary bed as a major site of vascular resistance and flow regulation62,63. Consistent with this view, a 20–30% reduction in pericyte number in Pdgfrb−/+ mice blunts NVC64, while a 60% subacute pericyte loss by Pdgfrb-targeted expression of the diphtheria toxin receptor leads to a 50% reduction in resting CBF65. In contrast, others found that pericyte depletion (20–30%) increases flow velocity in precapillary arterioles with preserved vasoreactivity to hypercapnia66. Furthermore, several studies failed to demonstrate pericyte vasoactivity and capillary dilatations as initial drivers of NVC45,67-69, and more realistic models based both on flow and vascular topology placed the major site of microvascular flow regulation at the level or arterioles not capillaries70. Subsequent investigations in which the activated vascular network was carefully reconstructed revealed that first-order branches of penetrating arterioles (precapillary arterioles)71 are the first vascular segments to relax during NVC67,72,73 and that the dilatation or flow increase in downstream capillary branches, if any45,68, is delayed and has been considered passive67,73. In precapillary arterioles, mural cells have ovoid cell bodies, typical of canonical pericytes71, but are also endowed with the contractile protein ACTA2, typical of SMCs, and may represent transitional mural cells with a hybrid SMC–pericyte phenotype, termed ensheathing pericytes71,74. ACTA2-positive precapillary sphincters located on first-order capillaries can dynamically regulate capillary flow distribution during NVC75. These structures are unlikely to relate to ensheathing pericytes since the vasomotor dynamics of ensheathing pericytes are slower than classical SMCs14. Single-cell transcriptomics studies have not provided molecular insight into the morphological diversity of pericytes, since these cells fall in a single cluster13,76. In this regard, in vivo optogenetic activation of carefully phenotyped pericytes in reconstructed microvascular networks showed that ACTA2-negative capillary pericytes are also contractile, but with a much slower time-scale than SMCs or even ensheathing pericytes14. Whether and how these capillary pericytes contribute to NVC requires further exploration, but their slow vasoactive dynamics suggest that they have a greater impact on resting microvascular perfusion than fast hemodynamic responses.
Concerning the remote response, activity-induced vasodilation of upstream vessels is thought to be mediated by retrograde propagation of vasodilation and local autoregulatory adjustments to the intravascular pressure changes induced by downstream vasodilatation1. In interconnected vascular networks, coordination of downstream and upstream vasodilation is needed to effectively increase flow while avoiding a flow steal from adjacent vascular territories77. Therefore, the local vascular response needs to be coordinated with a remote response upstream. During NVC, endothelial cells sense and transmit neurovascular signals upstream to SMCs, which are the effectors responsible for the vasodilation that increases blood flow78. A proposed mechanism for the retrograde propagation is that K+ ions released during neural activity activate endothelial inward rectifier KIR2.1 channels, which results in endothelial hyperpolarization79 that spreads retrogradely to adjacent endothelial cells, perhaps through gap junctions80 (Fig. 4b). Another pathway includes Ca2+ signals generated by transient receptor potential ankyrin-1 (TRPA1) channels in capillary endothelial cells, propagated upstream via endothelial pannexin-1 and purinergic signaling81. At the level of arterioles, the hyperpolarization process propagates from the endothelium to electrically coupled SMCs via myoendothelial junctions, thereby resulting in vasodilation80, although SMC KIR channels also seem to be involved81. Caveolae, which are invaginations of the endothelial membrane and are abundant in arterioles, and endothelial NO synthase have recently been implicated in NVC through independent mechanisms82. Furthermore, endothelial cells express neurotransmitter receptor subunits13 and could be targeted by neurotransmitters. In support of this hypothesis, endothelial downregulation of the NMDA receptor subunit GluN1 suppresses NVC73. It remains unclear whether caveolae and endothelial NMDA receptors participate in local and/or remote responses. Therefore, NVC is initiated in the substance of the brain and requires not only brain cells at the site of activation but also local and remote mechanisms engaging all the cells of the vascular wall.
The retrograde vasodilatation of larger vessels upstream would flood their entire territory unless the hemodynamic response is restricted to the activated area9. Interneurons or neurovascular projections from the locus coeruleus could focus the hemodynamic response to the activated area by inducing localized vasoconstriction55,83,84, but vascular mechanisms could also be at play. In the retina, pericytes located on adjacent capillary networks are connected by thin membrane extensions (nanotubes) terminating in gap junctions that allow intercellular Ca2+ fluxes85. Through these nanotubes, light-induced pericyte relaxation and capillary dilatation is coupled to simultaneous contraction of the paired pericyte and capillary constriction in the adjacent network85. These pericytes bridges, as well as ACTA-positive pericytes located at capillary branch points86, could contribute to limit the spatial extent of the vasodilatation response.
Subcortical pathways.
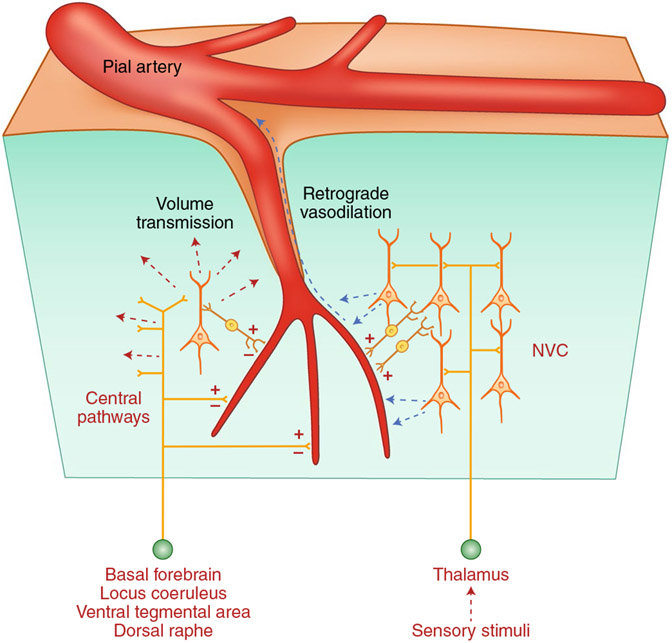
Subcortical pathways originating in the basal forebrain87, the raphe10, the locus coeruleus30 and the ventral tegmental area88 have profound effects on CBF (Fig. 5). These hemodynamic effects result from different mechanisms, including direct neurovascular innervation, activation of local neurons and volume transmission of neurotransmitter released89. The functional role of these pathways is not clear, but they have been implicated in global changes in CBF during sleep and arousal (see the section “Integrated vascular responses”), focusing NVC to the activated areas83, modulation of BBB permeability90, cross-hemispheric neurovascular synchronization of cortical vasomotion33 and in anticipatory changes in CBF before activation91,92.
Fig. 5 ∣. Sources and targets of brain intrinsic vasoactive signals.
Central pathways arising from the basal forebrain and brainstem nuclei contact cerebral blood vessels directly or through interposed interneurons (intrinsic innervation) and can either increase (+) or decrease (−) CBF diffusely. Neurotransmitters released from these pathways may also affect more distant vessels through volume transmission. Somatosensory or visual stimuli originating from the thalamus produce localized increases in blood flow by activating local neurons, which in turn release vasoactive agents (NVC; see Fig. 4 for details).
Collectively, these observations indicate that the cerebrovascular network is regulated by segment-specific mechanisms that can be engaged either by systemic factors originating in the periphery and reaching into the brain or by factors arising from the brain and propagating backward toward the periphery (Fig. 6).
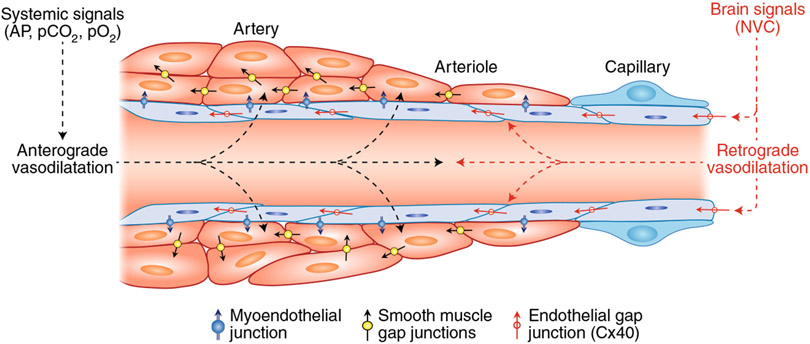
Fig. 6 ∣. Central and peripheral vasoactive signals regulating CBF.
Vasoactive signals arising from the periphery (systemic signals) exert anterograde effects on all segments of the cerebrovascular tree by acting mainly on SMCs (anterograde vasodilatation). Brain-intrinsic vasoactive signals evoked by NVC and, possibly, central pathways arise within the substance of the brain and propagate retrogradely to microvascular SMCs and beyond via inter-endothelial junctions (retrograde vasodilatation). During activities of daily living, in which major changes in AP and blood gases occur, maintenance of the perfusion and homeostatic balance of the brain depends on the dynamic and coordinated interaction of these vasoactive signals engaging all segments of the neurovascular complex.
Interaction of systemic and brain factors
The mechanisms mentioned above do not work in isolation but are highly interactive93. These complex interactions are critically important in the moment-to-moment regulation of CBF in behaving humans, which is discussed next.
Integrated vascular responses.
The interaction of neurovascular regulatory mechanisms is best appreciated in the CBF changes induced by activities of daily living. Exercise and sleep, conditions associated with profound changes in systemic metabolic and hemodynamic variables, as well as brain function, exemplify the intricate central and peripheral coordination needed to assure adequate cerebral perfusion.
Exercise.
In humans, exercise increases heart rate, cardiac output, arterial and venous pressure, sympathetic nerve activity, whole-body O2 and glucose metabolism, intracranial pressure and brain activity94. The three- to sixfold increase in cardiac output serves to provide the musculature with the blood supply needed to support its increased work94. Maintaining adequate cerebral perfusion to sustain brain activity in the face of these profound hemodynamic and metabolic changes requires the fine coordination of systemic and cerebral regulatory mechanisms. In humans, moderate exercise, <60% of maximal O2 uptake, leads to an ~25% increase in CBF95. These CBF changes are likely to result from increased brain activity through NVC and from the rise in pCO2 induced by moderate exercise96. In addition, cerebrovascular autoregulatory adjustments are needed to counteract the concomitant increase in AP. Interestingly, despite these marked hemodynamic and metabolic changes, neural activity is still able to further increase blood flow velocity97. With intense exercise, CBF returns to normal despite persistent increases in brain activity and AP96. The mechanisms of CBF reduction may involve, at least in part, a reduction in pCO2 caused by the hyperventilation associated with intense exercise96 and sympathetic activation leading to constriction of cerebral arteries (but see ref. 98). Therefore, the interaction between autoregulation, CO2 reactivity and NVC is critical for the maintenance of cerebral perfusion during exercise. In addition, a role of breathing in cerebral oxygenation was recently identified in ambulatory mice99, thereby providing additional evidence of the intricate relationship between central and peripheral factors in cerebrovascular function.
Sleep.
Cerebrovascular function during sleep is vitally important for the clearance of metabolites needed to maintain the homeostasis of internal milieu of the brain100. At sleep onset in humans (stage I–II), neural activity shifts from the alpha rhythm to the slower theta rhythm (alpha–theta transition) associated with a reduction in AP and an increase in CBF101. In stage III/IV non-rapid eye movement (REM) sleep, CBF and brain metabolic activity are reduced, despite increased pCO2 and reduced pO2 that would tend to counteract the CBF reduction102,103. This paradox may be explained by the observation that the CBF reactivity to hypoxia and hypercapnia are suppressed at this sleep stage104,105 so that respiratory gases have little impact on CBF. Progressing to REM sleep, CBF and brain activity return to normal103,106. During awakening (theta–alpha transition), there is a reduction in CBF even though AP increases101. Overall, the neurovascular changes during sleep cannot be explained by any single factor regulating CBF, and results from an integrated response involving not only systemic factors (AP and pCO2) and NVC102 but also sympathetic perivascular nerves107 and, possibly, central pathways arising from the basal forebrain, the raphe, the ventral tegmental area, the locus coeruleus and the parabrachial nucleus, which are involved in the neural mechanisms underlying sleep108 and can broadly modulate CBF (Fig. 5).
These observations exemplify the integrated regulation of CBF during common human activities, in which systemic and brain intrinsic factors work in concert to safeguard cerebral perfusion while maintaining the metabolic and energetic homeostasis of the whole body.
Functional brain imaging implications
Systemic variables can confound the assessment of neuronal connectivity by fMRI33. Very low frequency oscillations in blood oxygen level-dependent (BOLD) signals are assumed to reflect hemodynamic correlates of neural activity, and synchrony of these oscillations across the brain is widely used to assess connectivity between different brain regions, for example, resting-state fMRI33. Aortic pulsatility and breathing-induced fluctuations in end-tidal CO2 and blood oxygenation have an impact on BOLD, causing very low frequency oscillations across the whole brain109-111. These in turn give rise to spurious white matter signals112 and may underlie sex-biased neuronal connectivity113. Synchronous BOLD signals linked to these systemic factors can be erroneously interpreted as neuronal and need to be considered in the interpretation of connectivity studies114. Another example is provided by the effect of AP increases on hemoglobin oxygenation, a proxy for the BOLD response, in the neonatal rat brain, in which autoregulation is not fully developed and AP can determine the direction of the signal change during activation115. Therefore, the potential impact of systemic variables needs careful consideration in brain imaging studies.
The concept of the neurovascular complex
These findings suggest that the cellular, molecular and functional diversity of the cerebrovascular tree cannot be adequately represented by a canonical NVU. First, diverse cell types and associated structures play a role in cerebrovascular function at different levels of the vascular network (Fig. 2a,b). Single-cell transcriptomics studies have shown that even the same cell type has a well-defined segmental molecular signature underlying diverse functions (Fig. 3a,b). Such molecular diversity is reflected in segmental differences in BBB permeability, vasoactivity and susceptibility to disruption and damage18,82,116-118. Second, neurovascular cells do not interact just with their immediate neighbors but reach out beyond their limited confines. While endothelial cells, pericytes and SMCs may propagate vasoactive signals along the vessel wall, cells in the perivascular space and vessel wall work in concert to enable trafficking of cells, proteins and interstitial fluid in and out the brain, which is essential for proteostasis, immune surveillance and hydrodynamic balance48,100,119,120. Third, the neurovascular network acts as a signaling source that regulates the homeostasis of neurons and glia throughout the entire brain in the normal state, in neuroinflammation and in neurodegeneration4,23,24,121. These effects involve different vascular and perivascular cells with exquisite segmental specificity. Therefore, there is no single NVU ‘clone’ replicated at all levels of the cerebral vasculature, but a complex of diverse neurovascular modules that reach all the way back to large extracranial vessels. At any given time, this heterogeneous neurovascular complex is regulated by systemic and brain-intrinsic vasoactive signals aimed to protect the structural and functional integrity of the brain.
The neurovascular complex in disease
The segmental heterogeneity of the cerebrovascular network and the integrated actions of systemic and brain intrinsic factors is also reflected in the cerebrovascular alterations occurring in disease states. Conditions in which central and peripheral pathogenic factors converge on different vascular segments leading to brain dysfunction and damage are examined next.
Neurodegenerative diseases.
Alzheimer’s disease (AD), a major cause of age-related cognitive impairment, is associated with neurovascular dysfunction that is considered an early pathogenic factor in the disease course122,123. The vascular dysfunction in AD target specific segments and cells of the vascular network with diverse pathogenic mechanisms. Amyloid-β (Aβ), a key culprit in AD, disrupts the major regulatory mechanisms of the cerebral circulation9. These effects are mediated by pial and intracerebral arterioles through activation of innate immunity receptors on perivascular and meningeal macrophages and production of free radicals by the enzyme NOX2 (ref. 124). Recent data also point to a role of capillary dysfunction in the vascular effects of Aβ. In mouse models of Aβ accumulation, transient occlusions of capillaries by circulating leukocytes mediate reductions in CBF and cognitive impairment125. Furthermore, Aβ may disrupt capillary flow distribution by targeting pericytes117. These effects may limit the equalization of flow in capillary networks (homogenization) required for efficient O2 delivery and cause dysfunction in energy-sensitive neural networks involved in learning and memory126. Hyperphosphorylated tau, the other major pathogenic factor in AD, selectively dampens arteriolar dilatation during NVC, an effect related to tau binding to the post-synaptic density leading to suppression of NO production during glutamatergic synaptic activity118. Human studies have also unveiled a key role of large cerebral arteries. Stiffness and increased pulsatility of large extracranial arteries feeding the brain is linked to AD, possibly by impairing their contribution to cerebrovascular resistance and CBF regulation122 (Fig. 1a). Furthermore, proteomics and single-nuclei RNA-seq data of AD have hinted at the contribution of atherosclerosis in large intracranial arteries by inducing synaptic dysfunction, independently of Aβ and tau127. Therefore, the vascular contribution to AD involves not only arterioles and capillaries but also large cerebral and extracerebral arteries.
In Parkinson disease (PD), a common movement disorder caused by a dopamine deficit and accumulation of the presynaptic protein α-synuclein, systemic factors have a well-established pathogenic impact. Alterations in breathing (hypoventilation and reduced sensitivity to hypoxia) and orthostatic hypotension (blood pressure drop while standing), which are linked to dysfunction of the autonomic nervous system128, are associated with worse deficits129-132 and cognitive impairment133,134. The negative impact of these systemic changes is compounded by blunting of cerebrovascular homeostatic mechanisms, such as NVC and CO2 reactivity130,135,136, which increase the susceptibility of the brain to injury. Similar central and peripheral alterations have been reported in other synucleinopathies, such as multisystem atrophy and dementia with Lewy bodies129,137. Sleep disorders are very common in these conditions and have been implicated in their pathobiology.
Sleep disorders.
Alterations in sleep are common in the general population, but have been closely associated with neurodegenerative diseases, particularly PD and other synucleinopathies138. Obstructive sleep apnea (OSA), which is characterized by frequent episodes of apnea during sleep139, provides an example of the impact of systemic factors on the brain. Apnea leads to increases in pCO2 and decreases in pO2, which would normally protect the brain against hypoxic damage by increasing the delivery of CBF. However, hypoxic and hypercapnic vasodilatation are suppressed during sleep (see the section “Integrated vascular responses”) so that the brain is unable to compensate for the reduced arterial pO2 by increasing CBF. As a result, the O2-carrying capacity of blood decreases during apneic episodes. Cyclic hypoxia-reoxygenation also leads to oxidative stress and increased expression of the potent vasoconstrictor endothelin in cerebral arterioles, thereby compromising NVC and endothelium-dependent vasodilation140. REM sleep behavior disorder, a prodromal sign of neurodegenerative diseases, particularly synucleinopathies, is characterized by dream-enacting behaviors and nightmares linked to REM sleep without the atonia (partial paralysis) that normally accompanies this sleep stage141. Although sleep disorders, particularly REM sleep behavior disorder, are often caused by dysfunction of brainstem centers controlling sleep, the bouts of apnea-induced hypoxia, sleep fragmentation and the disturbances in cerebrospinal fluid hydrodynamics from increased intrathoracic pressure, are thought to promote neurodegenerative pathology in vulnerable brain regions139,141. Thus, sleep disorders represent another example of the interaction of central and peripheral vascular pathomechanisms leading to brain dysfunction and damage.
Neurovascular diseases and traumatic brain injury.
Changes in AP, hormones and other systemic variables have a profound effect on the outcome of stroke and trauma. Ischemic and hemorrhagic strokes are often associated with an acute hypertensive response (AP > 140/90 mmHg), which, owing to the suppression of cerebrovascular autoregulation and BBB dysfunction, promotes cerebral edema, aggravates ischemic brain injury and is uniformly associated with worse outcome142,143. Similarly, autoregulation and CBF response to hypercapnia and hypoxia are blunted in brain trauma, and alterations in AP and blood gases play a pivotal role in the outcome for patients144. These conditions highlight the contribution of extracerebral vascular and metabolic factors to the outcome of brain injury.
Conclusions and future directions
The data presented above indicate that the neurovascular complex comprises heterogenous vascular modules regulated by factors intrinsic and extrinsic to the brain through segment-specific mechanisms. A previously unappreciated molecular diversity in vascular and perivascular cells has also emerged.
These observations have notable implications for neurovascular research. For example, co-cultures of NVU, including organoids and microfluidic devices in which three-dimensional cultures are subjected to shear stress, simulating the effect of blood flow145, are well suited to provide insight into neurovascular communication and disease mechanisms in patient-derived induced pluripotent stem cells146. While these are valuable insights, the diverse molecular signature of endothelial cells, mural cells and perivascular cells13,147,148 needs to be congruent with the vascular segment of interest. Therefore, using generic vascular cells, be they immortalized, primary or derived from induced pluripotent stem cells, may not reflect the unique molecular and functional phenotype of specific microvascular segment to be modeled.
Unbiased approaches to genotype neurovascular cells, for example, RNA-seq, have unveiled remarkable molecular diversity in vascular and perivascular cells. These efforts have generated lists of genes that the segmental localization and biological significance of which can only be inferred indirectly from generic GO lists. These transcriptomics data, if experimentally verified, would become a valuable resource for proteomics, metabolomics, structural and functional studies to more accurately phenotype functionally heterogenous neurovascular cells, for example, mural cells, and to gain a better understanding of their integrated role in the neurovascular complex.
The application of advanced brain imaging tools in concert with genetically encoded sensors and cell-specific markers have unveiled new features of neurovascular function related not only to blood flow and NVC but also to brain clearance, cerebrospinal fluid hydrodynamics, neuroplasticity, neurogenesis and BBB permeability18,23,24,48,120,149. The increasing use of awake behaving mice48,50,82,99 provides the opportunity to investigate the integrated control of the cerebral microcirculation and the influence of systemic factors, such as AP, blood gases and breathing, on cerebrovascular function99.
However, these studies need to account for the influence of systemic variables, such as AP and blood gases, which have not been routinely monitored150. This problem is particularly relevant to studies using awake, head-fixed behaving mice, in which changes in these variables are likely to occur and may affect the results. Habituation to head restraint may help minimize stress-induced arousal and autonomic responses, but it does not eliminate the effects of changes in systemic variables evoked by the stimuli delivered or accompanying the behavior being studied. A caveat is that monitoring AP and blood gases requires vascular access, which is invasive and could preclude behavioral testing and confound cerebrovascular assessments. Measurement of these variables in separate mice under identical experimental conditions could rule out major changes, but dynamic changes linked to behaviors could be missed. Noninvasive approaches to monitor in real-time the physiological state of the mice would be a welcome addition to our experimental toolbox.
Advances in noninvasive human brain imaging and assessment of large vessel function provide the unique opportunity to examine the integrated regulation of the neurovascular complex in humans in health and disease35 and avoid important confounders (see the section “Implications for functional brain imaging”). These studies may illuminate the functional interaction between extracranial and intracranial vessels and the influence of systemic factors, which may not be feasible in small laboratory animals. The combination of these approaches will also provide insight into the neurovascular correlates of complex behaviors and their alterations in disease, which may have diagnostic and therapeutic value.
In conclusion, the introduction of the NVU has led to advances in the understanding of the signaling mechanisms linking neurons and glia with the local microvasculature. However, the NVU concept does not account for the coordinated interaction of intracerebral microvascular events with larger arteries upstream and with vasoactive signals arising from the periphery, which are critical for the dynamic regulation of cerebrovascular function. These considerations, in concert with the segmental molecular diversity of vascular cells, suggest the concept of a neurovascular complex composed of distinct functional modules encompassing the entire cerebrovascular tree and regulated by factors intrinsic and extrinsic to the brain. Efforts to elucidate the mechanisms governing the neurovascular complex may provide an illuminating look at the integrated regulation of cerebrovascular function with major implications for the vascular pathobiology of human diseases affecting the brain.
Supplementary Material
Acknowledgements
We thank A. Gupta for providing the image of human brain vessel (Fig. 1a) and J. Anrather for input on the transcriptomics analysis. Supported by NIH grants R01-NS34179, R01-NS100447, R37-NS089323, R01-NS095441 and R01-NS/HL37853 to C.I.
Footnotes
Competing interests
C.I. serves on the Scientific Advisory Board of Broadview Ventures. S.S. has no conflicts to declare.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41593-021-00904-7.
References
- 1.Iadecola C The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves de Lima K, Rustenhoven J & Kipnis J Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu. Rev. Immunol 38, 597–620 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Koizumi T, Kerkhofs D, Mizuno T, Steinbusch HWM & Foulquier S Vessel-associated immune cells in cerebrovascular diseases: from perivascular macrophages to vessel-associated microglia. Front. Neurosci 13, 1291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraco G et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 574, 686–690 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paredes I, Himmels P & Ruiz de Almodovar C Neurovascular communication during CNS development. Dev. Cell 45, 10–32 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Tsai HH et al. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science 351, 379–384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attwell D et al. Glial and neuronal control of brain blood flow. Nature 468, 232–243 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attwell D & Iadecola C The neural basis of functional brain imaging signals. Trends Neurosci. 25, 621–625 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Iadecola C Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci 5, 347–360 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Cohen ZVI, Bonvento G, Lacombe P & Hamel E Serotonin control of the regulation of the brain microcirculation. Prog. Neurobiol 50, 335–362 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Kaplan L, Chow BW & Gu C Neuronal regulation of the blood–brain barrier and neurovascular coupling. Nat. Rev. Neurosci 21, 416–432 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannocks M-J et al. Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow. Metab 38, 669–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanlandewijck M et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Hartmann DA et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci 24, 633–645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders A et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lendahl U, Nilsson P & Betsholtz C Emerging links between cerebrovascular and neurodegenerative diseases-a special role for pericytes. EMBO Rep. 20, e48070 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalucka J et al. Single-cell transcriptome atlas of murine endothelial cells. Cell 180, 764–779.e20 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Santisteban MM et al. Endothelium–macrophage crosstalk mediates blood–brain barrier dysfunction in hypertension. Hypertension 76, 795–807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu X, De Silva TM, Chen J & Faraci FM Cerebral vascular disease and neurovascular injury in ischemic stroke. Circ. Res 120, 449–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen MB et al. Brain endothelial cells are exquisite sensors of age-related circulatory cues. Cell Rep. 30, 4418–4432.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson-Leary J et al. Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress. Transl. Psychiatry 7, e1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkhelfa M et al. The involvement of neuroinflammation and necroptosis in the hippocampus during vascular dementia. J. Neuroimmunol 320, 48–57 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Shen J et al. Neurovascular coupling in the dentate gyrus regulates adult hippocampal neurogenesis. Neuron 103, 878–890.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan C et al. Endothelium-derived semaphorin 3G regulates hippocampal synaptic structure and plasticity via neuropilin-2/plexinA4. Neuron 101, 920–937.e13 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Kierdorf K, Masuda T, Jordão MJC & Prinz M Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci 20, 547–562 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Silver R & Curley JP Mast cells on the mind: new insights and opportunities. Trends Neurosci. 36, 513–521 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Weller RO, Sharp MM, Christodoulides M, Carare RO & Möllgård K The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 135, 363–385 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Dorrier CE et al. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration. Nat. Neurosci 24, 234–244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iadecola C et al. Nitric oxide synthase-containing neural processes on large cerebral arteries and cerebral microvessels. Brain Res. 606, 148–155 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Toussay X, Basu K, Lacoste B & Hamel E Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J. Neurosci 33, 3390–3401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaucher E, Tong XK, Cholet N, Lantin S & Hamel E GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J. Comp. Neurol 421, 161–171 (2000). [PubMed] [Google Scholar]
- 32.Cauli B et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci 24, 8940–8949 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drew PJ, Mateo C, Turner KL, Yu X & Kleinfeld D Ultra-slow oscillations in fMRI and resting-state connectivity: neuronal and vascular contributions and technical confounds. Neuron 107, 782–804 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraci FM & Heistad DD Regulation of large cerebral arteries and cerebral microvascular pressure. Circ. Res 66, 8–17 (1990). [DOI] [PubMed] [Google Scholar]
- 35.Claassen J, Thijssen DHJ, Panerai RB & Faraci FM Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol. Rev 10.1152/physrev.00022.2020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes JN & Charkoudian N Integrative cardiovascular control in women: regulation of blood pressure, body temperature, and cerebrovascular responsiveness. FASEB J. 35, e21143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith BA, Clayton EW & Robertson D Experimental arrest of cerebral blood flow in human subjects: The Red Wing Studies revisited. Perspect. Biol. Med 54, 121–131 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawano Y Diurnal blood pressure variation and related behavioral factors. Hypertens. Res 34, 281–285 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Lidington D, Kroetsch JT & Bolz S-S Cerebral artery myogenic reactivity: the next frontier in developing effective interventions for subarachnoid hemorrhage. J. Cereb. Blood Flow. Metab 38, 17–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoiland RL, Fisher JA & Ainslie PN Regulation of the cerebral circulation by arterial carbon dioxide. Compr. Physiol 9, 1101–1154 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Lewis NCS, Messinger L, Monteleone B & Ainslie PN Effect of acute hypoxia on regional cerebral blood flow: effect of sympathetic nerve activity. J. Appl. Physiol 116, 1189–1196 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willie CK et al. Regional brain blood flow in man during acute changes in arterial blood gases. J. Physiol 590, 3261–3275 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iadecola C Does nitric oxide mediate the increases in cerebral blood flow elicited by hypercapnia? Proc. Natl Acad. Sci. USA 89, 3913–3916 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faraci FM et al. Acid-sensing ion channels: novel mediators of cerebral vascular responses. Circ. Res 125, 907–920 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei HS et al. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron 91, 851–862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golanov EV & Reis DJ Contribution of oxygen-sensitive neurons of the rostral ventrolateral medulla to hypoxic cerebral vasodilatation in the rat. J. Physiol 495, 201–216 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hrvatin S et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci 21, 120–129 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Veluw SJ et al. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron 105, 549–561.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu M, Ackerman JJ & Yablonskiy DA Body and brain temperature coupling: the critical role of cerebral blood flow. J. Comp. Physiol. B 179, 701–710 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kedarasetti RT et al. Functional hyperemia drives fluid exchange in the paravascular space. Fluids Barriers CNS 17, 52 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoiland RL et al. Nitric oxide is fundamental to neurovascular coupling in humans. J. Physiol 598, 4927–4939 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Mishra A et al. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci 19, 1619–1627 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krawchuk MB, Ruff CF, Yang X, Ross SE & Vazquez AL Optogenetic assessment of VIP, PV, SOM and NOS inhibitory neuron activity and cerebral blood flow regulation in mouse somato-sensory cortex. J. Cereb. Blood Flow. Metab 40, 1427–1440 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Echagarruga C, Gheres KW, Norwood JN & Drew PJ nNOS-expressing interneurons control basal and behaviorally-evoked arterial dilation in somatosensory cortex of mice. eLife 9, e6053 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee L et al. Key aspects of neurovascular control mediated by specific populations of inhibitory cortical interneurons. Cereb. Cortex 30, 2452–2464 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacroix A et al. COX-2-derived prostaglandin E2 produced by pyramidal neurons contributes to neurovascular coupling in the rodent cerebral cortex. J. Neurosci 35, 11791–11810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JH et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anenberg E, Chan AW, Xie Y, LeDue JM & Murphy TH Optogenetic stimulation of GABA neurons can decrease local neuronal activity while increasing cortical blood flow. J. Cereb. Blood Flow. Metab 35, 1579–1586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vazquez AL, Fukuda M & Kim SG Inhibitory neuron activity contributions to hemodynamic responses and metabolic load examined using an inhibitory optogenetic mouse model. Cereb. Cortex 28, 4105–4119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma Y et al. Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proc. Natl Acad. Sci. USA 113, E8463–E8471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang G, Huard JM, Beitz AJ, Ross ME & Iadecola C Stellate neurons mediate functional hyperemia in the cerebellar molecular layer. J. Neurosci 20, 6968–6973 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall CN et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gould IG, Tsai P, Kleinfeld D & Linninger A The capillary bed offers the largest hemodynamic resistance to the cortical blood supply. J. Cereb. Blood Flow. Metab 37, 52–68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kisler K et al. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci 20, 406–416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikolakopoulou AM et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci 22, 1089–1098 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson AN et al. Mild pericyte deficiency is associated with aberrant brain microvascular flow in aged PDGFRβ+/− mice. J. Cereb. Blood Flow. Metab 40, 2387–2400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rungta RL, Chaigneau E, Osmanski BF & Charpak S Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron 99, 362–375.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill RA et al. Regional blood flow in the normal and ischemic brain Is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J & Lindauer U Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl Acad. Sci. USA 107, 22290–22295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmid F, Tsai PS, Kleinfeld D, Jenny P & Weber B Depth-dependent flow and pressure characteristics in cortical microvascular networks. PLoS Comput. Biol 13, e1005392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grant RI et al. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J. Cereb. Blood Flow. Metab 39, 411–425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai C et al. Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses. Proc. Natl Acad. Sci. USA 115, E5796–E5804 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogan-Cann AD, Lu P & Anderson CM Endothelial NMDA receptors mediate activity-dependent brain hemodynamic responses in mice. Proc. Natl Acad. Sci. USA 116, 10229–10231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uemura MT, Maki T, Ihara M, Lee VMY & Trojanowski JQ Brain microvascular pericytes in vascular cognitive impairment and dementia. Front. Aging Neurosci 12, 80 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grubb S et al. Precapillary sphincters maintain perfusion in the cerebral cortex. Nat. Commun 11, 395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hariharan A et al. The ion channel and GPCR toolkit of brain capillary pericytes. Front. Cell Neurosci 14, 601324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Secomb TW Theoretical models for regulation of blood flow. Microcirculation 15, 765–775 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen BR, Kozberg MG, Bouchard MB, Shaik MA & Hillman EM A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J. Am. Heart Assoc 3, e000787 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Longden TA et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci 20, 717–726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zechariah A et al. Intercellular conduction optimizes arterial network function and conserves blood flow homeostasis during cerebrovascular challenges. Arterioscler. Thromb. Vasc. Biol 40, 733–750 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thakore P et al. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. eLife 10, e63040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chow BW et al. Caveolae in CNS arterioles mediate neurovascular coupling. Nature 579, 106–110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bekar LK, Wei HS & Nedergaard M The locus coeruleus–norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J. Cereb. Blood Flow. Metab 32, 2135–2145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devor A et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J. Neurosci 28, 14347–14357 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alarcon-Martinez L et al. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature 36, 451–455 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Gonzales AL et al. Contractile pericytes determine the direction of blood flow at capillary junctions. Proc. Natl Acad. Sci. USA 117, 27022–27033 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang F, Xu S & Iadecola C Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience 69, 1195–1204 (1995). [DOI] [PubMed] [Google Scholar]
- 88.Kolodziej A et al. SPECT-imaging of activity-dependent changes in regional cerebral blood flow induced by electrical and optogenetic self-stimulation in mice. NeuroImage 103, 171–180 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Lecrux C & Hamel E Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states. Philos. Trans. R. Soc. Lond. B Biol. Sci 371, 20150350 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raichle ME, Hartman BK, Eichling JO & Sharpe LG Central noradrenergic regulation of cerebral blood flow and vascular permeability. Proc. Natl Acad. Sci. USA 72, 3726–3730 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tan CO Anticipatory changes in regional cerebral hemodynamics: a new role for dopamine? J. Neurophysiol 101, 2738–2740 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sirotin YB & Das A Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 457, 475–479 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Willie CK, Tzeng YC, Fisher JA & Ainslie PN Integrative regulation of human brain blood flow. J. Physiol 592, 841–859 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Green DJ, Hopman MT, Padilla J, Laughlin MH & Thijssen DH Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol. Rev 97, 495–528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Braz ID & Fisher JP The impact of age on cerebral perfusion, oxygenation and metabolism during exercise in humans. J. Physiol 594, 4471–4483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith KJ & Ainslie PN Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol 102, 1356–1371 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Willie CK et al. Neurovascular coupling and distribution of cerebral blood flow during exercise. J. Neurosci. Methods 198, 270–273 (2011). [DOI] [PubMed] [Google Scholar]
- 98.Brassard P, Tymko MM & Ainslie PN Sympathetic control of the brain circulation: appreciating the complexities to better understand the controversy. Auton. Neurosci 207, 37–47 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Zhang Q et al. Cerebral oxygenation during locomotion is modulated by respiration. Nat. Commun 10, 5515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fultz NE et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 366, 628–631 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kotajima F, Meadows GE, Morrell MJ & Corfield DR Cerebral blood flow changes associated with fluctuations in alpha and theta rhythm during sleep onset in humans. J. Physiol 568, 305–313 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Corfield DR & Meadows GE Control of cerebral blood flow during sleep and the effects of hypoxia. Adv. Exp. Med. Biol 588, 65–73 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Townsend RE, Prinz PN & Obrist WD Human cerebral blood flow during sleep and waking. J. Appl. Physiol 35, 620–625 (1973). [DOI] [PubMed] [Google Scholar]
- 104.Meadows GE, Dunroy HM, Morrell MJ & Corfield DR Hypercapnic cerebral vascular reactivity is decreased, in humans, during sleep compared with wakefulness. J. Appl. Physiol 94, 2197–2202 (2003). [DOI] [PubMed] [Google Scholar]
- 105.Meadows GE, O’Driscoll DM, Simonds AK, Morrell MJ & Corfield DR Cerebral blood flow response to isocapnic hypoxia during slow-wave sleep and wakefulness. J. Appl Physiol 97, 1343–1348 (2004). [DOI] [PubMed] [Google Scholar]
- 106.Madsen PL et al. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. J. Appl Physiol 70, 2597–2601 (1991). [DOI] [PubMed] [Google Scholar]
- 107.Ozbay PS et al. Sympathetic activity contributes to the fMRI signal. Commun. Biol 2, 421 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scammell TE, Arrigoni E & Lipton JO Neural circuitry of wakefulness and sleep. Neuron 93, 747–765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hussein A et al. The association between resting-state functional magnetic resonance imaging and aortic pulse-wave velocity in healthy adults. Hum. Brain Mapp 41, 2121–2135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Birn RM, Diamond JB, Smith MA & Bandettini PA Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage 31, 1536–1548 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Wise RG, Ide K, Poulin MJ & Tracey I Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. NeuroImage 21, 1652–1664 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Özbay PS et al. Contribution of systemic vascular effects to fMRI activity in white matter. NeuroImage 176, 541–549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lynch CJ et al. Prevalent and sex-biased breathing patterns modify functional connectivity MRI in young adults. Nat. Commun 11, 5290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Das A, Murphy K & Drew PJ Rude mechanicals in brain haemodynamics: non-neural actors that influence blood flow. Philos. Trans. R. Soc. Lond. B Biol. Sci 376, 20190635 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kozberg MG, Chen BR, DeLeo SE, Bouchard MB & Hillman EMC Resolving the transition from negative to positive blood oxygen level-dependent responses in the developing brain. Proc. Natl Acad. Sci. USA 110, 4380–4385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mathiesen Janiurek M, Soylu-Kucharz R, Christoffersen C, Kucharz K & Lauritzen M Apolipoprotein M-bound sphingosine-1-phosphate regulates blood–brain barrier paracellular permeability and transcytosis. eLife 8, 13–22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nortley R et al. Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 365, eaav9518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park L et al. Tau induces PSD95–neuronal NOS uncoupling and neurovascular dysfunction independent of neurodegeneration. Nat. Neurosci 23, 1079–1089 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wardlaw JM et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat. Rev. Neurol 89, 137–153 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Mestre H et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367, eaax7171 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ajami B et al. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci 21, 541–551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cortes-Canteli M & Iadecola C Alzheimer’s disease and vascular aging: JACC Focus Seminar. J. Am. Coll. Cardiol 75, 942–951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iturria-Medina Y et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun 7, 11934 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park L et al. Brain perivascular macrophages initiate the neurovascular dysfunction of Alzheimer Aβ peptides. Circ. Res 121, 258–269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cruz-Hernández JC et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci 22, 413–420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ostergaard L Blood flow, capillary transit times, and tissue oxygenation: the centennial of capillary recruitment. J. Appl. Physiol 129, 1413–1421 (2020). [DOI] [PubMed] [Google Scholar]
- 127.Wingo AP et al. Shared proteomic effects of cerebral atherosclerosis and Alzheimer’s disease on the human brain. Nat. Neurosci 383, 696–700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goldstein DS Dysautonomia in Parkinson disease. Compr. Physiol 4, 805–826 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.D’Arrigo A et al. Respiratory dysfunction in Parkinson’s disease: a narrative review. ERJ Open Res. 6, 00165–2020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gutteridge DS, Saredakis D, Badcock NA, Collins-Praino LE & Keage HAD Cerebrovascular function during cognition in Parkinson’s disease: a functional transcranial Doppler sonography study. J. Neurol. Sci 408, 116578 (2020). [DOI] [PubMed] [Google Scholar]
- 131.McDonald C, Newton JL & Burn DJ Orthostatic hypotension and cognitive impairment in Parkinson’s disease: causation or association? Mov. Disord 31, 937–946 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Serebrovskaya T et al. Hypoxic ventilatory responses and gas exchange in patients with Parkinson’s disease. Respiration 65, 28–33 (1998). [DOI] [PubMed] [Google Scholar]
- 133.Anang JB et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 83, 1253–1260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Longardner K, Bayram E & Litvan I Orthostatic hypotension is associated with cognitive decline in Parkinson disease. Front. Neurol 11, 897 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rosengarten B et al. Neurovascular coupling in Parkinson’s disease patients: effects of dementia and acetylcholinesterase inhibitor treatment. J. Alzheimers Dis 22, 415–421 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Camargo CH et al. Abnormal cerebrovascular reactivity in patients with Parkinson’s disease. Parkinsons Dis. 2015, 523041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng W et al. Spatial patterns of decreased cerebral blood flow and functional connectivity in multiple system atrophy (cerebellar-type): a combined arterial spin labeling perfusion and resting state functional magnetic resonance imaging study. Front. Neurosci 13, 777 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Malhotra RK Neurodegenerative disorders and sleep. Sleep. Med. Clin 13, 63–70 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Andrade AG, Bubu OM, Varga AW & Osorio RS The relationship between obstructive sleep apnea and Alzheimer’s disease. J. Alzheimers Dis 64, S255–S270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Capone C et al. Endothelin 1-dependent neurovascular dysfunction in chronic intermittent hypoxia. Hypertension 60, 106–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu MT REM sleep behavior disorder (RBD). Neurobiol. Dis 143, 104996 (2020). [DOI] [PubMed] [Google Scholar]
- 142.Qureshi AI Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation 118, 176–187 (2008). [DOI] [PubMed] [Google Scholar]
- 143.Malhotra K et al. Association of blood pressure with outcomes in acute stroke thrombectomy. Hypertension 75, 730–739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeiler FA et al. Continuous cerebrovascular reactivity monitoring in moderate/severe traumatic brain injury: a narrative review of advances in neurocritical care. Br. J. Anaesth 124, 440–453 (2020). [DOI] [PubMed] [Google Scholar]
- 145.Bhalerao A et al. In vitro modeling of the neurovascular unit: advances in the field. Fluids Barriers CNS 17, 22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao Z et al. Central role for PICALM in amyloid-β blood–brain barrier transcytosis and clearance. Nat. Neurosci 18, 978–987 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Batiuk MY et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun 11, 1220 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Utz SG et al. Early fate defines microglia and non-parenchymal brain macrophage development. Cell 181, 557–573.e18 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Ouellette J et al. Vascular contributions to 16p11.2 deletion autism syndrome modeled in mice. Nat. Neurosci 560, 1090–1101 (2020). [DOI] [PubMed] [Google Scholar]
- 150.Grutzendler J & Nedergaard M Cellular control of brain capillary blood flow: in vivo imaging veritas. Trends Neurosci. 42, 528–536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.