Abstract
In the 3rd year of the SARS-CoV-2 pandemic, much has been learned about the long-term effects of COVID-19 pneumonia on the lungs. Approximately one-third of patients with moderate-to-severe pneumonia, especially those requiring intensive care therapy or mechanical ventilation, have residual abnormalities at chest CT 1 year after presentation. Abnormalities range from parenchymal bands to bronchial dilation to frank fibrosis. Less is known about the long-term pulmonary vascular sequelae, but there appears to be a persistent, increased risk of venothromboembolic events in a small cohort of patients. Finally, the associated histologic abnormalities resulting from SARS-CoV-2 infection are similar to those seen in patients with other causes of acute lung injury.
© RSNA, 2022
Summary
Some patients with moderate-to-severe COVID-19 have chest CT abnormalities that persist at least a year after infection and may be associated with symptoms; these CT abnormalities show similarities to those described in the 2002–2003 severe acute respiratory syndrome epidemic.
Essentials
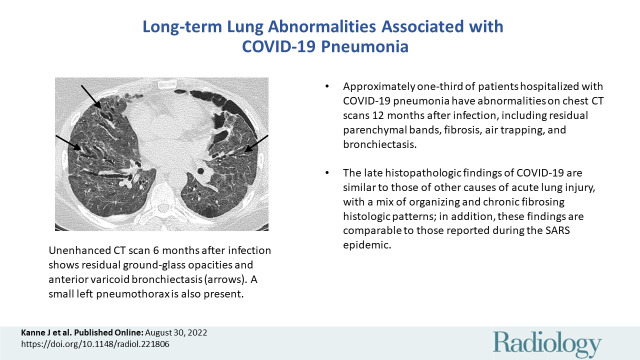
■ Approximately one-third of patients hospitalized with COVID-19 pneumonia had abnormalities on chest CT scans 12 months after infection.
■ CT abnormalities ranged from residual parenchymal bands to fibrosis, as well as air trapping and bronchiectasis.
■ A very small number of patients had a persistently elevated risk of venothromboembolic disease after acute infection.
■ The late-disease histopathologic findings of COVID-19 were similar to those of other causes of acute lung injury, with a mix of organizing and chronic fibrosing histologic patterns, and were comparable to those reported for the severe acute respiratory syndrome epidemic.
Introduction
Into the 3rd year of the SARS-CoV-2 pandemic and after the most recent wave of the Omicron variant in early 2022, much of the world has shifted to an endemic mode of dealing with COVID-19, albeit unofficially, as the World Health Organization has not declared the pandemic over at the time of this writing. Vaccines are readily available in many countries and much of the worldwide population presumably has some degree of immunity from vaccination, previous infection, or both. Unlike SARS-CoV-1, which has not been reported in the community since mid-2003 (1), SARS-CoV-2 does not appear to be fading to extinction. Even newer variants of SARS-CoV-2 have been shown to escape neutralizing antibodies from previous infection and vaccination (2), contributing to the new infections and reinfections globally.
As the pool of individuals who have suffered one or more episodes of COVID-19 rapidly grows, the proportion of the population with long-term symptoms and chronic lung findings of the disease increases. Post–COVID-19 conditions, also referred to as “long COVID,” “long-haul COVID,” or “post-acute sequelae of COVID-19,” consist of a lengthy list of signs and symptoms ranging from shortness of breath to depression and sleep disturbance (3–5) and are reported to occur in up to 10% of patients (6–8). While no universally agreed upon definitions exist, The British Medical Journal guidelines define “long COVID” as persistent symptoms after 4 weeks and “post-COVID syndrome” as symptoms continuing beyond 12 weeks (3). Because the causes of persistent symptoms are likely multifactorial and currently not well understood, the growing radiology literature on chronic lung findings in COVID-19 may eventually facilitate understanding of long-term respiratory issues and imaging correlates in affected individuals.
Familiarity with the typical long-term sequelae of COVID-19 pneumonia at chest imaging is important in evaluating potential causes of chronic respiratory symptoms in survivors, assessing improvement at follow-up imaging, and distinguishing expected post–COVID-19 findings from other lung conditions. This article summarizes current knowledge of post–COVID-19 lung parenchymal, airway, pulmonary vascular, and histopathologic findings.
Lung Parenchymal Abnormalities
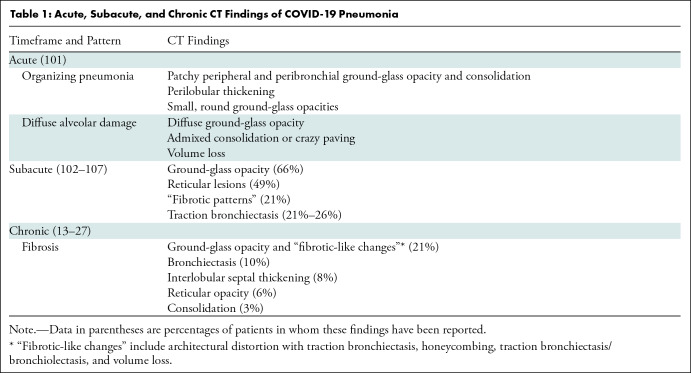
The acute and subacute CT lung parenchymal findings of COVID-19 pneumonia have been well described and are summarized in Table 1. These patterns of lung injury are similar to those of SARS-CoV-1 infection (9,10) and hemagglutinin type 1 and neuraminidase type 1 (H1N1) influenza (11,12).
Table 1:
Acute, Subacute, and Chronic CT Findings of COVID-19 Pneumonia
Several prospective observational studies have evaluated the long-term chest CT changes of patients with COVID-19 pneumonia approximately 12 months after illness (13–27). However, these studies are limited by small cohorts with a wide variety of illness severity. Further complicating matters are differences in follow-up paradigms and CT evaluation methods.
Fortunately, a recent systematic review and meta-analysis by Watanabe et al (28) provides better understanding of the observed chest CT findings approximately 12 months after COVID-19 pneumonia. The authors aggregated study data from 15 observational (21) studies, providing data on 3134 individuals. Of note, the populations of these studies were heterogeneous (heterogeneity statistic, I2 = 93%), with 11 studies from China, three from Italy, and one from the United Kingdom. In the combined pool of 3134 patients, 1801 patients had CT scans performed at 12 months. Twelve of the 15 studies provided data on the proportion of patients with any residual lung abnormalities at CT, estimated to be 33%. Ground-glass opacity and “fibrotic-like changes” were the most common findings (21% for both), followed by bronchiectasis in 10%, interlobular septal thickening in 8%, reticular opacity in 6%, and consolidation in 3% of patients. “Fibrotic-like changes” varied across studies and included “architectural distortion with traction bronchiectasis, honeycombing, or both” (Figs 1–3) (15), “traction bronchiectasis/bronchiolectasis, volume loss, or both” (26), “evidence of stripe-like fibrosis but not reticular opacity” (21), and “the presence of honeycombing, reticulation, and traction bronchiectasis” (27).
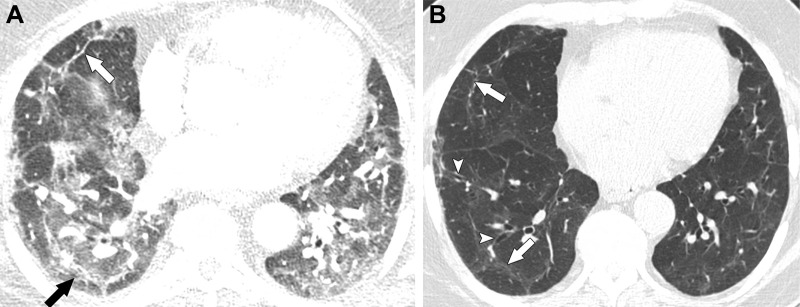
Figure 1:
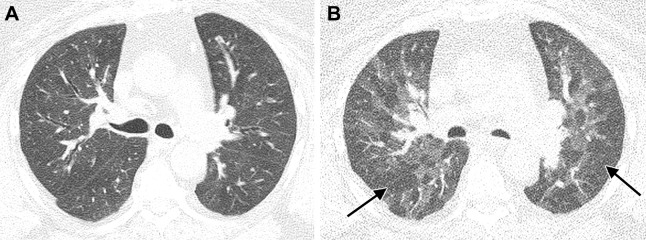
Images in a 63-year-old man with residual lung abnormalities from SARS-CoV-2 infection. (A) Contrast-enhanced axial CT image at presentation shows peripheral and peribronchial ground-glass opacity and consolidation along with perilobular thickening (arrows). (B) Unenhanced axial CT image 1 year later shows patchy residual ground-glass opacity, persistent perilobular thickening (arrows), and mild bronchial dilation (arrowheads) in areas of ground-glass opacity.
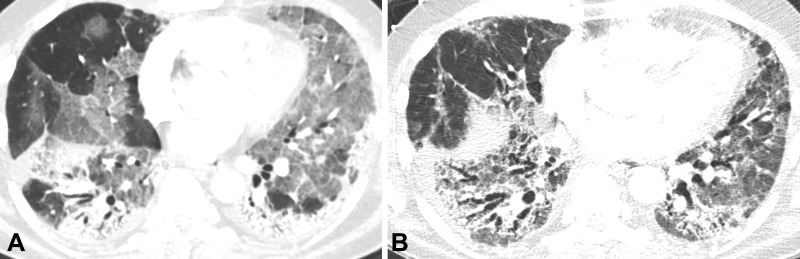
Figure 3:
Images in a 56-year-old man with fibrosis resulting from SARS-CoV-2 infection. (A) Contrast-enhanced axial CT image early during infection shows extensive ground-glass opacity with posterior and peripheral predominant consolidation and some areas of crazy paving. (B) Unenhanced axial CT image from 10 months later shows lower lobe predominant reticulation, traction bronchiectasis, and ground-glass opacity with lower lobe volume loss.
Figure 2:
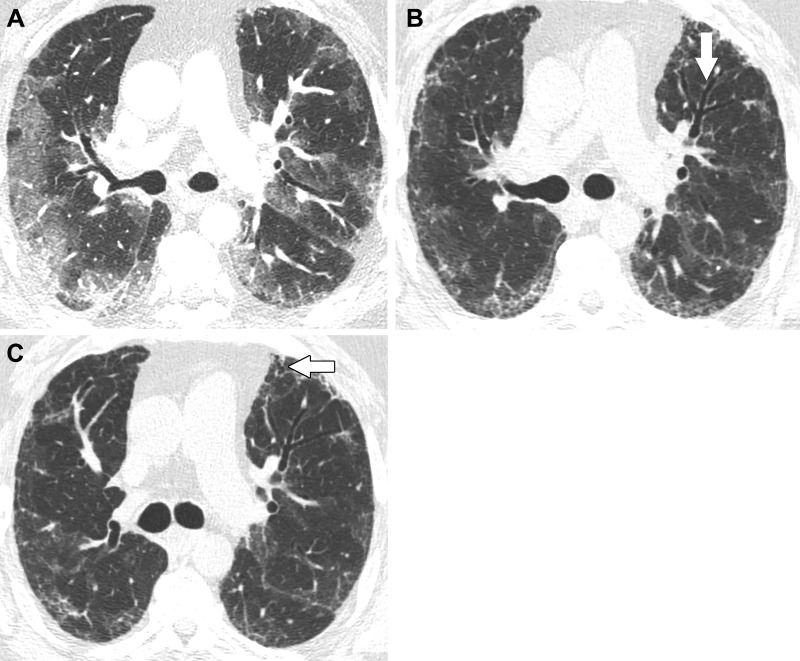
Images in a 57-year-old man with fibrosis resulting from SARS-CoV-2 infection. (A) Contrast-enhanced axial CT image at presentation shows peripheral predominant ground-glass opacity with a small amount of consolidation. (B) Unenhanced axial CT image 3 months later shows marked clearing of ground-glass opacity but development of reticulation and mild bronchial dilation (arrow). (C) Unenhanced axial CT image 6 months after infection shows further decrease of ground-glass opacity and a lesser extent of reticulation. The area of bronchial dilation in the left upper lobe has resolved, although there is a small peripheral area of traction bronchiectasis (arrow).
Twelve of the 15 studies reported the proportion of abnormal chest CT findings at 12 months according to COVID-19 severity. In this subanalysis, 85% (950 of 1112) of patients with severe-to-critical COVID-19 and 87% (560 of 641) with mild-to-moderate COVID-19 were included. In the severe-to-critical group, 38% (278 of 816) of patients had residual CT abnormalities, including ground-glass opacity, “fibrotic-like changes,” bronchiectasis, and interlobular septal thickening. In the mild-to-moderate group, 24% (91 of 378) of patients had residual CT findings consisting mostly of ground-glass opacity. The results of this systematic review and meta-analysis are similar to results published in 2003 from the SARS-CoV-1 epidemic that showed 30%–40% of survivors of severe acute respiratory syndrome had radiologic abnormalities 6–12 months after recovery. Those with residual abnormalities at 12 months had similar findings 15 years later (29,30).
Confounding full understanding of the long-term chest CT findings of COVID-19 are the many biases and shortcomings in these longitudinal observational studies. Because many studies focus on chest CT findings over time, it is not surprising that study cohorts favor patients with more severe disease as they were more likely to undergo chest CT at the time of diagnosis, and patients with mild or no residual abnormalities may not have undergone further imaging. Patients in many of these studies were more likely to be hospitalized and require intensive care unit admission and mechanical ventilation.
Another confounder is that these studies primarily involve patients who contracted COVID-19 in the earlier part of the pandemic. The virus has evolved over time with the more recent, more contagious Omicron variant (BA.1, BA.1.1, BA.2, BA.3, BA.4, and BA.5 lineages), which is associated with milder disease than the initial variant and the more severe Delta variant (B.1.617.2 and AY lineages). A recent study of 106 hospitalized patients with COVID-19, 40 with the Omicron variant (earlier lineage) and 66 with the Delta variant, showed lower CT severity scores in the cohort with the Omicron variant (31). Yoon et al (32) retrospectively reviewed CT scans of 176 hospitalized patients, 88 with the Delta variant and 88 with the early-lineage Omicron variant. Patients with the Omicron variant had a less severe extent of disease and more of a peribronchial distribution (rather than peripheral) than patients infected with the Delta variant.
The definition of “fibrosis” at chest CT used in these studies is also problematic. As highlighted in the systematic review and meta-analysis by Watanabe et al (28), the definition of “fibrotic-like abnormalities” used in some studies varied. Because tissue confirmation of fibrosis was not obtained (appropriately so), the presence of fibrosis was only assumed based on CT findings. Another potential confounder is that patients with residual interstitial lung abnormalities on follow-up CT scans may have had those abnormalities before COVID-19 pneumonia. These abnormalities have been reported to occur in up to 10% of the population, especially older individuals, who make up the majority of patients with more severe COVID-19 pneumonia (33).
Effects on Airways
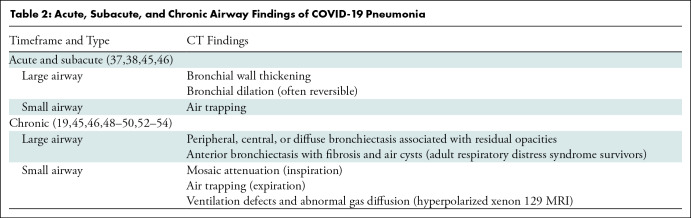
Large and small airway abnormalities can be seen in survivors of COVID-19 pneumonia, with frequency and severity correlating with the severity of the acute disease. The acute and subacute CT airway findings of COVID-19 pneumonia are summarized in Table 2. Findings of small airway disease, such as mosaic attenuation and air trapping, have been seen at paired inspiratory and expiratory CT, and studies of hyperpolarized xenon 129 (129Xe) MRI show abnormal ventilation and perfusion patterns in patients with long-term COVID-19 respiratory symptoms, even with a normal CT scan.
Table 2:
Acute, Subacute, and Chronic Airway Findings of COVID-19 Pneumonia
Airway abnormalities seen in sequela of previous major respiratory viral outbreaks provide context for the COVID-19 pandemic. In avian-origin influenza (H7N9), bronchiectasis was common at 12-month follow-up CT and present in 24% of patients (10 of 41), while restrictive or obstructive pulmonary function test abnormalities were found in 55% (11 of 20) of patients for whom 12-month follow-up examinations were available (34). Bronchiectasis as a long-term consequence of infection was also seen in Middle East respiratory syndrome and SARS-CoV-1 (35). Air trapping at CT was described as a common finding in survivors of SARS-CoV-1 pneumonia, found in 93% (37 of 40) of patients at a mean follow-up of 51.8 days and in 80% (16 of 20) of patients at a mean follow-up of 140.7 days (35), and in 23% (11 of 47) in another study of 6-month CT in children with SARS-CoV-1 (36).
Large Airway Abnormalities
Bronchial abnormalities such as wall thickening and dilation are common in patients with COVID-19 pneumonia in the acute and early convalescent phases, decreasing in frequency and severity over time (37). Bronchial dilation persists in a subset of patients after recovery from COVID-19 pneumonia, more frequently in patients with more severe disease, and often as traction bronchiectasis accompanied by other signs of fibrosis. Bronchiectasis after COVID-19 is often peripheral and associated with reticulation or bandlike opacities. Besutti et al (38) found bronchiectasis at CT in 13% (52 of 405) of patients when performed 5–7 months after discharge for severe COVID-19 pneumonia. Of those, 85% (44 of 52) of patients had a peripheral distribution, while only 2% (one of 52) had a central distribution and 13% (seven of 52) had both a central and peripheral distribution. As in idiopathic interstitial pneumonias, traction bronchiectasis may be important to recognize because of a correlation with functional impairment. In one study of COVID-19 survivors, traction bronchiectasis was inversely associated with the diffusing capacity of lung for carbon monoxide percent predicted (R = −0.49, P < .001) and forced vital capacity percent predicted (R = −0.23, P = .04), and was directly correlated with cough scale score (R = 0.25, P = .03) (39).
Although traction bronchiectasis associated with fibrosis may be an important chronic finding in COVID-19 survivors, existing studies often fail to distinguish traction bronchiectasis (suggesting features of fibrosis) from bronchiectasis broadly construed, which can be caused by any airway injury (Figs 4–6). For example, in a prospective CT scan study of patients 6 months after discharge for moderate or severe COVID-19 pneumonia, Caruso et al (40) reported “fibrosis-like changes” defined as “reticulation and/or honeycombing” in 72% (85 of 118) of patients, and bronchiectasis in 25% (29 of 118); the percentage of patients with traction bronchiectasis was not reported. The meta-analysis by Watanabe et al (28) similarly includes studies in which the frequencies of traction bronchiectasis and other types of bronchiectasis are unclear. These potentially overlapping categories make it difficult to know if bronchiectasis in survivors of COVID-19 represents a primary finding of fibrosis (traction bronchiectasis), airway damage from viral infection or barotrauma, or some combination of these etiologies.
Figure 4:
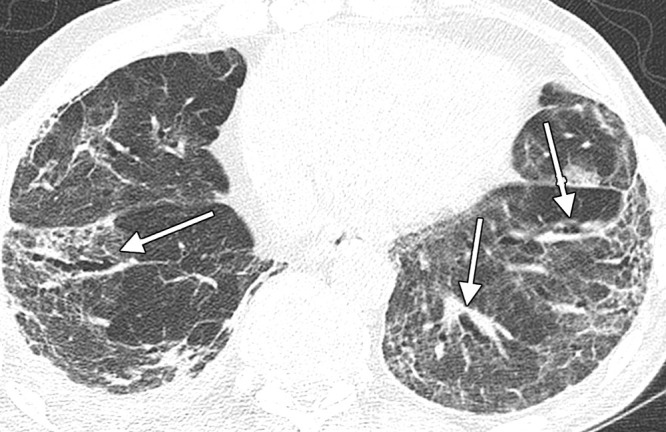
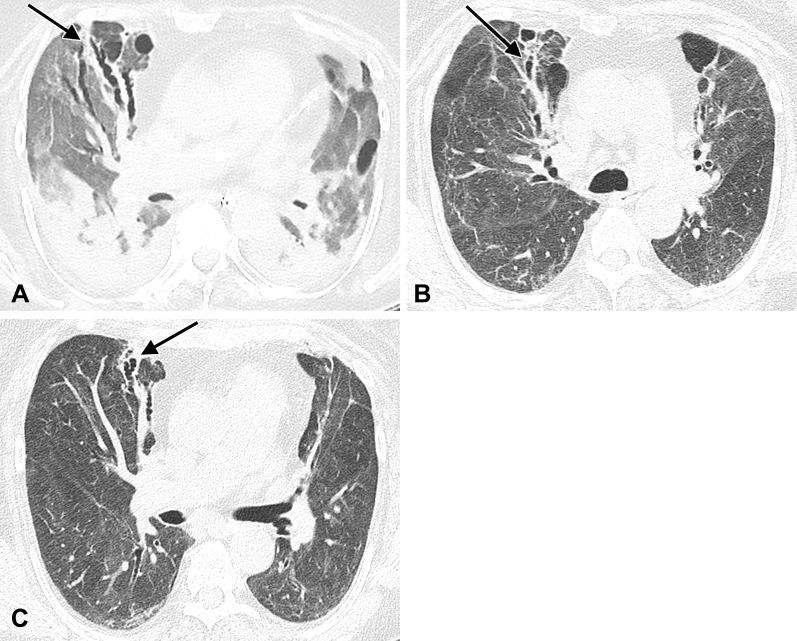
Image in a 74-year-old man with history of SARS-CoV-2 infection. Unenhanced axial CT image 5 months after acute infection shows bilateral residual peripheral ground-glass opacity and bandlike opacities. Varicoid traction bronchiectasis and bronchiolectasis occurs within areas of reticulation and architectural distortion, in keeping with fibrosis (arrows).
Figure 6:
Images in a 51-year-old woman with history of SARS-CoV-2 infection, noninvasive positive pressure ventilation, and chronic dyspnea requiring home oxygen therapy. (A) Contrast-enhanced axial CT image during acute infection shows bilateral ground-glass opacity with peripheral predominance (arrows). (B) Contrast-enhanced axial CT image after discharge, 2 months after presentation, shows diffuse ground-glass opacity and architectural distortion with diffuse varicoid bronchial dilation (arrows). (C) Unenhanced axial CT image 6 months after presentation shows decrease in ground-glass opacity but persistent diffuse varicoid bronchiectasis (arrows); a small left pneumothorax is also present.
Figure 5:
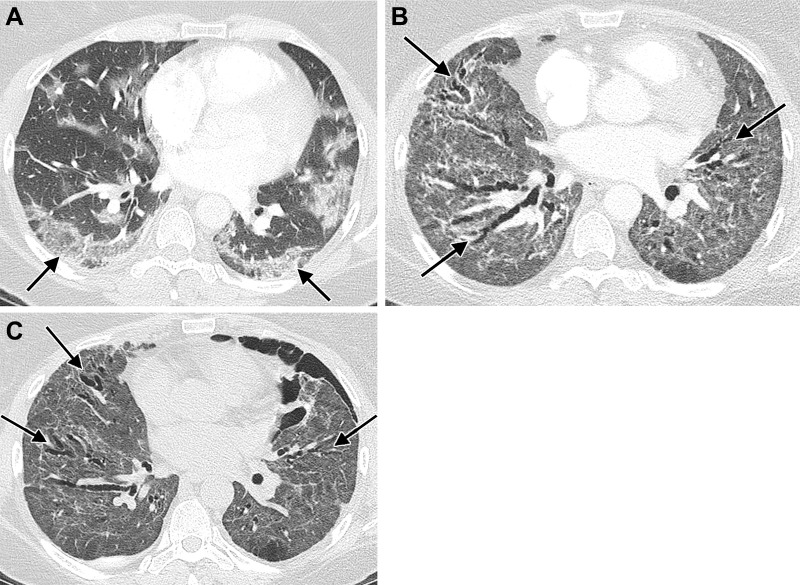
Images in a 77-year-old woman hospitalized with acute respiratory distress syndrome resulting from SARS-CoV-2 infection. (A) Unenhanced axial CT image during acute infection and mechanical ventilation shows typical findings of alveolar damage, with dependent consolidation and ground-glass opacity throughout the remainder of the lungs. Varicoid bronchial dilation and an air cyst have developed within the anterior right lung (arrow). (B, C) Unenhanced axial CT images 10 months after infection show anterior predominant varicoid bronchiectasis (arrows), slightly decreased in severity and accompanied by reticulation and architectural distortion. A background of residual ground-glass opacity, peripheral parenchymal bands, and reticulation is also present.
Bronchiectasis has long been recognized as a common finding in acute respiratory distress syndrome (ARDS) caused by conditions other than COVID-19. Often most extensive in the anterior lungs and accompanied by reticulation and architectural distortion, ARDS-related bronchiectasis is thought to be a product of barotrauma in the setting of mechanical ventilation, with severity correlating with duration of ventilation and high inspiratory pressures (41,42). Of the 7% (28 of 405) of patients with fibrotic abnormalities in a study of survivors of severe COVID-19 pneumonia, 36% (10 of 28) had “post-ventilatory fibrosis,” defined as anterior predominant subpleural cystic spaces and reticulation, and 90% (nine of 10) of these had traction bronchiectasis (38). Traction bronchiectasis may be primarily due to ARDS and mechanical ventilation. One study of patients hospitalized with moderate COVID-19 pneumonia that excluded patients with ARDS, mechanical ventilation, or both found bronchiectasis or bronchiolectasis on CT scans at 3 and 12 months in only 2% (two of 84) of patients, while “traction bronchiectasis/bronchiolectasis” as a finding of fibrosis was not identified on CT scans in any patient at 3 months and had developed in only 2% (two of 84) of patients at 12 months (43).
Bronchial dilation can be completely reversible, even from COVID-19 pneumonia complicated by ARDS, which underscores the need for caution in interpreting acute or subacute bronchial dilation as a sign of parenchymal fibrosis or lasting airway damage. In a study of 41 survivors of COVID-19 pneumonia with ARDS, Hu et al (44) compared CT scans obtained 1–4 weeks after onset of symptoms with those obtained at least 4 months after infection. Twenty-eight of the 41 patients (68%) had developed varicoid dilation of bronchi (“traction bronchiectasis”) within parenchymal opacities in the 1st month, which resolved in the majority (21 of 28, 75%) and significantly improved in the remaining seven patients (17% of the study sample). In a study of patients hospitalized for COVID-19, Pan et al (13) found dilated bronchi at CT performed at discharge in 27% (57 of 209) of patients and at 12 months after symptom onset in 11% (24 of 209), with resolution of bronchial dilation in 33 patients. Luger et al (45) found bronchial dilation in 11% (eight of 76) of patients with mild-to-severe COVID-19 pneumonia at baseline and in 9% (eight of 91) at 12-month follow-up CT.
Small Airway Abnormalities
Recent studies have used paired inspiratory and expiratory CT scans to evaluate the possible contribution of small airway disease to persistent symptoms in long-term COVID-19. Air trapping is defined as the presence of lobules or regions with a less than normal increase in attenuation and a lack of decreased volume at end-expiratory CT (46). Although an obstructive deficit at spirometry is much less common than limitations in diffusing capacity (diffusing capacity of lung for carbon monoxide) in survivors of COVID-19, some patients show evidence of small airway disease at pulmonary function tests, and air trapping at CT may herald small airway disease below the threshold of detection with pulmonary function tests (19,37).
Air trapping is a common finding in acute respiratory infections and has been reported in COVID-19 (47). Air trapping has also been reported as a long-term finding in COVID-19 survivors in several studies. In a study of 205 patients previously hospitalized for COVID-19 pneumonia, air trapping was seen at expiratory CT in 29%, with significantly higher quantitative measures of air trapping in the severe pneumonia group than in the mild pneumonia group (48). Additional studies have examined the incidence of air trapping at CT in symptomatic patients with long-term COVID-19. Franquet et al (37) used paired inspiratory and expiratory CT to assess patients with persistent respiratory symptoms at least 30 days after COVID-19 symptom onset (median, 72.5 days). Air trapping was the most common abnormality (37 of 48 patients, 77%) (Fig 7), more common than findings such as ground-glass opacity (19 of 48 patients, 40%), reticulation (18 of 48 patients, 38%), or traction bronchiectasis (nine of 48 patients, 19%). In addition, air trapping was more commonly seen in male patients and increased with age. In a prospective study of patients with postacute sequelae of COVID-19 who remained symptomatic for at least 30 days following diagnosis, Cho et al (49) identified air trapping by qualitative inspection in 58% (50 of 86) of patients. The authors also used quantitative CT with a supervised machine learning method to assess the percentage of air trapping within the lungs, finding similar mean values for patients treated in the ambulatory setting (25%), hospitalized patients (25%), and patients who required the intensive care unit (27%). However, patients with COVID-19 had significantly greater mean air trapping than healthy controls (7%, P < .001).
Figure 7:
Images in a 58-year-old woman with history of SARS-CoV-2 infection, ongoing dyspnea after infection, and history of sleep apnea. (A) Unenhanced axial CT image at full inspiration performed 2 years after acute infection shows subtle diffuse mosaic attenuation. (B) Paired expiratory axial CT image shows extensive lobular and regional low attenuation indicative of air trapping (arrows).
It is uncertain whether air trapping is a manifestation of reversible airway inflammation, primary airway damage due to COVID-19, postinfectious bronchiolitis obliterans, sequela of diffuse alveolar damage (DAD), or some other process. Studies of air trapping in COVID-19 survivors have been limited by a lack of comparison CT scans before the onset of infection, precluding the exclusion of preexisting small airway disease. In addition, the presence of air trapping as a common finding in asymptomatic individuals without evidence of small airway disease has been well documented (50).
Hyperpolarized 129Xe MRI has also recently emerged as a technique for investigating heterogeneity in ventilation and gas transfer in patients with long-term COVID-19 symptoms, such as breathlessness. Hyperpolarized 129Xe gas rapidly diffuses across alveolar membranes and into red blood cells, allowing reconstruction of gas, tissue and plasma, and red blood cell phase images that depict regional ventilation and pulmonary perfusion (51). In a study of 76 COVID-19 survivors (a mean of 13.8 weeks after the index positive COVID-19 test) with persistent respiratory symptoms and nine healthy volunteers without a history of COVID-19, Kooner et al (52) found significantly greater mean ventilation defect percentages in 23 patients previously hospitalized with COVID-19 (8%) than in 53 patients without hospitalization (4%); both groups had significantly higher ventilation defect percentages than the healthy volunteers (1%). The same study showed abnormal ratios of residual volume to total lung capacity in 14 of 38 patients (37%) for whom it was measured, suggesting small airway obstruction as a cause. However, other hyperpolarized 129Xe MRI studies have found relatively normal ventilation measured at the gas phase, with significant gas exchange deficits evidenced by abnormal red blood cell phase images and a significantly decreased red blood cell–to–tissue plasma ratio, a marker of gas diffusion across alveolar epithelium (51,53). The relative contributions of small airway disease and alveolar vascular disease are yet to be determined and may vary among individuals and across clinical circumstances.
Pulmonary Vascular Abnormalities
The presence of pulmonary vascular abnormalities was recognized early in the COVID-19 pandemic. Dilated pulmonary vasculature in regions of pneumonia was described in initial case series (54,55). Shortly thereafter, elevated risks of pulmonary emboli and in situ thrombosis of the pulmonary arteries were noted, especially in patients with severe disease. Over the subsequent 3 years of the pandemic, the spectrum of recognized COVID-19–associated pulmonary vascular disease greatly broadened, impacting current medical practice.
In this section, we will review contemporary evidence and insights regarding the long-term pulmonary vascular manifestations of SARS-CoV-2 infection with a focus on pulmonary vascular disease in “long COVID.” Evolving data related to pulmonary vascular disease in acute COVID-19 are summarized in Table 3. A common thread is pulmonary endotheliitis (56–58), which is an important feature of acute COVID-19 that can persist in convalesce for an uncertain duration.
Table 3:
Acute Pulmonary Vascular Manifestations of COVID-19 Infection
“Long COVID” includes a variety of conditions, including pulmonary embolism (PE), that seem to occur at a higher rate among people previously diagnosed with COVID-19. Bull-Otterson et al (59), in a retrospective matched cohort study of adults from a national electronic health record data set with more than 63 million records (March 2020–November 2021), followed cohorts for 30–365 days after the index encounter for 26 incident conditions described to be associated with “long COVID.” The study cohorts of 353 164 patients with COVID-19 and 1 640 776 without COVID-19 were stratified by age. The COVID-19 cohort had significantly more incident conditions compared with the non–COVID-19 cohort, with 38% (35.4% for ages 18–64 years, 45.4% for 65 years and older) versus 16% (14.6% for ages 18–64 years, 18.5% for 65 years and older). The highest risk ratio was for PE, with 2.1 and 2.2 for younger and older ages, respectively.
The risk of “long COVID” for patients with breakthrough infections was studied by Al-Aly et al (60) in a retrospective cohort study from the Veterans Affairs database. Those with breakthrough COVID-19 were studied for a variety of incident conditions described to be associated with “long COVID” and for mortality. The breakthrough COVID-19 group was compared with contemporary, historical, and unvaccinated controls and patients with seasonal influenza. Between 30 days and 6 months after breakthrough COVID-19 infection, patients had elevated risk (hazard ratio [HR], 1.5) for postacute COVID-19 conditions, with the highest risk for PE (HR, approximately 4). This risk was worst for patients requiring the intensive care unit compared with inpatients and outpatients, both overall and for PE. Patients with breakthrough COVID-19 also had a higher risk of death (HR, 1.75); however, compared with unvaccinated patients with COVID-19, these patients had lower risk (“long COVID” HR, 0.85; death HR, 0.66). When patients hospitalized with influenza were compared with patients hospitalized with breakthrough COVID-19, patients with COVID-19 had higher risk of “long-COVID–associated” conditions (HR, 1.27) and death (HR, 2.43).
It is important to distinguish between extremely rare vaccine-associated thrombotic adverse events, including PE and PE associated with COVID-19, breakthrough COVID-19, and long-term COVID-19. Vaccine-induced immune thrombotic thrombocytopenic purpura (VITT) is caused by the development of antibodies to platelet factor 4 polyanion complexes and has been reported for all four of the major SARS-CoV-2 vaccines in recent use (Pfizer, Moderna, Johnson & Johnson, and AstraZeneca), and most frequently for ChADOx1 nCoV-19 (AstraZeneca) (61–64). Symptoms typically develop within 4 weeks of initial vaccination. Recognition of VITT has key therapeutic implications, as heparin is avoided due to the similar mechanism of immune-mediated heparin-induced thrombocytopenia.
The long-term pulmonary vascular manifestations of COVID-19 remain incompletely understood. The current consensus favors endotheliitis (56,65,66) and extension of the pulmonary inflammatory process (67) rather than vasculitis as the dominant explanation for the wide-ranging COVID-19–associated pulmonary vascular abnormalities. These include a persistently elevated risk of PE and possibly the development of chronic thromboembolic pulmonary hypertension (68) and pulmonary hypertension (69). A variety of intriguing but uncommonly described pulmonary vascular abnormalities have occasionally been reported to be associated with COVID-19 and are of unclear importance. In a small series of patients who required the intensive care unit, Brito-Azevedo et al (70) described intrapulmonary vascular dilation with shunt placement at echocardiography, and the authors suggested this may at least in part be responsible for COVID-19–associated hypoxemia and dilated vessels at CT, with a mechanism similar to hepatopulmonary syndrome.
Dhawan et al (71) proposed using lung ventilation-perfusion (V/Q) scintigraphy, preferably with SPECT, as the first-line imaging test to assess for residual clot and small pulmonary vessel disease for patients who have recovered from COVID-19 but still have persistent respiratory symptoms. Their rationale is that V/Q scanning plays a leading role in the evaluation of pulmonary small vessel disease, which may be suboptimally demonstrated on CT pulmonary angiographs. The authors highlighted the expected patterns of small vessel disease in addition to PE and lung parenchymal disease and suggested that V/Q scanning should play a clinical and research role in elucidating the evolution of postacute COVID-19 vascular disease. Along with V/Q scanning, longitudinal data from spectral CT should continue to shed light on the long-term pulmonary vascular sequela of COVID-19 (72).
Pathologic Findings of Long-term COVID-19
As the COVID-19 pandemic has progressed, pathologic findings in the lung associated with SARS-CoV-2 infection have slowly materialized. Some of the very first reports of the histopathologic changes in COVID-19 pneumonia from living patients were reported from Wuhan, China, where patients undergoing lung cancer surgery were also found to have COVID-19 (73). These early reports, unsurprisingly, described changes in acute or early-organizing DAD or other patterns of acute lung injury. Now, in the 3rd year of the pandemic, a clearer picture of the histopathologic changes associated with COVID-19 has emerged.
SARS-CoV-2 infects cells of the human respiratory tract by binding to the angiotensin-converting enzyme 2 (ACE2) receptor (74). In the acute setting, patients with SARS-CoV-2 and respiratory failure typically have histopathologic findings of DAD. Other forms of acute lung injury, including organizing pneumonia and acute fibrinous and organizing pneumonia, have been reported but are less commonly encountered than DAD. The histopathologic features and pathophysiologic mechanisms of acute COVID-19 pneumonia are beyond the scope of this review and have been well described (75–82). Some authors are of the opinion that acute COVID-19 pathologic findings are similar to those of other forms of acute lung injury (83,84), but others suggest that there are findings more commonly present in patients with acute lung injury secondary to COVID-19. While pulmonary microthrombi are often observed as a component of DAD of any cause, they are frequently mentioned as being a prominent finding in acute COVID-19 or occurring more often than other viral pneumonias (77,85–87). Other vascular lesions have been described, including old recanalized thrombi, vascular congestion, and hemangiomatosis-like lesions (Figs 8, 9), in areas without features of acute lung injury (88). While the exact pathophysiologic mechanisms are still debated, these findings suggest the possibility of a distinct vascular phenotype of lung injury occurring in patients with COVID-19.
Figure 8:
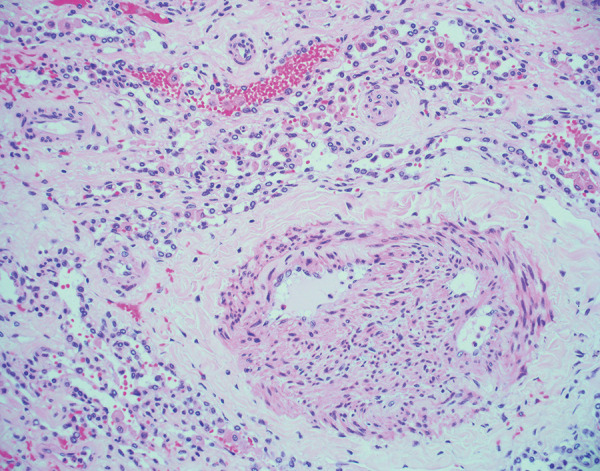
Histologic image (hematoxylin and eosin stain, 200× magnification) shows recanalized pulmonary arteriole with neolumen formation. This finding was observed in an explanted lung 4 months after acute COVID-19.
Figure 9:
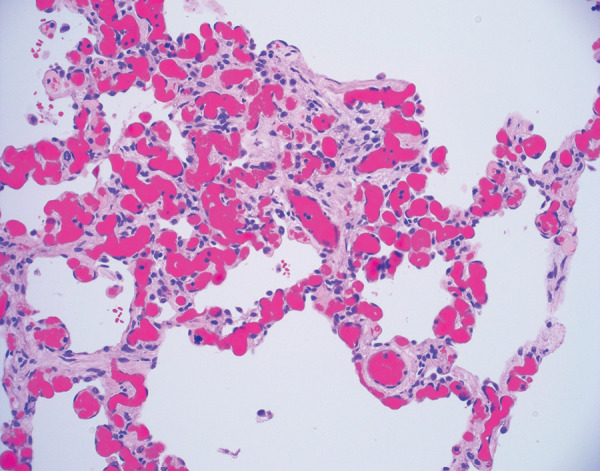
Histologic image (hematoxylin and eosin stain, 200× magnification) of alveolar septa shows a vascular congestion and hemangiomatosis-like lesion. Notice the absence of acute lung injury, inflammation, and hyaline membrane formation. While originally described in the acute setting, this vascular congestion and hemangiomatosis-like lesion was identified in an explanted lung approximately 4 months after acute COVID-19 pneumonia.
A patient with DAD, either secondary to COVID-19 or another cause, typically progresses through an acute phase characterized by hyaline membrane formation, then to an organizing phase with fibroblast proliferation (Fig 10) (89). A dichotomy exists in how the lung resolves DAD. While most patients with DAD will have some long-term respiratory symptoms, there may be a gradual resolution of DAD or the DAD may progress to a fibrotic phase (89,90).
Figure 10:
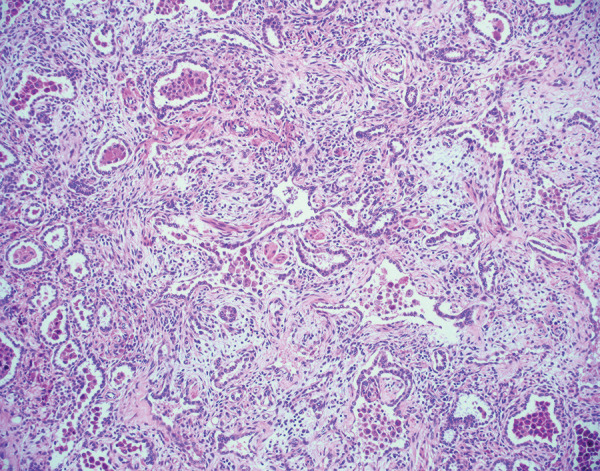
Histologic image (hematoxylin and eosin stain, 100× magnification) of pulmonary parenchyma shows organizing diffuse alveolar damage. There are residual alveolar spaces with marked increase in the interstitium by cellular fibroblastic proliferations. Some fibroblastic proliferations are also likely within alveoli. Type 2 pneumocyte hyperplasia is present. These findings were observed in an explanted lung approximately 6 months after acute COVID-19.
In the ongoing pandemic, most patients show a complete resolution of pulmonary abnormalities without histologic evidence of identifiable disease and are unlikely to be imaged further (91). It is now known that this is not true for all patients. Long-term pulmonary sequelae of acute COVID-19 may manifest as organizing pneumonia weeks after initial infection, and this may spontaneously resolve (92). Some of the earliest reports of pulmonary histopathologic changes associated with fibrosis in patients with severe COVID-19 were from explanted lungs from patients who underwent transplant (93,94). The authors of these studies identified diffuse interstitial fibrosis with uniform collagenous thickening of alveolar septa. Honeycomb change was also identified, along with cystic spaces lined by histiocytes and giant cells. Some of these findings have also been observed in a lung explant specimen in a preprint article and from an autopsy (95,96). In a series of transbronchial biopsies from Brazil, septal thickening and airway remodeling were identified (97). Beyond airway remodeling, there are reports of chronic bronchiolitis and peribronchiolar metaplasia (98,99). These aforementioned cases (93,94,97) probably represent the fibrotic phase of DAD, which is well described in an autopsy study from China and from a small series of explanted lungs with very similar findings in the United States (Fig 11) (98,100).
Figure 11:
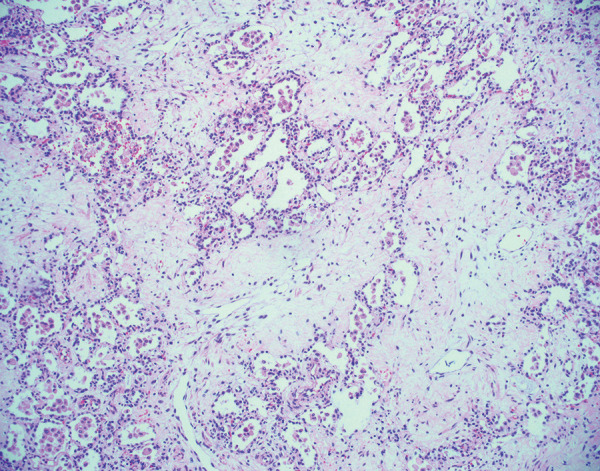
Histologic image (hematoxylin and eosin stain, 100× magnification) shows diffuse pulmonary fibrosis in an explanted lung 6 months after acute COVID-19. There is deposition of paucicellular, eosinophilic material within the pulmonary interstitium. Some residual alveolar spaces are present but appear compressed. These findings have been previously described in explanted lungs and likely represent the fibrotic phase of diffuse alveolar damage.
Complicating the histopathologic picture, another series of cases based on surgical lung biopsies identified usual interstitial pneumonia as the pattern of fibrosis in patients with persistent interstitial lung disease following COVID-19 (99). These authors also found other patterns of lung injury, including DAD superimposed upon usual interstitial pneumonia, desquamative interstitial pneumonia, and acute organizing pneumonia. Lastly, it should be noted that acute lung injury, especially DAD, is frequently encountered by the pathologist in overlapping stages (ie, acute on chronic or acute and organizing) (89). Secondary infection can also occur at this phase (Fig 12) (93). At the time of this writing, the pulmonary pathology community is actively studying the histopathologic findings in long-term COVID-19, and much will be said in this area in the near future.
Figure 12:
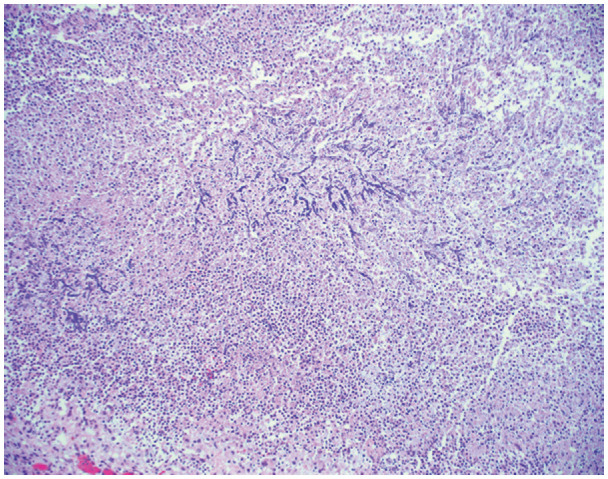
Histologic image (hematoxylin and eosin stain, 100× magnification) shows numerous fungal hyphae with acute angle branching and septations. These fungal hyphae are favored to represent Aspergillus, but no culture data were available. The fungi are seen in a background of marked neutrophilia, consistent with a necrotizing abscess.
Conclusion
The scientific and medical communities have learned much about diagnosing and treating COVID-19 over the 2.5 years since the first cases were reported in Wuhan, China. Although many studies have been published on the late-term effects of COVID-19, important limitations exist, including small case numbers for some described entities and publication biases toward positive imaging studies and severe spectrum disease. Furthermore, “big data” electronic health record studies are prone to selection bias and information bias. The meta-analyses discussed in this review offer some clarity on the data, yet are ultimately impacted by the variable studies they include.
No consensus currently exists for imaging management of patients with long-term sequelae of COVID-19 pneumonia. A reasonable approach may include inspiratory thin-section chest CT to characterize suspected parenchymal disease, with expiratory imaging as deemed appropriate for assessment of small airway disease. Imaging for suspected acute or chronic pulmonary embolism can be performed with chest CT pulmonary angiography or ventilation-perfusion scanning. Hyperpolarized 129Xe MRI has shown early promise as a tool for detecting abnormalities in patients with chronic symptoms and otherwise normal imaging, although it is considered a research modality and is not widely available. Imaging decisions should be based on patient signs and symptoms, careful clinical evaluation, and the specific question(s) needing to be answered.
Disclosures of conflicts of interest: J.P.K. No relevant relationships. B.P.L. No relevant relationships. J.J.S. No relevant relationships. A.H. No relevant relationships. L.B.H. No relevant relationships.
Abbreviations:
- ARDS
- acute respiratory distress syndrome
- DAD
- diffuse alveolar damage
- HR
- hazard ratio
- PE
- pulmonary embolism
References
- 1. Centers for Disease Control and Prevention . CDC SARS Response Timeline . 2013. . https://www.cdc.gov/about/history/sars/timeline.htm. Accessed June 1, 2022 .
- 2. Hachmann NP , Miller J , Collier AY , et al . Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5 . N Engl J Med 2022. ; 387 ( 1 ): 86 – 88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahase E . Covid-19: What do we know about “long covid”? BMJ 2020. ; 370 : m2815 . [DOI] [PubMed] [Google Scholar]
- 4. Hope AA , Evering TH . Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection . Infect Dis Clin North Am 2022. ; 36 ( 2 ): 379 – 395 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Long COVID or Post-COVID conditions 2022 . https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed June 27, 2022 .
- 6. Sudre CH , Murray B , Varsavsky T , et al . Attributes and predictors of long COVID . Nat Med 2021. ; 27 ( 4 ): 626 – 631 [Published correction appears in Nat Med 2021;27(6):1116.] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenhalgh T , Knight M , A’Court C , Buxton M , Husain L . Management of post-acute covid-19 in primary care . BMJ 2020. ; 370 : m3026 . [DOI] [PubMed] [Google Scholar]
- 8. Xie Y , Bowe B , Al-Aly Z . Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status . Nat Commun 2021. ; 12 ( 1 ): 6571 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Müller NL , Ooi GC , Khong PL , Zhou LJ , Tsang KW , Nicolaou S . High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission . AJR Am J Roentgenol 2004. ; 182 ( 1 ): 39 – 44 . [DOI] [PubMed] [Google Scholar]
- 10. Ketai L , Paul NS , Wong KT . Radiology of severe acute respiratory syndrome (SARS): the emerging pathologic-radiologic correlates of an emerging disease . J Thorac Imaging 2006. ; 21 ( 4 ): 276 – 283 . [DOI] [PubMed] [Google Scholar]
- 11. Ajlan AM , Quiney B , Nicolaou S , Müller NL . Swine-origin influenza A (H1N1) viral infection: radiographic and CT findings . AJR Am J Roentgenol 2009. ; 193 ( 6 ): 1494 – 1499 . [DOI] [PubMed] [Google Scholar]
- 12. Marchiori E , Zanetti G , D’Ippolito G , et al . Swine-origin influenza A (H1N1) viral infection: thoracic findings on CT . AJR Am J Roentgenol 2011. ; 196 ( 6 ): W723 – W728 . [DOI] [PubMed] [Google Scholar]
- 13. Pan F , Yang L , Liang B , et al . Chest CT Patterns from Diagnosis to 1 Year of Follow-up in Patients with COVID-19 . Radiology 2022. ; 302 ( 3 ): 709 – 719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu X , Liu X , Zhou Y , et al . 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study . Lancet Respir Med 2021. ; 9 ( 7 ): 747 – 754 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Han X , Fan Y , Alwalid O , et al . Fibrotic Interstitial Lung Abnormalities at 1-year Follow-up CT after Severe COVID-19 . Radiology 2021. ; 301 ( 3 ): E438 – E440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y , Ding C , Yu L , et al . One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection . BMC Med 2021. ; 19 ( 1 ): 191 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang L , Yao Q , Gu X , et al . 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study . Lancet 2021. ; 398 ( 10302 ): 747 – 758 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shang L , Wang L , Zhou F , Li J , Liu Y , Yang S . Long-term effects of obesity on COVID-19 patients discharged from hospital . Immun Inflamm Dis 2021. ; 9 ( 4 ): 1678 – 1685 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao Y , Yang C , An X , et al . Follow-up study on COVID-19 survivors one year after discharge from hospital . Int J Infect Dis 2021. ; 112 : 173 – 182 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhan Y , Zhu Y , Wang S , et al . SARS-CoV-2 immunity and functional recovery of COVID-19 patients 1-year after infection . Signal Transduct Target Ther 2021. ; 6 ( 1 ): 368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao T , Meng D , Xiong L , et al . Long-Term Effects of COVID-19 on Health Care Workers 1-Year Post-Discharge in Wuhan . Infect Dis Ther 2022. ; 11 ( 1 ): 145 – 163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gamberini L , Mazzoli CA , Prediletto I , et al . Health-related quality of life profiles, trajectories, persistent symptoms and pulmonary function one year after ICU discharge in invasively ventilated COVID-19 patients, a prospective follow-up study . Respir Med 2021. ; 189 : 106665 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bellan M , Baricich A , Patrucco F , et al . Long-term sequelae are highly prevalent one year after hospitalization for severe COVID-19 . Sci Rep 2021. ; 11 ( 1 ): 22666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou F , Tao M , Shang L , et al . Assessment of Sequelae of COVID-19 Nearly 1 Year After Diagnosis . Front Med (Lausanne) 2021. ; 8 : 717194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y , Han X , Huang J , et al . Follow-up study of pulmonary sequelae in discharged COVID-19 patients with diabetes or secondary hyperglycemia . Eur J Radiol 2021. ; 144 : 109997 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vijayakumar B , Tonkin J , Devaraj A , et al . CT Lung Abnormalities after COVID-19 at 3 Months and 1 Year after Hospital Discharge . Radiology 2022. ; 303 ( 2 ): 444 – 454 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zangrillo A , Belletti A , Palumbo D , et al . One-Year Multidisciplinary Follow-Up of Patients With COVID-19 Requiring Invasive Mechanical Ventilation . J Cardiothorac Vasc Anesth 2022. ; 36 ( 5 ): 1354 – 1363 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watanabe A , So M , Iwagami M , et al . One-year follow-up CT findings in COVID-19 patients: A systematic review and meta-analysis . Respirology 2022. ; 27 ( 8 ): 605 – 616 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang P , Li J , Liu H , et al . Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study . Bone Res 2020. ; 8 ( 1 ): 8 [Published correction appears in Bone Res 2020;8:34.] . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hui DS , Joynt GM , Wong KT , et al . Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors . Thorax 2005. ; 60 ( 5 ): 401 – 409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsakok MT , Watson RA , Saujani SJ , et al . Chest CT and Hospital Outcomes in Patients with Omicron Compared with Delta Variant SARS-CoV-2 Infection . Radiology 2022. . 10.1148/radiol.220533. Published online June 21, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoon SH , Lee JH , Kim BN . Chest CT Findings in Hospitalized Patients with SARS-CoV-2: Delta versus Omicron Variants . Radiology 2022. . 10.1148/radiol.220676. Published online June 28, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hata A , Schiebler ML , Lynch DA , Hatabu H . Interstitial Lung Abnormalities: State of the Art . Radiology 2021. ; 301 ( 1 ): 19 – 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen J , Wu J , Hao S , et al . Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection . Sci Rep 2017. ; 7 ( 1 ): 17275 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang YC , Yu CJ , Chang SC , et al . Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT . Radiology 2005. ; 236 ( 3 ): 1067 – 1075 . [DOI] [PubMed] [Google Scholar]
- 36. Chu WC , Li AM , Ng AW , et al . Thin-Section CT 12 Months After the Diagnosis of Severe Acute Respiratory Syndrome in Pediatric Patients . AJR Am J Roentgenol 2006. ; 186 ( 6 ): 1707 – 1714 . [DOI] [PubMed] [Google Scholar]
- 37. Franquet T , Giménez A , Ketai L , et al . Air trapping in COVID-19 patients following hospital discharge: retrospective evaluation with paired inspiratory/expiratory thin-section CT . Eur Radiol 2022. ; 32 ( 7 ): 4427 – 4436 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Besutti G , Monelli F , Schirò S , et al . Follow-Up CT Patterns of Residual Lung Abnormalities in Severe COVID-19 Pneumonia Survivors: A Multicenter Retrospective Study . Tomography 2022. ; 8 ( 3 ): 1184 – 1195 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McGroder CF , Zhang D , Choudhury MA , et al . Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length . Thorax 2021. ; 76 ( 12 ): 1242 – 1245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caruso D , Guido G , Zerunian M , et al . Post-Acute Sequelae of COVID-19 Pneumonia: Six-month Chest CT Follow-up . Radiology 2021. ; 301 ( 2 ): E396 – E405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Treggiari MM , Romand JA , Martin JB , Suter PM . Air cysts and bronchiectasis prevail in nondependent areas in severe acute respiratory distress syndrome: a computed tomographic study of ventilator-associated changes . Crit Care Med 2002. ; 30 ( 8 ): 1747 – 1752 . [DOI] [PubMed] [Google Scholar]
- 42. Desai SR , Wells AU , Rubens MB , Evans TW , Hansell DM . Acute respiratory distress syndrome: CT abnormalities at long-term follow-up . Radiology 1999. ; 210 ( 1 ): 29 – 35 . [DOI] [PubMed] [Google Scholar]
- 43. Bocchino M , Lieto R , Romano F , et al . Chest CT-based Assessment of 1-year Outcomes after Moderate COVID-19 Pneumonia . Radiology 2022. . 10.1148/radiol.220019. Published online May 10, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu Q , Liu Y , Chen C , et al . Reversible Bronchiectasis in COVID-19 Survivors With Acute Respiratory Distress Syndrome: Pseudobronchiectasis . Front Med (Lausanne) 2021. ; 8 : 739857 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luger AK , Sonnweber T , Gruber L , et al . Chest CT of Lung Injury 1 Year after COVID-19 Pneumonia: The CovILD Study . Radiology 2022. ; 304 ( 2 ): 462 – 470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hansell DM , Bankier AA , MacMahon H , McLoud TC , Müller NL , Remy J . Fleischner Society: glossary of terms for thoracic imaging . Radiology 2008. ; 246 ( 3 ): 697 – 722 . [DOI] [PubMed] [Google Scholar]
- 47. Costa RD , Zanon M , Watte G , et al . Expiratory CT scanning in COVID-19 patients: can we add useful data? J Bras Pneumol 2022. ; 48 ( 2 ): e20210204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jia X , Han X , Cao Y , et al . Quantitative inspiratory-expiratory chest CT findings in COVID-19 survivors at the 6-month follow-up . Sci Rep 2022. ; 12 ( 1 ): 7402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cho JL , Villacreses R , Nagpal P , et al . Quantitative Chest CT Assessment of Small Airways Disease in Post-Acute SARS-CoV-2 Infection . Radiology 2022. ; 304 ( 1 ): 185 – 192 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tanaka N , Matsumoto T , Miura G , et al . Air trapping at CT: high prevalence in asymptomatic subjects with normal pulmonary function . Radiology 2003. ; 227 ( 3 ): 776 – 785 . [DOI] [PubMed] [Google Scholar]
- 51. Grist JT , Collier GJ , Walters H , et al . Lung Abnormalities Depicted with Hyperpolarized Xenon MRI in Patients with Long COVID . Radiology 2022. . 10.1148/radiol.220069. Published online May 24, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kooner HK , McIntosh MJ , Matheson AM , et al . 129Xe MRI ventilation defects in ever-hospitalised and never-hospitalised people with post-acute COVID-19 syndrome . BMJ Open Respir Res 2022. ; 9 ( 1 ): e001235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grist JT , Chen M , Collier GJ , et al . Hyperpolarized 129Xe MRI Abnormalities in Dyspneic Patients 3 Months after COVID-19 Pneumonia: Preliminary Results . Radiology 2021. ; 301 ( 1 ): E353 – E360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li Y , Xia L . Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management . AJR Am J Roentgenol 2020. ; 214 ( 6 ): 1280 – 1286 . [DOI] [PubMed] [Google Scholar]
- 55. Lang M , Som A , Carey D , et al . Pulmonary Vascular Manifestations of COVID-19 Pneumonia . Radiol Cardiothorac Imaging 2020. ; 2 ( 3 ): e200277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alnima T , Mulder MMG , van Bussel BCT , Ten Cate H . COVID-19 Coagulopathy: From Pathogenesis to Treatment . Acta Haematol 2022. ; 145 ( 3 ): 282 – 296 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fogarty H , Townsend L , Morrin H , et al . Persistent endotheliopathy in the pathogenesis of long COVID syndrome . J Thromb Haemost 2021. ; 19 ( 10 ): 2546 – 2553 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ambrosino P , Calcaterra I , Molino A , et al . Persistent Endothelial Dysfunction in Post-Acute COVID-19 Syndrome: A Case-Control Study . Biomedicines 2021. ; 9 ( 8 ): 957 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bull-Otterson L , Baca S , Saydah S , et al . Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years — United States, March 2020–November 2021 . Atlanta, Ga: : Centers for Disease Control and Prevention; , 2022. . [Google Scholar]
- 60. Al-Aly Z , Bowe B , Xie Y . Long COVID after breakthrough SARS-CoV-2 infection . Nat Med 2022. ; 28 ( 7 ): 1461 – 1467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tobaiqy M , MacLure K , Elkout H , Stewart D . Thrombotic Adverse Events Reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 Vaccines: Comparison of Occurrence and Clinical Outcomes in the EudraVigilance Database . Vaccines (Basel) 2021. ; 9 ( 11 ): 1326 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matar RH , Than CA , Nakanishi H , et al . Outcomes of patients with thromboembolic events following coronavirus disease 2019 AstraZeneca vaccination: a systematic review and meta-analysis . Blood Coagul Fibrinolysis 2022. ; 33 ( 2 ): 90 – 112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gangi A , Mobashwera B , Ganczakowski M , Ayto R . Imaging and Hematologic Findings in Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 (AstraZeneca) Vaccination . Radiology 2021. ; 301 ( 2 ): E416 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Curcio R , Gandolfo V , Alcidi R , et al . Vaccine-induced massive pulmonary embolism and thrombocytopenia following a single dose of Janssen Ad26.COV2.S vaccination . Int J Infect Dis 2022. ; 116 : 154 – 156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thacker VV , Sharma K , Dhar N , Mancini GF , Sordet-Dessimoz J , McKinney JD . Rapid endotheliitis and vascular damage characterize SARS-CoV-2 infection in a human lung-on-chip model . EMBO Rep 2021. ; 22 ( 6 ): e52744 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Korompoki E , Gavriatopoulou M , Fotiou D , Ntanasis-Stathopoulos I , Dimopoulos MA , Terpos E . Late-onset hematological complications post COVID-19: An emerging medical problem for the hematologist . Am J Hematol 2022. ; 97 ( 1 ): 119 – 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Giryes S , Bragazzi NL , Bridgewood C , De Marco G , McGonagle D . COVID-19 Vasculitis and vasculopathy-Distinct immunopathology emerging from the close juxtaposition of Type II Pneumocytes and Pulmonary Endothelial Cells . Semin Immunopathol 2022. ; 44 ( 3 ): 375 – 390 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cueto-Robledo G , Roldan-Valadez E , Graniel-Palafox LE , et al . Chronic Thromboembolic Pulmonary Hypertension (CTEPH): A Review of Another Sequel of Severe Post-Covid-19 Pneumonia . Curr Probl Cardiol 2022. . 10.1016/j.cpcardiol.2022.101187. Published online March 25, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tudoran C , Tudoran M , Lazureanu VE , et al . Evidence of Pulmonary Hypertension after SARS-CoV-2 Infection in Subjects without Previous Significant Cardiovascular Pathology . J Clin Med 2021. ; 10 ( 2 ): 199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brito-Azevedo A , Pinto EC , de Cata Preta Corrêa GA , Bouskela E . SARS-CoV-2 infection causes pulmonary shunt by vasodilatation . J Med Virol 2021. ; 93 ( 1 ): 573 – 575 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dhawan RT , Gopalan D , Howard L , et al . Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19 . Lancet Respir Med 2021. ; 9 ( 1 ): 107 – 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salerno D , Oriaku I , Darnell M , et al . Association of abnormal pulmonary vasculature on CT scan for COVID-19 infection with decreased diffusion capacity in follow up: A retrospective cohort study . PLoS One 2021. ; 16 ( 10 ): e0257892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tian S , Hu W , Niu L , Liu H , Xu H , Xiao SY . Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer . J Thorac Oncol 2020. ; 15 ( 5 ): 700 – 704 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lan J , Ge J , Yu J , et al . Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor . Nature 2020. ; 581 ( 7807 ): 215 – 220 . [DOI] [PubMed] [Google Scholar]
- 75. Borczuk AC . Pulmonary pathology of COVID-19: a review of autopsy studies . Curr Opin Pulm Med 2021. ; 27 ( 3 ): 184 – 192 . [DOI] [PubMed] [Google Scholar]
- 76. Wiersinga WJ , Rhodes A , Cheng AC , Peacock SJ , Prescott HC . Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review . JAMA 2020. ; 324 ( 8 ): 782 – 793 . [DOI] [PubMed] [Google Scholar]
- 77. Bösmüller H , Matter M , Fend F , Tzankov A . The pulmonary pathology of COVID-19 . Virchows Arch 2021. ; 478 ( 1 ): 137 – 150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Peiris S , Mesa H , Aysola A , et al . Pathological findings in organs and tissues of patients with COVID-19: A systematic review . PLoS One 2021. ; 16 ( 4 ): e0250708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Satturwar S , Fowkes M , Farver C , et al . Postmortem Findings Associated With SARS-CoV-2: Systematic Review and Meta-analysis . Am J Surg Pathol 2021. ; 45 ( 5 ): 587 – 603 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Vasquez-Bonilla WO , Orozco R , Argueta V , et al . A review of the main histopathological findings in coronavirus disease 2019 . Hum Pathol 2020. ; 105 : 74 – 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Calabrese F , Pezzuto F , Fortarezza F , et al . Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists . Virchows Arch 2020. ; 477 ( 3 ): 359 – 372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Borczuk AC , Salvatore SP , Seshan SV , et al . COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City . Mod Pathol 2020. ; 33 ( 11 ): 2156 – 2168 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Konopka KE , Nguyen T , Jentzen JM , et al . Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD . Histopathology 2020. ; 77 ( 4 ): 570 – 578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McMullen P , Pytel P , Snyder A , et al . A series of COVID-19 autopsies with clinical and pathologic comparisons to both seasonal and pandemic influenza . J Pathol Clin Res 2021. ; 7 ( 5 ): 459 – 470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hariri LP , North CM , Shih AR , et al . Lung Histopathology in Coronavirus Disease 2019 as Compared With Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review . Chest 2021. ; 159 ( 1 ): 73 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Parra-Medina R , Herrera S , Mejia J . Systematic Review of Microthrombi in COVID-19 Autopsies . Acta Haematol 2021. ; 144 ( 5 ): 476 – 483 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jonigk D , Werlein C , Lee PD , Kauczor HU , Länger F , Ackermann M . Pulmonary and Systemic Pathology in COVID-19-Holistic Pathological Analyses . Dtsch Arztebl Int 2022. ; (Forthcoming):arztebl.m2022.0231 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. De Michele S , Sun Y , Yilmaz MM , et al . Forty Postmortem Examinations in COVID-19 Patients . Am J Clin Pathol 2020. ; 154 ( 6 ): 748 – 760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beasley MB . The pathologist’s approach to acute lung injury . Arch Pathol Lab Med 2010. ; 134 ( 5 ): 719 – 727 . [DOI] [PubMed] [Google Scholar]
- 90. Katzenstein AL , Bloor CM , Leibow AA . Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review . Am J Pathol 1976. ; 85 ( 1 ): 209 – 228 . [PMC free article] [PubMed] [Google Scholar]
- 91. Diaz A , Bujnowski D , McMullen P , et al . Pulmonary Parenchymal Changes in COVID-19 Survivors . Ann Thorac Surg 2022. ; 114 ( 1 ): 301 – 310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Funk GC , Nell C , Pokieser W , Thaler B , Rainer G , Valipour A . Organizing pneumonia following Covid19 pneumonia . Wien Klin Wochenschr 2021. ; 133 ( 17-18 ): 979 – 982 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Aesif SW , Bribriesco AC , Yadav R , et al . Pulmonary Pathology of COVID-19 Following 8 Weeks to 4 Months of Severe Disease: A Report of Three Cases, Including One With Bilateral Lung Transplantation . Am J Clin Pathol 2021. ; 155 ( 4 ): 506 – 514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hall DJ , Schulte JJ , Lewis EE , et al . Successful Lung Transplantation for Severe Post-COVID-19 Pulmonary Fibrosis . Ann Thorac Surg 2022. ; 114 ( 1 ): e17 – e19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bharat A , Querrey M , Markov NS , et al . Lung transplantation for pulmonary fibrosis secondary to severe COVID-19 . medRxiv.2020. Posted October 27, 2020. Accessed June 15, 2022. [Google Scholar]
- 96. Schwensen HF , Borreschmidt LK , Storgaard M , Redsted S , Christensen S , Madsen LB . Fatal pulmonary fibrosis: a post-COVID-19 autopsy case . J Clin Pathol 2020. ; 74 ( 6 ): 400 – 402 . [DOI] [PubMed] [Google Scholar]
- 97. Baldi BG , Fabro AT , Franco AC , et al . Clinical, radiological, and transbronchial biopsy findings in patients with long COVID-19: a case series . J Bras Pneumol 2022. ; 48 ( 3 ): e20210438 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Flaifel A , Kwok B , Ko J , et al . Pulmonary Pathology of End-Stage COVID-19 Disease in Explanted Lungs and Outcomes After Lung Transplantation . Am J Clin Pathol 2022. ; 157 ( 6 ): 908 – 926 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Konopka KE , Perry W , Huang T , Farver CF , Myers JL . Usual Interstitial Pneumonia is the Most Common Finding in Surgical Lung Biopsies from Patients with Persistent Interstitial Lung Disease Following Infection with SARS-CoV-2 . EClinicalMedicine 2021. ; 42 : 101209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li , Wu J , Wang S , et al . Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China . Histopathology 2021. ; 78 ( 4 ): 542 – 555 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kanne , Bai H , Bernheim A , et al . COVID-19 Imaging: What We Know Now and What Remains Unknown . Radiology 2021. ; 299 ( 3 ): E262 – E279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lerum , Aaløkken TM , Brønstad E , et al . Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19 . Eur Respir J 2021. ; 57 ( 4 ): 2003448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Froidure , Mahsouli A , Liistro G , et al . Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae . Respir Med 2021. ; 181 : 106383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Balbi M , Conti C , Imeri G , et al . Post-discharge chest CT findings and pulmonary function tests in severe COVID-19 patients . Eur J Radiol 2021. ; 138 : 109676 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van den Borst B , Peters JB , Brink M , et al . Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19) . Clin Infect Dis 2021. ; 73 ( 5 ): e1089 – e1098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhao YM , Shang YM , Song WB , et al . Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery . EClinicalMedicine 2020. ; 25 : 100463 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. González J , Benítez ID , Carmona P , et al . Pulmonary Function and Radiologic Features in Survivors of Critical COVID-19: A 3-Month Prospective Cohort . Chest 2021. ; 160 ( 1 ): 187 – 198 . [DOI] [PMC free article] [PubMed] [Google Scholar]