Abstract
Nucleotide-binding, leucine-rich repeat receptors (NLRs) perceive pathogen effectors to trigger plant immunity. The direct recognition mechanism of pathogen effectors by coiled-coil NLRs (CNLs) remains unclear. We demonstrate that the Triticum monococcum CNL Sr35 directly recognizes the pathogen effector AvrSr35 from Puccinia graminis f. sp. tritici and report a cryo–electron microscopy structure of Sr35 resistosome and a crystal structure of AvrSr35. We show that AvrSr35 forms homodimers that are disassociated into monomers upon direct recognition by the leucine-rich repeat domain of Sr35, which induces Sr35 resistosome assembly and the subsequent immune response. The first 20 amino-terminal residues of Sr35 are indispensable for immune signaling but not for plasma membrane association. Our findings reveal the direct recognition and activation mechanism of a plant CNL and provide insights into biochemical function of Sr35 resistosome.
Direct recognition of AvrSr35 induces assembly of Sr35 resistosome and activation of immune response.
INTRODUCTION
To resist pathogen infection, plants have evolved a sophisticated two-branched innate immune system consisting of pathogen-associated molecular pattern-triggered immunity and effector-triggered immunity (ETI) (1). Nucleotide-binding, leucine-rich repeat (LRR) receptors (NLRs) act as intracellular immune receptors in ETI. NLRs share a conserved multidomain architecture comprising a variable N-terminal domain, the central nucleotide-binding and oligomerization domain (NOD), and the C-terminal LRR domain. On the basis of the feature of their N termini, plant NLRs are categorized into two main groups, namely, the coiled-coil (CC)–NLRs (CNLs) and Toll/interleukin-1 receptor (TIR)–NLRs (2–4). NLRs directly or indirectly detect pathogen effectors and subsequently initiate a robust immune response and local cell death termed “hypersensitive response” (HR) (5), thus restricting pathogen proliferation.
Wheat stem rust, caused by the fungus Puccinia graminis f. sp. tritici (Pgt), is a devastating disease that causes severe losses in wheat crop yields (6). Stem rust resistance gene Sr35 from the wheat species Triticum monococcum encodes a CNL that confers immunity against most virulent races of the fungus Pgt, including Ug99. AvrSr35 was identified as a pathogen avirulence gene encoding the secreted protein AvrSr35 that triggers host immune response (7, 8). Although Sr35-dependent resistance has been linked to the recognition of the Pgt effector AvrSr35, the physiological and potential virulence functions of AvrS35 remain elusive. A previous study has revealed that Sr35 colocalizes with AvrSr35 in the Nicotiana benthamiana leaf epidermal cells (7). However, it is unclear whether Sr35 binds AvrSr35 directly or indirectly and the molecular basis for activation and assembly of Sr35 resistosome and subsequent immune response remains unknown. In this study, we determined a cryo–electron microscopy (cryo-EM) structure of AvrSr35-induced Sr35 resistosome and a crystal structure of AvrSr35. Supported by in vivo and in vitro biochemical and functional analysis, our structural data reveal the molecular mechanism for direct recognition and activation of a plant CNL by pathogen effector and provide valuable information for the function and downstream signaling of plant resistosome.
RESULTS
Sr35 directly interacts with AvrSr35 in vitro
To investigate the interaction between Sr35 and AvrSr35, we obtained recombinant Sr35 and AvrSr35 and used them to perform interaction assays in vitro. Glutathione S-transferase (GST) pull-down assay, microscale thermophoresis (MST), and gel filtration chromatography indicated that Sr35 and AvrSr35 directly and strongly interact [Kd (dissociation constant) = 7.8 μM in buffer containing 500 mM NaCl] (fig. S1, A to C), which is in agreement with previously published data (7). Moreover, gel filtration analysis shows that the Sr35-AvrSr35 complex further oligomerizes in the presence of adenosine 5′-triphosphate (ATP) into Sr35 resistosome (fig. S1D), which is consistent with other NLRs such as HOPZ-ACTIVATED RESISTANCE 1 (ZAR1). While the structure of the CNL ZAR1 resistosome whose formation is induced indirectly by pathogen effector has been solved (9), the mechanisms for direct recognition of a pathogen effector by a CNL and subsequent resistosome assembly remain unclear.
Overall structure of Sr35 resistosome
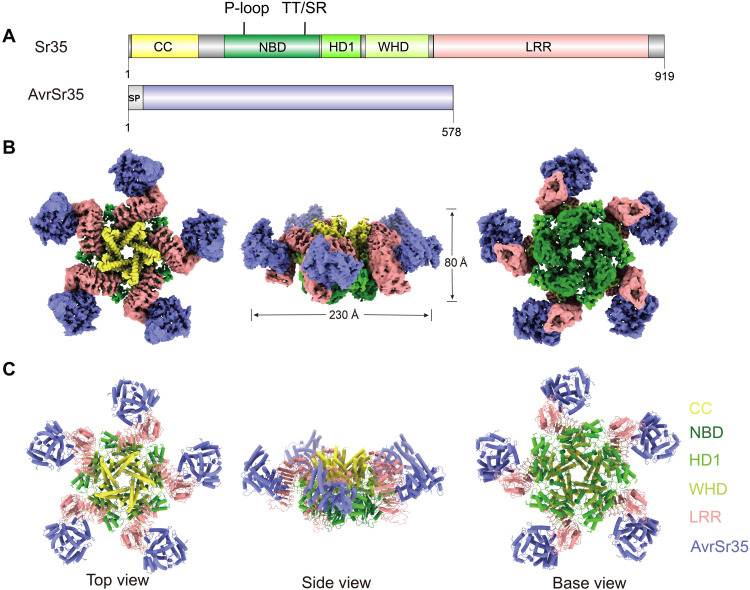
AvrSr35 is an uncharacterized pathogen effector protein with an N-terminal signal peptide (Fig. 1A). Sr35 consists of the N-terminal CC domain, the central NOD, and the C-terminal LRR domain. The NOD is further divided into three subdomains: the nucleotide-binding domain (NBD), the helical domain 1 (HD1), and the winged-helix domain (WHD) (Fig. 1A). To gain insight into the structural basis for AvrSr35-induced Sr35 oligomerization, we visualized the Sr35 resistosome using cryo-EM with the overall resolution of 3.33 Å (fig. S2). The cryo-EM density map corresponding to the C terminus of LRR domain of Sr35 and its interface with AvrSr35 was relatively poorly defined, so we used the local refinement method to improve the resolution and enable model building, allowing clear presentation of the Sr35-AvrSr35 interaction interface. The three-dimensional (3D) reconstruction did not show a clear location of the N-terminal residues 1 to 23.
Fig. 1. Overall structure of the Sr35 resistosome.
(A) Schematic depiction of the domain organization of Sr35 and AvrSr35. (B and C) Composite density map of the Sr35 resistosome from three cryo-EM reconstructions (B) and corresponding atomic model (C) shown in three orthogonal views.
The 3D reconstruction shows that Sr35 resistosome contains five protomers of Sr35-AvrSr35 complex, forming a wheel-like structure measuring ~230 Å in diameter and ~80 Å in height (Fig. 1, B and C). NOD of each protomer laterally packs against each other, forming the central platform and the base surface of the resistosome wheel. The LRR domains extend from the tail of the WHD subdomain to form five separate arms bent in the same direction with AvrSr35 bound on the inner side. The CC domains of five Sr35 protomers, each composed of a trimeric α-helical bundle, are arranged in a linear fashion from the narrow end to the wide end of a funnel-shaped formation located at the top side of NOD platform (Fig. 1, B and C, and fig. S3, A and B).
Oligomerization of Sr35 resistosome is mediated by Sr35NOD
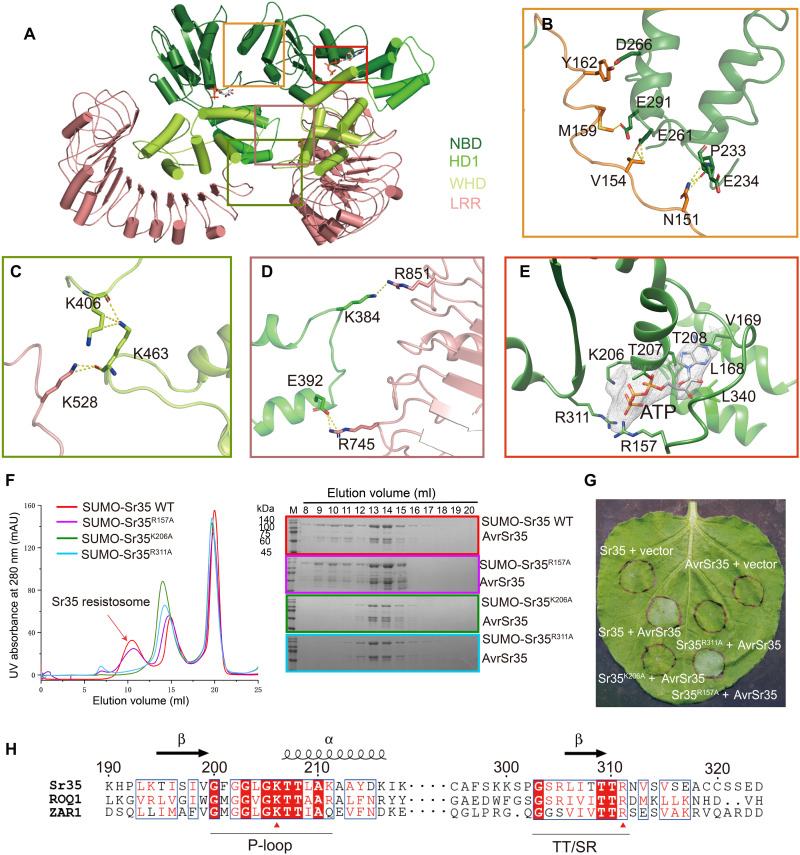
The wheel-like structure of Sr35 resistosome is stabilized by formation of the same three interacting regions between adjacent Sr35 protomers (Fig. 2A). In the first region, the long loop region connecting Sr35CC to Sr35NBD forms several hydrogen bonds with three α helices from the neighboring Sr35NBD (Fig. 2B). The second region includes hydrogen bonds between K528 from Sr35LRR and the K463 from the adjacent Sr35WHD (Fig. 2C). The third region involves polar interactions between residues K384 and E392 from Sr35HD1 and residues R851 and R745 from the other Sr35LRR, respectively (Fig. 2D). An ATP molecule with well-defined electron density is stabilized at the nucleotide-binding pocket of each Sr35NOD by hydrophobic and polar contacts (Fig. 2E). The β- and γ-phosphates of ATP form extensive interactions with residues K206 from the P-loop motif and R311 from the TT/SR motif (10). Moreover, the side chain of R157 from long loop region (residues 145 to 146) extends toward the γ-phosphate group and forms hydrogen bonds (Fig. 2E).
Fig. 2. Oligomerization of Sr35 resistosome.
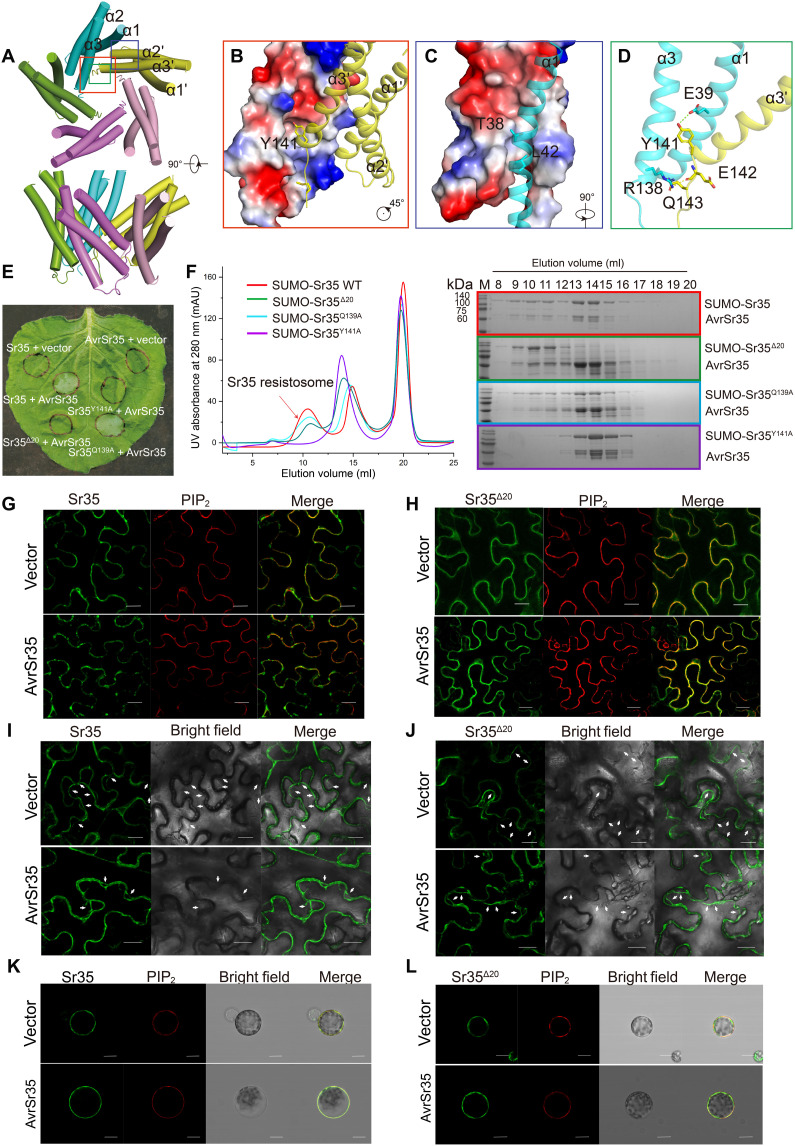
(A) An overall view of two adjacent Sr35 protomers in the resistosome. Perspective of presentation highlight the interfaces mediating the lateral dimer. (B to D) Close-up views of the NBD-NBD, LRR-WHD, and HD1-LRR interfaces, respectively. Hydrogen bonds are shown as yellow dashed lines. (E) Interaction between ATP and Sr35. Cryo-EM density of ATP is shown as gray mesh. (F) Mutations of residues in the ATP-binding pocket affect the formation of Sr35 resistosome. Left: Gel filtration profiles of wild-type (WT) Sr35 or its mutant variants and AvrSr35 incubated in the presence of ATP. Curves are indicated in different colors, and the peak corresponding to resistosome is indicated by arrow. Right: The peak fractions in the left panel were visualized by SDS–polyacrylamide gel electrophoresis (PAGE) and Coomassie blue staining. Gels are color-coded as the left panel. UV, ultraviolet; mAU, milli–absorbance units. (G) Coinfiltration of N. benthamiana with WT and mutant Sr35 and AvrSr35 constructs. The images were taken at 36 to 48 hours postinfection (hpi). The experiment was performed three times. (H) Local sequence alignment between the Sr35, ROQ1, and ZAR1. Positions of P-loop and TT/SR motif are labeled along the sequence using underscore. Evolutionary conserved amino acids that interact with ATP are marked with a red triangle.
To verify the structural observations, we examined the oligomerization capabilities of Sr35 mutants Sr35R157A, Sr35K206A, and Sr35R311A using gel filtration column. The Sr35K206A and Sr35R311A mutants were unable to oligomerize, whereas the Sr35R157A mutant largely retained the oligomerization ability (Fig. 2F). Likewise, coinjection of AvrSr35 with Sr35K206A and Sr35R311A into N. benthamiana did not cause discernible changes, whereas coinjection of Sr35R157A and AvrSr35 resulted in necrosis (Fig. 2G). Moreover, sequence alignment indicated that the Sr35 residues K206 and R311, but not R157, are highly conserved among plant NLRs including ZAR1and ROQ1 (RECOGNITION OF XOPQ 1), which is supported by the structural similarity of their NODs (Fig. 2H and fig. S3C). Together, our data support the crucial role of NOD-mediated Sr35 oligomerization in resistance against Pgt.
Recognition of AvrSr35 by Sr35LRR
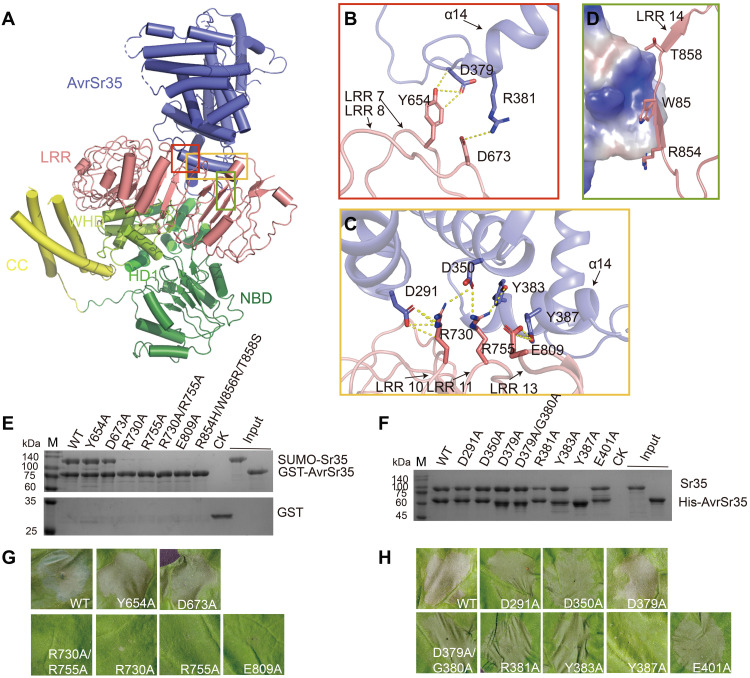
In the Sr35 resistosome, the helix α14 of AvrSr35 tightly packs against the inner lateral side of Sr35LRR predominantly via several polar interactions (Fig. 3A). Residues AvrSr35D379 and AvrSr35R381 from the N terminus of helix α14 hydrogen bond with residues Sr35Y654 and Sr35D673 from the loop region of the seventh and eighth α/β horseshoe fold repeats of Sr35 LRR (Fig. 3B). The side chains of Sr35R730, Sr35R755, and Sr35E809 form a network of hydrogen bonds with the side chains of AvrSr35D291, AvrSr35D350, AvrSr35Y383, and AvrSr35Y387 located near helix α14 of AvrSr35 (Fig. 3C).
Fig. 3. The C-terminal LRR domain mediates Sr35 interaction with AvrSr35.
(A) Overall structure of Sr35-AvrSr35 complex in resistosome shown in cartoon mode. The interaction regions between the two proteins are highlighted with different colored frames. (B) Detailed interactions of the N terminus of the AvrSr35 helix α14 with Sr35 LRRs 7 and 8. Hydrogen bonds are shown as yellow dashed lines. (C) Detailed interactions of AvrSr35 α14 with Sr35 LRRs 11 and 13. Hydrogen bonds are shown as yellow dashed lines. (D) Surface contacts between the R854, W856, and T858 of Sr35LRR, as well as AvrSr35, represented by its coulombic surface potential. (E) Sr35 or (F) AvrSr35 mutations reduce Sr35-AvrSr35 interaction in vitro. Purified N-terminal 6×His-SUMO–tagged WT or mutant Sr35 was incubated with GST-tagged AvrSr35, or Sr35 was incubated with 6×His-tagged WT or mutant AvrSr35, followed by purification using Glutathione Sepharose 4B or Ni–nitrilotriacetic acid (Ni-NTA) beads. The proteins were visualized by SDS-PAGE with Coomassie brilliant blue staining. CK in (E), control containing GST protein and SUMO-Sr35; CK in (F), control group containing Sr35. (G) Sr35 or (H) AvrSr35 mutations abolish disease resistance in N. benthamiana leaves. The images were taken at 36 to 48 hpi. The experiment was performed three times.
In line with structural observations, the mutants Sr35R730A, Sr35R755A, and Sr35E809A, but not Sr35Y654A and Sr35D673A, severely impaired the Sr35-AvrSr35 interaction, whereas the double mutant Sr35R730A/R755A abolished the interaction (Fig. 3E). The triple mutant Sr35R854H/W856R/T858S also abolished Sr35-AvrSr35 interaction (Fig. 3E), possibly because of the electrostatic repulsion formed between the positively charged AvrSr35 loop and the bulky positively charged side chain of arginine in place of W856 (Fig. 3D). Our data provide an explanation to why the Ug99-susceptible wheat strain M1120 containing these three residue mutations fails to elicit a resistance response (7). Moreover, the AvrSr35 mutant AvrSr35R381A exhibited substantially diminished binding to Sr35, whereas the mutant AvrSr35Y387A was completely unable to bind Sr35 (Fig. 3F).
We then tested the impact of Sr35 or AvrSr35 mutants on AvrSr35-mediated cell death in N. benthamiana. Almost all aforementioned Sr35 mutants that severely weakened or abrogated the Sr35-AvrSr35 interaction did not cause mesophyll cell death (Fig. 3, G and H), whereas the AvrSr35R381A with reduced Sr35-binding activity elicited weaker cell death compared to wild-type (WT) AvrSr35 coexpressed with Sr35. Collectively, our data support an essential role of Sr35LRR in recognition of AvrSr35.
Mechanism for AvrSr35-induced Sr35 activation
To understand how AvrSr35 induces the activation of Sr35, we also determined the crystal structure of AvrSr35 lacking the signal peptide (residues 1 to 26) at 2.0-Å resolution. Two AvrSr35 molecules exist in an asymmetric unit. The buried surface area of their interface is 1430.2 Å2, suggesting that AvrSr35 might form a homodimer (Fig. 4A and table S3). To validate our structural observations, we performed a GST pull-down assay using GST-tagged and His-tagged AvrSr35 and analytical ultracentrifugation (AUC). His-tagged AvrSr35 coeluted with GST-tagged AvrSr35 in the pull-down assay (Fig. 4B), and the molecular weight of AvrSr35 calculated from the sedimentation coefficient (S) was approximately 100 kDa (fig. S3D). These results indicate that AvrSr35 forms a dimer in solution.
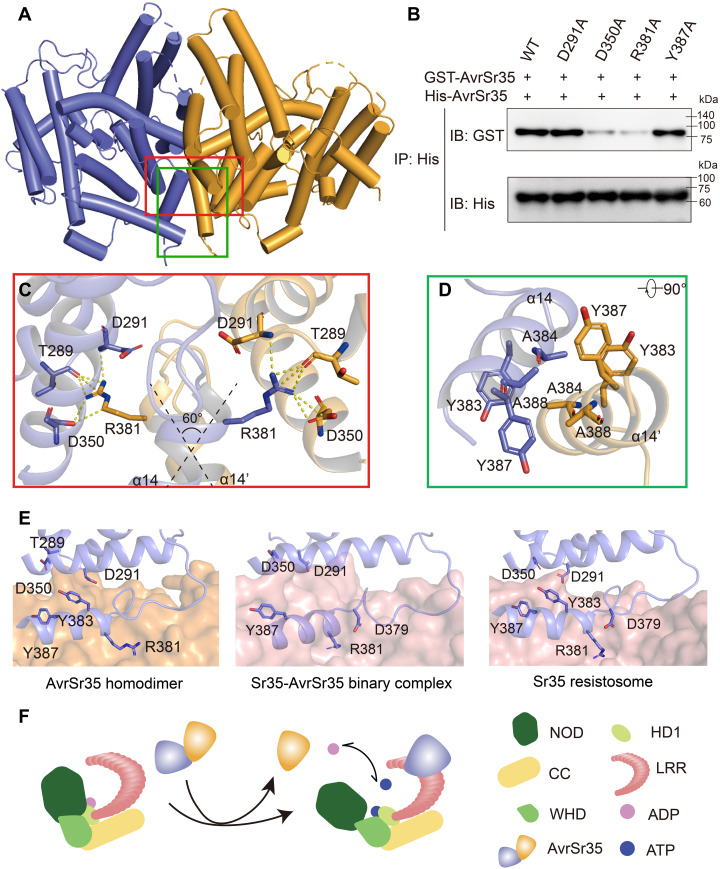
Fig. 4. Mechanism of Sr35 activation by AvrSr35.
(A) The crystal structure of the AvrSr35 dimer is shown in ribbon representation with subunits A and B colored slate and orange, respectively. (B) AvrSr35 mutants around interaction interface shown in (C) and (D) diminish the homodimerization interaction. IP: immunoprecipitation; IB: immunoblotting. (C) Detailed view of hydrophilic interactions between two copies of AvrSr35. (D) Bottom view of hydrophobic interactions between two copies of AvrSr35. (E) Comparison of the interface between different states of Sr35 activated by AvrSr35. Left: AvrSr35 dimerization interface that one protomer represented in orange surface and the other protomer shown in slate cartoon. Middle: Interaction surface of the intermediate state of AvrSr35 and Sr35 complex in the absence of ATP. Sr35 LRR is shown as salmon surface, and AvrSr35 is shown as slate cartoon. Right: Interaction surface of the activated state of Sr35 resistosome. Sr35LRR is shown as salmon surface, and AvrSr35 is shown as slate cartoon. Interacting residues are shown as sticks and labeled accordingly. (F) Diagram depicting the binding of AvrSr35 with Sr35.
In AvrSr35 dimer, two protomers pack against each other at a ~60° angle, and the interaction is mediated by helix α14 (residues 381 to 390) (Fig. 4C). A cluster of aromatic residues (Y383, A384, Y387, and A388 of both protomers) forms a stable hydrophobic core (Fig. 4D). Concurrently, the positively charged residue R381 from the N terminus of helix α14 hydrogen bonds with residues T289, D291, and D350 of another AvrSr35, thereby further stabilizing the conformation of the AvrSr35 dimer (Fig. 4C). Consistent with these observations, the AvrSr35D350A and AvrSr35R381A mutants severely impaired the dimerization capacity, whereas the AvrSr35D291A and AvrSr35Y387A mutants did not (Fig. 4B). Furthermore, the retention volumes of the AvrSr35D350A and AvrSr35R381A mutants in gel filtration increased compared to that of WT AvrSr35, and the molecular weight of AvrSr35D350A determined by AUC was approximately 65 kDa (fig. S3, D and E). Together, these results demonstrate that AvrSr35 is a bona fide dimer in solution.
However, Sr35 and AvrSr35 bind at a 1:1 stoichiometry, implying that AvrSr35 homodimer dissociates into monomers upon recognition by Sr35. To further explore the mechanism by which AvrSr35 activates Sr35, we determined the cryo-EM structure of the AvrSr35-Sr35 binary complex formed in the absence of ATP (fig. S4 and table S1). The structure clearly shows that AvrSr35 is tightly bound to the Sr35LRR as in the structure of Sr35 resistosome (Fig. 4E and fig. S4, E and F). Structural alignment of dimerized AvrSr35 with AvrSr35 from Sr35-AvrSr35 binary complex and AvrSr35 from Sr35 resistosome indicated notable similarity with root mean square deviation values of 1.277 and 1.061 Å, respectively (fig. S3F). Notably, further comparison indicates that the AvrSr35 dimerization interface and the AvrSr35-Sr35 interface completely overlap (Fig. 4E). The residues that participate in AvrSr35 dimerization, i.e., D291, D350, Y383, and Y387, are recognized by Sr35 residues R730, R755, and E809. Moreover, the residue AvrSr35 Y387, which hydrogen bonds with E809 of Sr35, plays a crucial role in Sr35-AvrS35 interaction but not in AvrSr35 dimerization (Figs. 3, E and F, and 4B). In addition, the AvrSr35 residue R381 does not seem to be essential for the Sr35-AvrSr35 interaction but plays a critical role in AvrSr35 dimerization (Figs. 3, E and F, and 4B). Characterization of AvrSr35R381A-induced resistosome indicated that AvrSr35R381A can also induce the assembly of Sr35-AvrSr35 complex, albeit at lower ligand binding ratio compared to the WT Sr35 resistosome (figs. S2C and S5C). The propensity for interaction between AvrSr35 and Sr35 can also be supported by a larger buried surface area of the AvrSr35-Sr35 interface (2371.7 Å2) compared to that of the AvrSr35 dimerization interface (1430.2 Å2) (table S3).
CC domain plays an integral role in Sr35-mediated immune signaling
In Sr35 resistosome, the three-helix bundles of Sr35 CC domains pack against each other, forming an inverted funnel-shaped structure (Figs. 1, B and C, and 5A).
Fig. 5. CC domain plays an integral role in Sr35-mediated immune signaling and triggering cell death.
(A) Top and side views of Sr35 CC domain in Sr35 resistosome. (B) Hydrophobic interaction between α3′ and α1, α2, α3. (C) Hydrophobic interactions between α1 and α2′ and α3′. (D) Detailed hydrophilic interaction in the center of the three parallel trimeric helical bundle of Sr35 CC domain. (E) Mutations in CC domain of Sr35 abolish HR in N. benthamiana leaves. The images were taken 36 to 48 hpi. (F) Mutations of amino acids involved in CC domain interactions affect the oligomerization of Sr35 resistosome. Left: Gel filtration profiles of Sr35 and AvrSr35 incubated with ATP. Right: The peak fractions in the top panel were visualized by SDS-PAGE and Coomassie blue staining. (G and H) Representative images displaying subcellular localization of WT Sr35 and mutant Sr35Δ20 mutant in the presence or absence of AvrSr35 in N. benthamiana. Sr35 was fused to the C-terminal fragment of enhanced green fluorescent protein (eGFP), and plasma membrane (PM) was indicated by PIP2::mCherry. Images were obtained using confocal microscope and pseudo-colored with Leica software package. Scale bars, 20 μm. (I and J) Subcellular localization of WT Sr35 and the Sr35Δ20 mutant in the presence or absence of AvrSr35 observed in N. benthamiana after plasmolysis. Retracted PM is indicated by white arrows. Scale bars, 20 μm. (K and L) Representative images displaying localization of WT Sr35 and Sr35Δ20 mutant in protoplasts in the presence or absence of AvrSr35. Sr35 was fused to the C-terminal fragment of eGFP, and the PM was indicated by PIP2::mCherry. Images were obtained using confocal microscope and pseudo-colored with Leica software package. Scale bars, 20 μm.
The pentamerization of the CC domains is mainly mediated by two hydrophobic interaction interfaces. In the first interface, the aromatic group of Y141 at the C terminus of the α3′ inserts obliquely into the hydrophobic pocket made of residues V46, L47, W65, A66, V69, and L134 from the neighboring subunit (Fig. 5B). Y141 also hydrogen bonds with residue E39 from adjacent CC domain (Fig. 5D). The second oligomerization surface includes an α1 packing against α2′ and α3′ from adjacent CC domain (Fig. 5C). In support of structural observations, the mutation Sr35Y141A greatly inhibited the assembly of resistosome in gel filtration column and abolished the death-inducing activity in mesophyll cells (Fig. 5, E and F). These results indicate that the oligomerization of CC domain is essential both for Sr35 resistosome assembly and the immune function of Sr35.
Considering that the amino acid sequence of the invisible N-terminal region (amino acids 1 to 23) of Sr35CC corresponds to the N-terminal helix α1 in ZAR1 and NREQUIREMENT GENE 1.1 (NRG1.1), which is required for immune function and plasma membrane (PM) association (9, 11, 12), we investigated the impact of the N-terminal region of Sr35 in mediating cell death. As expected, the mutants Sr35Δ12 and Sr35Δ20 lacking the N-terminal residues 1 to 12 and 1 to 20 that respectively abolished the cell death activity of Sr35 in N. benthamiana confirm results from a previous study (Fig. 5E and fig. S6A) (13). Sr35Δ20 mutant had a negligible effect on the assembly of Sr35 resistosome in gel filtration column (Fig. 5, E and F). These findings indicate that the N-terminal region (residues 1 to 20) is essential for the Sr35 immune function but not for resistosome assembly.
To further investigate the relationship between the N-terminal region of Sr35 and immune activity, we observed the subcellular localization of enhanced green fluorescent protein (eGFP)–tagged WT Sr35 (Sr35::eGFP) and its mutants in N. benthamiana (Fig. 5 and fig. S7). The location of PM was indicated by the aquaporin phosphatidylinositol 4,5-bisphosphate (PIP2) fused to mCherry (PIP2::mCherry) (11, 14). Ectopically expressed Sr35::eGFP displayed colocalization with PIP2::mCherry in the presence and the absence of AvrSr35 (Fig. 5, G, I, and K). The oligomerization-deficient mutant Sr35K206A was also observed on PM and in cytoplasm in the presence and in the absence of AvrSr35. These observations were further supported by the results of plasmolysis and protoplast assays (fig. S7), indicating that Sr35 is membrane-associated, although distribution in cytoplasm cannot be ruled out. Unexpectedly, PM association was also observed for the Sr35Δ20 mutant in the presence of AvrSr35 (Fig. 5, H, J, and L), which is similar to NRG1.1 but differs from ZAR1, which, upon deletion of the first N-terminal helix, exhibits impaired membrane localization (9, 12). Together, these findings indicate that the N-terminal residues 1 to 20 of Sr35 are required for the Sr35-mediated HR, but not for PM association.
DISCUSSION
The importance of CC domain in Sr35-mediated cell death
Self-association of CC domain plays an important role in functional activation. The N termini of some activated NLRs such as MLA10 (15, 16), Sr33 (17), Sr50 (17), and RPS5 (18) undergo self-association, which is required for their immune response. The TIR domain assembly of RECOGNITION OF PERONOSPORA PARASITICA 1 (RPP1) and ROQ1 resistosomes is necessary for nicotinamide adenine dinucleotide hydrolysis (10, 19), and the oligomerization of the ZAR1 CC domain is required for PM association and formation of cation channel (11). The structures of Sr35 and ZAR1 resistosomes bear notable similarity in the central pore, suggesting that Sr35 resistosome might exhibit ZAR1-like ion channel activity. However, in ZAR1, a series of acidic residues lining the inner channel render its surface predominantly electronegative, whereas the corresponding surface in Sr35 is more neutral (fig. S6, B and C). Moreover, the narrowest point at the aperture in Sr35 (radius, 2.1 Å) is notably wider than the Ca2+ permissive channel in ZAR1 (radius, 1.7 Å) (fig. S6, D and E). Similarly, a residue equivalent to the acidic residue E11 of ZAR1, which plays a key role in calcium-permeable channel activity, might not exist in the corresponding region of Sr35 according to our experimental data (fig. S6A). This is somewhat similar to NLR-REQUIRED FOR CELL DEATH 4 (NRC4), which does not require N-terminal acidic residues for its function (15). Together, the activated Sr35 resistosome may perform an unknown channel activity to induce cell death, but direct experimental evidence for this hypothesis remains to be provided.
The conserved NOD rearrangement is required for oligomerization of NLRs
The NOD in NLRs is believed to function as an intramolecular switch, with adenosine 5′-diphosphate (ADP)– and ATP-bound forms dictating the activation of NLRs (4, 20). Previously reported structures of some NLRs have clearly shown that their NOD undergoes structural remodeling during effector-induced activation, including in ZAR1 (21) and NRC1 (22). Previous studies have demonstrated that replacing the ADP-binding residue D503 with valine makes Sr35 self-activating, consistent with that of other ADP-bound NLRs (13). The binary structure of Sr35-AvrSr35 complex in the absence of ATP shows that both Sr35LRR and AvrSr35 adopt a similar conformation to that of Sr35-AvrSr35 resistosome but the CC domain and NOD are not visible (fig. S4), indicating that Sr35 CC domain and NOD might adopt a dynamic conformation in an intermediate state. Moreover, we generated the structure of Sr35 in an inactive state using SWISS-MODEL (23) and superimposed this inactive model to the active Sr35-AvrSr35 complex structure from Sr35 resistosome based on Sr35 LRR domain, showing the extensive steric clashes between AvrSr35 and inactive Sr35NBD (fig. S8). This means that the binding of AvrSr35 triggers the deflection of NBD domain. On the basis of these observations, we propose that Sr35 might undergo a conformational remodeling in which AvrSr35 binding triggers a conformational change in NBD subdomain to promote ADP-to-ATP exchange similar to the allosteric mechanism of ZAR1.
The immune evasion mechanism of AvrSr35
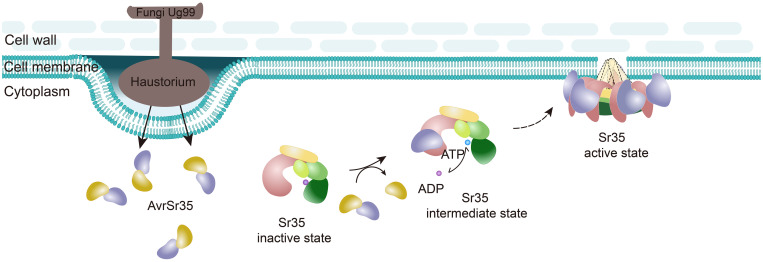
Pathogen effectors interfere with plant immunity in different ways to evade immune receptor recognition (24). The finding that AvrSr35 naturally exists as a homodimer can help us understand how AvrSr35 may conceal the recognition interface by dimerization. Nevertheless, the Sr35-AvrSr35 interaction negates the concealment, which allows activation of the immune response. This implies that the host NLRs also coevolve with corresponding Avr proteins throughout the course of the unceasing evolutionary arms race between plants and pathogens. In summary, our data support a model of the race-specific direct recognition of the Ug99 effector AvrSr35 by the wheat resistance gene Sr35 (Fig. 6), which sheds new light on CNL-mediated immune signaling.
Fig. 6. Model of AvrSr35-induced assembly of the Sr35 resistosome.
In resting state, Sr35 maintains an inactive state through autoinhibitory intramolecular interaction of various domains. Once AvrSr35 is injected by Pgt into host cells, Sr35 directly binds AvrSr35 via its LRR domain, inducing conformational changes in NOD that promote the exchange of ADP to ATP, which activates Sr35. The activated AvrSr35-bound Sr35 self-assembles into pentamer that is associated with PM, probably inserting into the cell membrane to form a channel similar to ZAR1.
MATERIALS AND METHODS
Recombinant protein expression and purification
For purification of AvrSr35, the sequence encoding predicted mature AvrSr35 protein (residues 27 to 578; the N-terminal signal peptide was deleted) was cloned into pMCSG7 vector with an N-terminal 6×His-tag and transformed into Escherichia coli Rosetta (DE3) cells. The recombinant strains were grown in Luria-Bertani broth at 37°C until the optical density at 600 nm (OD600) reached 0.8 to 1.0, after which protein overexpression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 16°C for 18 hours. The cells were harvested by centrifugation at 4000 rpm for 15 min at 4°C and resuspended in the lysis buffer [25 mM tris-HCl (pH 7.5), 150 mM NaCl, and 3 mM β-mercaptoethanol]. After cell sonication and centrifugation, the supernatant containing target protein was purified using Ni–nitrilotriacetic acid (Ni-NTA) (QIAGEN) column, followed by further purification with cation-exchange chromatography (SP FF, GE Healthcare) and gel filtration chromatography (Superdex 200, 10/300GL, GE Healthcare) in buffer containing 25 mM tris-HCl (pH 7.5), 150 mM NaCl, and 2 mM dithiothreitol (DTT).
For purification of Sr35, open reading frame encoding full-length Sr35 (residues 1 to 919) was cloned into the pFastBac1 vector with an N-terminal 6×His-SUMO (small ubiquitin-like modifier) tag and expressed in SF9 insect cells (Thermo Fisher Scientific) at 28°C. After 72 hours of recombinant baculovirus infection, the cells were harvested by centrifugation at 4000 rpm for 15 min at 4°C and resuspended in the lysis buffer [25 mM tris-HCl (pH 7.5), 1 M NaCl, and 3 mM β-mercaptoethanol]. After sonication and centrifugation, supernatant was purified using Ni-NTA (QIAGEN) column. Target protein was eluted with lysis buffer containing 200 mM imidazole, and the SUMO tag was removed using PreScission protease (1:100, w/w). Sr35 was then further purified using gel filtration (Superdex 200, 10/300GL, GE Healthcare) in buffer containing 25 mM tris-HCl (pH 7.5), 500 mM NaCl, and 2 mM DTT.
Reconstitution of the Sr35 resistosome
Purified Sr35 was mixed with AvrSr35 (residues 27 to 578) at a molar ratio of ~1:2 and 1 mM ATP and incubated at 4°C overnight. The mixture was then purified using gel filtration (Superose 6, 10/300GL, GE Healthcare). For cryo-EM experiments, the purified Sr35 resistosome was concentrated to ~3 mg/ml in buffer containing 25 mM tris-HCl (pH 7.5), 150 mM NaCl, and 2 mM DTT.
Cryo-EM sample preparation and data collection
Frozen-hydrated specimens were prepared using a Vitrobot Mark IV plunger (Thermo Fisher Scientific). Aliquots of 5-μl Sr35-AvrSr35 complex at a concentration of 0.25 mg/ml were applied to glow-discharged (30 s at 15 mA) holey carbon grids (QUANTIFOIL Au, R1.2/1.3, 300 mesh). The excess solution from the grid was blotted for 4 s at 100% humidity and 4°C before the grid was plunged into liquid ethane. We transferred the grid to the precooled box and kept it in liquid nitrogen preparing for data collection by transmission electron microscopes. Sr35 resistosome induced by AvrSr35 and AvrSr35R381A at the concentration of 0.5 mg/ml were loaded onto a freshly glow-discharged (30 s at 15 mA) holey carbon grid (QUANTIFOIL Cu R1.2/1.3), respectively. The following process of frozen sample preparation of resistosome is same as the binary complex mentioned above.
FEI Titan Krios transmission electron microscope equipped with a Gatan K2/K3 Summit direct detector and Gatan BioQuantum energy filter was used for data collection. Data of Sr35-AvrSr35 complex were collected using SerialEM at a nominal magnification of ×165,000 with a pixel size of 0.84 Å, and the images were recorded with a defocus range from −1.2 to −1.8 μm in superresolution mode with 32 frames per movie at a total dose of 50 electrons/Å2. In addition, data of Sr35 resistosome (induced by AvrSr35 and AvrSr35R381A) were collected respectively with a K3 summit electron direct detector in superresolution mode. The FEI automated imaging software EPU was used to control the 300-kV transmission electron microscope operated at nominal magnification of ×81,000 (with a calibrated pixel size of 1.095 Å per pixel) with a defocus range from −1.0 to −1.6 μm. A dose rate of 20 electrons per pixel per second was used, generating 32 movie frames with a total dose of 50 electrons/Å2. Detailed data collection is listed in table S1.
Image processing and 3D reconstruction
The 32 frames of each movie in a superresolution model were aligned, dose-weighted, and summed using MotionCor2 with a binning factor of 2 resulted in summed micrographs. Motion-corrected images were import into Relion/3.1.2 and the contrast transfer function (CTF) parameters were estimated using CTFFIND-4.1.
For Sr35-AvrSr35 complex, 3194 micrographs with a resolution within 4 Å were selected for the autopicking with a Laplacian-of-Gaussian filter. Then, 558,301 particles were subjected to three rounds of reference-free 2D classification. About 320,391 multiorientations with high-resolution particles were picked from 2D classification and subject to 3D classification into four classes (C1 symmetry). The particles extracted from 2D classification were imported to cryoSPARC for a reconstruction of the initial model and used as an initial reference model with a low-pass filter resolution of 40 Å for 3D classification. Multiple rounds of 3D classification (C1 symmetry) without angular sampling to reduce particle heterogeneity were carried out.
From 3D classification, four major classes can be discerned. Class III adopts a rigid structure with clear structural features, while classes I, II, and IV have poor structural features. The particles from optimal class III were selected for 3D refinement, converging at a resolution of 3.8 Å. The resulting dataset with 85,070 particles was used for CTF refinement and 3D refinement. The density maps from the refinement were obtained at 3.6 Å on the basis of the gold-standard Fourier shell correlation (FSC) 0.143 criterion with the high-resolution noise substitution.
For Sr35 resistosome induced by AvrSr35, about 30,000 particles selected from the initial picking with Laplacian-of-Gaussian filter were subjected to one round of reference-free 2D classification that generated template images for reference 2D classification of all particles. After two rounds of reference 2D classification, 150,000 particles were selected from well-defined particle images and used for the generation of initial 3D model. 3D classification was performed without imposing symmetry by using the initial reference model. All particles were classified into four classes, and the optimal class with intact Sr35 resistosome was further selected for the 3D classification with an imposing symmetry C5. Then, the particles were selected for CTF refinement and 3D refinement, converging at a resolution of 3.3 Å. The density maps from the refinement were obtained at 3.3 Å on the basis of the gold-standard FSC 0.143 criterion with the high-resolution noise substitution. To enhance the resolution of C terminal domain of Sr35 that interacted with Avr35, a local mask was created for the particle subtraction and was performed in further 3D classification. After three rounds of 3D classification, 3D refinements of local electron density map were carried out. The reconstruction resolutions at 3.6 Å were determined on the basis of the gold-standard FSC 0.143 criterion with the high-resolution noise substitution.
For the Sr35 resistosome induced by AvrSr35R381A dataset, a similar strategy displayed in Sr35 resistosome induced by AvrSr35 was applied. 3D refinement was performed against the final dataset containing 30,530 particles, converging at a resolution of 3.6 Å. Detailed data collection and refinement statistics are listed in table S1.
Model building and refinement
Initial model building was performed in SWISS-MODEL server using the structure of ZAR1 resistosome [Protein Data Bank (PDB) ID: 6J5T] as a template. The structure of ZAR1 resistosome was docked into the EM density of Sr35 resistosome in UCSF Chimera (25), followed by docking of five ATP molecules into the density using COOT (26). The model of five ATP-bound Sr35-AvrSr35 molecules was then refined in PHENIX (27) on the basis of the EM map in real space with secondary structure and geometric constraints. The structural model was verified by MolProbity included in the PHENIX package. Table S1 summarizes the model statistics.
The model of binary structure of Sr35 and AvrSr35 and the model of Sr35 resistosome induced by AvrSr35R381A were using the structure of Sr35 resistosome served as an initial model. We docked the Sr35 resistosome model in the map using Chimera (25) and modified it in COOT (26) to properly fit the density. Table S1 summarizes the model statistics.
Crystallization, data collection, and determination of the AvrSr35 structure
Crystallization and optimization screens were performed with commercial and homemade sparse-matrix screens using the hanging drop vapor diffusion method at 16°C. Best AvrSr35 crystals were grown in 0.1 M sodium malonate (pH 6.0) and 12% (v/v) polyethylene glycol 3350 at the protein concentration of 12 mg/ml. Harvested crystals were cryoprotected in 20% (v/v) ethylene glycol and flash-frozen in liquid nitrogen.
All x-ray diffraction data were collected at the beamline BL-19 U1 of the Shanghai Synchrotron Radiation Facility using a charge-coupled device detector. The diffraction images were processed using HKL2000 (28). Phasing and model building of SeMet-labeled AvrSr35 were performed with the single-wavelength anomalous diffraction method with program AutoSol in PHENIX (27). All structural figures of were generated in PyMol (http://pymol.org). Data collection and refinement statistics are listed in table S2.
Plant materials and constructs
Infection assays in this study were performed using N. benthamiana. All N. benthamiana plants were grown for 4 to 5 weeks at 25°C and 70% relative humidity in 14-hour light period/10-hour dark period cycles.
WT Sr35 and AvrSr35 sequences amplified by polymerase chain reaction (PCR) were inserted into Gateway pDONR207 vector using Gateway BP Clonase II enzyme mix (Thermo Fisher Scientific). Single or, if necessary, multiple rounds of PCR-based site-directed mutagenesis were performed with the WT pDONR207 construct as template to prepare single-point and multiple mutant constructs. WT Sr35 and AvrSr35 and their mutant variants were transferred to the pK7FWG2 (eGFP–C-terminal) and pK7GWF2 (eGFP–N-terminal) binary vectors using Gateway LR Clonase II enzyme mix (Thermo Fisher Scientific).
Transient expression in N. benthamiana
Plant transformation was performed with Agrobacterium tumefaciens GV3101. Transformed GV3101 was cultured overnight at 28°C in yeast extract peptone (YEP) broth containing rifampin (50 μg/ml) and spectinomycin (50 μg/ml) until the logarithmic growth phase (OD600 = 0.5 to 0.6). Bacteria were then collected by centrifugation at 4000 rpm for 10 min resuspended in the infiltration solution [10 mM MgCl2, 10 mM MES, and 150 μM acetosyringone (pH 5.6)] at the concentration of OD600 = 1.0. For Sr35-AvrSr35 coinfiltration experiments, A. tumefaciens cultures were adjusted to the OD600 value of 1.0 and mixed at 1:1 ratio. Agrobacteria mixtures were infiltrated into young leaves of 4 to 6 weeks old N. benthamiana WT plants using a 1-ml needleless syringe. Leaf appearance was observed at 36 to 48 hours postinfection (hpi). To inhibit cell death, 2 mM LaCl3 in distilled water was infiltrated into leaves at 26 hours after agroinfiltration.
Transient expression in Arabidopsis protoplasts
Arabidopsis protoplasts and transfections were performed as described (29). Transfected protoplasts were incubated at room temperature under weak light (1.5 μmol·m−2·s−1) for 12 to 16 hours for expressing target proteins.
Sr35-AvrSr35 pull-down assays
For analysis of interactions between WT or mutated Sr35 and AvrSr35, sequence encoding AvrSr35 (residues 27 to 578) was cloned into PGEX-6p-1 vector and expressed in E.coli Rosetta (DE3) at 16°C. After overexpression with 1 mM IPTG for 18 hours, the cells were collected and lysed by ultrasonication. Supernatant from the cell lysate was purified using Glutathione Sepharose 4B beads (QIAGEN). After washing beads with 10 column volumes of wash buffer [25 mM tris-HCl (pH 7.5), 150 mM NaCl, and 3 mM β-mercaptoethanol], the beads were eluted with elution buffer [25 mM tris-HCl (pH 7.5), 150 mM NaCl, 3 mM β-mercaptoethanol, and 15 mM reduced glutathione]. Purified target proteins were incubated with AvrSr35 at a molar ratio of ~1:2 at 4°C for 2 hours, followed by loading of the protein mixture onto GST beads. Elution was performed using the elution buffer after beads were washed with 10 column volumes of the wash buffer. Eluted fractions were stained by Coomassie brilliant blue staining and resolved with SDS–polyacrylamide gel electrophoresis (PAGE).
For analysis of interactions between WT or mutated AvrSr35 and Sr35, purified Sr35 was incubated with 6×His-tagged AvrSr35 and then purified using Ni-NTA beads. After washing beads with 10 column volumes of wash buffer [25 mM tris-HCl (pH 7.5), 150 mM NaCl, 3 mM β-mercaptoethanol, 20 mM imidazole, and 0.2% Triton X-100], the beads were eluted with elution buffer [25 mM tris-HCl (pH 7.5), 150 mM NaCl, 3 mM β-mercaptoethanol, 0.2% Triton X-100, and 300 mM imidazole]. Eluted fractions were stained by Coomassie brilliant blue and analyzed with SDS-PAGE.
Confocal microscopy
We used an agroinfiltration approach to transiently expressing proteins in N. benthamiana for confocal imaging. Leaves were imaged for protein location at 36 to 48 hpi with the confocal laser microscope (Leica Microsystems SP8, Germany). eGFP was detected using a 488-nm laser collecting emission between 495 and 545 nm; mCherry was excited using a 552-nm laser with an emission spectrum of 580 to 630 nm.
Analytical ultracentrifugation
Sedimentation velocity experiments were used to measure the molecular sizes of WT AvrSr35 and its mutants. The experiments were performed in Beckman XL-A analytical ultracentrifuge equipped with absorbance optics and an An-60 Ti rotor (Beckman Coulter Inc., Fullerton, CA). The samples were diluted to an optical density of 1 at 280 nm (OD280) in a 1.2-cm optical path. The rotor speed for analysis of all samples was set to 35,000g. Differential sedimentation coefficient c(s), frictional coefficients, and molecular mass were calculated using the SEDFIT software (30).
Microscale thermophoresis
MST is a biophysical technique that measures the strength of the interaction between two molecules by detecting changes in the fluorescence signal caused by temperature changes induced by an infrared laser. For protein labeling, we adjusted the concentration of Sr35 to 2 μM using the labeling buffer and mixed the Sr35 with nano blue dye in a 1:1 volume ratio to the final volume of 200 μl. We incubated the mixture for 30 min at room temperature in the dark and then loaded it to column B. We collected the eluate in 100- to 150-μl fraction termed as labeled Sr35. Labeled Sr35 was mixed in equal volumes with different concentration gradients of protein AvrSr35 and tested on CMI Monolith NT.115pico instrument with an excitation wavelength of 460 to 490 nm. Simple analysis and regular fitting were done using the MO.Affinity Analysis software (31).
Acknowledgments
We thank Y. Miao and W. Ma at the College of Life Sciences, Fujian Agriculture and Forestry University for providing the vectors pDonr207, pK7FWG2, and pK7WGF2 and the seeds of N. benthamiana. We thank Y.-H. Chen at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for exploring the channel function of activated Sr35. We thank H. Cui at Plant Immunity Center, Fujian Agriculture and Forestry University for guidance on protoplast experiments. We thank the Cryo-EM Center of Southern University of Science and Technology for providing server used for our data analysis work. We thank the beamline BL19-U1 of Shanghai Synchrotron Radiation Facility (SSRF) for the x-ray diffraction data collection. We thank our colleague V. Perčulija for scientific and language editing of the article.
Funding: This work was supported by the National Key Research and Development Program of China (2021YFC2301403), National Natural Science Foundation of China grants (82225028, 82172287), Chinese Academy of Sciences (SKF2020NO1), and China Postdoctoral Science Foundation (2020 M682067).
Author contributions: Design and supervision: S.O. Protein expression and purification: Y.-B.Z., T.-T.C., Y.-Z.L., and L.-R.T. AvrSr35 crystallization and structure determination: Z.-K.L. Data collection and refinement of Sr35 resistosome cryo-EM structure: P.W., M.-X.L., X.M., and Z.-K.L. Fluorescence colocalization: Y.-B.Z. and T.-T.C. N. benthamiana assays: Y.-B.Z. and S.-R.Z. Protoplast assays: Z.Z., L.C., and Q.C. Writing the manuscript with input from all authors: Y.-B.Z., M.-X.L., and T.-T.C.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The EM density maps of Sr35 resistosome and Sr35 resistosome induced by AvrSr35R381A generated in this study have been deposited in the EMDB under accession codes EMD-33153 and EMD-33498, respectively. The atomic coordinate has been deposited in the PDB under the accession codes 7XE0 for Sr35 resistosome and 7XX2 for Sr35 resistosome induced by AvrSr35R381A. The EM density maps of binary complex of Sr35 and AvrSr35 have been deposited in the EMDB under accession codes EMD-33486. The atomic coordinate has been deposited in the PDB under the accession codes 7XVG. The atomic coordinate of AvrSr35 x-ray structure has been deposited in the PDB under accession codes 7XDS. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S8
Tables S1 to S8
REFERENCES AND NOTES
- 1.Jones J. D., Dangl J. L., The plant immune system. Nature 444, 323–329 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Maruta N., Burdett H., Lim B. Y. J., Hu X., Desa S., Manik M. K., Kobe B., Structural basis of NLR activation and innate immune signalling in plants. Immunogenetics 74, 5–26 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burdett H., Kobe B., Anderson P. A., Animal NLRs continue to inform plant NLR structure and function. Arch. Biochem. Biophys. 670, 58–68 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Maekawa T., Kufer T. A., Schulze-Lefert P., NLR functions in plant and animal immune systems: So far and yet so close. Nat. Immunol. 12, 817–826 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Kourelis J., van der Hoorn R. A. L., Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 30, 285–299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pretorius Z. A., Singh R. P., Wagoire W. W., Payne T. S., Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis. f. sp. tritici in Uganda. Plant Dis. 84, 203 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Salcedo A., Rutter W., Wang S., Akhunova A., Bolus S., Chao S., Anderson N., De Soto M. F., Rouse M., Szabo L., Bowden R. L., Dubcovsky J., Akhunov E., Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science 358, 1604–1606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saintenac C., Zhang W., Salcedo A., Rouse M. N., Trick H. N., Akhunov E., Dubcovsky J., Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 341, 783–786 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Hu M., Wang J., Qi J., Han Z., Wang G., Qi Y., Wang H.-W., Zhou J.-M., Chai J., Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364, eaav5870 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Ma S., Lapin D., Liu L., Sun Y., Song W., Zhang X., Logemann E., Yu D., Wang J., Jirschitzka J., Han Z., Schulze-Lefert P., Parker J. E., Chai J., Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 370, eabe3069 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Bi G., Su M., Li N., Liang Y., Dang S., Xu J., Hu M., Wang J., Zou M., Deng Y., Li Q., Huang S., Li J., Chai J., He K., Chen Y.-H., Zhou J.-M., The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell 184, 3528–3541.e12 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Jacob P., Kim N. H., Wu F., El-Kasmi F., Chi Y., Walton W. G., Furzer O. J., Lietzan A. D., Sunil S., Kempthorn K., Redinbo M. R., Pei Z.-M., Wan L., Dangl J. L., Plant “helper” immune receptors are Ca2+-permeable nonselective cation channels. Science 373, 420–425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolus S., Akhunov E., Coaker G., Dubcovsky J., Dissection of cell death induction by wheat stem rust resistance protein Sr35 and its matching effector AvrSr35. Mol. Plant Microbe Interact. 33, 308–319 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinière A., Lavagi I., Nageswaran G., Rolfe D. J., Maneta-Peyret L., Luu D.-T., Botchway S. W., Webb S. E. D., Mongrand S., Maurel C., Martin-Fernandez M. L., Kleine-Vehn J., Friml J., Moreau P., Runions J., Cell wall constrains lateral diffusion of plant plasma-membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 109, 12805–12810 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adachi H., Contreras M. P., Harant A., Wu C.-h., Derevnina L., Sakai T., Duggan C., Moratto E., Bozkurt T. O., Maqbool A., Win J., Kamoun S., An N-terminal motif in NLR immune receptors is functionally conserved across distantly related plant species. eLife 8, e49956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maekawa T., Cheng W., Spiridon L. N., Töller A., Lukasik E., Saijo Y., Liu P., Shen Q.-H., Micluta M. A., Somssich I. E., Takken F. L. W., Petrescu A.-J., Chai J., Schulze-Lefert P., Coiled-coil domain-dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe 9, 187–199 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Cesari S., Moore J., Chen C., Webb D., Periyannan S., Mago R., Bernoux M., Lagudah E. S., Dodds P. N., Cytosolic activation of cell death and stem rust resistance by cereal MLA-family CC–NLR proteins. Proc. Natl. Acad. Sci. U.S.A. 113, 10204–10209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ade J., DeYoung Brody J., Golstein C., Innes R. W., Indirect activation of a plant nucleotide binding site–Leucine-rich repeat protein by a bacterial protease. Proc. Natl. Acad. Sci. U.S.A. 104, 2531–2536 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin R., Qi T., Zhang H., Liu F., King M., Toth C., Nogales E., Staskawicz B. J., Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Science 370, eabd9993 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leipe D. D., Koonin E. V., Aravind L., STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: Multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J. Mol. Biol. 343, 1–28 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Wang J., Hu M., Wu S., Qi J., Wang G., Han Z., Qi Y., Gao N., Wang H.-W., Zhou J.-M., Chai J., Ligand-triggered allosteric ADP release primes a plant NLR complex. Science 364, eaav5868 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Steele J. F. C., Hughes R. K., Banfield M. J., Structural and biochemical studies of an NB-ARC domain from a plant NLR immune receptor. PLOS ONE 14, e0221226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F. T., de Beer T. A. P., Rempfer C., Bordoli L., Lepore R., Schwede T., SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes Fischer N., Naseer N., Shin S., Brodsky I. E., Effector-triggered immunity and pathogen sensing in metazoans. Nat. Microbiol. 5, 14–26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E., UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L.-W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., Zwart P. H., PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otwinowski Z., Minor W., Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Yoo S. D., Cho Y. H., Sheen J., Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Schuck P., Gillis R. B., Besong T. M. D., Almutairi F., Adams G. G., Roweb A. J., Harding S. E., SEDFIT–MSTAR: Molecular weight and molecular weight distribution analysis of polymers by sedimentation equilibrium in the ultracentrifuge. Analyst 139, 79–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhont J. K. G., Wiegand S., Duhr S., Braun D., Thermodiffusion of charged colloids: Single-particle diffusion. Langmuir 23, 1674–1683 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S8
Tables S1 to S8